Abstract
Extracellular vesicles (EVs) are a group of secretory vesicles with cell-derived membrane and contents. Due to the cargo delivery capability, EVs can be designed as drug delivery platforms for cancer therapy. Biocompatibility and immune compatibility endow EVs with unique advantages compared with other nanocarriers. With the development of this field, multiple ingenious modification methods have been developed to obtain engineered EVs with desired performance. Application of engineered EVs in cancer therapy has gradually shifted from monotherapy to combinational therapy to fight against heterogeneous cancer cells and complex tumor microenvironment. In addition, the strong plasticity and load capacity of engineered EV make it potential to achieve various combinations of cancer treatment methods. In this review, we summarize the existing schemes of cancer combination therapy realized by engineered EVs, highlight the mechanisms and representative examples of these schemes and provide guidance for the future application of engineered EVs to design more effective cancer combination treatment plans.
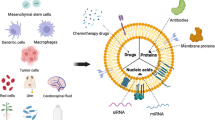
Graphical Abstract

Similar content being viewed by others
Background
Extracellular vesicles (EVs) with cell-like structures originate from various cells [1]. It has been proved that they play essential roles in cell-to-cell communication under different physiological and pathological conditions [2, 3]. Based on the vesicle structure and the mechanism of intercellular communication, EVs are developed as drug delivery vectors and therapeutic agents for cancer therapy [4,5,6]. Compared with other nano-drug delivery platforms, natural EVs have better biocompatibility, prolonged blood circulation life span, lower immunogenicity and systemic toxicity. Moreover, features from parent cells endow EVs with unique targeting capability to their source cells [5, 7, 8]. However, natural EVs also have some unsatisfactory performance in cancer therapy. Up to now, no standardized protocol for isolation methods has been established. Also, large-scale manufacturing production and purification of EVs is still complicated [4]. The targeting ability of natural EVs would be weakened as trapped in some major organs such as liver or spleen [9, 10]. The anti-cancer immune response caused by natural EVs derived from tumor cells or immune cells is not strong enough [11, 12]. Low drug delivery efficiency, insufficient infiltration and penetration of natural EVs limit their anti-cancer efficiency [13]. To overcome the above-mentioned challenges of natural EVs, increasing studies are focusing on engineering EVs to obtain ideal anti-cancer effects. By various engineering methods such as loading functional cargoes and membrane modification, engineered EVs will show better performance and become more suitable in cancer therapy.
Combination therapy is a potential strategy to improve the therapeutic effect of cancer. Up to now, cancer is still a considerable threat to human health. Monotherapy is hard to fight against all kinds of carcinogenic factors in the complex tumor and tumor microenvironment (TME) [14]. Tumor heterogeneity determines the necessity of combination therapy. Recently, the research trend of cancer treatment has gradually removed from monotherapy to combination therapy. Combining two or more therapeutic approaches is expected to maximize the therapeutic efficacy of each method and minimize their disadvantages and side effects. For example, chemotherapy-involved combination therapy combats the multi-drug resistance (MDR) and endows chemotherapy drugs with higher performance in cancer treatments [15]. In addition, combination therapy with immune checkpoint blockade (ICB) can overcome the resistance and broaden the clinical application of ICB [16]. More and more clinical trials have shown that combinational therapy is more beneficial to patients than single therapy [17, 18]. Applying EVs in cancer treatment has been studied for decades. The researches range from the carrier property of natural EVs to the current use of engineered EVs that carry various anti-cancer substances and produce cancer vaccines. Recently, increasing studies are attempting to use engineered EVs to achieve cancer combination therapy. Engineered EVs with biocompatibility and targeting ability can be used for the targeted co-delivery of two and more anticancer materials. Their lipid bilayer membrane can also be modified to conduct combinational therapy. Based on these unique characteristics, engineered EVs have great potentials to be promising platforms for cancer combination therapy.
In this review, we begin by introducing the cell biology of EVs and the strategies of engineering EVs. Next, we summarize the existing schemes of cancer combination therapy based on engineered EVs (Fig. 1). We focus on understanding how these engineered EV-based combination schemes achieve better therapeutic effects in cancer, which can provide guidelines and references for further use of engineered EVs in cancer combination therapy and be helpful to deeply understand the future development of engineered EVs in cancer treatment.
Existing schemes of cancer combination therapy based on engineered EVs. Chemotherapy-related combination, immunotherapy-related combination, and visual therapy are three main categories. Other schemes, such as the combination of new and traditional therapy, combination of three or more drugs, are increasingly mentioned in recent studies. PDT photodynamic therapy; PTT photothermal therapy; ICI immune checkpoint inhibitors; ICB immune checkpoint blockade
EV biology
According to sizes and origins, EVs can be classified into exosomes, microvesicles (i.e., microparticles), and apoptotic bodies. Exosome, whose size is from 40 to 200 nm, is generated when multivesicular bodies and plasma membranes fuse [19]. Microvesicles are particles ranging from 100–200 nm to 1 μm, generaing from membrane budding [20]. Apoptotic body is from the dying cell and has a diameter of 800 nm to 5 μm. Currently, apoptotic body is rarely used in cancer therapy due to its unsatisfactory size, so the knowledge of apoptotic bodies is not covered in this review.
Exosomes and microvesicles have different biogenetic mechanisms (Fig. 2). Exosomes are formed as intraluminal vesicles (ILVs) in the endosome circulation [21]. Vesicles derived from the endocytosis pathway or trans-Golgi network (TGN) form the early endosomes. During the following evolution process, early endosomes will develop into the multivesicular endosomes (MVEs) with part of endosomal membrane invagination into the lumen to form ILVs. Then some MVEs will fuse with the cell plasma membrane and release the ILVs into the extracellular environment in the form of exosomes [19, 22, 23]. As for microvesicles, understanding of their molecular mechanisms of biogenesis is still limited. It is known that microvesicles originate by direct budding at the plasma membrane [19, 23]. After biogenesis and release, EVs can enter the circulation and follow the blood flow to various positions in the body, completing the cargo delivering and intracellular communication mission. Due to the liposolubility, an essential feature of EVs is crossing a physical barrier such as the blood–brain barrier (BBB) [24]. It is one of Evs’ advantages as drug delivery system compared to other carriers [8]. Moreover, EVs are vesicles derived from autologous cells and regarded as “self” by the immune system, so they have good biocompatibility. Except for these features, EVs have also shown the capability for targeting recipient cells, which is closely related to the protein profile on EV surfaces. The targeting property is thought to be achieved by specific recognition and binding between the proteins at the EV surface and the ligands at the plasma membrane of the recipient cells [19]. Notably, the recipient cell can also be parent cell because the protein profile of EVs is similar to the producing cells, which is the reason to use tumor-derived EVs for cancer targeting therapy [12]. After EVs dock on the surface of the target cells, one possible outcome is that EVs activate the signaling pathway of recipient cells to generate corresponding responses through the binding of receptor and ligand. The other is that EVs release their cargoes into recipient cells through endocytosis or direct membrane fusion [19].
Brief biology of exosomes and microvesicles. Exosomes are formed in the endosome circulation as intraluminal vesicles (ILVs), which are derived from the multivesicular endosomes (MVEs). Microvesicles originate by a direct budding at the plasma membrane. After biogenesis, some exosomes and microvesicles will target the recipient cells. Through the binding of receptor and ligand, endocytosis pathway, or direct membrane fusion, exosomes and microvesicles will complete the cargo delivering and intracellular communication mission
In conclusion, the EVs biology is deeply understood, making it possible to use engineered EVs for cancer therapy. Further exploration of the unknown points will provide more theoretical evcidence for developing better design and engineering of EVs for therapeutic applications.
Strategies of EV engineering
To broaden the application of EVs, various engineering strategies have been developed to improve EVs performance in cancer therapy [25] (Fig. 3). There is no best strategy and standard method to modify EVs. Each strategy has its own advantages and usage. Of note, the way of engineering EV can be based on diverse strategies. Combination therapy for cancer usually requires the simultaneous application of multiple engineering strategies.
Strategies of EV engineering. EV modification (right) is a direct method to obtain engineered EVs, including passive loading cargo, EV membrane modification, hybridization, and biomimetic EV production. Producing cell modification (left) is the indirect method to get engineered EVs through engineering parent cells by active loading cargo or genetic manipulation
EV modification (direct method)
Passive loading cargo
Due to EVs’ poor active uptake ability, the loading quantity of simply mixing drug with EVs is usually unsatisfactory. Various passive loading methods have been developed to improve the cargo loading efficiency, such as electroporation [26], freeze and thaw cycles [27], sonication [28], and extrusion [29]. The principle of these physical methods is to increase the gap of the EV membrane or apply external force to make cargo enter the EVs. The point worthy to notice is that these passive methods may undermine EV membrane and affect EV integrity.
Modified EV membrane
The EV membrane structure is composed of lipids and proteins, which can be linked to some substances through chemical reactions to reduce clearance and enhance tumor-targeting capability. Click chemistry that utilizes copper-catalyzed azide-alkyne cyclo-addition reaction to attach specific molecules to EV membrane is one of the representatives [30,31,32]. Interlinkage between sulfhydryl and maleimide can also be employed to engineer EV membrane [33,34,35]. In these cases, chemical bonds contribute to the strong connection between target substances and EV membrane.
Hybridization
Hybrid vesicles can be designed by fusing EVs with other lipid nanovesicles like liposomes [36, 37]. The similar lipid membrane structure is beneficial to their fusion and the hybrid vesicles not only remain the characteristics of EVs but also obtain the advantages of other lipid nanovesicles.
Biomimetic EV production
Using individual biomimetic molecules such as EV membrane lipids and proteins to develop EV-based mimetics is feasible to produce better drug delivery platforms [38]. Encapsulating inorganic or organic nanoparticles with active EV membrane is a typical example of biomimetic EVs [39,40,41]. These mimetics have structure similar to natural EVs, which means they can perform excellent pharmacokinetics and biocompatibility.
Producing cell modification (indirect method)
Active loading cargo
Majority of cells are able to perform active uptake of drugs in extracellular space. To obtain the EVs loaded with ideal cargo, co-incubation with producing cells is one of commonly used approaches. Materials with smaller sizes are more suitable for this strategy. Adjusting the conditions of co-incubation could influence the cargo loading efficiency and EV generation [42].
Genetic manipulation
Genetic manipulation through transfection tools like viral vectors to deliver cargo into producing cells is an effective approach for engineering parental cells, especially for loading nucleic acid [43, 44]. Donor cells that up-regulate nucleic acid expression can generate the target EVs with abundant nucleic acid. Future advances in genetic manipulation will further improve transfection efficiency to obtain ideal engineered EVs.
Simultaneous modification of producing cell and EV
In some cases, target EVs for cancer combinational therapy requires multiple modifications to obtain more functions. Just engineering EVs or producing cells alone is hard to meet the design requirements. Combining both modified donor cells and EVs properly is regarded as a potential choice. For instance, Lv et al. prepared genetically engineered exosomes by transfecting parent cells. Then the exosomes were fused with thermosensitive liposomes and formed the hybrid nanoparticles. This simultaneous modification scheme successfully produced the target engineered EVs for cancer chemoimmunotherapy [45].
Engineered EVs for chemotherapy-related combination therapy
Chemotherapy is a traditional method routinely used in cancer therapy for a long time. The outcomes of mono-chemotherapy are usually far from satisfactory in malignant tumors, so combinational strategies have been applied to obtain a higher performance [15]. Our previous research had used the microparticles derived from autologous tumors to achieve targeted chemotherapy in 2019 [46]. With the development of EVs, more and more studies focus on how to improve tumor-killing efficiency and reduce adverse reactions of chemotherapy through chemotherapy combinations with engineered EVs (Table 1).
Engineered EVs for the combination of chemotherapy with anti-drug resistance treatment
MDR is the primary issue accounting for the failure of chemotherapy [47]. The biological background of MDR is very complicated, which contains inherent or acquired mechanisms of cancer cells [48]. Extensive studies have developed various strategies to overcome these MDR mechanisms, such as the usage of multidrug resistance inhibitors, RNA interference therapy and new anticancer drugs that can evade efflux reaction [49]. Engineered EVs as delivery platforms can realize co-delivery of these anti-MDR substances and chemotherapeutic drugs to suppress MDR and improve the chemotherapeutic effects.
One of the most essential mechanisms of MDR is the overexpression of efflux transporters in tumor cell membrane, and P-glycoprotein (P-gp) is a prominent example of such drug efflux pumps [50]. Therefore, the combined delivery of P-gp siRNA and chemotherapeutic agents by engineered EVs is anticipated to enable the effective inhibition of drug-resistant tumors (Fig. 4). Wang et al. employed the alternative strategy of breaking and self-assembly to acquire the engineered mimic vesicles that replace the natural EVs. They achievied P-gp silencing combined chemotherapy strategy by loading P-gp siRNA and doxorubicin (DOX) into these mimic vesicles. The results demonstrated that these engineered drug carriers were able to overcome drug resistance and synergistically kill MDR tumors through P-gp silencing and DOX-induced growth inhibition [51]. Besides RNAi therapy, researchers found that tumor cell-derived EVs can directly down-regulate P-gp expression [52, 53]. Although the mechanism is not clear, this finding indicates that engineering tumor cell-derived EVs has the potential to overcome MDR and improve the chemotherapeutic effects. To realize that, Yong et al. developed biocompatible tumor exosome-based porous silicon nanoparticles loaded with DOX (DOX@E-PSiNPs) and showed DOX@E-PSiNOs could reduce the expression of P-gp and enhance cytotoxicity of DOX to cancer stem cells [54]. Liu et al. also used the functional EVs engineered with lipid-grafted hyaluronic acid (DOX@lipHA-hEVs) to reverse cancer drug resistance effectively [55]. All these studies showed that engineered EVs are potent tools to inhibit P-gp expression and enhance chemotherapeutic effect.
Engineered EVs for the combination of chemotherapy with anti-drug resistance treatment. Mono-chemotherapy often induces multi-drug resistance (MDR) in cancer cells. Engineered EVs as delivery platforms can realize co-delivery of anti-MDR substances like P-glycoprotein (P-gp) siRNA and chemotherapeutic drugs to suppress MDR and improve the chemotherapeutic effects
Dysregulated microRNAs (miRNAs) expression is another crucial pathological mechanism that contributes to the drug-resistance of cancer cells [56,57,58,59]. Transportation of antisense inhibitors against such miRNAs is a potential strategy for anti-drug resistance treatment. As mentioned above, EVs are promising platforms for RNA transportation. Therefore, researchers develop engineered EVs to deliver anti-miRNA and chemotherapeutic drugs to improve the effect of chemotherapy. For example, Liang et al. exploited engineered exosomes loaded with 5-Fluorouracil (5-FU) and miRNA-21inhibitor (miR-21i) via electroporation, successfully overcoming drug resistance in colon cancer cells and enhancing the cytotoxicity of 5-FU [60]. Wang et al. showed that exosomes carrying anti-miR214 could effectively reverse chemoresistance to cisplatin in gastric cancer [61]. Rajendran et al. demonstrated that anti-miR-21 delivery mediated by tumor cell-derived EVs could attenuate DOX resistance in breast cancer cells with a three-fold higher cell kill efficiency than in cells treated with DOX alone [62]. These prominent results suggest that delivery of anti-miRNA and chemotherapeutics based on engineered EVs is feasible to reverse drug resistance and improve chemotherapy performance.
Engineered EVs for combinational chemo-photothermal therapy
Photothermal therapy (PTT) is an emerging method that applies photothermal agents (PTAs) to convert optical energy into heat to kill cancer cells [63]. As the temperature rises, most chemotherapy drugs will become more effective. Hyperthermic intraperitoneal chemotherapy is one of the most typical examples [64]. In addition, appropriate temperature rise can boost the susceptibility of cancer cells to chemotherapy and reduce their drug resistance [65, 66]. Therefore, combining chemotherapy and PTT can improve the synergistic efficacy of comprehensive therapy and reduce the dose of chemotherapeutic drugs to minimize the side effect.
As novel vehicles, engineered EVs can co-delivery chemotherapeutic drugs and PTAs (Fig. 5). The targeting capability and enhanced internalization of engineered EVs will further decrease the dose of chemotherapeutic drugs without limiting efficacy, avoid the distribution of PTA to normal tissues and cause fewer adverse effects. Engineered EVs significantly improve the feasibility and safety of combinational chemo-photothermal therapy. Zhang et al. had achieved this combinational chemo-photothermal strategy through generating an EVs-based self-grown gold nanostructure encapsulated with DOX (EVdox@AuNP). They proved that the popcorn-like EVdox@AuNP was capable of completing photothermal transduction and release DOX for chemotherapy, enhancing antitumor efficacy with tumor inhibitory rate up to 98.6% and reducing side effects [67]. Wang et al. also developed an engineered cell-derived microparticle for synergistic photothermal/lose-dose chemotherapy. To get higher load efficiency without disrupting the membrane integrity of EVs, they proposed an alternative approach that the parent cells were firstly engineered with transfer PTT agent Bi2Se3 and DOX, then irradiated with UV light to produce the ideal microparticles (Bi2Se3/DOX@MPs). Their results indicated that injection of Bi2Se3/DOX@MPs into tumor-bearing mice resulted in remarkably synergistic antitumor efficacy under 808 nm laser irradiation by combining PTT with low-dose chemotherapy [68].
Engineered EVs for combinational chemo-photothermal therapy. Engineered EVs can co-delivery chemotherapeutic drugs and photothermal agents (PTAs) to tumor sites. Under near-infrared (NIR) irradiation, PTAs convert optical energy into heat for photothermal therapy (PTT). The elevated temperature can induce the collapse of the structure of engineered EVs and the controllable release of chemotherapeutic drugs to realize chemotherapy. Chemotherapy combined with PTT induces cancer cell death
Highly controlled release is another outstanding advantage of applying engineered EVs for combinational chemo-photothermal therapy. In normal conditions, chemotherapeutic drugs and photothermal materials are packaged in the membrane of engineered EVs. After the target area is irradiated, the heat generated by the PTT will increase the local temperature rapidly. The elevated temperature can induce the collapse of the structure of engineered EVs and the controllable release of therapeutic agents to realize precise cancer therapy. Zhu et al. utilized the electroporation technique to establish such a biocompatible delivery system. They prepared the microvesicles (MVs) loaded with DOX and photosensitizer indocyanine green (ICG). They showed that the ambient temperature increased by external near-infrared (NIR) laser irradiation induced the crack of MVs and the controllable release of DOX and ICG. In this case, tumor cells would be killed by the synergistic effect of chemotherapy and PTT [69]. Tian et al. also developed a controllable delivery system for co-loading DOX and ICG. In their research, tumor-derived exosomes were mixed with mesoporous silica nanoparticles (MSNs) loaded with DOX and ICG then exosome-camouflaged MSNs (ID@E-MSNs) was constructed through extrusion. Likewise, their outcomes demonstrated that ID@E-MSNs could produce hyperthermia to collapse nano-vehicles and accelerate drug release, achieving effective chemo-photothermal therapy under 808 nm NIR irradiation [70]. In short, all the researches above indicate that engineered EVs as delivery platforms perform strong load capacity and good biocompatibility, which means they have great potential in combinational chemo-photothermal therapy.
Chemotherapy combined with gene therapy based on engineered EVs
Gene therapy is a promising approach for delivering therapeutic nucleic acids into cells to correct or modify genetic information [71]. Genetic mutation is the primary cause of cancer. Some mutated genes have been proved to be able to affect cancer therapeutic effect. Using gene therapy to target these mutated genes at the genetic level can fundamentally improve the effectiveness of cancer treatment, including chemotherapy. As an ideal nano-delivery platform, engineered EVs are capable of co-delivering of therapeutic nucleic acids and chemotherapeutic drugs. Moreover, the targeted transport by engineered EVs can reduce chemotoxicity and genetic contamination to other normal cells while achieving cancer combination therapy.
The clustered regularly interspersed short palindromic repeats (CRISPR) and CRISPR-associated proteins 9 (Cas9) system is a powerful tool for genomic editing [72]. Kim et al. explored the cancer-derived exosome as an intracellular delivery system for CRISPR/Cas9. They used the CRISPR/Cas9-loaded exosomes to suppress the expression of poly (ADP-ribose) polymerase-1 (PARP-1), which was closely related to the repair of chemotherapy-induced DNA damage in cancer cells. They showed that the inhibition of PARP-1 by this engineered delivery system with genome editing tool enabled synergistic cytotoxicity effect on ovarian cancer cells when combined with platinum-based chemotherapy [73].
Oncogene B-cell lymphoma-2 (Bcl-2) is involved in the control of cell anti-apoptotic defense mechanism to resist anti-cancer therapy [74]. To inhibit Bcl-2 expression and potentiate chemotherapy, Zhu et al. established the folate-engineered microvesicles for packaging Bcl-2 siRNA and paclitaxel (BFMVs@Bcl-2 siRNA@Paclitaxel). Compared with single model therapy, this MV-based drug delivery system performed high tumor-targeting capability, down-regulated Bcl-2 expression level, and significantly improved the synergistic antitumor efficacy of chemotherapy and gene therapy [75].
Engineered EVs for the combinational delivery of nanoparticles and chemotherapeutic drugs
Nanoparticles (NPs) are widely used in drug delivery due to their unique physiological properties [76]. Smaller size and larger specific surface area provide NPs with high efficient loading capacity [77]. In the TME, NPs can prevent the degradation of drugs from the acidic and proteolytic environment [78]. More importantly, NPs show increased accumulation in the tumor site through the enhanced permeability and retention (EPR) effect [77, 79, 80]. Therefore, combining nanoparticles with cancer chemotherapy is an ideal strategy in theory. However, some obstacles limit the application of NPs in chemotherapy, including insufficient targeting property, potential toxicity, and high clearance in body. Engineered EVs may be a possible solution to these limitations of NPs. Studies have developed a strategy that modifies NPs by using EV membrane to assist NPs in evading immune clearance and targeting tumor cells. Such engineered biomimetic EVs can realize high loading efficiency, low toxicity, long circulation time, effective targeting, and enhanced accumulation for better chemotherapy.
Many studies have made breakthrough progress in this area. For example, Wang et al. packaged the reactive oxygen species (ROS)-responsive thioether-linked paclitaxel-linoleic acid conjugates (PTX-S-LA) and cucurbitacin B (CuB) into nano-sized polymeric micelles and further used exosome membrane to decorate these nanoparticles to obtain a sequential-bioactivating prodrug nanoplatform (EMPCs) [81]. They proved that EMPCs exhibited amplified prodrug bioactivation, prolonged blood circulation, selective targeting of tumor cells, and enhanced tumor penetration. Pan et al. also developed a biomimetic nano-vector Exo-poly (isobutylene-alt-maleic anhydride) /Fe3O4-human serum albumin (HSA)@DOX with urinary exosome membrane. It could modify Fe3O4 nanoparticle, DOX, and HSA as a chemo-chemodynamic nano-platform for targeted homologous prostate cancer therapy [39]. To further improve the targeting ability, Li et al. developed macrophage-derived exosomes-coated poly (lactic-co-glycolic acid) nanoplatforms and further modified them with peptides that can target tumor cells. Their study revealed that the engineered exosomes-coated nanoparticle not only significantly prolonged the circulation time of DOX, but also achieved higher tumor targetability and better accumulation in tumor tissues during triple-negative breast cancer chemotherapy [82]. Collectively, all these studies show that applying engineered EVs for combinational delivery of nanoparticles and chemotherapeutic drugs is a feasible approach to combine the advantages of nanoparticles and EVs for high-efficiency chemotherapy.
Engineered EVs for immunotherapy-related cancer combination therapy
Cancer immunotherapy that utilizes immune system to combat tumors is a validated and critical approach for cancer treatment. The significant clinical benefits achieved by immunotherapy have revolutionized the treatment of many advanced cancers. However, low response rates and immune-related adverse events limit the broad application of cancer immunotherapy. Adopting combinational strategy is a potential way to overcome these limitations [83, 84]. Applications of engineered EV in this field have made some remarkable breakthroughs to realize immunotherapy-related combination therapy (Table 2).
Notably, immune cell-derived EVs play critical roles in this field. Recent studies have shown that immune cell-derived EVs can facilitate the immune response process by mediating crosstalk between different cells in the cancer-immunity cycle [11, 85, 86]. Applying immune cell-derived EVs can regulate the state of target immune cells to promote anti-cancer immunity. For example, Choo et al. showed that exosome-mimetic nanovesicles derived from M1 macrophages could repolarize M2 tumor-associated macrophages (TAMs) to M1 macrophages and induce antitumor immune responses in vitro and in vivo [87]. Matsumoto et al. proved that the small EVs originating from mature dendritic cells (DCs) could repolarize macrophages and activate DCs for tumor antigen-based cancer immunotherapy [88]. In addition, compared with parent cells, immune cell-derived EVs have some unique features. Their membrane area is smaller. Thus, they have higher density of functional signaling molecules. Their nano-scale particle size and non-cellular activity make them penetrate tumors easily, retent longer, and less susceptible to the tumor immunosuppressive microenvironment [89, 90]. These features make immune cell-derived EVs more potent in immunotherapy-related cancer treatment. Take T cell as an example, Fu et al. proved that EVs released by T cells expressing chimeric antigen receptor (CAR) are relatively safe and effective compared with CAR-T therapy for solid tumors. CAR exosomes have the potential to overcome the flaws of CAR-T cells to accumulate and replicate in TME [91]. Despite these advantages of immune cell-derived EVs, we should also pay attention to the duality of their effects on the immune system. That is, they may exhibit both immune-stimulatory and immunosuppressive roles. The specific effect of immune cell-derived EVs is closely related to the state of parent cells [4, 11]. Therefore, adjusting parent cells to an appropriate status is important when applying immune cell-derived EVs to immunotherapy-related cancer treatment.
Engineered EVs for the combination of immunotherapy with chemotherapy
Immunotherapy functions mainly through immune effector cells. In the TME, immune effector cells lose anti-tumor abilities and changed to immunosuppressive phenotype due to complex mechanisms [92]. For instance, M1 macrophages inhibit tumor growth while M2 macrophages in the TME, such as TAMs, suppress anti-cancer immunity and promote tumor growth [93]. Therefore, rewiring immunosuppressive cells to generate anti-cancer immunity is essential for immunotherapy. Studies demonstrate that chemotherapeutic agents can produce immunostimulatory functions to reactivate immune effector cells [94]. Meanwhile, chemotherapy can induce immunogenic cell death (ICD) then expose more tumor antigens, facilitating better immunotherapy [95]. Given these functions of chemotherapy, combining immunotherapy with chemotherapy to increase the synergistic anti-tumor effects of monotherapy is reasonable. Engineered EVs act as drug delivery platforms can be used for co-delivering immunomodulatory materials and chemotherapeutic drugs (Fig. 6). Enhanced tumor site accumulation and endocytosis phagocytosis effects of engineered EVs are beneficial to transport immunomodulatory drugs into immunosuppressive cells to produce better immune reprogramming effects.
Engineered EVs for the combination of immunotherapy with chemotherapy. Immunomodulatory materials and chemotherapeutic drugs are co-delivered to the tumor microenvironment by engineered EVs. Immunomodulatory materials reprogram immunosuppressive cells to immune effector cells. Chemotherapy can induce immunogenic cell death (ICD) and expose more tumor antigens, facilitating better immunotherapy
For instance, to improve the treatment of metastatic peritoneal carcinoma (mPC) by chemoimmunotherapy, Lv et al. designed genetically engineered exosomes-thermosensitive liposomes hybrid nanoparticles (gETL NPs) for targeted delivery of granulocyte–macrophage colony-stimulating factor (GM-CSF) and docetaxel (DTX). They prepared genetically engineered exosomes through transducing lentiviral vectors into fibroblasts for overexpression of CD47 and GM-CSF. CD47 mediated phagocytosis enhancement and GM-CSF promoted repolarization of macrophages towards M1 profile. Then G/D-gETL NPs were generated by the fusion of these engineered exosomes and thermosensitive DTX-loaded liposomes. They showed that G/D-gETL NPs effectively repolarized the M2 phenotype macrophages to M1 phenotype and inhibited tumor development in mice through combining immunotherapy and chemotherapy [45]. In another research, to enhance immunotherapy and reprogram tumor microenvironment in pancreatic ductal adenocarcinoma (PDAC), Zhou et al. constructed an exosome-based delivery system (iEXO-OXA) that loaded with galectin-9 siRNA and surficially modified with oxaliplatin (OXA) to reverse immunosuppression and trigger ICD. They demonstrated that iEXO-OXA improved tumor targeting efficacy and increased drug accumulation in the tumor site. OXA-mediated cytotoxicity promoted tumor cells death and induced ICD. Galectin-9 siRNA-mediated immunotherapy elicited anti-tumor immunity through tumor-suppressive macrophage polarization, cytotoxic T lymphocytes recruitment, and regulatory T cells downregulation. IEXO-OXA, combining immunotherapy and chemotherapy, achieved significant therapeutic efficacy in orthotopic PDAC mice [96]. Both studies proved that immunotherapy combined with chemotherapy based on engineered EVs could broaden the application of immunotherapy and produce stronger anti-cancer immunity.
Engineered EVs for the combination of immunotherapy with photodynamic therapy or photothermal therapy
Similar to chemotherapy, photodynamic therapy (PDT) and PTT can induce ICD and stimulate adaptive immune responses, which will cause long-term immunological memory. Converting immunologically “cold” into “hot” tumor by PDT or PTT could effectively increase the sensitivity of immunotherapy [97, 98]. However, PDT or PTT application in cancer treatment is limited by the low tumor selectivity and significant adverse effects of photosensitizers (PS) or PTA. To overcome these obstacles, engineered EVs with superior delivery functions are explored to deliver PS or PTA. The targeted capability of engineered EVs can reduce the damage of these foreign materials to normal cells and achieve precise PDT or PTT. At the same time, the immunostimulatory effects of PDT or PTT will promote immunotherapy and achieve a combined therapeutic effect on cancer.
In the Pinto et al. study, the PS meta(tetrahydroxyphenyl)-chlorin (mTHPC) was encapsulated into EVs derived from mesenchymal stem/stromal cells (MSCs) to form the fourth generation of immune active PS vectors (EVs-mTHPC). Turbulence, as a pioneering method, was developed for large-scale production of EVs-mTHPC that was compatible with requirements of clinical translation while preserving the topology and integrity of natural EVs. The results of this study showed that high yield EVs-mTHPC performed higher tumor selectivity, induced tumor necrosis, promoted antitumor immune cell infiltration, and finally achieved immune reprogramming precision photodynamic therapy in carcinomatosis [99]. In another typical study conducted by Cheng et al. hybrid therapeutic nanovesicles (I/R@hGLV) were generated by fusing the CD47-overexpressed exosomes with thermosensitive liposomes loaded with photothermal agent ICG and immune adjuvant R837. The overexpression of CD47 on the engineered exosomes was in favor of avoiding the immune clearance by the mononuclear phagocyte system and prolonging the blood circulation time. Moreover, the CD47-expression could competitively bind with SIRPα, prior to tumor cells, leading to enhancing tumor cell phagocytosis by macrophages. The cargo ICG could achieve better PTT and induce ICD, triggering strong immune responses with the help of adjuvant R837. In tumor-bearing mice, I/R@hGLV efficiently inhibited tumors by combining cancer immunotherapy and PTT [100]. These two studies showed that immunotherapy combined with PDT or PTT based on engineered EVs is a feasible approach to obtain more potent anti-tumor effects.
Engineered EVs for the combination of immune checkpoint inhibitor (ICI) with anti-ICI resistance treatment
Immune checkpoint inhibitors (ICI) play key roles in cancer immunotherapy and the significant clinical effects achieved by ICI have greatly improved the outcome of many advanced cancers. However, despite the success of ICI, resistance to these agents restricts the production of immune responses. Mechanisms of the ICI resistance are complex, which include lack of sufficient T cell infiltration and tumor immunosuppressive microenvironment [101]. Anti-ICI resistance treatment is required to increase the potential response rates. Repolarizing TAMs is one of the promising strategies to improve ICI resistance by enhancing T-cell antitumor immunity and ameliorating tumor immunosuppression [102, 103]. As mentioned earlier, engineered EVs are potent tools to reset M2-like TAMs to M1 phenotype. In the Choo et al. research, exosome-mimetic nanovesicles derived from M1 macrophages (M1NVs) were used to reset M2 TAMs to M1 macrophages, which showed the potential anti-cancer efficacy of ICI [87]. To better target and repolarize TAMs, Wei et al. further engineered macrophage-derived microparticles through mannose modification and loading metformin (Met@Man-MPs). In Met@Man-MPs, mannose could target CD206/MRC1, the highly expressed receptor of M2 TAM, and the cargo metformin contributed to repolarizing TAMs to M1 profile through the AMPK-NF-κB signaling pathway. They proved that Met@Man-MPs could degrade tumor collagen to support the infiltration of CD8 + T cells into tumor interiors and enhance tumor penetration of ICI, boosting immune checkpoint inhibition therapy and improving anticancer efficacy and long-term memory immunity after combinational treatment [104]. Although the number of such research is limited, the available data have shown that engineered EVs are suitable platforms for combining ICI with anti-ICI resistance treatment to increase the response rates of immune checkpoint blockade. Future development of engineered EVs relevant to anti-ICI resistance methods combined with ICI will enrich this combinational scheme.
Engineered EVs for the combination of therapeutic cancer vaccine with immune checkpoint blockade
Therapeutic cancer vaccine has emerged as a powerful therapy strategy by stimulating adaptive immunity against specific tumor antigens to control over tumor growth and induce regression of tumors [105]. Cancer vaccines usually comprise four key components: tumor antigens, formulations, immune adjuvants, and delivery vehicles [106]. Recently, EVs derived from tumor cells or immune cells have been engineered to cover these four components, which has become an attractive method to develop cancer vaccines. Tumor-derived EVs retaining multiple tumor antigens, coupled with their own delivery function, only need to be modified with adjuvants before they used as suitable tumor vaccines. For example, Morishita et al. made use of exosomes derived from murine melanoma cells combined with adjuvant CpG to generate a cancer vaccine, successfully induced tumor antigen-specific immune response in mice [107]. EV originating from DC is another promising candidate for tumor vaccine. Antigen-loaded and matured DC-derived EVs with high expression of major histocompatibility complex and co-stimulatory molecules CD80 and CD86 are capable of directly stimulating antigen-reactive T cell responses [108, 109]. In murine tumor models, DC-derived EVs have been demonstrated to activate strong adaptive immune responses against cancers [110].
Although therapeutic tumor vaccines can induce anti-tumor immunity, they often failed to produce actual anti-tumor effects in many trials. With a better understanding of host immunity and TME, the immunosuppressive barriers in the TME has been proved to be a critical reason for the failure of cancer vaccines. “Cold” tumors have poor capacity to attract immune cell infiltration and immunosuppressive factors, which will weaken the functions of immune cells and make vaccine-induced immunity hard to form [111]. Combinational therapy provides a choice to overcome the problem, especially when tumor vaccines are combined with ICB therapy. Cancer vaccines promote anti-cancer immunity priming, and then ICI boost and maintain the vaccine’s effect to produce persistent immune responses. A series of studies have indicated the effectiveness of therapeutic cancer vaccine combined with immune checkpoint blockade therapy [112, 113]. Engineered EVs are also explored to apply in this combinational scheme (Fig. 7). In the Zhao et al. research, tumor-derived antigenic microparticles (T-MPs) were employed to load nano-Fe3O4 and then adjuvant CpG-loaded liposome arrays were bound to the surface of T-MPs to form the cancer vaccines (Fe3O4/T-MPs-CpG/Lipo). The combination of Fe3O4/T-MPs-CpG/Lipo vaccine and immune checkpoint PD-L1 blockade specifically inhibited ∼83% progression of B16F10-bearing mice [34]. In another typical study by Phung et al., exosomes derived from ovalbumin-pulsed, activated DCs were modified with anti-CTLA-4 antibody to generate bifunctional exosomes (EXO-OVA-mAb) combining vaccination and ICB. Their study showed that the functionalized EXO-OVA-mAb could synergize cancer vaccine efficacy and immune checkpoint inhibition to generate potent antitumor immune responses against the tumor [114]. These preliminary research results have shown that engineered EVs with inherent vaccine properties have great potential to realize the combination of a therapeutic cancer vaccine with ICB.
Engineered EVs for the combination of cancer vaccine with immune checkpoint blockade. EVs derived from mature dendritic cells are modified with immune checkpoint inhibitors. These engineered EVs can realize the combination of cancer vaccine and immune checkpoint blockade, effectively activating T cells to inhibit tumor growth
Engineered EVs for visual therapy combining imaging and cancer treatment
Visual therapy is an ideal pattern of cancer therapeutics which can achieve both imaging and treatment simultaneously. It will significantly benefit the clinical work in the whole treatment process. Before the treatment, accurate imaging provides the location, size, and distribution of the tumor, determining whether it is suitable for therapy. In the treatment process, real-time imaging can track the distribution and metabolism of drugs, which can guide therapy precisely. After the treatment, curative effects can be monitored through the changes in the images of the lesion. Therefore, unexpected damage to normal tissues can be found and avoided promptly. Considering these strengths of visual therapy, researchers are committed to achieving the combination of cancer imaging and treatment. One of the most potent approaches to visual therapy is utilizing engineered EVs (Fig. 8). The load capacity and biocompatibility of engineered EVs are conducive to delivering imaging materials for multimodal imaging. Their precise targeting ability can further help label tumor cells and achieve targeted visual therapy. For example, in the glioma research of Jia et al. a new type of exosome for targeted imaging and therapy was designed. The engineered exosomes (RGE-Exo-SPION/Cur) were loaded with superparamagnetic iron oxide nanoparticles (SPION) and curcumin (Cur), and then their membrane was conjugated with neuropilin-1-targeted peptide (RGE) to obtain glioma-targeting ability. The study proved that RGE-Exo-SPION/Cur could penetrate the BBB smoothly and provided good results for targeted imaging, SPION-mediated magnetic flow hyperthermia therapy, and Cur-mediated therapy [32]. In another research by Fan et al. the strategy of using functionalized DNA to anchor quantum dots (QDs) onto EV surface was developed for tumor imaging and therapy. The labeling property of luminophores QDs endowed QD-engineered EVs with imaging function. When this strategy was applied to artificial vesicles of M1 macrophages loaded with DOX, it realized targeted label and treatment for visual therapy of tumor in mice [35]. Cao et al. also used the features of QDs to develop engineered exosomes for visual therapy. They first synthesized the vanadium carbide QDs modified with cell nucleus-target TAT peptides (V2C-TAT), then encapsulated the V2C-TAT QDs into exosomes engineered with cell target-RDG peptide. They formed a dual-target system of cell membrane and nucleus of cancer (V2C-PEG-TAT@Ex-RGD). Results showed that V2C-PEG-TAT@Ex-RGD could target the cell and enter the nucleus to accomplish low-temperature PTT and realized multimodal imaging, including fluorescent imaging, photoacoustic imaging, and magnetic resonance imaging [115]. All these prominent results suggest that engineered EVs are potential platforms for delivering imaging and therapeutic agents. Their noteworthy performance is helpful to achieve visual therapy for cancer precision treatment.
Engineered EVs for other schemes of cancer combination therapy
Recently, researchers have developed some emerging cancer treatment methods and tried to combine them with traditional therapy to obtain better cancer treatment effects. Engineered EVs are also explored to apply in these schemes of combinational therapy. For example, carbon monoxide (CO), which can traverse tumor stroma and enter tumor cells to affect metabolism and energy production, has drawn growing concerns for cancer therapy [116]. The function of sensitizing other treatments such as radiotherapy makes CO have great potential to be combined with other cancer therapies. Zhu et al. used tumor-derived exosomes to deliver manganese carbonyl, which could react with H2O2 in the TME to produce CO, finally achieving the combination of cancer gas therapy and low dose radiotherapy to inhibit tumor growth in 4T1 mice [117].
Besides the various dual combinations of cancer treatment approaches mentioned above, some studies reported that engineered EVs could realize three or more combinational schemes in cancer treatment. For instance, Ding et al. indicated an engineered self-activatable photo-EV for synergistic trimodal anticancer therapy. In this study, M1 macrophage-derived EVs were prepared and simultaneously loaded with bis[2,4,5-trichloro-6-(pentyloxycarbonyl) phenyl] oxalate (CPPO), photosensitizer chlorin e6 (Ce6), and chemotherapy drugs prodrug aldoxorubicin (Dox-EMCH). M1 EVs with tumor-homing capability could target tumor sites and repolarize M2 to M1 macrophage to realize immunotherapy and produce H2O2. Then H2O2 would react with CPPO to generate chemical energy that activates Ce6, producing singlet oxygen (1O2) for PDT. Meanwhile, 1O2 brought up the rupture of EV membrane and induced the release of Dox-EMCH for chemotherapy. After injection into 4T1 tumor-bearing mice, these engineered EVs performed a perfect combination of immunotherapy, PDT, and chemotherapy in TME. It significantly suppressed tumor growth and prolonged the survival of tumor-bearing mice [118]. In another study by Wang et al., DOX was loaded into the exosomes coated with magnetic nanoparticles conjugated with molecular beacons. Under NIR radiation, these engineered exosomes could generate thermal energy to realize PTT and trigger cargoes release. The released molecular beacon could target the miR-21 for gene therapy, and DOX could perform chemotherapeutic effect. Synthetic chemo/gene/photothermal therapy of these engineered exosomes applied in tumor-bearing mice could reduce tumor size by 97.57% [119]. These innovative researches suggest that engineered EVs can display great diversity and achieve various combinational schemes in cancer treatment.
Perspectives and challenges
The application of engineered EVs has emerged as a promising way to fight against cancer. Much progress has been made and the development trend mainly focused on combinational treatment in recent years. Therefore, deeper understanding of the EVs biology and better improvements in engineering strategies diminished the barrier for the application of engineered EVs to cancer combination therapy. A great number of studies have shown that engineered EVs with strong load capacity, high plasticity, good biocompatibility, and precise targeting capabilities are promising platforms to implement cancer combinational therapy, especially for the combinations related to chemotherapy or immunotherapy. Moreover, engineered EVs is very potential to further optimize the synthetic effect of the combination therapy to reach the desired therapeutic effect.
Nevertheless, some critical issues need to be resolved in this field. The first one is how to overcome the difficulty of mass production of engineered EVs. Up to now, it still costs much time to produce enough EVs for cancer treatment. The incomplete modification will further reduce the utilization ratio of EVs, which significantly limits the wide application in clinical trials. The second problem is the heterogeneity of EVs. The isolated EVs are very heterogeneous due to different sizes or contents. During the engineering process, different degrees of modification in each EV will lead to obvious heterogeneity. In addition, safety and targeted delivery are other issues that we should pay attention to when engineered EVs are applied in cancer combination therapy.
The issues above are particularly prominent in immune cell-derived EVs. Unlike tumor cells, human autologous immune cells are hard to obtain sufficient parent cells in vitro in short time. Coupled with the difficulty in mass production of EVs, it is laborious and costly to produce healing amounts of immune cell-derived EVs in practice. Moreover, primary human immune cells are difficult to isolate to 100% homogeneity, and their activation is often asynchronous and incomplete. Therefore, the obtained EVs from these immune cells often have significant heterogeneity, including immunostimulatory and tolerogenic EVs. These problems limit the effect of immune cell-derived EVs in this field.
Recently, some new technologies and methods have been developed to provide potential solutions to the above problems. For EVs mass production, there are two main development directions. One is to optimize the culture of source cells, and the other is to find more suitable stimulation to increase EV production per cell [120]. Multiple bioreactors like “flask” bioreactor and stirred tank bioreactors were designed for large-scale culture of source cells [121,122,123]. Mechanical stress was also proven and developed for the mass production of EVs [124]. As for the heterogeneity of EVs, single-vesicle and single-molecule analysis techniques to precisely analyze each EV and its cargo is a promising strategy. In addition, new advanced devices or techniques such as atomic force microscopy [125], super-resolution fluorescence microscopy [126], fluorescence correlation spectroscopy [127], nanoflow cytometry [128, 129], and single-particle interferometric reflectance imaging sensing (SP-IRIS) [130] were developed for obtaining more accurate characterization of each EV and its cargo [131]. For the EVs targeting ability, further revealing the mechanism of targeted delivery in EVs biology will be helpful to provide the theoretical basis for improving EV targeting capabilities [19].
Although most studies about engineered EVs and cancer combination therapy are still in the pre-clinical stage, technological advances and solutions to the problems mentioned above will shed light on the translation of engineered EVs from the bench to the bedside for cancer combination therapy in the future. More and better combinational schemes based on engineered EVs will also be developed to break the dilemma of cancer therapy.
Conclusions
Engineered EVs have great potential in cancer combination therapy. In this review, we introduced current combinational schemes of engineered EVs and other cancer therapies. It is predictable that this area will be promising and more breakthroughs in cancer treatment will be achieved through more diverse combinational strategies of engineered EVs.
Availability of data and materials
Not applicable.
Abbreviations
- EV:
-
Extracellular vesicle
- TME:
-
Tumor microenvironment
- MDR:
-
Multi-drug resistance
- ICB:
-
Immune checkpoint blockade
- ILV:
-
Intraluminal vesicle
- TGN:
-
Trans-Golgi network
- MVE:
-
Multivesicular endosome
- BBB:
-
Blood–brain barrier
- P-gp:
-
P-glycoprotein
- DOX:
-
Doxorubicin
- miRNA:
-
MicroRNA
- 5-FU:
-
5-fluorouracil
- PTT:
-
Photothermal therapy
- PTA:
-
Photothermal agents
- ICG:
-
Indocyanine green
- NIR:
-
Near-infrared
- MSNs:
-
Mesoporous silica nanoparticles
- CRISPR:
-
The clustered regularly interspersed short palindromic repeats
- Cas9:
-
CRISPR-associated proteins 9
- PARP-1:
-
Poly (ADP-ribose) polymerase-1
- Bcl-2:
-
B-cell lymphoma-2
- NP:
-
Nanoparticle
- EPR:
-
Enhanced permeability and retention
- ROS:
-
Reactive oxygen species
- PTX:
-
Paclitaxel
- CuB:
-
Cucurbitacin B
- HSA:
-
Human serum albumin
- TAM:
-
Tumor-associated macrophage
- CAR:
-
Chimeric antigen receptor
- ICD:
-
Immunogenic cell death
- mPC:
-
Metastatic peritoneal carcinoma
- gETL NPs:
-
Genetically engineered exosomes-thermosensitive liposomes hybrid nanoparticles
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- DTX:
-
Docetaxel
- PDAC:
-
Pancreatic ductal adenocarcinoma
- OXA:
-
Oxaliplatin
- PDT:
-
Photodynamic therapy
- PS:
-
Photosensitizers
- MSCs:
-
Mesenchymal stem/stromal cells
- ICI:
-
Immune checkpoint inhibitors
- DC:
-
Dendritic cell
- T-MPs:
-
Tumor-derived antigenic microparticles
- SPION:
-
Superparamagnetic iron oxide nanoparticle
- Cur:
-
Curcumin
- QDs:
-
Quantum dots
- CO:
-
Carbon monoxide
- Ce6:
-
Chlorin e6
- SP-IRIS:
-
Single-particle interferometric reflectance imaging sensing
References
Sullivan R, Maresh G, Zhang X, Salomon C, Hooper J, Margolin D, Li L. The emerging roles of extracellular vesicles as communication vehicles within the tumor microenvironment and beyond. Front Endocrinol. 2017;8:194.
Maacha S, Bhat AA, Jimenez L, Raza A, Haris M, Uddin S, Grivel JC. Extracellular vesicles-mediated intercellular communication: roles in the tumor microenvironment and anti-cancer drug resistance. Mol Cancer. 2019;18:55.
Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17.
Pirisinu M, Pham TC, Zhang DX, Hong TN, Nguyen LT, Le MT. Extracellular vesicles as natural therapeutic agents and innate drug delivery systems for cancer treatment: recent advances, current obstacles, and challenges for clinical translation. Semin Cancer Biol. 2020. https://doi.org/10.1016/j.semcancer.2020.08.007.
Zhang X, Zhang H, Gu J, Zhang J, Shi H, Qian H, Wang D, Xu W, Pan J, Santos HA. Engineered extracellular vesicles for cancer therapy. Adv Mater. 2021;33:e2005709.
Vader P, Mol E, Pasterkamp G, Schiffelers R. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148–56.
Liu C, Su C. Design strategies and application progress of therapeutic exosomes. Theranostics. 2019;9:1015–28.
Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SE, Vader P. Extracellular vesicles as drug delivery systems: why and how? Adv Drug Deliv Rev. 2020;159:332–43.
Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mäger I, Vader P, Lee Y, Sork H, Seow Y, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316.
Tsoi KM, MacParland SA, Ma XZ, Spetzler VN, Echeverri J, Ouyang B, Fadel SM, Sykes EA, Goldaracena N, Kaths JM, et al. Mechanism of hard-nanomaterial clearance by the liver. Nat Mater. 2016;15:1212–21.
Veerman RE, Güçlüler Akpinar G, Eldh M, Gabrielsson S. Immune cell-derived extracellular vesicles—functions and therapeutic applications. Trends Mol Med. 2019;25:382–94.
Ma J, Zhang H, Tang K, Huang B. Tumor-derived microparticles in tumor immunology and immunotherapy. Eur J Immunol. 2020;50:1653–62.
Liang Q, Bie N, Yong T, Tang K, Shi X, Wei Z, Jia H, Zhang X, Zhao H, Huang W, et al. The softness of tumour-cell-derived microparticles regulates their drug-delivery efficiency. Nat Biomed Eng. 2019;3:729–40.
Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021;221:107753.
Qin SY, Cheng YJ, Lei Q, Zhang AQ, Zhang XZ. Combinational strategy for high-performance cancer chemotherapy. Biomaterials. 2018;171:178–97.
Patel SA, Minn AJ. Combination cancer therapy with immune checkpoint blockade: mechanisms and strategies. Immunity. 2018;48:417–33.
Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, Garon EB, Lee P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895–903.
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28.
Ridger VC, Boulanger CM, Angelillo-Scherrer A, Badimon L, Blanc-Brude O, Bochaton-Piallat ML, Boilard E, Buzas EI, Caporali A, Dignat-George F, et al. Microvesicles in vascular homeostasis and diseases. Position Paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb Haemost. 2017;117:1296–316.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020. https://doi.org/10.1126/science.aau6977.
Teng F, Fussenegger M. Shedding light on extracellular vesicle biogenesis and bioengineering. Adv Sci. 2020;8:2003505.
Rufino-Ramos D, Albuquerque PR, Carmona V, Perfeito R, Nobre RJ, de Almeida LP. Extracellular vesicles: novel promising delivery systems for therapy of brain diseases. J Control Release. 2017;262:247–58.
Armstrong JP, Holme MN, Stevens MM. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. 2017;11:69–83.
Kooijmans SAA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJA, Schiffelers RM, Raemdonck K, Vader P. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J Control Release. 2013;172:229–38.
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30.
Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12:655–64.
Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release. 2015;205:35–44.
Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–79.
Hu L, Wickline SA, Hood JL. Magnetic resonance imaging of melanoma exosomes in lymph nodes. Magn Reson Med. 2015;74:266–71.
Jia G, Han Y, An Y, Ding Y, He C, Wang X, Tang Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302–16.
Pan X, Liang Z, Li J, Wang S, Kong F, Xu K, Tang B. Active-site-matched fluorescent probes for rapid and direct detection of vicinal-sulfydryl-containing peptides/proteins in living cells. Chemistry. 2015;21:2117–22.
Zhao H, Zhao B, Wu L, Xiao H, Ding K, Zheng C, Song Q, Sun L, Wang L, Zhang Z. Amplified cancer immunotherapy of a surface-engineered antigenic microparticle vaccine by synergistically modulating tumor microenvironment. ACS Nano. 2019;13:12553–66.
Fan Z, Xiao K, Lin J, Liao Y, Huang X. Functionalized DNA enables programming exosomes/vesicles for tumor imaging and therapy. Small. 2019;15:e1903761.
Lin Y, Wu J, Gu W, Huang Y, Tong Z, Huang L, Tan J. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci. 2018;5:1700611.
Piffoux M, Silva AKA, Wilhelm C, Gazeau F, Tareste D. Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS Nano. 2018;12:6830–42.
Vázquez-Ríos AJ, Molina-Crespo Á, Bouzo BL, López-López R, Moreno-Bueno G, de la Fuente M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. J Nanobiotechnol. 2019;17:85.
Pan S, Zhang Y, Huang M, Deng Z, Zhang A, Pei L, Wang L, Zhao W, Ma L, Zhang Q, Cui D. Urinary exosomes-based engineered nanovectors for homologously targeted chemo-chemodynamic prostate cancer therapy via abrogating EGFR/AKT/NF-kB/IkB signaling. Biomaterials. 2021;275:120946.
Cheng G, Li W, Ha L, Han X, Hao S, Wan Y, Wang Z, Dong F, Zou X, Mao Y, Zheng SY. Self-assembly of extracellular vesicle-like metal-organic framework nanoparticles for protection and intracellular delivery of biofunctional proteins. J Am Chem Soc. 2018;140:7282–91.
Liu C, Zhang W, Li Y, Chang J, Tian F, Zhao F, Ma Y, Sun J. Microfluidic sonication to assemble exosome membrane-coated nanoparticles for immune evasion-mediated targeting. Nano Lett. 2019;19:7836–44.
Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. 2015;199:145–55.
Wang B, Yao K, Huuskes BM, Shen HH, Zhuang J, Godson C, Brennan EP, Wilkinson-Berka JL, Wise AF, Ricardo SD. Mesenchymal stem cells deliver exogenous microRNA-let7c via exosomes to attenuate renal fibrosis. Mol Ther. 2016;24:1290–301.
Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21:185–91.
Lv Q, Cheng L, Lu Y, Zhang X, Wang Y, Deng J, Zhou J, Liu B, Liu J. Thermosensitive exosome-liposome hybrid nanoparticle-mediated chemoimmunotherapy for improved treatment of metastatic peritoneal cancer. Adv Sci. 2020;7:2000515.
Guo M, Wu F, Hu G, Chen L, Xu J, Xu P, Wang X, Li Y, Liu S, Zhang S, et al. Autologous tumor cell-derived microparticle-based targeted chemotherapy in lung cancer patients with malignant pleural effusion. Sci Transl Med. 2019. https://doi.org/10.1126/scitranslmed.aat5690.
Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S. Drug resistance in cancer: an overview. Cancers. 2014;6:1769–92.
Kunjachan S, Rychlik B, Storm G, Kiessling F, Lammers T. Multidrug resistance: physiological principles and nanomedical solutions. Adv Drug Deliv Rev. 2013;65:1852–65.
Saraswathy M, Gong S. Different strategies to overcome multidrug resistance in cancer. Biotechnol Adv. 2013;31:1397–407.
Dong J, Qin Z, Zhang WD, Cheng G, Yehuda AG, Ashby CR Jr, Chen ZS, Cheng XD, Qin JJ. Medicinal chemistry strategies to discover P-glycoprotein inhibitors: an update. Drug Resist Updat. 2020;49:100681.
Wang T, Luo Y, Lv H, Wang J, Zhang Y, Pei R. Aptamer-based erythrocyte-derived mimic vesicles loaded with siRNA and doxorubicin for the targeted treatment of multidrug-resistant tumors. ACS Appl Mater Interfaces. 2019;11:45455–66.
Ma J, Zhang Y, Tang K, Zhang H, Yin X, Li Y, Xu P, Sun Y, Ma R, Ji T, et al. Reversing drug resistance of soft tumor-repopulating cells by tumor cell-derived chemotherapeutic microparticles. Cell Res. 2016;26:713–27.
Jin X, Ma J, Liang X, Tang K, Liu Y, Yin X, Zhang Y, Zhang H, Xu P, Chen D, et al. Pre-instillation of tumor microparticles enhances intravesical chemotherapy of nonmuscle-invasive bladder cancer through a lysosomal pathway. Biomaterials. 2017;113:93–104.
Yong T, Zhang X, Bie N, Zhang H, Zhang X, Li F, Hakeem A, Hu J, Gan L, Santos HA, Yang X. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun. 2019;10:3838.
Liu J, Ye Z, Xiang M, Chang B, Cui J, Ji T, Zhao L, Li Q, Deng Y, Xu L, et al. Functional extracellular vesicles engineered with lipid-grafted hyaluronic acid effectively reverse cancer drug resistance. Biomaterials. 2019;223:119475.
Kurozumi S, Yamaguchi Y, Kurosumi M, Ohira M, Matsumoto H, Horiguchi J. Recent trends in microRNA research into breast cancer with particular focus on the associations between microRNAs and intrinsic subtypes. J Hum Genet. 2017;62:15–24.
Wang S, Su X, Bai H, Zhao J, Duan J, An T, Zhuo M, Wang Z, Wu M, Li Z, et al. Identification of plasma microRNA profiles for primary resistance to EGFR-TKIs in advanced non-small cell lung cancer (NSCLC) patients with EGFR activating mutation. J Hematol Oncol. 2015;8:127.
Valeri N, Gasparini P, Braconi C, Paone A, Lovat F, Fabbri M, Sumani KM, Alder H, Amadori D, Patel T, et al. MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating human DNA MutS homolog 2 (hMSH2). Proc Natl Acad Sci USA. 2010;107:21098–103.
Penna E, Orso F, Taverna D. miR-214 as a key hub that controls cancer networks: small player, multiple functions. J Invest Dermatol. 2015;135:960–9.
Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K, Sun B, Chen B, Xiao Z. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnol. 2020;18:10.
Wang X, Zhang H, Bai M, Ning T, Ge S, Deng T, Liu R, Zhang L, Ying G, Ba Y. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol Ther. 2018;26:774–83.
Bose RJC, Uday Kumar S, Zeng Y, Afjei R, Robinson E, Lau K, Bermudez A, Habte F, Pitteri SJ, Sinclair R, et al. Tumor cell-derived extracellular vesicle-coated nanocarriers: an efficient theranostic platform for the cancer-specific delivery of anti-miR-21 and imaging agents. ACS Nano. 2018;12:10817–32.
Lyu Y, Li J, Pu K. Second near-infrared absorbing agents for photoacoustic imaging and photothermal therapy. Small Methods. 2019. https://doi.org/10.1002/smtd.201900553.
Spiliotis J, Halkia E, de Bree E. Treatment of peritoneal surface malignancies with hyperthermic intraperitoneal chemotherapy-current perspectives. Curr Oncol. 2016;23:e266-275.
Zeng X, Luo M, Liu G, Wang X, Tao W, Lin Y, Ji X, Nie L, Mei L. Polydopamine-modified black phosphorous nanocapsule with enhanced stability and photothermal performance for tumor multimodal treatments. Adv Sci. 2018;5:1800510.
Zhang Z, Wang J, Chen C. Near-infrared light-mediated nanoplatforms for cancer thermo-chemotherapy and optical imaging. Adv Mater. 2013;25:3869–80.
Zhang D, Qin X, Wu T, Qiao Q, Song Q, Zhang Z. Extracellular vesicles based self-grown gold nanopopcorn for combinatorial chemo-photothermal therapy. Biomaterials. 2019;197:220–8.
Wang D, Yao Y, He J, Zhong X, Li B, Rao S, Yu H, He S, Feng X, Xu T, et al. Engineered cell-derived microparticles Bi(2)Se(3)/DOX@MPs for imaging guided synergistic photothermal/low-dose chemotherapy of cancer. Adv Sci. 2020;7:1901293.
Zhu L, Wang C, Pang DW, Zhang ZL. Controlled release of therapeutic agents with near-infrared laser for synergistic photochemotherapy toward cervical cancer. Anal Chem. 2019;91:6555–60.
Tian R, Wang Z, Niu R, Wang H, Guan W, Chang J. Tumor exosome mimicking nanoparticles for tumor combinatorial chemo-photothermal therapy. Front Bioeng Biotechnol. 2020;8:1010.
Zhou Z, Liu X, Zhu D, Wang Y, Zhang Z, Zhou X, Qiu N, Chen X, Shen Y. Nonviral cancer gene therapy: delivery cascade and vector nanoproperty integration. Adv Drug Deliv Rev. 2017;115:115–54.
Zhuo C, Zhang J, Lee JH, Jiao J, Cheng D, Liu L, Kim HW, Tao Y, Li M. Spatiotemporal control of CRISPR/Cas9 gene editing. Signal Transduct Target Ther. 2021;6:238.
Kim SM, Yang Y, Oh SJ, Hong Y, Seo M, Jang M. Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J Control Release. 2017;266:8–16.
Montero J, Letai A. Why do BCL-2 inhibitors work and where should we use them in the clinic? Cell Death Differ. 2018;25:56–64.
Zhu L, Dong D, Yu ZL, Zhao YF, Pang DW, Zhang ZL. Folate-engineered microvesicles for enhanced target and synergistic therapy toward breast cancer. ACS Appl Mater Interfaces. 2017;9:5100–8.
Kemp JA, Shim MS, Heo CY, Kwon YJ. “Combo” nanomedicine: co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv Drug Deliv Rev. 2016;98:3–18.
Gupta J, Safdari HA, Hoque M. Nanoparticle mediated cancer immunotherapy. Semin Cancer Biol. 2021;69:307–24.
Liu J, Zhang R, Xu ZP. Nanoparticle-based nanomedicines to promote cancer immunotherapy: recent advances and future directions. Small. 2019;15:e1900262.
Green JJ, Elisseeff JH. Mimicking biological functionality with polymers for biomedical applications. Nature. 2016;540:386–94.
Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71–9.
Wang K, Ye H, Zhang X, Wang X, Yang B, Luo C, Zhao Z, Zhao J, Lu Q, Zhang H, et al. An exosome-like programmable-bioactivating paclitaxel prodrug nanoplatform for enhanced breast cancer metastasis inhibition. Biomaterials. 2020;257:120224.
Li S, Wu Y, Ding F, Yang J, Li J, Gao X, Zhang C, Feng J. Engineering macrophage-derived exosomes for targeted chemotherapy of triple-negative breast cancer. Nanoscale. 2020;12:10854–62.
Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, Mueller B, Quaratino S, Sabatos-Peyton C, Petruzzelli L, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17:286–301.
Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13:143–58.
Lindenbergh MFS, Stoorvogel W. Antigen presentation by extracellular vesicles from professional antigen-presenting cells. Annu Rev Immunol. 2018;36:435–59.
Yan W, Jiang S. Immune cell-derived exosomes in the cancer-immunity cycle. Trends Cancer. 2020;6:506–17.
Choo YW, Kang M, Kim HY, Han J, Kang S, Lee JR, Jeong GJ, Kwon SP, Song SY, Go S, et al. M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano. 2018;12:8977–93.
Matsumoto A, Asuka M, Takahashi Y, Takakura Y. Antitumor immunity by small extracellular vesicles collected from activated dendritic cells through effective induction of cellular and humoral immune responses. Biomaterials. 2020;252:120112.
Nikfarjam S, Rezaie J, Kashanchi F, Jafari R. Dexosomes as a cell-free vaccine for cancer immunotherapy. J Exp Clin Cancer Res. 2020;39:258.
Pitt JM, André F, Amigorena S, Soria JC, Eggermont A, Kroemer G, Zitvogel L. Dendritic cell-derived exosomes for cancer therapy. J Clin Invest. 2016;126:1224–32.
Fu W, Lei C, Liu S, Cui Y, Wang C, Qian K, Li T, Shen Y, Fan X, Lin F, et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat Commun. 2019;10:4355.
Ugel S, Canè S, De Sanctis F, Bronte V. Monocytes in the tumor microenvironment. Annu Rev Pathol. 2021;16:93–122.
DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369–82.
Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74–88.
Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111.
Zhou W, Zhou Y, Chen X, Ning T, Chen H, Guo Q, Zhang Y, Liu P, Zhang Y, Li C, et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268:120546.
Chang M, Hou Z, Wang M, Li C, Lin J. Recent advances in hyperthermia therapy-based synergistic immunotherapy. Adv Mater. 2021;33:e2004788.
Alzeibak R, Mishchenko TA, Shilyagina NY, Balalaeva IV, Vedunova MV, Krysko DV. Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future. J Immunother Cancer. 2021. https://doi.org/10.1136/jitc-2020-001926.
Pinto A, Marangon I, Méreaux J, Nicolás-Boluda A, Lavieu G, Wilhelm C, Sarda-Mantel L, Silva AKA, Pocard M, Gazeau F. Immune reprogramming precision photodynamic therapy of peritoneal metastasis by scalable stem-cell-derived extracellular vesicles. ACS Nano. 2021;15:3251–63.
Cheng L, Zhang X, Tang J, Lv Q, Liu J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials. 2021;275:120964.
Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49.
Jones KI, Tiersma J, Yuzhalin AE, Gordon-Weeks AN, Buzzelli J, Im JH, Muschel RJ. Radiation combined with macrophage depletion promotes adaptive immunity and potentiates checkpoint blockade. EMBO Mol Med. 2018. https://doi.org/10.15252/emmm.201809342.
Wu Q, Zhou W, Yin S, Zhou Y, Chen T, Qian J, Su R, Hong L, Lu H, Zhang F, et al. Blocking triggering receptor expressed on myeloid cells-1-positive tumor-associated macrophages induced by hypoxia reverses immunosuppression and anti-programmed cell death ligand 1 resistance in liver cancer. Hepatology. 2019;70:198–214.
Wei Z, Zhang X, Yong T, Bie N, Zhan G, Li X, Liang Q, Li J, Yu J, Huang G, et al. Boosting anti-PD-1 therapy with metformin-loaded macrophage-derived microparticles. Nat Commun. 2021;12:440.
Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21:360–78.
Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18:168–82.
Morishita M, Takahashi Y, Matsumoto A, Nishikawa M, Takakura Y. Exosome-based tumor antigens-adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials. 2016;111:55–65.
Damo M, Wilson DS, Simeoni E, Hubbell JA. TLR-3 stimulation improves anti-tumor immunity elicited by dendritic cell exosome-based vaccines in a murine model of melanoma. Sci Rep. 2015;5:17622.
Wahlund CJE, Güclüler G, Hiltbrunner S, Veerman RE, Näslund TI, Gabrielsson S. Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci Rep. 2017;7:17095.
Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600.
Abdulrahman Z, de Miranda N, van Esch EMG, de Vos van Steenwijk PJ, Nijman HW, Marij JPW, van Poelgeest MIE, van der Burg SH. Pre-existing inflammatory immune microenvironment predicts the clinical response of vulvar high-grade squamous intraepithelial lesions to therapeutic HPV16 vaccination. J Immunother Cancer. 2020. https://doi.org/10.1136/jitc-2020-000563.
Ott PA, Hu-Lieskovan S, Chmielowski B, Govindan R, Naing A, Bhardwaj N, Margolin K, Awad MM, Hellmann MD, Lin JJ, et al. A Phase Ib trial of personalized neoantigen therapy plus anti-PD-1 in patients with advanced melanoma, non-small cell lung cancer, or bladder cancer. Cell. 2020;183:347-362.e324.
Koerner J, Horvath D, Herrmann VL, MacKerracher A, Gander B, Yagita H, Rohayem J, Groettrup M. PLGA-particle vaccine carrying TLR3/RIG-I ligand Riboxxim synergizes with immune checkpoint blockade for effective anti-cancer immunotherapy. Nat Commun. 2021;12:2935.
Phung CD, Pham TT, Nguyen HT, Nguyen TT, Ou W, Jeong JH, Choi HG, Ku SK, Yong CS, Kim JO. Anti-CTLA-4 antibody-functionalized dendritic cell-derived exosomes targeting tumor-draining lymph nodes for effective induction of antitumor T-cell responses. Acta Biomater. 2020;115:371–82.
Cao Y, Wu T, Zhang K, Meng X, Dai W, Wang D, Dong H, Zhang X. Engineered exosome-mediated near-infrared-II region V(2)C quantum dot delivery for nucleus-target low-temperature photothermal therapy. ACS Nano. 2019;13:1499–510.
Zhou Y, Yu W, Cao J, Gao H. Harnessing carbon monoxide-releasing platforms for cancer therapy. Biomaterials. 2020;255:120193.
Zhu D, Liu Z, Li Y, Huang Q, Xia L, Li K. Delivery of manganese carbonyl to the tumor microenvironment using tumor-derived exosomes for cancer gas therapy and low dose radiotherapy. Biomaterials. 2021;274:120894.
Ding J, Lu G, Nie W, Huang LL, Zhang Y, Fan W, Wu G, Liu H, Xie HY. Self-activatable photo-extracellular vesicle for synergistic trimodal anticancer therapy. Adv Mater. 2021;33:e2005562.
Wang J, Chen P, Dong Y, Xie H, Wang Y, Soto F, Ma P, Feng X, Du W, Liu BF. Designer exosomes enabling tumor targeted efficient chemo/gene/photothermal therapy. Biomaterials. 2021;276:121056.
Grangier A, Branchu J, Volatron J, Piffoux M, Gazeau F, Wilhelm C, Silva AKA. Technological advances towards extracellular vesicles mass production. Adv Drug Deliv Rev. 2021;176:113843.
Mitchell JP, Court J, Mason MD, Tabi Z, Clayton A. Increased exosome production from tumour cell cultures using the Integra CELLine Culture System. J Immunol Methods. 2008;335:98–105.
de Almeida FM, Bernardes N, Oliveira FD, Costa AC, Fernandes-Platzgummer A, Farinha JP, Rodrigues CAV, Jung S, Tseng RJ, Milligan W, et al. Scalable production of human mesenchymal stromal cell-derived extracellular vesicles under serum-/xeno-free conditions in a microcarrier-based bioreactor culture system. Front Cell Dev Biol. 2020;8:553444.
Haraszti RA, Miller R, Stoppato M, Sere YY, Coles A, Didiot MC, Wollacott R, Sapp E, Dubuke ML, Li X, et al. Exosomes produced from 3D cultures of mscs by tangential flow filtration show higher yield and improved activity. Mol Ther. 2018;26:2838–47.
Piffoux M, Silva AKA, Lugagne JB, Hersen P, Wilhelm C, Gazeau F. Extracellular vesicle production loaded with nanoparticles and drugs in a trade-off between loading, yield and purity: towards a personalized drug delivery system. Adv Biosyst. 2017;1:e1700044.
Yuana Y, Oosterkamp TH, Bahatyrova S, Ashcroft B, Garcia Rodriguez P, Bertina RM, Osanto S. Atomic force microscopy: a novel approach to the detection of nanosized blood microparticles. J Thromb Haemost. 2010;8:315–23.
Nizamudeen Z, Markus R, Lodge R, Parmenter C, Platt M, Chakrabarti L, Sottile V. Rapid and accurate analysis of stem cell-derived extracellular vesicles with super resolution microscopy and live imaging. Biochim Biophys Acta Mol Cell Res. 2018;1865:1891–900.
Corso G, Heusermann W, Trojer D, Görgens A, Steib E, Voshol J, Graff A, Genoud C, Lee Y, Hean J, et al. Systematic characterization of extracellular vesicle sorting domains and quantification at the single molecule—single vesicle level by fluorescence correlation spectroscopy and single particle imaging. J Extracell Vesicles. 2019;8:1663043.
Choi D, Montermini L, Jeong H, Sharma S, Meehan B, Rak J. Mapping subpopulations of cancer cell-derived extracellular vesicles and particles by nano-flow cytometry. ACS Nano. 2019;13:10499–511.
Morales-Kastresana A, Telford B, Musich TA, McKinnon K, Clayborne C, Braig Z, Rosner A, Demberg T, Watson DC, Karpova TS, et al. Labeling extracellular vesicles for nanoscale flow cytometry. Sci Rep. 1878;2017:7.
Daaboul GG, Gagni P, Benussi L, Bettotti P, Ciani M, Cretich M, Freedman DS, Ghidoni R, Ozkumur AY, Piotto C, et al. Digital detection of exosomes by interferometric imaging. Sci Rep. 2016;6:37246.
Silva AM, Lázaro-Ibáñez E, Gunnarsson A, Dhande A, Daaboul G, Peacock B, Osteikoetxea X, Salmond N, Friis KP, Shatnyeva O, Dekker N. Quantification of protein cargo loading into engineered extracellular vesicles at single-vesicle and single-molecule resolution. J Extracell Vesicles. 2021;10:e12130.
Acknowledgements
Not applicable
Funding
This work was supported by Grants from the National Major Scientific and Technological Special Project for “Significant New Drugs Development” (No.: 2019ZX09301-001), the National Science Foundation of China (No.: 81770096, 81802113), Key Laboratory Open Fund of Hubei Province (No.: F016.02004.20003.082).
Author information
Authors and Affiliations
Contributions
JC, QT, ZY were major contributor in writing the manuscript. YJ directed the writing and revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, J., Tan, Q., Yang, Z. et al. Engineered extracellular vesicles: potentials in cancer combination therapy. J Nanobiotechnol 20, 132 (2022). https://doi.org/10.1186/s12951-022-01330-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-022-01330-y