Abstract
Background
The triglyceride glucose (TyG) index is a cutting-edge and highly effective marker of insulin resistance, a crucial factor in the development and exacerbation of diabetic kidney disease (DKD). To date, there has been limited research on how the triglyceride-glucose (TyG) index affects the outlook for patients suffering from DKD.
Methods
In this multicenter retrospective cohort study, the analysis recruited 2,203 DKD patients from the National Health and Nutrition Examination Survey (NHANES) dataset, which covers the US from 2001 to 2018. The research applied a Cox proportional hazards model with multiple variables to investigate the association of the TyG index with mortality outcomes. Restricted cubic splines (RCS) and methods for analyzing threshold effects were employed to identify possible non-linear relationships.
Results
Over nearly 19 years of follow-up, this study captured data on 753 all-cause and 231 cardiovascular disease-specific fatalities. Sophisticated statistical methods, including RCS and smoothing curve adjustments via penalized splines, helped identify distinctive patterns: The baseline TyG index was observed to have a U-shaped pattern related to overall mortality and an L-shape with cardiovascular diseases(CVD) mortality among individuals with DKD. Notably, TyG index below 9.15 for overall mortality and 9.27 for CVD mortality were linked to reduced death rates (HR = 0.65, 95% CI = 0.52–0.82 for all-cause; HR = 0.58, 95% CI = 0.43–0.83 for CVD). On the other hand, TyG index exceeding these benchmarks (greater than 9.15 for all-cause and 9.27 for CVD) correlated with increased all-cause mortality risks (HR = 1.21, 95% CI = 1.02–1.43) and showed a non-significant change in CVD mortality risks (HR = 1.07, 95% CI = 0.83–1.38).
Conclusions
This study emphasizes the non-linear linkage involving the TyG index and death rates due to CVD and other factors in patients with DKD, demonstrating its effectiveness in estimating potential adverse events within this demographic.
Similar content being viewed by others
Background
Type 2 diabetes mellitus (T2DM) has emerged as a significant global public health challenge. Epidemiological studies reveal that by 2021, the worldwide diabetes population had grown to 537 million. Predictions suggest this figure will escalate to 783 million by 2045 [1]. Furthermore, diabetic kidney disease (DKD), affecting 20–50% of individuals with T2DM, is a prevalent and costly complication arising from prolonged diabetes [2]. Moreover, DKD significantly increases the likelihood of all-cause mortality and cardiovascular disease (CVD) in contrast to individuals who do not have diabetes [3]. Specifically, DKD notably contributes to increased mortality in T2DM patients, representing an exceptionally high risk for younger individuals and accounting for a substantial portion of the increased risk in older patients [4, 5]. Therefore, identifying residual risk factors for DKD patients is critical in reducing death rates, especially from cardiovascular events. Additionally, insulin resistance (IR), a state of reduced insulin effectiveness, is linked to increased CVD risk in T2DM individuals [6, 7] and strongly correlates with DKD, thereby intensifying the associated CVD risk [8,9,10]. Given these findings, numerous studies have highlighted IR as a crucial prognostic indicator for adverse outcomes in DKD patients [11, 12].
Patients with IR often have compromised fasting blood glucose (FBG) and triglycerides (TG) levels. Introduced by Simental-Mendía et al. in 2008, the TyG index, which integrates fasting plasma glucose and triglyceride measurements, is critical in evaluating IR [13]. Its efficacy is on par with or surpasses conventional techniques like the hyperinsulinemic-euglycemic clamp test and Homeostatic Model Assessment for Insulin Resistance(HOMA-IR) [14, 15]. Moreover, the TyG index stands out for its accessibility, affordability, and reliability.
Despite its potential, the use of the TyG index to indicate IR and predict outcomes in DKD patients is still uncertain. The study aimed to assess its predictive power for overall mortality associated with cardiovascular disease in DKD individuals.
Methods
Participant demographics and study framework
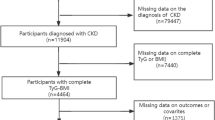
The National Health and Nutrition Examination Survey (NHANES) is carried out biennially as a cross-sectional analysis that mirrors the entire demographic range of Americans. The core focus is scrutinizing American adults' and children's wellness and dietary conditions through detailed interviews, physical assessments, nutritional evaluations, and laboratory investigations [16]. A sophisticated multi-stage, stratified sampling method was employed to select the study cohort, ensuring its national representation [17]. The execution of the NHANES was a collaborative effort between the Centers for Disease Control and Prevention (CDC) [16] and the National Center for Health Statistics (NCHS). The NCHS Research Ethics Review Board granted ethical clearance for the NHANES protocol, and written consent was secured from every individual involved. Between 2001 and 2018, 9 NHANES cycles saw the enrollment of 102,321 individuals. After excluding individuals under 20 years old (n = 45,402), pregnant individuals (n = 1,328), and those either without DKD or lacking relevant data on this condition (n = 50,986), a total of 4,695 adults with DKD were initially identified. Subsequently, after removing participants without TyG index data (n = 2,377) and those missing mortality information (n = 25), the final cohort included 2,203 eligible individuals (Fig. 1).
Definition of diabetic kidney disease and TyG
Diabetes mellitus was diagnosed following the guidelines established by the American Diabetes Association (ADA), encompassing:
-
1.
A prior diagnosis by a healthcare professional.
-
2.
FBG levels of 7. 0 mmol/L or higher.
-
3.
Glycosylated hemoglobin A1c (HbA1c) levels of 6. 5% or higher.
-
4.
Current use of diabetes medications or insulin.
Per the KDIGO 2021 Guidelines, DKD was characterized by the presence of diabetes mellitus along with a urinary albumin-to-creatinine ratio (UACR) ≧of 30 mg/g and/or an estimated glomerular filtration rate (eGFR) less than 60 mL/min/1. 73 m2 [18]. The TyG index was determined using the formula: TyG index = Ln [TG, (mg/dL) × FBG (mg/dL) / 2] [13, 19]. Participants in the study were divided into quartiles (Q1, Q2, Q3, and Q4) according to their scores on the TyG index, designating Q1 as the reference group.
Definition of mortality from any cause and cardiovascular-related Deaths
Participant survival was determined based on records of all-cause and CVD mortality sourced from the National Death Index (NDI) controlled by the CDC up to December 31, 2019, in the US [20]. Causes of death in these records adhere to the International Classification of Diseases, Tenth Revision (ICD-10). All-cause mortality includes fatalities due to any fundamental reason, while deaths attributed to cardiovascular issues are explicitly categorized using ICD-10 codes I00-I09, I11, I13, and I20-I51.
Covariates
Initial demographic data collection encompassed sex (male, female), age, among other variables. A questionnaire was administered to collect 'yes' or 'no' responses regarding smoking, alcohol consumption, hypertension, diabetic retinopathy, and CVD. Clinical parameters such as HbA1c, FBG, lipid profiles, and liver and kidney function indicators were obtained from the NHANES laboratory data. Calculation of the eGFR utilized the CKD-EPIscr (eGFRscr) formula [21]. For comprehensive details on demographic data, lipid profiles, liver and kidney function metrics, the formulas for BMI and its classification criteria, the equation for eGFRscr, definitions of CVD, and specific categories for smoking and alcohol use, please consult supplementary material 1.
Statistical analysis
Considering NHANES's intricate sampling structure, the study utilized sample weights, clustering, and stratification, adhering to the standards for NHANES data analysis [22].To mitigate potential bias due to missing data, the analysis utilized a multilevel approach designed for survey data to conduct multiple imputations [9, 10]. This study employed a Gibbs sampling procedure to generate ten interpolated datasets, preceded by 500 iterations of burn-in and followed by 100 updates. This process was designed to ensure random independence between the interpolated datasets.
To assess the normality of continuous variables, the Kolmogorov–Smirnov test was employed. Variables following a normal distribution were expressed as mean ± standard deviation (SD), whereas those not fitting a normal distribution were described using the median and interquartile range (IQR). Categorical variables were represented as numbers and percentages. For comparisons among continuous variables, the Wilcoxon rank sum test or t-test was employed, and categorical variables were analyzed using either the Pearson chi-square test or Fisher's exact test. To ascertain the TyG index's independent predictive capability, the analysis constructed a multivariate Cox proportional hazards regression model with four distinct models for adjusting confounding variables. The initial model was crude and unadjusted, while Model 1 incorporated adjustments for age, sex, race, and BMI. Model 2 further included adjustments for alcohol intake, smoking, retinopathy, hypertension, and CVD, building upon Model 1. Model 3 added HbA1c, LDL-C, eGFR, and ACR adjustments to Model 2. Additionally, this research utilized multiple Cox regression hazard models incorporating restricted cubic splines and curve smoothing (applying the penalized spline technique) to examine potential non-linear correlations linking the TyG index with mortality stemming from any cause or cardiovascular occurrences.
After identifying the non-linear association, the analysis determined the inflection points of the TyG index concerning both overall and CVD mortality. A recursive algorithm was used to do this. Subsequently, a bi-phasic approach to Cox proportional hazards was implemented at each key turning point, examining how the TyG index correlates with mortality risk from all causes and CVD. In sensitivity assessments, analyses were stratified by sex, age (either below 60 or 60 and older), ethnic background (White, Black, Mexican, or Other), and BMI categories (Normal weight, Low weight, Overweight, or Obese), as well as HbA1c levels (either < 6.5 or ≥ 6.5) and presence of CVD. All statistical analyses were conducted on R version 4.3.0 and the Empower (R)(www. empowerstats.com, X&Y Solutions, Inc., Boston MA), with findings deemed statistically significant at a two-sided P value less than 0.05.
Results
Subject characteristics
This cohort study enrolled 2,203 subjects aged 20 years and above, with an average age of approximately 66 years, and about 58% of them were male. Table 1 displayed the basic parameters organized by TyG index quartiles, showing an average TyG index of 9.26 ± 0.79 for participants. Laboratory characteristics segmented by TyG index quartiles were presented in Table 2. The observations indicated that participants with elevated TyG index were generally younger, were predominantly of Mexican ethnicity, and exhibited a greater prevalence of hypertension in contrast to their counterparts in the lowest quartile. Significant variations in biochemical parameters were also noted among the groups. Individuals with higher TyG index exhibited elevated concentrations of FBG, HbA1c, FINS, LDL-C, TC, TG, UACR, eGFR, AST, and ALT compared to their counterparts with lower TyG index, with all differences being statistically significant (P < 0. 01). Furthermore, notable differences were observed in Scr and BUN levels across the four groups (all P < 0. 05).
Correlations between TyG index values and mortality outcomes
Table 3 detailed the recording of 753 instances of all-cause mortality and 231 cases of deaths related to CVD throughout the follow-up period. To ascertain the TyG index's standalone correlation with mortality risks, the analysis utilized three distinct Cox regression models. Specifically, in Model 3, after adjusting for multiple confounders, the adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality across the TyG index quartiles varied from 1. 00 (reference) to 0. 79 (0. 64–0. 97), 0. 76 (0. 61–0. 93), and 0. 94 (0. 74–1. 18), resulting in a P trend of 0. 47. Regarding CVD mortality, the HRs for these quartiles stood at 0. 78 (0. 54–1. 12), 0. 59 (0. 40–0. 87), and 0. 84 (0. 55–1. 28), with a P trend of 0. 24.
Identifying non-linear associations
In the initial multivariate analysis regarding the TyG index, a non-linear relationship emerged between mortality threats from all causes and CVD. Additional analysis of this correlation was performed using a Cox proportional hazards regression analysis that included RCS and curve smoothing, explicitly employing the penalized spline technique. Intriguingly, the adjusted plots revealed distinct patterns: a U-shaped pattern related to mortality from all causes (as depicted in Fig. 2A) and an L-shaped correlation for CVD mortality (Fig. 2B).
Association between TyG index and all-cause (A) and CVD mortality (B) in T2DM patients with diabetic kidney disease. Each hazard ratio was computed with a TyG index level of A 9.15 and B 9.27 as the reference. Adjusted for age, sex, race, BMI, alcohol intake, smoking, retinopathy, Hypertension, CVD, HbA1c, LDL-C, eGFR and UACR. The solid line and blue area represent the estimated values and their corresponding 95% CIs, respectively (TyG index: triglyceride-glucose index; CI: Confidence interval)
The analysis employed the conventional Cox proportional hazards regression model and a two-piece variant to measure these associations. The latter identified critical inflection points at 9.15 for all-cause mortality and 9.27 for mortality due to CVD, both significant with log-likelihood ratio P-values under 0. 05, as detailed in Table 4. After accounting for factors such as age, sex, race, BMI, alcohol intake, smoking, retinopathy, hypertension, CVD, HbA1c, LDL-C, eGFR, and ACR, this study observed significant trends. Specifically, for every unit rise in the TyG index, there was a 35% decrease in the likelihood of all-cause mortality (HR 0.65, 95% CI = 0.52–0.82) and a 42% decrease in the likelihood of CVD mortality (HR 0.58, 95% CI = 0.43–0.83), until reaching the inflection points.
Furthermore, as illustrated in Table 4 and Fig. 2, the lowest mortality risks for all-cause and CVD were observed when the baseline TyG index reached these threshold values. Nonetheless, once the baseline TyG index surpassed 9. 15, a notable positive correlation with the likelihood of overall mortality was observed (HR 1. 21, 95% CI = 1. 02–1. 43). Conversely, for CVD mortality, no significant correlation was detected when the baseline TyG index surpassed 9. 27 (HR 1. 07, 95% CI = 0. 83–1. 38).
Subgroup and sensitivity analyses
Subgroup analyses comparing higher (≧ 9.15 for all-cause mortality and ≧ 9.27 for CVD mortality) and lower (< 9.15 for all-cause mortality and < 9.27 for CVD mortality) TyG index thresholds in DKD patients showed consistent survival impacts across all subgroups, including variations in sex, age, ethnicity, BMI, and HbA1c levels. Details were provided in Tables 5 and 6.
Moreover, the analysis found no significant interactions involving the TyG index with the factors used for stratification.
Discussion
This study is pioneering in investigating how the TyG index relates to mortality due to any cause and CVD in those with DKD. The findings highlight a notable non-linear relationship between initial TyG index measurements and the likelihood of mortality from any cause and cardiovascular complications in this patient group. It shows a U-shaped pattern for overall mortality and an L-shaped pattern for CVD mortality. Additionally, this research determined their respective turning points by threshold effect analysis (all-cause mortality: 9. 15, CVD mortality: 9. 27). Briefly, this research highlighted the TyG index's utility in predicting the likelihood of overall mortality among DKD patients, helping to identify those at higher risk and possibly guiding more targeted tests and treatments.
Extensive studies has recognized the TyG index as an important indicator of poor health outcomes in both the general population and diverse patient groups [23,24,25]. For instance, a meta-analysis of 12 cohort studies suggested that higher TyG index levels may be connected to an increased risk of CVD among the overall population. However, no link was identified with all-cause mortality [26]. Furthermore, a study conducted by Chen et al. found that the TyG index was positively correlated with cardiovascular mortality in individuals under the age of 65, while a nonlinear relationship was observed concerning all-cause mortality [27]. Regarding the specific population of patients with DKD, existing studies have underscored the TyG index's role in predicting and assessing the risk of DKD development within T2DM cohorts [28,29,30]. Nevertheless, its capacity to forecast long-term adverse outcomes in DKD patients remains inadequately investigated.
To address this research gap, this study examined 2,203 patients with prevalent DKD from a nationally representative U.S. sample. The research sought to identify the relationship of the TyG index with both overall and cardiovascular-specific mortality in this group. These findings indicated that a TyG index meeting or exceeding the cutoff is significantly linked to increased all-cause mortality among DKD patients, in contrast to those with lower TyG index values. This emphasized the significance of the TyG index for identifying those at greater risk of mortality within this demographic. Known as a novel and economic marker for IR—a well-documented risk factor for CVD in general and diabetic populations—the TyG index continues to prove its significance. It also forecasts cardiovascular outcomes in CVD patients [31, 32]. IR is likely a crucial mechanism in this context, as it is a notable marker for various conditions, including obesity, hypertension, and dyslipidemia, alongside other metabolic syndrome features and metabolic disturbances triggered by inflammation and oxidative stress, all contributing to diverse adverse health outcomes [33,34,35]. Moreover, IR intensifies platelet activity and the expression of thromboxane A2-dependent tissue factor, fostering thrombosis and increased inflammation [36]. It also accelerates TG hydrolysis, leading to excessive reactive oxygen species (ROS) production, mitochondrial damage, and functional decline, all implicated in the genesis and progression of adverse health events [37].
IR is critical in the development and progression of DKD. T2DM is inherently associated with IR, and studies indicate that individuals with T2DM who also have DKD exhibit a higher likelihood of IR than those without DKD [38]. Beyond the traditional mechanisms by which IR can worsen DKD (such as hyperglycemia, hyperinsulinemia, and hyperlipidemia), recent research has unveiled that IR can directly impair kidney function at the cellular level [39]. Specifically, it can disrupt normal insulin signaling in glomerular podocytes and key insulin-responsive cells in the kidneys, leading to irreversible renal damage [40]. These findings recognize the TyG index as an autonomous marker of negative outcomes in DKD patients, underscoring its importance in prognostic evaluations.
Unexpectedly, These findings showed that lower TyG index values (below 9.15 for all-cause deaths and 9.27 for CVD-related deaths) significantly impacted the association of the TyG index with mortality risks from both all causes and CVD. After adjusting for other variables, each increment in the TyG index corresponded to an approximately 35% reduction in all-cause mortality risk and a 42% decrease in the likelihood of CVD-related death. A lower TyG index in patients with DKD might indicate low blood sugar or lipid levels rather than just IR. Several plausible explanations could account for this counterintuitive relationship. Initially, studies suggest that lower TG or fasting plasma glucose levels are associated with negative health outcomes within specific ranges, with deficient levels possibly reflecting inadequate nutritional status [41,42,43].
Additionally, hypoglycemia may induce arrhythmias, thrombosis, vascular inflammation, and vasoconstriction, which could escalate the risk of cardiovascular incidents or mortality [44]. Moreover, low total cholesterol levels have been recognized as a crucial indicator of cardiogenic death in heart failure patients [45]. Thus, evaluating the TyG index, especially in those at the extreme ends of the spectrum, can enhance risk stratification for adverse events within this group.
However, once the TyG index surpassed the 9.27 threshold, its connection to CVD mortality was not apparent in the DKD group. While the TyG index's capability in assessing CVD mortality across different populations is widely recognized, some studies have yet to reach consistent conclusions. A case in point is a study with 3,614 hypertensive subjects that did not establish a significant correlation with CVD mortality in middle-aged and senior hypertensive populations [42, 46, 47]. The analysis suspects several factors could explain these discrepancies: Firstly, the average age of the DKD population in this study was 65.97, and the elderly naturally have a higher susceptibility to CVD. Secondly, as this inaugural US cohort study reveals an L-shaped connection of the TyG index related to CVD in patients with DKD, insights into the TyG index's linkage to CVD mortality within this demographic are scant and naturally limited by the study's sample size. Finally, the retrospective design of this research, despite adjustments for confounders, might only account for some unmeasured confounding variables, suggesting the necessity for broader studies to deepen the understanding.
Strengths and limitations
The study's merit is grounded in the novel examination of how the TyG index correlates with outcomes for those affected by DKD. With a longer follow-up and a significant sample size, the analysis discerned both U-shaped and L-shaped patterns as critical points for DKD patients. Nonetheless, it is essential to consider certain inherent limitations of this research: First, given that this study is a retrospective cohort study, the inherent characteristics of retrospective studies may encounter specific biases, including the potential for unmeasured confounding factors. Second, the study's need for ongoing tracking of the TyG index limits the assessment of how changes in this biomarker might influence its connection with particular mortality causes over time. Prior research has implied that the aggregate and fluctuating levels of TyG could be pivotal in recognizing individuals at elevated cardiovascular risk [48, 49]. Third, this study only included DKD patients from the US, so future research needs to verify the generalizability of these findings to patients in other countries.
Conclusions
The results underscore the significance of the TyG index as a crucial measure for overall and cardiovascular mortality in individuals with DKD, revealing a non-linear linkage with mortality rates. Consequently, utilizing the TyG index for risk assessment in these individuals could provide new perspectives and guide future investigations focused on forecasting adverse events in the DKD population.
Availability of data and materials
The datasets generated and analyzed during the current study are available in the NHANES repository (https://www.cdc.gov/nchs/nhanes/index.htm).
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- ADA :
-
American Diabetes Association
- ALT:
-
Alanine aminotransferase
- AST :
-
Aspartate aminotransferase
- BMI:
-
Body Mass Index
- BUN :
-
Blood Urea Nitrogen
- CI :
-
Confidence interval
- CVD :
-
Cardiovascular Disease
- CDC :
-
Centers for Disease Control and Prevention
- DKD :
-
Diabetic Kidney Disease
- eGFR :
-
Estimated Glomerular Filtration Rate
- FBG:
-
Fasting blood glucose
- FINS:
-
Fasting insulin
- HbA1c :
-
Glycosylated hemoglobin A1c
- HDL-C :
-
High-density lipoprotein cholesterol
- HR:
-
Hazard ratio
- HOMA-IR:
-
Homeostatic Model Assessment for Insulin Resistance
- IR :
-
Insulin Resistance
- IQR:
-
Interquartile Range
- ICD-10 :
-
International Classification of Diseases, Tenth Revision
- LDL-C :
-
Low-density lipoprotein cholesterol
- NDI :
-
National Death Index
- NCHS :
-
National Center for Health Statistics
- NHANES :
-
National Health and Nutrition Examination Survey
- PIR:
-
Poverty Income Ratio
- RCS :
-
Restricted Cubic Splines
- ROS :
-
Reactive Oxygen Species
- Scr :
-
Serum creatinine
- SD :
-
Standard Deviation
- TyG :
-
Triglyceride-glucose
- T2DM :
-
Type 2 diabetes mellitus
- TG :
-
Triglycerides
- TC :
-
Total Cholesterol
- UACR :
-
Urinary Albumin-to-creatinine Ratio
References
Diabetes around the world in 2021: The IDF Diabetes Atlas 10th Edition ed: International Diabetes Federation:Available from https://diabetesatlas.org/.
Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183: 109119. https://doi.org/10.1016/j.diabres.2021.109119. [publishedOnlineFirst:20211206].
Rao KondapallySeshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–41. https://doi.org/10.1056/NEJMoa1008862.
Tancredi M, Rosengren A, Svensson A-M, et al. Excess Mortality among Persons with Type 2 Diabetes. N Engl J Med. 2015;373(18):1720–32. https://doi.org/10.1056/NEJMoa1504347.
Penno G, Solini A, Bonora E, et al. Defining the contribution of chronic kidney disease to all-cause mortality in patients with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian Multicenter Study. Acta Diabetol. 2018;55(6):603–12. https://doi.org/10.1007/s00592-018-1133-z. [publishedOnlineFirst:20180324].
Patel TP, Rawal K, Bagchi AK, et al. Insulin resistance: an additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail Rev. 2016;21(1):11–23. https://doi.org/10.1007/s10741-015-9515-6.
Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293–302. https://doi.org/10.1038/nrendo.2014.29.
Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol. 2016;311(6):F1087–108. https://doi.org/10.1152/ajprenal.00340.2016.
Quartagno M, Carpenter JR, Goldstein H. Multiple imputation with survey weights: a multilevel approach. J Surv Stat Methodol. 2020;8(5):965–89. https://doi.org/10.1093/jssam/smz036.
Quartagno M, Grund S, Carpenter J. jomo: A Flexible Package for Two-level Joint Modelling Multiple Imputation. R Journal 2019;11(2) https://doi.org/10.32614/rj-2019-028.
Penno G, Solini A, Orsi E, et al. Insulin resistance, diabetic kidney disease, and all-cause mortality in individuals with type 2 diabetes: a prospective cohort study. BMC Med. 2021;19(1):66. https://doi.org/10.1186/s12916-021-01936-3.
Nerpin E, Riserus U, Ingelsson E, et al. Insulin sensitivity measured with euglycemic clamp is independently associated with glomerular filtration rate in a community-based cohort. Diabetes Care. 2008;31(8):1550–5. https://doi.org/10.2337/dc08-0369[publishedOnlineFirst:20080528].
Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. https://doi.org/10.1089/met.2008.0034.
Chamroonkiadtikun P, Ananchaisarp T, Wanichanon W. The triglyceride-glucose index, a predictor of type 2 diabetes development: a retrospective cohort study. Prim Care Diabetes. 2020;14(2):161–7. https://doi.org/10.1016/j.pcd.2019.08.004. [publishedOnlineFirst:20190826].
Park HM, Lee HS, Lee Y-J, et al. The triglyceride–glucose index is a more powerful surrogate marker for predicting the prevalence and incidence of type 2 diabetes mellitus than the homeostatic model assessment of insulin resistance. Diabetes Research and Clinical Practice 2021;180 https://doi.org/10.1016/j.diabres.2021.109042.
Prevention CfDCa. Available from: https://www.cdc.gov/nchs/nhanes/index.htm.
Wolffenbuttel BHR, Heiner-Fokkema MR, Green R, et al. Relationship between serum B12 concentrations and mortality: experience in NHANES. BMC Med. 2020;18(1):307. https://doi.org/10.1186/s12916-020-01771-y. [publishedOnlineFirst:20201009].
Rovin BH, Adler SG, Barratt J, et al. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):S1–276. https://doi.org/10.1016/j.kint.2021.05.021.
Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51. https://doi.org/10.1210/jc.2010-0288. [published Online First: 20100519].
Files. P-ULM. Available from: https://www.cdc.gov/nchs/data-linkage/mortality-public.htm.
Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. https://doi.org/10.7326/0003-4819-150-9-200905050-00006.
(NHANES) Analytic and Reporting Guidelines Centers for Disease Control Prevention Atlanta. GA: CDC: The National Health and Nutrition Examination Survey, 2006.
Dang K, Wang X, Hu J, et al. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003–2018. Cardiovasc Diabetol. 2024;23(1) https://doi.org/10.1186/s12933-023-02115-9.
Xiong S, Chen Q, Zhang Z, et al. A synergistic effect of the triglyceride-glucose index and the residual SYNTAX score on the prediction of intermediate-term major adverse cardiac events in patients with type 2 diabetes mellitus undergoing percutaneous coronary intervention. Cardiovasc Diabetol. 2022;21(1) https://doi.org/10.1186/s12933-022-01553-1.
Cai W, Xu J, Wu X, et al. Association between triglyceride-glucose index and all-cause mortality in critically ill patients with ischemic stroke: analysis of the MIMIC-IV database. Cardiovasc Diabetol. 2023;22(1) https://doi.org/10.1186/s12933-023-01864-x.
Liu X, Tan Z, Huang Y, et al. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1) https://doi.org/10.1186/s12933-022-01546-0.
Chen J, Wu K, Lin Y, et al. Association of triglyceride glucose index with all-cause and cardiovascular mortality in the general population. Cardiovasc Diabetol. 2023;22(1):320. https://doi.org/10.1186/s12933-023-02054-5. [publishedOnlineFirst:20231122].
Li HF, Miao X, Li Y. The Triglyceride Glucose (TyG) Index as a Sensible Marker for Identifying Insulin Resistance and Predicting Diabetic Kidney Disease. Med Sci Monit. 2023;29:e939482. https://doi.org/10.12659/MSM.939482. [publishedOnlineFirst:20230708].
Liu L, Xia R, Song X, et al. Association between the triglyceride-glucose index and diabetic nephropathy in patients with type 2 diabetes: a cross-sectional study. J Diabetes Investig. 2021;12(4):557–65. https://doi.org/10.1111/jdi.13371. [publishedOnlineFirst:20200907].
Kassab HS, Osman NA, Elrahmany SM. Assessment of triglyceride-glucose index and ratio in patients with type 2 diabetes and their relation to microvascular complications. Endocr Res. 2023;48(4):94–100. https://doi.org/10.1080/07435800.2023.2245909.
Son DH, Lee HS, Lee YJ, et al. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2022;32(3):596–604. https://doi.org/10.1016/j.numecd.2021.11.017. [publishedOnlineFirst:20211207].
Nam KW, Kwon HM, Lee YS. High triglyceride-glucose index is associated with early recurrent ischemic lesion in acute ischemic stroke. Sci Rep. 2021;11(1):15335. https://doi.org/10.1038/s41598-021-94631-5. [publishedOnlineFirst:20210728].
Tao L-C, Xu J-n, Wang T-t, et al. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1) https://doi.org/10.1186/s12933-022-01511-x.
Yang Q, Vijayakumar A, Kahn BB. Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cell Biol. 2018;19(10):654–72. https://doi.org/10.1038/s41580-018-0044-8.
Zhang Y, Ding X, Hua B, et al. Predictive effect of triglyceride‑glucose index on clinical events in patients with type 2 diabetes mellitus and acute myocardial infarction: results from an observational cohort study in China. Cardiovasc Diabetol 2021;20(1) https://doi.org/10.1186/s12933-021-01236-3.
Gerrits AJ, Koekman CA, van Haeften TW, et al. Platelet tissue factor synthesis in type 2 diabetic patients is resistant to inhibition by insulin. Diabetes. 2010;59(6):1487–95. https://doi.org/10.2337/db09-1008. [publishedOnlineFirst:20100303].
Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301(6):H2181–90. https://doi.org/10.1152/ajpheart.00554.2011.
Parvanova AI, Trevisan R, Iliev IP, et al. Insulin Resistance and Microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006;55(5):1456–62. https://doi.org/10.2337/db05-1484.
Filippone EJ, Gupta A, Farber JL. Normoglycemic diabetic nephropathy: the role of insulin resistance. Case Rep Nephrol Urol. 2014;4(2):137–43. https://doi.org/10.1159/000364901[publishedOnlineFirst:20140628].
Jiang A, Song A, Zhang C. Modes of podocyte death in diabetic kidney disease: an update. J Nephrol. 2022;35(6):1571–84. https://doi.org/10.1007/s40620-022-01269-1.
Abbasi F, Reaven GM. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides x glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism. 2011;60(12):1673–6. https://doi.org/10.1016/j.metabol.2011.04.006. [publishedOnlineFirst:20110531].
Zhang Q, Xiao S, Jiao X, et al. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001–2018. Cardiovasc Diabetol. 2023;22(1) https://doi.org/10.1186/s12933-023-02030-z.
Li H, Jiang Y, Su X, et al. The triglyceride glucose index was U-shape associated with all-cause mortality in population with cardiovascular diseases. Diabetol Metab Syndr. 2023;15(1) https://doi.org/10.1186/s13098-023-01153-3.
Rist PM, Buring JE, Ridker PM, et al. Lipid levels and the risk of hemorrhagic stroke among women. Neurology 2019;92(19) https://doi.org/10.1212/wnl.0000000000007454.
Kozdag G, Ertas G, Emre E, et al. Low serum triglyceride levels as predictors of cardiac death in heart failure patients. Tex Heart Inst J. 2013;40(5):521–8.
Liu Q, Zhang Y, Chen S, et al. Association of the triglyceride-glucose index with all-cause and cardiovascular mortality in patients with cardiometabolic syndrome: a national cohort study. Cardiovasc Diabetol. 2024;23(1) https://doi.org/10.1186/s12933-024-02152-y.
Pang J, Qian L, Che X, et al. TyG index is a predictor of all-cause mortality during the long-term follow-up in middle-aged and elderly with hypertension. Clin Exp Hypertens. 2023;45(1) https://doi.org/10.1080/10641963.2023.2272581.
Zheng H, Chen G, Wu K, et al. Relationship between cumulative exposure to triglyceride-glucose index and heart failure: a prospective cohort study. Cardiovasc Diabetol. 2023;22(1) https://doi.org/10.1186/s12933-023-01967-5.
Tai S, Fu L, Zhang N, et al. Association of the cumulative triglyceride-glucose index with major adverse cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2022;21(1) https://doi.org/10.1186/s12933-022-01599-1.
Acknowledgements
The authors sincerely thank the participants and the staff of the NHANES for their essential contributions to this research.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
HZ conceived the study design. LW, YS, and QZ were involved in data curation, while HZ and LW conducted the data analysis. HZ and QY wrote and edited the manuscript. JB and MC contributed to interpreting the results, revising the manuscript, and finalizing it. All authors have reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol for NHANES received approval from the National Center for Health Statistics and its Ethics Review Board. All participants gave written informed consent. Since the data are publicly accessible, the need for an ethical approval statement and informed consent was exempted for this research.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, H., Wang, L., Zhang, Q. et al. Non-linear association of triglyceride-glucose index with cardiovascular and all-cause mortality in T2DM patients with diabetic kidney disease: NHANES 2001–2018 retrospective cohort study. Lipids Health Dis 23, 253 (2024). https://doi.org/10.1186/s12944-024-02249-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02249-z