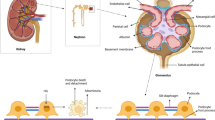
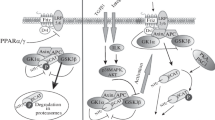
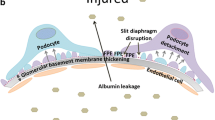
Abstract
Diabetic kidney disease (DKD) accounts for a large proportion of end-stage renal diseases that require renal replacement therapies including dialysis and transplantation. Therefore, it is critical to understand the occurrence and development of DKD. Podocytes are mainly injured during the development of DKD, ultimately leading to their extensive death and loss. In turn, the injury and death of glomerular podocytes are also the main culprits of DKD. This review introduces the characteristics of podocytes and summarizes the modes of their death in DKD, including apoptosis, autophagy, mitotic catastrophe (MC), anoikis, necroptosis, and pyroptosis. Apoptosis is characterized by nuclear condensation and the formation of apoptotic bodies, and it exerts a different effect from autophagy in mediating DKD-induced podocyte loss. MC mediates a faulty mitotic process while anoikis separates podocytes from the basement membrane. Moreover, pyroptosis activates inflammatory factors to aggravate podocyte injuries whilst necroptosis drives signaling cascades, such as receptor-interacting protein kinases 1 and 3 and mixed lineage kinase domain-like, ultimately promoting the death of podocytes. In conclusion, a thorough knowledge of the modes of podocyte death in DKD can help us understand the development of DKD and lay the foundation for strategies in DKD disease therapy.
Graphical abstract




Similar content being viewed by others
Abbreviations
- AGEs:
-
Advanced glycation end products
- Ang II:
-
Angiotensin II
- AOPPs:
-
Advanced oxidation protein products
- AMPK:
-
AMP -activated protein kinase
- BASP1:
-
Brain acid-soluble protein 1
- CB1R:
-
Cannabinoid receptor 1
- Cdk5:
-
Cyclin-dependent kinase 5
- CD2AP:
-
CD2-associated protein
- DKD:
-
Diabetic kidney disease
- EMT:
-
Epithelial-mesenchymal transformation
- ERS:
-
Endoplasmic reticulum stress
- EVs:
-
Extracellular vesicles
- FOXO4:
-
Forkhead box O4
- FSGS:
-
Focal segmental glomerulosclerosis
- FSP1:
-
Fibroblast-specific protein 1
- GBM:
-
Glomerular basement membrane
- GSDMD:
-
Gasdermin D
- GSK3β:
-
Glycogen synthase kinase-3β
- HG:
-
High glucose
- LN:
-
Lupus nephritis
- LRP6:
-
Lipoprotein receptor-related protein 6
- MAD:
-
Mitotic arrest deficiency
- MC:
-
Mitotic catastrophe
- MCNS:
-
Minimal change nephrotic syndrome
- MDM2:
-
Murine double minute 2
- miRNAs:
-
MicroRNAs
- MLKL:
-
Mixed lineage kinase domain-like
- mTOR:
-
Mammalian target of rapamycin
- NLRP3:
-
Nucleotide-oligomerization domain-like receptor 3
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- PI3-K:
-
Phosphoinositide 3-kinase
- RAGE:
-
Receptors for AGEs
- RARRES1:
-
Retinoic acid receptor responder protein 1
- RIPK1:
-
Receptor-interacting protein kinase 1
- ROS:
-
Reactive oxygen species
- RIOK1:
-
RIO kinase 1
- TGF-β:
-
Transforming growth factor-β
- VEGF:
-
Vascular endothelial growth factor
- Wnt:
-
Wingless‐type
- WT1:
-
Wilms' tumor 1 transcription factor
References
Brosius FC, Tuttle KR, Kretzler M (2016) JAK inhibition in the treatment of diabetic kidney disease. Diabetologia 59(8):1624–1627. https://doi.org/10.1007/s00125-016-4021-5
Zhou D, Zhou M, Wang Z et al (2019) PGRN acts as a novel regulator of mitochondrial homeostasis by facilitating mitophagy and mitochondrial biogenesis to prevent podocyte injury in diabetic nephropathy. Cell Death Dis 10(7):524. https://doi.org/10.1038/s41419-019-1754-3
Denhez B, Lizotte F, Guimond MO et al (2015) Increased SHP-1 protein expression by high glucose levels reduces nephrin phosphorylation in podocytes. J Biol Chem 290(1):350–358. https://doi.org/10.1074/jbc.M114.612721
Manda G, Checherita AI, Comanescu MV, Hinescu ME (2015) Redox signaling in diabetic nephropathy: hypertrophy versus death choices in mesangial cells and podocytes. Mediators Inflamm 2015:604208. https://doi.org/10.1155/2015/604208
Li JJ, Kwak SJ, Jung DS et al (2007) Podocyte biology in diabetic nephropathy. Kidney Int Suppl 106:S36-42. https://doi.org/10.1038/sj.ki.5002384
Wang X, Liu J, Zhen J et al (2014) Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney Int 86(4):712–725. https://doi.org/10.1038/ki.2014.111
Tuncdemir M, Ozturk M (2011) The effects of angiotensin-II receptor blockers on podocyte damage and glomerular apoptosis in a rat model of experimental streptozotocin-induced diabetic nephropathy. Acta Histochem 113(8):826–832. https://doi.org/10.1016/j.acthis.2010.12.003
Altintas MM, Reiser J (2019) Podocytes: way to go. Am J Pathol 189(2):226–228. https://doi.org/10.1016/j.ajpath.2018.11.003
Lin JS, Susztak K (2016) Podocytes: the weakest link in diabetic kidney disease? Curr Diab Rep 16(5):45. https://doi.org/10.1007/s11892-016-0735-5
Mathew S, Chen X, Pozzi A, Zent R (2012) Integrins in renal development. Pediatr Nephrol 27(6):891–900. https://doi.org/10.1007/s00467-011-1890-1
Pavenstadt H, Kriz W, Kretzler M (2003) Cell biology of the glomerular podocyte. Physiol Rev 83(1):253–307. https://doi.org/10.1152/physrev.00020.2002
Saleem MA, O’Hare MJ, Reiser J et al (2002) A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13(3):630–638. https://doi.org/10.1681/ASN.V133630
Moreno JA, Sanchez-Nino MD, Sanz AB et al (2008) A slit in podocyte death. Curr Med Chem 15(16):1645–1654. https://doi.org/10.2174/092986708784911542
Liu M, Liang K, Zhen J et al (2017) Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat Commun 8(1):413. https://doi.org/10.1038/s41467-017-00498-4
Anil Kumar P, Welsh GI, Saleem MA, Menon RK (2014) Molecular and cellular events mediating glomerular podocyte dysfunction and depletion in diabetes mellitus. Front Endocrinol (Lausanne) 5:151. https://doi.org/10.3389/fendo.2014.00151
Wolf G, Chen S, Ziyadeh FN (2005) From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 54(6):1626–1634. https://doi.org/10.2337/diabetes.54.6.1626
Hostetter TH (2003) Hyperfiltration and glomerulosclerosis. Semin Nephrol 23(2):194–199. https://doi.org/10.1053/anep.2003.50017
Langham RG, Kelly DJ, Cox AJ et al (2002) Proteinuria and the expression of the podocyte slit diaphragm protein, nephrin, in diabetic nephropathy: effects of angiotensin converting enzyme inhibition. Diabetologia 45(11):1572–1576. https://doi.org/10.1007/s00125-002-0946-y
Minakawa A, Fukuda A, Sato Y et al (2019) Podocyte hypertrophic stress and detachment precedes hyperglycemia or albuminuria in a rat model of obesity and type2 diabetes-associated nephropathy. Sci Rep 9(1):18485. https://doi.org/10.1038/s41598-019-54692-z
Dobrinskikh E, Okamura K, Kopp JB et al (2014) Human podocytes perform polarized, caveolae-dependent albumin endocytosis. Am J Physiol Renal Physiol 306(9):F941-951. https://doi.org/10.1152/ajprenal.00532.2013
Agrawal S, Smoyer WE (2017) Role of albumin and its modifications in glomerular injury. Pflugers Arch 469(7–8):975–982. https://doi.org/10.1007/s00424-017-2029-4
Qin XS, Tsukaguchi H, Shono A et al (2009) Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J Am Soc Nephrol 20(12):2534–2545. https://doi.org/10.1681/ASN.2009010011
Tossidou I, Teng B, Menne J et al (2010) Podocytic PKC-alpha is regulated in murine and human diabetes and mediates nephrin endocytosis. PLoS ONE 5(4):e10185. https://doi.org/10.1371/journal.pone.0010185
Teng B, Schroder P, Muller-Deile J et al (2016) CIN85 deficiency prevents nephrin endocytosis and proteinuria in diabetes. Diabetes 65(12):3667–3679. https://doi.org/10.2337/db16-0081
Okamura K, Dummer P, Kopp J et al (2013) Endocytosis of albumin by podocytes elicits an inflammatory response and induces apoptotic cell death. PLoS ONE 8(1):e54817. https://doi.org/10.1371/journal.pone.0054817
Castrop H, Schiessl IM (2017) Novel routes of albumin passage across the glomerular filtration barrier. Acta Physiol (Oxf) 219(3):544–553. https://doi.org/10.1111/apha.12760
D’Arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43(6):582–592. https://doi.org/10.1002/cbin.11137
Xu X, Lai Y, Hua ZC (2019) Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep 39:1. 10.1042/BSR20180992
Hockenbery D (1995) Defining apoptosis. Am J Pathol 146(1):16–19
Chen YQ, Wang XX, Yao XM et al (2011) MicroRNA-195 promotes apoptosis in mouse podocytes via enhanced caspase activity driven by BCL2 insufficiency. Am J Nephrol 34(6):549–559. https://doi.org/10.1159/000333809
Chuang PY, Yu Q, Fang W et al (2007) Advanced glycation endproducts induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int 72(8):965–976. https://doi.org/10.1038/sj.ki.5002456
Chuang PY, Dai Y, Liu R et al (2011) Alteration of forkhead box O (foxo4) acetylation mediates apoptosis of podocytes in diabetes mellitus. PLoS ONE 6(8):e23566. https://doi.org/10.1371/journal.pone.0023566
Yu J, Wu H, Liu ZY et al (2017) Advanced glycation end products induce the apoptosis of and inflammation in mouse podocytes through CXCL9-mediated JAK2/STAT3 pathway activation. Int J Mol Med 40(4):1185–1193. https://doi.org/10.3892/ijmm.2017.3098
Ha TS, Hong EJ, Han GD (2015) Diabetic conditions downregulate the expression of CD2AP in podocytes via PI3-K/Akt signalling. Diabetes Metab Res Rev 31(1):50–60. https://doi.org/10.1002/dmrr.2562
Susztak K, Raff AC, Schiffer M, Böttinger EP (2006) Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55(1):225–233
Eid AA, Ford BM, Bhandary B et al (2013) Mammalian target of rapamycin regulates Nox4-mediated podocyte depletion in diabetic renal injury. Diabetes 62(8):2935–2947. https://doi.org/10.2337/db12-1504
Chen X, Liu W, Xiao J et al (2020) FOXO3a accumulation and activation accelerate oxidative stress-induced podocyte injury. FASEB J 34(10):13300–13316. https://doi.org/10.1096/fj.202000783R
Cao Y, Hao Y, Li H et al (2014) Role of endoplasmic reticulum stress in apoptosis of differentiated mouse podocytes induced by high glucose. Int J Mol Med 33(4):809–816. https://doi.org/10.3892/ijmm.2014.1642
Cao AL, Wang L, Chen X et al (2016) Ursodeoxycholic acid and 4-phenylbutyrate prevent endoplasmic reticulum stress-induced podocyte apoptosis in diabetic nephropathy. Lab Invest 96(6):610–622. https://doi.org/10.1038/labinvest.2016.44
Zhang Y, Gao X, Chen S et al (2017) Cyclin-dependent kinase 5 contributes to endoplasmic reticulum stress induced podocyte apoptosis via promoting MEKK1 phosphorylation at Ser280 in diabetic nephropathy. Cell Signal 31:31–40. https://doi.org/10.1016/j.cellsig.2016.12.009
Lim SK, Park SH (2012) The high glucose-induced stimulation of B1R and B2R expression via CB(1)R activation is involved in rat podocyte apoptosis. Life Sci 91(19–20):895–906. https://doi.org/10.1016/j.lfs.2012.07.020
Wang L, Li H (2020) MiR-770-5p facilitates podocyte apoptosis and inflammation in diabetic nephropathy by targeting TIMP3. Biosci Rep 40:4. 10.1042/BSR20193653
Guo J, Han J, Liu J, Wang S (2020) MicroRNA-770-5p contributes to podocyte injury via targeting E2F3 in diabetic nephropathy. Braz J Med Biol Res 53(9):e9360. https://doi.org/10.1590/1414-431x20209360
Zhou Z, Wan J, Hou X et al (2017) MicroRNA-27a promotes podocyte injury via PPARgamma-mediated beta-catenin activation in diabetic nephropathy. Cell Death Dis 8(3):e2658. https://doi.org/10.1038/cddis.2017.74
Bai X, Geng J, Li X et al (2018) Long noncoding RNA LINC01619 regulates MicroRNA-27a/Forkhead box protein O1 and endoplasmic reticulum stress-mediated podocyte injury in diabetic nephropathy. Antioxid Redox Signal 29(4):355–376. https://doi.org/10.1089/ars.2017.7278
Zhao SM, Zhang T, Qiu Q et al (2019) MiRNA-337 leads to podocyte injury in mice with diabetic nephropathy. Eur Rev Med Pharmacol Sci 23(19):8485–8492. https://doi.org/10.26355/eurrev_201910_19161
Zha F, Bai L, Tang B et al (2019) MicroRNA-503 contributes to podocyte injury via targeting E2F3 in diabetic nephropathy. J Cell Biochem 120(8):12574–12581. https://doi.org/10.1002/jcb.28524
Yang H, Wang Q, Li S (2016) MicroRNA-218 promotes high glucose-induced apoptosis in podocytes by targeting heme oxygenase-1. Biochem Biophys Res Commun 471(4):582–588. https://doi.org/10.1016/j.bbrc.2016.02.028
Qian X, Tan J, Liu L et al (2018) MicroRNA-134-5p promotes high glucose-induced podocyte apoptosis by targeting bcl-2. Am J Transl Res 10(3):989–997
Gao F, Yao M, Shi Y et al (2013) Notch pathway is involved in high glucose-induced apoptosis in podocytes via Bcl-2 and p53 pathways. J Cell Biochem 114(5):1029–1038. https://doi.org/10.1002/jcb.24442
Peixoto EB, Papadimitriou A, Teixeira DA et al (2015) Reduced LRP6 expression and increase in the interaction of GSK3beta with p53 contribute to podocyte apoptosis in diabetes mellitus and are prevented by green tea. J Nutr Biochem 26(4):416–430. https://doi.org/10.1016/j.jnutbio.2014.11.012
Chen A, Feng Y, Lai H et al (2020) Soluble RARRES1 induces podocyte apoptosis to promote glomerular disease progression. J Clin Invest 130(10):5523–5535. https://doi.org/10.1172/JCI140155
Zhang Y, Xu C, Ye Q et al (2021) Podocyte apoptosis in diabetic nephropathy by BASP1 activation of the p53 pathway via WT1. Acta Physiol (Oxf) 232(1):e13634. https://doi.org/10.1111/apha.13634
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451(7182):1069–1075. https://doi.org/10.1038/nature06639
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290(5497):1717–1721. https://doi.org/10.1126/science.290.5497.1717
Parzych KR, Klionsky DJ (2014) An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal 20(3):460–473. https://doi.org/10.1089/ars.2013.5371
Yoshii SR, Mizushima N (2017) Monitoring and measuring autophagy. Int J Mol Sci 18:9. https://doi.org/10.3390/ijms18091865
Hartleben B, Gödel M, Meyer-Schwesinger C et al (2010) Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120(4):1084–1096. https://doi.org/10.1172/jci39492
Xin W, Li Z, Xu Y et al (2016) Autophagy protects human podocytes from high glucose-induced injury by preventing insulin resistance. Metabolism 65(9):1307–1315. https://doi.org/10.1016/j.metabol.2016.05.015
Woo CY, Kc R, Kim M et al (2020) Autophagic flux defect in diabetic kidney disease results in megamitochondria formation in podocytes. Biochem Biophys Res Commun 521(3):660–667. https://doi.org/10.1016/j.bbrc.2019.10.132
Li Z, Yuan Y, Meng Y et al (2017) Autophagy upregulation ameliorates cell injury in Sequestosome 1 knockout podocytes in vitro. Biochem Biophys Res Commun 490(2):98–103. https://doi.org/10.1016/j.bbrc.2017.05.102
Godel M, Hartleben B, Herbach N et al (2011) Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest 121(6):2197–2209. https://doi.org/10.1172/JCI44774
Dai H, Liu Q, Liu B (2017) Research progress on mechanism of podocyte depletion in diabetic nephropathy. J Diabetes Res 2017:2615286. https://doi.org/10.1155/2017/2615286
Ji J, Zhao Y, Na C et al (2019) Connexin 43autophagy loop in the podocyte injury of diabetic nephropathy. Int J Mol Med 44(5):1781–1788. https://doi.org/10.3892/ijmm.2019.4335
Xiao T, Guan X, Nie L et al (2014) Rapamycin promotes podocyte autophagy and ameliorates renal injury in diabetic mice. Mol Cell Biochem 394(1–2):145–154. https://doi.org/10.1007/s11010-014-2090-7
Zhao X, Chen Y, Tan X et al (2018) Advanced glycation end-products suppress autophagic flux in podocytes by activating mammalian target of rapamycin and inhibiting nuclear translocation of transcription factor EB. J Pathol 245(2):235–248. https://doi.org/10.1002/path.5077
Liu WJ, Gan Y, Huang WF et al (2019) Lysosome restoration to activate podocyte autophagy: a new therapeutic strategy for diabetic kidney disease. Cell Death Dis 10(11):806. https://doi.org/10.1038/s41419-019-2002-6
Liu Y, Zhang J, Wang Y, Zeng X (2017) Apelin involved in progression of diabetic nephropathy by inhibiting autophagy in podocytes. Cell Death Dis 8(8):e3006. https://doi.org/10.1038/cddis.2017.414
Liu J, Li QX, Wang XJ et al (2016) beta-Arrestins promote podocyte injury by inhibition of autophagy in diabetic nephropathy. Cell Death Dis 7:e2183. https://doi.org/10.1038/cddis.2016.89
Hou Y, Lin S, Qiu J et al (2020) NLRP3 inflammasome negatively regulates podocyte autophagy in diabetic nephropathy. Biochem Biophys Res Commun 521(3):791–798. https://doi.org/10.1016/j.bbrc.2019.10.194
Swanson PE, Carroll SB, Zhang XF, Mackey MA (1995) Spontaneous premature chromosome condensation, micronucleus formation, and non-apoptotic cell death in heated HeLa S3 cells. Ultrastructural observations Am J Pathol 146(4):963–971
Castedo M, Perfettini JL, Roumier T et al (2004) Cell death by mitotic catastrophe: a molecular definition. Oncogene 23(16):2825–2837. https://doi.org/10.1038/sj.onc.1207528
Mulay SR, Thomasova D, Ryu M et al (2013) Podocyte loss involves MDM2-driven mitotic catastrophe. J Pathol 230(3):322–335. https://doi.org/10.1002/path.4193
Galluzzi L, Vitale I, Aaronson SA et al (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25(3):486–541. https://doi.org/10.1038/s41418-017-0012-4
Migliorini A, Angelotti ML, Mulay SR et al (2013) The antiviral cytokines IFN-alpha and IFN-beta modulate parietal epithelial cells and promote podocyte loss: implications for IFN toxicity, viral glomerulonephritis, and glomerular regeneration. Am J Pathol 183(2):431–440. https://doi.org/10.1016/j.ajpath.2013.04.017
Liapis H, Romagnani P, Anders HJ (2013) New insights into the pathology of podocyte loss: mitotic catastrophe. Am J Pathol 183(5):1364–1374. https://doi.org/10.1016/j.ajpath.2013.06.033
Thomasova D, Anders HJ (2015) Cell cycle control in the kidney. Nephrol Dial Transplant 30(10):1622–1630. https://doi.org/10.1093/ndt/gfu395
Hagen M, Pfister E, Kosel A et al (2016) Cell cycle re-entry sensitizes podocytes to injury induced death. Cell Cycle 15(14):1929–1937. https://doi.org/10.1080/15384101.2016.1191710
Lasagni L, Ballerini L, Angelotti ML et al (2010) Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells 28(9):1674–1685. https://doi.org/10.1002/stem.492
Lasagni L, Lazzeri E, Shankland SJ et al (2013) Podocyte mitosis - a catastrophe. Curr Mol Med 13(1):13–23. https://doi.org/10.2174/1566524011307010013
Shankland SJ (2006) The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69(12):2131–2147. https://doi.org/10.1038/sj.ki.5000410
Hara M, Oohara K, Dai DF, Liapis H (2019) Mitotic catastrophe causes podocyte loss in the urine of human diabetics. Am J Pathol 189(2):248–257. https://doi.org/10.1016/j.ajpath.2018.10.016
Nagata M, Nakayama K, Terada Y et al (1998) Cell cycle regulation and differentiation in the human podocyte lineage. Am J Pathol 153(5):1511–1520. https://doi.org/10.1016/s0002-9440(10)65739-2
Tang H, Lei CT, Ye C et al (2017) MDM2 is implicated in high-glucose-induced podocyte mitotic catastrophe via Notch1 signalling. J Cell Mol Med 21(12):3435–3444. https://doi.org/10.1111/jcmm.13253
Su H, Wan Q, Tian XJ et al (2015) MAD2B contributes to podocyte injury of diabetic nephropathy via inducing cyclin B1 and Skp2 accumulation. Am J Physiol Renal Physiol 308(7):F728-736. https://doi.org/10.1152/ajprenal.00409.2014
Gilmore AP (2005) Anoikis. Cell Death Differ 12(Suppl 2):1473–1477. https://doi.org/10.1038/sj.cdd.4401723
Weil EJ, Lemley KV, Yee B et al (2011) Podocyte detachment in type 2 diabetic nephropathy. Am J Nephrol 33(Suppl 1):21–24. https://doi.org/10.1159/000327047
Reddy GR, Kotlyarevska K, Ransom RF, Menon RK (2008) The podocyte and diabetes mellitus: is the podocyte the key to the origins of diabetic nephropathy? Curr Opin Nephrol Hypertens 17(1):32–36. https://doi.org/10.1097/MNH.0b013e3282f2904d
Regoli M, Bendayan M (1997) Alterations in the expression of the alpha 3 beta 1 integrin in certain membrane domains of the glomerular epithelial cells (podocytes) in diabetes mellitus. Diabetologia 40(1):15–22. https://doi.org/10.1007/s001250050637
Chen HC, Chen CA, Guh JY et al (2000) Altering expression of alpha3beta1 integrin on podocytes of human and rats with diabetes. Life Sci 67(19):2345–2353. https://doi.org/10.1016/s0024-3205(00)00815-8
Sawada K, Toyoda M, Kaneyama N et al (2016) Upregulation of α3β1-integrin in podocytes in early-stage diabetic nephropathy. J Diabetes Res 2016:9265074. https://doi.org/10.1155/2016/9265074
Nakamura T, Ushiyama C, Suzuki S et al (2000) Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant 15(9):1379–1383. https://doi.org/10.1093/ndt/15.9.1379
Petermann AT, Krofft R, Blonski M et al (2003) Podocytes that detach in experimental membranous nephropathy are viable. Kidney Int 64(4):1222–1231. https://doi.org/10.1046/j.1523-1755.2003.00217.x
Yamaguchi Y, Iwano M, Suzuki D et al (2009) Epithelial-mesenchymal transition as a potential explanation for podocyte depletion in diabetic nephropathy. Am J Kidney Dis 54(4):653–664. https://doi.org/10.1053/j.ajkd.2009.05.009
Inoki K, Mori H, Wang J et al (2011) mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest 121(6):2181–2196. https://doi.org/10.1172/JCI44771
Xu Y, Gao H, Hu Y et al (2019) High glucose-induced apoptosis and necroptosis in podocytes is regulated by UCHL1 via RIPK1/RIPK3 pathway. Exp Cell Res 382(2):111463. https://doi.org/10.1016/j.yexcr.2019.06.008
Zhang Y, Chen X, Gueydan C, Han J (2018) Plasma membrane changes during programmed cell deaths. Cell Res 28(1):9–21. https://doi.org/10.1038/cr.2017.133
Khoury MK, Gupta K, Franco SR, Liu B (2020) Necroptosis in the Pathophysiology of Disease. Am J Pathol 190(2):272–285. https://doi.org/10.1016/j.ajpath.2019.10.012
Grootjans S, Vanden Berghe T, Vandenabeele P (2017) Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ 24(7):1184–1195. https://doi.org/10.1038/cdd.2017.65
He S, Huang S, Shen Z (2016) Biomarkers for the detection of necroptosis. Cell Mol Life Sci 73(11–12):2177–2181. https://doi.org/10.1007/s00018-016-2192-3
Sosna J, Voigt S, Mathieu S et al (2013) The proteases HtrA2/Omi and UCH-L1 regulate TNF-induced necroptosis. Cell Commun Signal 11:76. https://doi.org/10.1186/1478-811x-11-76
Cheng Q, Pan J, Zhou ZL et al (2021) Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy. Acta Pharmacol Sin 42(6):954–963. https://doi.org/10.1038/s41401-020-00525-z
Shi J, Gao W, Shao F (2017) Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci 42(4):245–254. https://doi.org/10.1016/j.tibs.2016.10.004
Li X, Zeng L, Cao C et al (2017) Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Exp Cell Res 350(2):327–335. https://doi.org/10.1016/j.yexcr.2016.12.006
Ding X, Jing N, Shen A et al (2021) MiR-21-5p in macrophage-derived extracellular vesicles affects podocyte pyroptosis in diabetic nephropathy by regulating A20. J Endocrinol Invest 44(6):1175–1184. https://doi.org/10.1007/s40618-020-01401-7
Li F, Mao X, Zhuang Q et al (2019) Inhibiting 4E-BP1 re-activation represses podocyte cell cycle re-entry and apoptosis induced by adriamycin. Cell Death Dis 10(3):241. https://doi.org/10.1038/s41419-019-1480-x
Qi YY, Zhou XJ, Cheng FJ et al (2018) Increased autophagy is cytoprotective against podocyte injury induced by antibody and interferon-alpha in lupus nephritis. Ann Rheum Dis 77(12):1799–1809. https://doi.org/10.1136/annrheumdis-2018-213028
Ogawa-Akiyama A, Sugiyama H, Kitagawa M et al (2020) Podocyte autophagy is associated with foot process effacement and proteinuria in patients with minimal change nephrotic syndrome. PLoS ONE 15(1):e0228337. https://doi.org/10.1371/journal.pone.0228337
Guo C, Fu R, Zhou M et al (2019) Pathogenesis of lupus nephritis: RIP3 dependent necroptosis and NLRP3 inflammasome activation. J Autoimmun 103:102286. https://doi.org/10.1016/j.jaut.2019.05.014
Kim SY, Park S, Lee SW et al (2021) RIPK3 Contributes to Lyso-Gb3-Induced Podocyte Death. Cells 10:2. https://doi.org/10.3390/cells10020245
Ding F, Wickman L, Wang SQ et al (2017) Accelerated podocyte detachment and progressive podocyte loss from glomeruli with age in Alport Syndrome. Kidney Int 92(6):1515–1525. https://doi.org/10.1016/j.kint.2017.05.017
Acknowledgements
None.
Funding
This study was financially supported by the National Natural Science Foundation of China grants (81770711, 81873602, 81800610, 81974096, 81961138007, 81974097, 81900629, 82000664, 82170773, 82100794, 82100729).
Author information
Authors and Affiliations
Contributions
CZ conceptualized the review. AJ wrote a draft of the review. CZ and AS revised the paper. The figures were drawn by AJ. CZ performed the final edits.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, A., Song, A. & Zhang, C. Modes of podocyte death in diabetic kidney disease: an update. J Nephrol 35, 1571–1584 (2022). https://doi.org/10.1007/s40620-022-01269-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-022-01269-1