Abstract
Background
The triglyceride glucose (TyG) index and triglyceride-to-high-density lipoprotein cholesterol (TG/HDL-C) ratio are recognized as simple non-insulin-based insulin resistance indices. Our study aimed to explore the relationship between these two indicators and heart failure (HF) in overweight or obesity individuals without diabetes.
Methods
This cross-sectional study selected 13,473 participants from the National Health and Nutrition Examination Survey (NHANES) 2001–2018 dataset. Weighted multivariable logistic regression and subgroup analysis were employed to evaluate the relationships between TyG index, TG/HDL-C ratio, and HF prevalence, respectively. Additionally, smooth curve fitting was utilized to analyze the dose–response relationships.
Results
A total of 13,473 obesity or overweight people without diabetes were included in this study through screening, among whom 291 (2.16%) had comorbid HF. The results of multivariable logistic regression suggested that the highest TyG index (OR = 2.4, 95% CI = 1.4–4.2, p = 0.002) and the highest TG/HDL-C ratio (OR = 1.2, 95% CI = 1.1–1.3, p < 0.001) both increased the prevalence of HF, especially in the non-Hispanic population. Dose–response relationships suggested nonlinear relationships between these two indicators and HF.
Conclusion
Our study demonstrated that elevated TyG index and TG/HDL-C ratio were closely associated with the prevalence of HF, and both exhibited nonlinear relationships with HF prevalence in overweight/obesity adults without diabetes. Based on these findings, additional prospective studies are needed for further validation.
Similar content being viewed by others
Introduction
With economic development, obesity has become an important social problem in many countries. In March 2023, the World Obesity Federation declared that by 2035, more than 4 billion people worldwide will be affected by overweight or obesity, constituting more than half of the global population [1]. Obesity causes insulin resistance (IR), and the resulting accumulation of insulin promotes fat synthesis, thus establishing a vicious cycle [2]. Overweight and obesity are closely related to many common diseases, including diabetes, hypertension, and coronary heart disease (CHD), and is also more common in heart failure (HF) patients, with the prevalence of overweight and obesity in HF patients reaching 40.3% and 31.2%, respectively [3].
As a manifestation of metabolic disorders, IR is strongly associated with HF and has been confirmed by numerous studies to be an independent risk factor for the development of HF. In addition, IR is prevalent in the HF population [4,5,6]. The main accurate and reliable method for assessing IR is the euglycemic insulin clamp. However, its clinical application is challenging due to technical complexity. The most commonly utilized alternative is the homeostatic model assessment of insulin resistance (HOMA-IR) index. Despite its widespread use, this method is not commonly employed in routine clinical practice due to factors such as complexity, time consumption, and cost. Currently, the triglyceride glucose (TyG) index and triglyceride/high-density lipoprotein (TG/HDL-C) ratio are considered convenient, inexpensive, and reliable non-insulin-based methods for estimating IR and have been shown to have high sensitivity [7, 8]. Numerous studies have assessed the relationship between these two indicators and the risk of developing cardiovascular diseases, including HF, and have consistently that they are strongly associated [9, 10]. We observed differences in the results of subgroup analyses among overweight/obesity patients with or without diabetes in studies investigating the association between IR and HF [11,12,13]. While existing studies have predominantly focused on analyzing risk factors for HF in overweight/obesity individuals with diabetes, there is a notable gap in research pertaining to overweight/ obesity populations without diabetes [14,15,16]. However, it is crucial to separately evaluate this population for HF risk factors because of the distinct disease progression of HF in populations without diabetes compared to that of populations with diabetes. Therefore, we explored the relationship between IR and HF using the TyG index and the TG/HDL-C ratio, two non-insulin-dependent indices of IR, using data from the National Health and Nutrition Examination Survey (NHANES) database. The objective of this study was to effectively guide subsequent relevant prospective studies by identifying the risk factors for HF in overweight/obesity patients without diabetes, thereby providing clinical evidence for risk stratification and individual management.
Methods
Study population
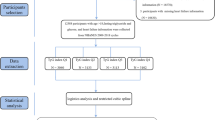
The National Health and Nutrition Examination Survey (NHANES) is a major epidemiological survey conducted by the National Center for Health Surveys (NCHS) of the U.S. Department of Health and Human Services (HHS) to assess the health and nutritional status of the U.S. population. For this study, we extracted population data (n = 100,908 individuals) from the 2001–2018 NHANES database. We excluded participants under 18 years of age (n = 38,930), as well as those with missing glucose (n = 298), blood lipid (n = 34,311), or HF questionnaire (n = 1616) data. We excluded 5386 patients with diabetes and 6894 individuals who had normal weight. A total of 13,473 adults were ultimately included in the analysis (Fig. 1).
Diabetes and overweight/obesity status
Diabetes status [17] was defined if the patients fulfilled at least one of the following criteria: (1) Fasting plasma glucose (FPG) ≥ 7 mmol/L, (2) HbA1c ≥ 6.5%, (3) 2-h plasma glucose during the OGTT ≥ 11.1 mmol/L, or (4) self-reported physician-diagnosed diabetes.
Overweight/obesity [18] was defined as a body mass index (BMI) ≥ 25 kg/m2.
TyG index and TG/HDL-C ratio
The TyG index was calculated using the following formula: Ln [fasting TG (mg/dL) × FPG (mg/dL)/2]. The TG/HDL-C ratio was calculated as TG (mg/dL) divided by HDL (mg/dL).
We classified the participants into four groups (Q1, Q2, Q3, and Q4) according to the quartiles of the TyG index and TG/HDL-C ratio, and the Q1 group served as the reference.
HF
In the NHANES, HF data were collected via a health questionnaire administered during a personal interview. In the self-report questionnaire, an affirmative answer to the following question was considered to indicate HF status: “Have you ever been told by a doctor or other health professional that you had congestive heart failure?”
Covariable definitions
We selected the following basic population characteristics for their potential to influence the relationships of the TyG index and TG/HDL-C ratio indices with HF in a population with obesity but without diabetes: age, sex, ethnicity (including Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, or Other), HbA1c, FPG, systolic blood pressure (SBP), diastolic blood pressure (DBP), albumin, creatinine, TC, HDL, LDL, TG, smoking status, stroke, CHD and impaired fasting glucose (IFG).
The definition of IFG according to the World Health Organization (WHO) is based on an FPG in the range of 6.1–6.9 mmol/L.
Statistical analysis
During the data analysis, we considered the weighting of the NHANES database. We categorized the total population into those with and without HF and analyzed the basic characteristics of the patients in both categories. Continuous variables are presented as the mean ± standard deviation or median (interquartile range) depending on their normal distribution, while categorical variables are presented as counts and percentages (%). A weighted linear regression model was used for continuous variables, and weighted chi-square tests were performed to analyze differences between categorical variables. The associations of the TyG index and the TG/HDL-C ratio with HF prevalence among adults with overweight/obesity but without diabetes were assessed by survey-weighted logistic regression analysis, and odds ratios (ORs) and 95% CIs were calculated via two models. Model 1 was adjusted for age, sex and ethnicity, and Model 2 was adjusted for age, sex, ethnicity, SBP, DBP, albumin, creatinine, LDL, TC, smoking status, CHD prevalence and stroke prevalence.
We then performed sensitivity analyses by stratified analysis and interaction tests with subgroups to test for interactions and effects of relevant confounding factors, including age (< 60 and ≥ 60), sex, ethnicity, SBP (< 140 mmHg and ≥ 140 mmHg), smoking status, stroke status, CHD status and IFG.
Dose–response relationships of the TyG index and TG/HDL-C ratio with the prevalence of HF were observed using generalized additive model (GAM) and fitted curve methods. If nonlinearity was detected, we first utilized smoothed curve fitting to assess whether the independent variable was partitioned into intervals. Then, we apply segmented regression, which employs separate line segments to fit each interval. A log-likelihood ratio test is conducted using a single-line model versus a segmented regression model to determine the presence of a threshold. The maximum likelihood is determined based on the model, and the inflection points of the connected segments are identified using a two-step recursive approach. Finally, we assessed the difference between the results of the two-part logistic regression model.
All the data analyses in this study were conducted using R software (http://www.R-project.org) and Empower (version 5.0; www.empowerstats.com; X&Y Solutions).
Results
Baseline characteristics
This study ultimately included 13,473 adults with a mean age of 47.82 ± 17.00 years, 47.57% of whom were male. Compared with the non-HF population, the HF population was older and had higher HbA1c levels, FPG levels, TyG index, TG/HDL-C ratio and creatinine levels as well as a greater prevalence of comorbidities such as stroke, CHD and IFG. The detailed results are presented in Table 1.
Associations of the TyG index and TG/HDL-C ratio with HF prevalence
In the multivariable logistic regression, both the TyG index and the TG/HDL-C ratio were divided into quartiles. After adjusting for age, sex, race, SBP, DBP, albumin, creatinine, LDL, TC, smoking status, CHD status, and stroke status in Model II, both the TyG index and TG/HDL-C ratio exhibited positive association with HF prevalence (TyG: OR = 2. 4, 95% CI: 1.4–4.2, p = 0.002; TG/HDL-C: OR = 1.2, 95% CI: 1.1–1.3, p < 0.001). The HF prevalence was greater in the Q4 subgroup than in the Q1 subgroup (TyG: OR = 3. 2, 95% CI: 1.7–5.9, p < 0.001; TG/HDL-C: OR = 3.4, 95% CI: 1.9-6.0, p < 0.001). The results are shown in Table 2.
We performed subgroup analyses (Fig. 2) of age (< 60 and ≥ 60 years), sex, race, SBP (< 140 mmHg and ≥ 140 mmHg), smoking status, stroke status, CHD status and IFG, and the associations of the TyG index and TG/HDL-C ratio with HF prevalence were stable. However, ethnicity influenced the associations between the TyG index and the TG/HDL-C ratio and HF prevalence (TyG: p for interaction = 0.019; TG/HDL-C: p for interaction = 0.024). The TyG index and TG/HDL-C ratio were strongly associated with HF prevalence in non-Hispanic populations, whereas there was no clear association in other races.
Subgroup analysis of the association between the prevalence of HF and TyG index (A), TG/HDL-C ratio (B). Adjusted for age, sex, ethnicity, systolic blood pressure (mmHg), diastolic blood pressure (mmHg), albumin(g/dl), creatinine (mg/dL), low density lipoprotein (mg/dl), total cholesterol (mg/dl), smoke statue, stroke and CHD, except the stratified factor itself. IFG, impaired fasting glucose; CHD, coronary heart disease; SBP, systolic blood pressure
Dose–response relationships
In addition, the results of the smoothed curve-fitting analysis confirmed a nonlinear positive association between the TyG index and the prevalence of HF (Fig. 3A), with an inflection point of 8.5 according to the threshold effect analysis (Table 3). The prevalence of HF increased with a TyG index greater than 8.5 (OR = 4.0, 95% CI = 2.4–6.4; p < 0.001). Smoothed curve-fitting analysis also suggested that the TG/HDL-C ratio was nonlinearly related to the prevalence of HF (Fig. 3B), with an inflection point of 6.2 according to threshold effect analysis (Table 4).
Discussion
This large-sample cross-sectional study based on data from the NHANES database was conducted to assess the associations of the TyG index and TG/HDL-C ratio with the prevalence of HF in an overweight/obesity population without diabetes. Within this population, we observed a strong association between an elevated TyG index and TG/HDL-C ratio and a high prevalence of HF. Subgroup analyses and interaction tests indicated that this association was present in non-Hispanic populations, with no clear association observed in other racial groups.
In the present study, the TyG index and TG/HDL-C ratio were utilized to assess IR, and both indices had a nonlinear relationship with the prevalence of HF; threshold analyses identified inflection points of 8.5 and 6.2 for the TyG index and TG/HDL-C ratio, respectively. The TyG index has been shown to associate well with the results of the euglycemic insulin clamp [7]. Some of these studies have indicated that a higher TyG index is a risk factor for HF prevalence [19, 20]. Hou et al. [21] reported that the prevalence of HF increased rapidly with an increasing TyG index exceeding 8.91, a threshold similar to our findings. The TG/HDL-C ratio, proposed as an indicator of atherosclerosis by Gaziano et al. [22], is associated with a greater proportion of small dense LDL particles, which are highly atherogenic [23]. Consequently, this indicator has been recognized as a predictor for atherosclerotic cardiovascular disease [24]. With further research, it has been discovered that the TG/HDL-C ratio can serve as a simple parameter for evaluating IR and is a useful indicator for identifying metabolic syndrome, particularly in overweight individuals [25,26,27]. This study is the first to explore the usefulness of the TG/HDL-C ratio in an HF population, thereby expanding the application of the TG/HDL-C ratio in heart disease populations. We hope that more studies will follow to further substantiate our conclusions.
The associations of diabetes, obesity, and overweight with HF are robust and often reciprocal. The current study revealed that, within the population without diabetes, non-insulin-based insulin resistance indices (the TyG index and TG/HDL-C ratio) were associated with a greater prevalecne of HF in individuals with obesity, and the results remained stable in subgroup analyses of individuals with abnormal glucose tolerance. Diabetes is a recognized metabolic factor that elevates the prevalence of HF, and other metabolic abnormalities, such as IR, similarly contribute to an increased prevalence of HF in obesity individuals without diabetes mellitus. IR is strongly associated with the risk, severity, and poor prognosis of HF. Using the HOMA-IR index, the ARIC Large Community Cohort Study revealed that IR was associated with the development of HF [28]. Metabolic syndrome and the development of HF are closely related to comorbid cardiovascular disease in patients without diabetes, and some of these effects are mediated by the HOMA-IR index [29]. IR results in disruptions to myocardial carbohydrate and lipid metabolism, mitochondrial and endoplasmic reticulum dysfunction, and endothelial dysfunction, ultimately leading to cardiac systolic and diastolic dysfunction [30]. On the other hand, hyperinsulinemia causes water and sodium retention, excites the sympathetic nervous system [31], and may induce cardiac remodeling, leading to impaired structural, functional [32], and metabolic changes that drive the progression of HF pathogenesis.
The presence of HF also predicts the development of IR, with glycemic abnormalities being highly prevalent in HF patients (43% of patients). Additionally, the prognosis for HF patients without diabetes is likely to be worse than that for patients with normal blood glucose and insulin levels [33,34,35]. An observational cohort study of 58,056 patients without diabetes revealed that HF patients had a significantly greater risk of developing diabetes later in life [36]. The increased risk of new-onset diabetes in patients with HF is primarily due to the progression of advancing IR [37, 38], the mechanisms of which are intricate. HF induces heightened sympathetic nerve activity, and this increased sympathetic nervous system activity can result in decreased insulin responsiveness, glucose utilization, and β-cell insulin secretion by impacting vasodilatory tone, free fatty acid levels, and oxidative stress [39]. Increased aldosterone impairs insulin secretion and sensitivity, which is a key factor in the development of diabetes [40]. Plasma aldosterone is strongly associated with BMI or an index of IR [41]. Angiotensin II (ANGII) can stimulate the blood vessel wall to generate reactive oxygen species (O2−), leading to inflammation, apoptosis, and reduced insulin formation and secretion [42]. Additionally, angiotensin II receptor 2 promotes pancreas-driven oxidative stress, apoptosis, and fibrosis, thereby affecting insulin production [43].
Subgroup analyses revealed that the effect of IR on HF prevalence was meaningful in non-Hispanic populations, regardless of sex (black or white). Differences in cardiovascular prevalence between non-Hispanic populations and other racial groups have been reported to some extent [44,45,46]. However, the exact reasons for these differences are currently unknown, and additional studies are needed to explore this further.
Limitations
Some limitations of this study should be considered. First, we were unable to establish a causal association between the TyG index or TG/HDL-C ratio and the onset of HF due to the cross-sectional design. Future multicenter prospective studies are necessary to confirm potential causal associations and identify respective inflection points. Second, the inclusion criteria for HF patients were self-reported, which may have led to an underestimation of the prevalence of HF. Third, although we adjusted for several potential confounders in our analysis, the results may still be influenced by other unknown factors. Finally, this study was based on a U.S. population and may not be generalizable to other countries.
Conclusion
Our study showed that an elevated TyG index and TG/HDL-C ratio were strongly and nonlinearly associated with a greater prevalence of HF in individuals with overweight or obesity but without diabetes.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- CI:
-
confidence interval
- OR:
-
odds ratio
References
Federation WO. World Obesity Atlas 2023. https://dataworldobesityorg/publications/?cat=19 2023.
Ye J. Mechanisms of insulin resistance in obesity. Front Med. 2013;7(1):14–24.
Kenchaiah S, Pocock SJ, Wang D, Finn PV, Zornoff LA, Skali H, Pfeffer MA, Yusuf S, Swedberg K, Michelson EL, et al. Body mass index and prognosis in patients with chronic heart failure: insights from the Candesartan in Heart failure: Assessment of reduction in mortality and morbidity (CHARM) program. Circulation. 2007;116(6):627–36.
Erqou S, Adler AI, Challa AA, Fonarow GC, Echouffo-Tcheugui JB. Insulin resistance and incident heart failure: a meta-analysis. Eur J Heart Fail. 2022;24(6):1139–41.
Son TK, Toan NH, Thang N, Le Trong Tuong H, Tien HA, Thuy NH, Van Minh H, Valensi P. Prediabetes and insulin resistance in a population of patients with heart failure and reduced or preserved ejection fraction but without diabetes, overweight or hypertension. Cardiovasc Diabetol. 2022;21(1):75.
Scherbakov N, Bauer M, Sandek A, Szabó T, Töpper A, Jankowska EA, Springer J, von Haehling S, Anker SD, Lainscak M, et al. Insulin resistance in heart failure: differences between patients with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail. 2015;17(10):1015–21.
Fritz J, Bjørge T, Nagel G, Manjer J, Engeland A, Häggström C, Concin H, Teleka S, Tretli S, Gylling B, et al. The triglyceride-glucose index as a measure of insulin resistance and risk of obesity-related cancers. Int J Epidemiol. 2020;49(1):193–204.
Giannini C, Santoro N, Caprio S, Kim G, Lartaud D, Shaw M, Pierpont B, Weiss R. The triglyceride-to-HDL cholesterol ratio: association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care. 2011;34(8):1869–74.
Zhang B, Dong C, Li S, Song X, Wei W, Liu L. Triglyceride to High-Density Lipoprotein Cholesterol Ratio is an important determinant of Cardiovascular Risk and Poor Prognosis in Coronavirus Disease-19: a retrospective Case Series Study. Diabetes Metabolic Syndrome Obesity: Targets Therapy. 2020;13:3925–36.
Khalaji A, Behnoush AH, Khanmohammadi S, Ghanbari Mardasi K, Sharifkashani S, Sahebkar A, Vinciguerra C, Cannavo A. Triglyceride-glucose index and heart failure: a systematic review and meta-analysis. Cardiovasc Diabetol. 2023;22(1):244.
Zheng H, Chen G, Wu K, Wu W, Huang Z, Wang X, Chen Z, Cai Z, Cai Z, Lan Y, et al. Relationship between cumulative exposure to triglyceride-glucose index and heart failure: a prospective cohort study. Cardiovasc Diabetol. 2023;22(1):239.
Huang R, Lin Y, Ye X, Zhong X, Xie P, Li M, Zhuang X, Liao X. Triglyceride-glucose index in the development of heart failure and left ventricular dysfunction: analysis of the ARIC study. Eur J Prev Cardiol. 2022;29(11):1531–41.
Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, Szklo M, Lima JA. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (multi-ethnic study of atherosclerosis) study. J Am Coll Cardiol. 2008;51(18):1775–83.
Höskuldsdóttir G, Sattar N, Miftaraj M, Näslund I, Ottosson J, Franzén S, Svensson AM, Eliasson B. Potential effects of bariatric surgery on the incidence of heart failure and atrial fibrillation in patients with type 2 diabetes Mellitus and obesity and on mortality in patients with Preexisting Heart failure: a Nationwide, Matched, Observational Cohort Study. J Am Heart Association. 2021;10(7):e019323.
Aminian A, Zajichek A, Arterburn DE, Wolski KE, Brethauer SA, Schauer PR, Kattan MW, Nissen SE. Association of metabolic surgery with major adverse Cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA. 2019;322(13):1271–82.
Horwich TB, Fonarow GC. Glucose, obesity, metabolic syndrome, and diabetes relevance to incidence of heart failure. J Am Coll Cardiol. 2010;55(4):283–93.
Diagnosis and Classification of Diabetes. Standards of Care in Diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S20–42.
Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27.
Li X, Chan JSK, Guan B, Peng S, Wu X, Lu X, Zhou J, Hui JMH, Lee YHA, Satti DI, et al. Triglyceride-glucose index and the risk of heart failure: evidence from two large cohorts and a mendelian randomization analysis. Cardiovasc Diabetol. 2022;21(1):229.
Xu L, Wu M, Chen S, Yang Y, Wang Y, Wu S, Tian Y. Triglyceride-glucose index associates with incident heart failure: a cohort study. Diabetes Metab. 2022;48(6):101365.
Zhang F, Hou X. Association between the triglyceride glucose index and heart failure: NHANES 2007–2018. Front Endocrinol. 2023;14:1322445.
Gaziano JM, Hennekens CH, O’Donnell CJ, Breslow JL, Buring JE. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation. 1997;96(8):2520–5.
Yokoyama K, Tani S, Matsuo R, Matsumoto N. Increased triglyceride/high-density lipoprotein cholesterol ratio may be associated with reduction in the low-density lipoprotein particle size: assessment of atherosclerotic cardiovascular disease risk. Heart Vessels. 2019;34(2):227–36.
Tani S. The ratio of triglyceride to high-density lipoprotein cholesterol as an Indicator of Risk Stratification for Atherosclerotic Cardiovascular Disease in a clinical setting. Intern Med (Tokyo Japan). 2020;59(21):2639–40.
Nie G, Hou S, Zhang M, Peng W. High TG/HDL ratio suggests a higher risk of metabolic syndrome among an elderly Chinese population: a cross-sectional study. BMJ open. 2021;11(3):e041519.
McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139(10):802–9.
Kosmas CE, Rodriguez Polanco S, Bousvarou MD, Papakonstantinou EJ, Peña Genao E, Guzman E, Kostara CE. The Triglyceride/High-Density Lipoprotein Cholesterol (TG/HDL-C) ratio as a risk marker for metabolic syndrome and Cardiovascular Disease. Diagnostics (Basel Switzerland) 2023, 13(5).
Vardeny O, Gupta DK, Claggett B, Burke S, Shah A, Loehr L, Rasmussen-Torvik L, Selvin E, Chang PP, Aguilar D, et al. Insulin resistance and incident heart failure the ARIC study (atherosclerosis risk in communities). JACC Heart Fail. 2013;1(6):531–6.
Burger PM, Koudstaal S, Dorresteijn JAN, Savarese G, van der Meer MG, de Borst GJ, Mosterd A, Visseren FLJ. Metabolic syndrome and risk of incident heart failure in non-diabetic patients with established cardiovascular disease. Int J Cardiol. 2023;379:66–75.
Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin. 2012;8(4):609–17.
Valensi P. Autonomic nervous system activity changes in patients with hypertension and overweight: role and therapeutic implications. Cardiovasc Diabetol. 2021;20(1):170.
Sundström J, Lind L, Nyström N, Zethelius B, Andrén B, Hales CN, Lithell HO. Left ventricular concentric remodeling rather than left ventricular hypertrophy is related to the insulin resistance syndrome in elderly men. Circulation. 2000;101(22):2595–600.
Suskin N, McKelvie RS, Burns RJ, Latini R, Pericak D, Probstfield J, Rouleau JL, Sigouin C, Solymoss CB, Tsuyuki R, et al. Glucose and insulin abnormalities relate to functional capacity in patients with congestive heart failure. Eur Heart J. 2000;21(16):1368–75.
Mamas MA, Deaton C, Rutter MK, Yuille M, Williams SG, Ray SG, New J, Gibson JM, Neyses L. Impaired glucose tolerance and insulin resistance in heart failure: underrecognized and undertreated? J Card Fail. 2010;16(9):761–8.
Zareini B, Rørth R, Holt A, Mogensen UM, Selmer C, Gislason G, Schou M, Køber L, Torp-Pedersen C, Lamberts M, et al. Heart failure and the prognostic impact and incidence of new-onset of diabetes mellitus: a nationwide cohort study. Cardiovasc Diabetol. 2019;18(1):79.
Nichols GA, Moler EJ. Cardiovascular disease, heart failure, chronic kidney disease and depression independently increase the risk of incident diabetes. Diabetologia. 2011;54(3):523–6.
Swan JW, Anker SD, Walton C, Godsland IF, Clark AL, Leyva F, Stevenson JC, Coats AJ. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. J Am Coll Cardiol. 1997;30(2):527–32.
Paolisso G, De Riu S, Marrazzo G, Verza M, Varricchio M, D’Onofrio F. Insulin resistance and hyperinsulinemia in patients with chronic congestive heart failure. Metab Clin Exp. 1991;40(9):972–7.
Kostis JB, Sanders M. The association of heart failure with insulin resistance and the development of type 2 diabetes. Am J Hypertens. 2005;18(5 Pt 1):731–7.
Luther JM, Brown NJ. The renin-angiotensin-aldosterone system and glucose homeostasis. Trends Pharmacol Sci. 2011;32(12):734–9.
Goodfriend TL, Egan B, Stepniakowski K, Ball DL. Relationships among plasma aldosterone, high-density lipoprotein cholesterol, and insulin in humans. Hypertens (Dallas Tex: 1979). 1995;25(1):30–6.
Joseph JJ, Echouffo Tcheugui JB, Effoe VS, Hsueh WA, Allison MA, Golden SH. Renin-angiotensin-aldosterone system, glucose metabolism and incident type 2 diabetes Mellitus: MESA. J Am Heart Association. 2018;7(17):e009890.
Graus-Nunes F, Souza-Mello V. The renin-angiotensin system as a target to solve the riddle of endocrine pancreas homeostasis. Biomed Pharmacotherapy = Biomedecine Pharmacotherapie. 2019;109:639–45.
Morgenstern LB, Smith MA, Lisabeth LD, Risser JM, Uchino K, Garcia N, Longwell PJ, McFarling DA, Akuwumi O, Al-Wabil A, et al. Excess stroke in Mexican americans compared with non-hispanic whites: the Brain Attack Surveillance in Corpus Christi Project. Am J Epidemiol. 2004;160(4):376–83.
Shulman E, Chudow JJ, Essien UR, Shanbhag A, Kargoli F, Romero J, Di Biase L, Fisher J, Krumerman A, Ferrick KJ. Relative contribution of modifiable risk factors for incident atrial fibrillation in hispanics, African americans and non-hispanic whites. Int J Cardiol. 2019;275:89–94.
Orlander PR, Goff DC, Morrissey M, Ramsey DJ, Wear ML, Labarthe DR, Nichaman MZ. The relation of diabetes to the severity of acute myocardial infarction and post-myocardial infarction survival in mexican-americans and non-hispanic whites. Corpus Christi Heart Project Diabetes. 1994;43(7):897–902.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization: DYC and CZ; collect the data: YC, GZQ and WXZ; statistical analysis and interpretation of results: DYC, CZ, and YC; writing original draft preparation: DYC; Revise the manuscript: ZHZ, YZ and PZ. All authors have read and approved the final version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the Research Ethics Review Board of the National Center for Health Statistics. The patients/participants provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cui, Dy., Zhang, C., Chen, Y. et al. Associations between non-insulin-based insulin resistance indices and heart failure prevalence in overweight/obesity adults without diabetes mellitus: evidence from the NHANES 2001–2018. Lipids Health Dis 23, 123 (2024). https://doi.org/10.1186/s12944-024-02114-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02114-z