Abstract
Background
Non-alcoholic fatty liver disease (NAFLD), often linked with obesity, can also affect individuals with normal weight, a condition known as “lean NAFLD”, imposing comparable burdens and adverse effects. However, the impact of diet on lean NAFLD remains underexplored. The objective of this study is to investigate the correlation between the Dietary Inflammatory Index (DII) and NAFLD among Americans, stratified by waist-to-height ratio (WHtR) and body mass index (BMI).
Methods
Five thousand one hundred fifty-two participants from the National Health and Nutrition Examination Survey (NHANES) 2003–2018 were comprised in the final analysis. NAFLD and advanced liver fibrosis were diagnosed by serological markers. Lean and abdominal lean individuals were identified using BMI and WHtR, separately. DII was determined by assigning scores to 28 distinct food parameters based on their inflammatory potential, obtained from the NAHNES website. Differences across DII quartiles were evaluated using the Kruskal-Wallis H Test, Chi-Square Test along with One-Way ANOVA. The correlation between DII and NAFLD was determined by multiple regression models and subgroup analyses.
Results
Among the 5152 subjects, 2503 were diagnosed with NAFLD, including 86 cases of lean NAFLD and 8 cases of abdominal lean NAFLD. DII was positively linked with NAFLD (Odds Ratio (OR) = 1.81 [1.48–2.21], P < 0.001) and advanced liver fibrosis (OR = 1.46 [1.02–2.07], P = 0.037). Further analysis revealed that this association was primarily observed in obese or abdominal obese participants (In BMI ≥ 25.00 kg/m^2, OR = 1.56 [1.23–1.98], P < 0.001. In WHtR> 0.50, OR = 1.48 [1.23–1.79], P < 0.001.), rather than their lean counterparts. Subgroup analyses indicated that female individuals, without a diagnosis of hypertension or diabetes appeared to be more sensitive to the rise in DII.
Conclusions
Our data demonstrated a significant positive correlation between DII and NAFLD in the general population. However, the impact of a pro-inflammatory diet was less prominent in lean individuals compared to obese ones.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is characterized by the accumulation of excess fat in the liver, in the absence of significant alcohol consumption or other long-term liver illnesses, including viral hepatitis or genetic liver disorders [1, 2]. Nowadays, NAFLD is the major cause of end-stage liver disease, primary liver carcinoma and demand for liver transplantation, placing a significant global burden [3]. In addition to its well-established association with obesity, ‘lean NAFLD’ refers to NAFLD affecting individuals with normal weight [4, 5]. The incidence of lean NAFLD varies across regions and races. A meta-analysis revealed that lean NAFLD accounted for approximately 19.20% of NAFLD patients worldwide (95% CI:3.70–7.00) [6]. Previous studies have indicated that lean NAFLD may exhibit comparable outcomes to conventional NAFLD, or potentially even worse liver-related events and overall mortality [7]. To date, genetics, epigenetics, dietary factors, and physical exercise have all been linked to the onset of NAFLD in lean individuals by influencing metabolic flexibility and adaptability [8, 9]. However, the specific mechanisms of lean NAFLD remain unclear. Additionally, specific guidelines for lean NAFLD are absent.
Recent studies have highlighted the critical role of inflammation in the pathogenesis of NAFLD [10, 11]. In humans, the inflammatory balance is maintained by cytokines including IL-6 and IL-1, along with tumor necrosis factor-alpha (TNF-α) and C-reactive protein (CRP) [12]. Disruption of this balance can lead to mild persistent inflammation and tissue damage. Diet plays a pivotal role in modifying the inflammatory state in humans and has been widely used in NAFLD management. Recommendations on NAFLD management include minimizing consumption of a typical Western eating style and advocating for the adoption of a Mediterranean diet. This dietary pattern contains higher intake of omega-3 and monounsaturated fatty acids, lower intake of carbohydrates, refined carbs, and sweets [13,14,15,16,17]. To provide the public with more precise dietary guidance, Shivappa et al. developed the Dietary Inflammatory Index (DII), which is now widely used to quantify the impact of an individual’s diet on inflammation [18]. Previous research has revealed the substantial link between DII and obesity [19], type 2 diabetes (T2DM) [20], hypertension [21] and metabolic dysfunction-associated fatty liver disease (MAFLD) [22]. However, research on the relationship between DII and NAFLD in individuals with diverse weights and body shapes is limited.
Nowadays, several non-invasive serological tests are widely employed in diagnosing NAFLD and advanced liver fibrosis. These tests include the Fatty Liver Index (FLI), the US Fatty Liver Index (USFLI), the Non-Alcoholic Fatty Liver Disease Fibrosis Score (NFS), the Fibrosis 4 Index (FIB-4), the Hepatic Steatosis Index (HSI) and the Aspartate Aminotransferase /Platelet Ratio Index (APRI). Numerous epidemiological studies [23,24,25,26,27,28,29] have confirmed the validity of these markers. The objective of this study is to explore associations between DII and NAFLD in different body mass index (BMI) and body shapes (defined by waist-to-height ratio (WHtR)), aiming to provide more detailed dietary advice for NAFLD management.
Methods
Population and study design
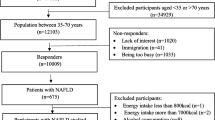
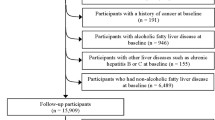
NHANES, a comprehensive database overseen by the Centers for Disease Control and Prevention (CDC), monitors the nutritional status and health of the Americans [30]. Data for this study were obtained from the eight NHANES cycles spanning from 2003 to 2018, as they included all of the relevant variables, readily available on the NHANES official website. The initial dataset consisted of 80,312 individuals. Participants were excluded if they were: (1) under the age of 18, (2) pregnant or were unable to submit a urine sample for testing, (3) had other chronic liver diseases (hepatitis B, C, and liver carcinoma), (4) engaged in excessive alcohol consumption, or (5) had incomplete information on crucial factors, including dietary data, demographic, laboratory and questionnaire. Following this screening procedure, the final research comprised 5152 individuals (Fig. 1 illustrates the study design).
Diagnostic criteria and definition
Definition of NAFLD
As previously stated, NAFLD was defined using FLI and USFLI together. The formulas are shown below:
Here, TG and GGT are the abbreviations of triglycerides and gamma-glutamyl transpeptidase, separately. In the calculation of USFLI, individuals are assigned to a value of 1 if they are classified as ‘non-Hispanic black’ or ‘Mexican American’ and 0 if they are not. Individuals with a FLI score ≥ 60 [31] or USFLI ≥30 [27] were defined as NAFLD.
The description of advanced liver fibrosis
Two groups of participants with NAFLD were categorized based on the NFS, FIB-4, and APRI scores. The formulas for these scores are as follows:
AST and ALT stand for aspartate transaminase and alanine aminotransferase, respectively, while PLT represents platelet count. Participants’ NFS values are calculated as follows: 0 if they do not have diabetes or impaired glucose tolerance (IFG), and 1 if they do. The typical upper limit of AST is denoted by ULN in the APRI computation. In NAFLD patients, NFS > 0.676 or FIB-4 > 2.67 or APRI> 1.0 [28] were considered indicators of advanced liver fibrosis.
Definition of lean/abdominal lean and obese/ abdominal obese individuals
The revised 2022 AGA Clinical Practice guidelines defined lean NAFLD as NAFLD occurring in individuals with a BMI < 25 kg/mˆ2 [32]. However, BMI alone may not provide a comprehensive assessment of body fat distribution [33]. Additional measures, such as the WHtR [8], body roundness index (BRI) [34], and a body shape index (ABSI) [35] were also incorporated to delineate abdominal obesity. To evaluate the correlation and reliability of these markers with NAFLD, ROC curves were created (Figs. 2, 3, 4, and 5). Among the measures considered, WHtR demonstrated both the best diagnostic performance and the simplest calculation method. Hence, a WHtR< 0.50 [36] was used as a measure of abdominal obesity, consistent with prior research. Finally, lean NAFLD was categorized as NAFLD with a BMI < 25.00 kg/mˆ2, while obese NAFLD was defined as having a BMI ≥ 25.00 kg/mˆ2. Additionally, abdominal-lean NAFLD was characterized by a WHtR< 0.5, whereas abdominal-obese NAFLD had a WHtR≥0.5.
Dietary assessment
The creation and validation of DII has been documented elsewhere [18, 37]. DII was computed using 45 dietary factors collected from 11 different communities globally. A Z-score was calculated by normalizing each parameter’s value through the removal of the database mean and division by the parameter’s standard deviation. Then, by doubling and removing one (from − 1 to + 1 and centering on 0), the Z-score was transformed into percentile values [18, 38]. Each central percentile was then multiplied by its associated inflammatory impact score. Then, the DII scores for each dietary parameter were added to calculate an individual’s DII.
Dietary information in NHANSE was collected via a 24-hour recall interview done at the mobile examination center (MEC). In this research, a total of 28 different food parameters, including dietary calorie intake, protein, carbohydrates, cholesterol, fat, fatty acids (saturated, monounsaturated and polyunsaturated), folic acid, beta-carotene, ω-3 and ω-6 fatty acids, niacin, fiber, alcohol, caffeine, and various vitamins (A, B1, B2, B6, B12, C, D, E), iron, zinc, selenium, and magnesium were employed in the calculation of DII, consistent with prior studies [39,40,41]. After gathering the data, the DII for each parameter was computed using the following formula:
After calculating the DII for each parameter, the DII scores of all parameters were summed to calculate an individual’s DII. Then, participants were divided into quartiles based on DII for further analyses. The quartiles were defined as follows: Quartile 1 (Q1): -5.20 < DII < − 0.09, Quartile 2 (Q2): -0.09 ≤ DII < 1.54, Quartile 3 (Q3): 1.54 ≤ DII < 2.90, and Quartile 4 (Q4): 2.90 ≤ DII < 5.52.
Covariates
The main covariates in this study included demographic data such as age, gender, race, smoking habits, family income-to-poverty ratio (PIR), educational level, as well as laboratory examinations including cholesterol (TC), TG, albumin (ALB), ALT, AST, GGT, high-density lipoprotein (HDL), PLT, and other metabolic diseases such as hypertension and diabetes. Hypertension was defined as either: (a) a history of hypertension, or (b) systolic blood pressure (SBP) ≥ 140 mmhg, or (c) diastolic blood pressure (DBP) ≥ 90 mmhg. Diabetes was diagnosed if any of the following criteria were met: (a) a prior diagnosis of diabetes, (b) a hemoglobin A1C concentration (HbA1c) above 6.4%, or (c) a fasting plasma glucose level (FPG) over 125 mg/dL, or (d) the use of insulin.
Statistical analysis
The statistical analysis in this study were conducted using R (4.3.1). Descriptive statistics were presented in various formats, including medians, averages, standard deviations, percentages and frequencies, depending on the data attributes. In Tables 1 and 2, the Chi-Square Test was utilized to examine the qualitative characteristics, while One-Way ANOVA and the Kruskal-Wallis H Test were performed to compare data from groups with normal or non-normal distributions, separately. Then, three logistic models were employed to calculate odds ratios (OR) and 95% confidence intervals (CI). Model 1 represented the original model with no confounding factors adjusted. Model 2 accounted for the impacts of age, gender, and races, while Model 3 further refined the education level, ratio of family income to poverty (PIR), hypertension, diabetes and smoking habits based on Model 2. Subgroup analyses based on age, gender, hypertension, and diabetes were conducted to assess the link between DII and NAFLD in diverse populations. All P-values were calculated on both sides and deemed statistically significant when below 0.05.
Results
The features of participants
Table 1 presents the baseline characteristics of the 5152 individuals grouped by DII quartiles. Among them, 1289 individuals were assigned to Group Q1, 1282 to Group Q2, 1293 to Group Q3 and 1288 to Group Q4. Significant variations were observed among the DII groups concerning gender, race, education level, PIR, smoking habits, BMI, waist circumstance, WHtR, SBP, TC, HDL, ALB, ALT, GGT, PLT, hypertension, diabetes, NAFLD as well as advanced liver fibrosis (P < 0.05). These disparities more pronounced with increasing DII scores.
Table 2 provides a comprehensive description of both lean and obese NAFLD. Among the 2503 NAFLD patients, 86 were classified as lean NAFLD while 2417 as obese NAFLD. Lean NAFLD patients tended to be older, with lower DBP, and higher levels of HDL and GGT. Additionally, they exhibited a higher percentage of diabetes (lean NAFLD vs obese NAFLD = 48.80% vs 36.80%), and a lower percentage of advanced liver fibrosis (lean NAFLD vs obese NAFLD = 9.50% vs 26.80%). Moreover, notable racial differences could be observed between lean and obese NAFLD. Non-Hispanic Asians exhibited a higher risk of being lean NAFLD, whereas Non-Hispanic Whites were more likely to suffer from obese NAFLD.
DII levels and NAFLD
Table 3 displays the detailed information on the association between DII and NAFLD in multivariable logistic regression models, as previously discussed. In general, NAFLD correlates positively with higher DII in all three models. The ORs of Q4 are 1.80 [1.54,2.10], 1.86 [1.57,2.19], 1.81 [1.48, 2.21], in the Model 1, Model 2, Model 3, separately. All P-values were below 0.05.
Furthermore, the correlation between DII and advanced liver fibrosis was assessed using the same methodology. Overall, a notable positive relationship was observed between higher DII and advanced liver fibrosis in all three models, especially in the highest DII group (Model 1: OR = 1.67 [1.28,2.17]; Model 2: OR = 1.73 [1.28, 2.34]; Model 3: OR = 1.46 [1.02,2.07]).
Subgroup analysis
Table 4 summarizes the findings of the subgroup analysis. Higher DII was related with an increased likelihood of NAFLD in adults both below and above the age of 60 (Q4: age ≤ 60, OR = 1.80 [1.39,2.33];age > 60, OR = 1.88 [1.37, 2.60]). Generally, a higher DII elevated the risk of NAFLD in both genders, particularly in females (in group Q4: male: OR = 1.35 [1.02,1.80], female: 2.35 [1.74,3.17]). Therefore, the findings imply that women may be more vulnerable to dietary inflammation than men. Surprisingly, among those without hypertension or diabetes, DII was more favorably related to the risk of NAFLD.
The link between DII and lean/abdominal lean NAFLD
Table 5 contains comprehensive information regarding the relationship between DII and lean or abdominal lean NAFLD across three logistic regression models. Model 1 remained unaltered, while Model 2 was modified for age and gender. Model 3 incorporated corrections for educational level, PIR, smoking habits, hypertension and diabetes based on the adjustments made in Model 2. Obviously, no statistical association could be observed between DII and lean or abdominal lean NAFLDs. However, a favorable correlation was identified between DII and obese or abdominal obese NAFLD (For BMI ≥25.00 kg/mˆ2, Q4: model 3: OR = 1.56 [1.23,1.98]. For WHtR≥0.5, Q4: model 3: OR = 1.48 [1.23,1.79]).
Discussion
Plenty of studies have demonstrated the adverse effects of pro-inflammatory diets on various metabolic diseases, including hypertension [42], heart failure [43], cognitive impairment [39] and diabetes [41]. More importantly, dietary inflammation has been implicated in the development of fatty liver disease [22, 44]. However, further investigation into the relationship of DII and NAFLD is warranted. On the one hand, it’s crucial to exclude excessive alcohol consumption as both a significant contributor to dietary inflammation and another leading cause of fatty liver disease. Neither Ting Tian [22] nor Mohsen Mazidi [44] definitively ruled out the direct effect of excessive alcohol intake in their studies due to their emphasis on a spectrum of fatty liver conditions. On the other hand, recent research has identified two subtypes of lean NAFLD. Type 1 is more common in those with abdominal obesity and insulin resistance (IR), while type 2 is more commonly observed in those with monogenic disorders [45, 46]. While adopting healthy eating habits is recommended for all forms of NAFLD [47], clinicians face challenges in selecting appropriate clinical assessments for patients with varied weights and body types. Yet, limited research has explored the effect of diet on NAFLD in individuals with varying body shapes. This knowledge gap inspired this study.
The main conclusions of this investigation are as follows: Firstly, individuals adhering to a pro-inflammatory diet are more susceptible to NAFLD and advanced liver fibrosis. Secondly, higher DII scores correlate with elevated BMI and WHtR. Moreover, the impact of dietary inflammation appears less pronounced in lean NAFLD compared to obese NAFLD. Finally, subgroup analysis indicates that female participants, and those without diabetes are particularly vulnerable to developing NAFLD when consuming a pro-inflammatory diet. The findings are consistent with earlier research revealing the induction of chronic inflammation by diet [13, 22, 48] and its role in the development of NAFLD [49].
In general, diets with higher DII are associated with processed foods containing increased calories, fat, cholesterol, and carbohydrates. More importantly, poor dietary habits often coincide with the accumulation of subcutaneous and visceral fat [40]. Initially, a pro-inflammatory diet stimulates adipose tissue to produce pro-inflammatory adipokines and cytokines, including TNF-α, IL-1, IL-6, etc. [50, 51]. These compounds contribute to persistent low-grade inflammation, a common etiology in both obesity and NAFLD. Subsequently, these pro-inflammatory mediators increase the production of reactive oxygen and nitrogen species [52], and induce immunological dysfunction by altering macrophages [53, 54], thereby exacerbating liver damage. Moreover, high DII diets have been linked to insulin resistance (IR) [41, 55] and the modification of gut flora [56]. Additionally, liver tissue exposure to prolonged free fatty acids (FFA) [57], one of the primary causes of NAFLD [58], is more common in obese individuals. Thus, it is hypothesized that obesity, particularly abdominal obesity, mediates the development of NAFLD driven by pro-inflammatory diets. Conversely, dietary factors have a lesser impact on lean NAFLD, highlighting the importance of genetic and epigenetic factors in the onset and progression of lean NAFLD. Previous studies have supported this assumption, demonstrating that certain genetic variations, such as the G variation in PNPLA3 and the T variant in TM6SF2, are more prevalent among lean NAFLD patients [59], potentially impacting processes related to inflammation, oxidative stress and lipid metabolism [60,61,62]. In a word, obese individuals may benefit more from modifying their dietary habits to prevent NAFLD, whereas lean individuals may require more targeted pharmacological therapies focusing on genes and downstream pathways rather than relying solely on dietary interventions. These therapies may include the use of certain anti-sense oligonucleotides, RNA interference, and medicines regulating gut flora [63]. Encouragingly, various drugs aimed at these processes are currently undergoing clinical trials, including traditional Chinese therapies such as Huazhi Fugan Granules [64], Fufang Zhenzhu Tiaozhi formula (FTZ) [65] and Chaihu-Shugan-San, Shen-Ling-Bai-Zhu-San [66].
In subgroup analyses, women were observed to have higher DII diets and be more vulnerable to the adverse effects of DII. This gender-related difference may be attributed to variations in dietary patterns and food choices. Besides, women are more likely to weight gain, especially around menopause, when estrogen levels decline, leading to increased fat storage [67]. Furthermore, research suggests that females are less likely to be physically active and are more prone to overeat owing to lifestyle and emotional factors [68, 69], highlighting the necessity of optimizing diet structure in women. Surprisingly, in this study, DII exhibits a stronger favorable correlation with NAFLD among participants without hypertension or diabetes. This phenomenon persists even after re-testing. One possible explanation is that those with hypertension and diabetes are already metabolically impaired, displaying decreased insulin sensitivity. Consequently, modifying the existing metabolic dysfunction remains challenging even with dietary improvements. Moreover, hypertension and diabetes serve as both causes and significant consequences of NAFLD, potentially leading to collinearity issues in data processing. This may also be due to predisposition and other lifestyle variables, such as varying levels of activity or quality of sleep and so on. To sum up, even individuals who have not been diagnosed with hypertension or diabetes should adopt a healthy diet pattern. This not only reduces the chance of acquiring hypertension and diabetes, but also mitigates the possibility of NAFLD.
Strengths and limitations
A major highlight of this study is its wide scope and thorough preparation, conducted within an organized multistage and cross-sectional project supervised by the NCHS. Furthermore, the program’s broad inclusiveness, which includes racial diversity, ensures a robust and representative sample, thereby enhancing the dependability and quality of this research. However, some restrictions should be acknowledged. Firstly, diagnostic uncertainty might arise owing to lack of a clear strategy or imaging data in detecting NAFLD and advanced liver fibrosis. Secondly, the use of questionnaires to collect data on dietary components may introduce recollection bias. Finally, despite the best efforts to adjusting for confounding variables, the potential influence of certain macronutrient-related confounders cannot be entirely avoided. However, the conclusions drawn in this study are considered valid and hold significant importance due to the researchers’ attempts to mitigate the impact of extraneous variables.
Conclusion
To sum up, this study demonstrated a notable positive connection between DII and NAFLD, as well as its’ progression to advanced liver fibrosis. Significantly, the impact of dietary inflammation on NAFLD was more pronounced in obese individuals compared to their lean counterparts. Furthermore, female participants, and those without a diagnose of hypertension and diabetes were more vulnerable to the negative effects of pro-inflammatory diets. The clinical significance or this study is multifaced: Firstly, it highlights the importance of dietary management in the general population, recommending diets with lower DII. Secondly, the study suggests that obese individuals may benefit more from adopting healthier eating patterns. Conversely, lean individuals may require more targeted pharmacological therapies on genes and their downstream pathways, indicating the need for further research in this area. Finally, the findings emphasize the importance of tailored dietary recommendations for specific demographic groups, such as female and those without hypertension or diabetes, to promote public health and prevent NAFLD.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
Hardy T, et al. Nonalcoholic fatty liver disease: pathogenesis and disease Spectrum. Ann Rev Pathol: Mechan Disease. 2016;11(1):451–96.
Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65(8):1038–48.
Powell EE, Wong VW-S, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397(10290):2212–24.
Ahadi M, et al. A review of non-alcoholic fatty liver disease in non-obese and lean individuals. J Gastroenterol Hepatol. 2020;36(6):1497–507.
Yip TCF, et al. Geographical similarity and differences in the burden and genetic predisposition of NAFLD. Hepatol. 2023;77(4):1404–27.
Ye Q, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(8):739–52.
Dao AD, et al. Prevalence, characteristics, and mortality outcomes of obese and nonobese MAFLD in the United States. Hepatol Int. 2023;17(1):225–36.
Eslam M, et al. Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat Rev Gastroenterol Hepatol. 2022;19(10):638–51.
Jang H, Kim W. Non-obese or lean nonalcoholic fatty liver disease matters, but is it preventable or inevitable in light of its risk factors? Clin Mol Hepatol. 2023;29(2):381–3.
Arrese M, et al. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61(5):1294–303.
Petrescu M, Vlaicu SI, Ciumărnean L, Milaciu MV, Mărginean C, Florea M, Vesa ȘC, Popa M. Chronic Inflammation-A Link between Nonalcoholic Fatty Liver Disease (NAFLD) and Dysfunctional Adipose Tissue. Medicina (Kaunas). 2022;58(5):641.
Xiong P, et al. Metaflammation in glucolipid metabolic disorders: pathogenesis and treatment. Biomed Pharmacother. 2023;161:114545.
Christ A, Lauterbach M, Latz E. Western diet and the immune system: an inflammatory connection. Immunity. 2019;51(5):794–811.
Malesza IJ, Malesza M, Walkowiak J, Mussin N, Walkowiak D, Aringazina R, Bartkowiak-Wieczorek J, Mądry E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells. 2021;10(11):3164.
Casas R, Castro-Barquero S, Estruch R, Sacanella E. Nutrition and Cardiovascular Health. Int J Mol Sci. 2018;19(12):3988.
Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56(1):255–66.
Ryan MC, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59(1):138–43.
Shivappa N, et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–96.
Kenđel Jovanović G, et al. The efficacy of an energy-restricted anti-inflammatory diet for the Management of Obesity in younger adults. Nutrients. 2020;12(11)
Tan J, et al. A Proinflammatory diet may increase mortality risk in patients with diabetes mellitus. Nutrients. 2022;14(10)
Zhou N, et al. The dietary inflammatory index and its association with the prevalence of hypertension: a cross-sectional study. Front Immunol. 2022;13:1097228.
Tian T, et al. Dietary quality and relationships with metabolic dysfunction-associated fatty liver disease (MAFLD) among United States adults, results from NHANES 2017-2018. Nutrients. 2022;14(21)
Younes R, et al. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease. J Hepatol. 2021;75(4):786–94.
Stern C, Castera L. Non-invasive diagnosis of hepatic steatosis. Hepatol Int. 2016;11(1):70–8.
Sourianarayanane A, McCullough AJ. Accuracy of steatosis and fibrosis NAFLD scores in relation to vibration controlled transient elastography: an NHANES analysis. Clin Res Hepatol Gastroenterol. 2022;46(7)
Sharma S. Non-invasive diagnosis of advanced fibrosis and cirrhosis. World J Gastroenterol. 2014;20(45)
Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and nutrition examination survey. Aliment Pharmacol Ther. 2015;41(1):65–76.
Cheah MC, McCullough AJ, Goh GB. Current Modalities of Fibrosis Assessment in Non-alcoholic Fatty Liver Disease. J Clin Transl Hepatol. 2017;5(3):261-71.
Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterol. 2019;156(5):1264–1281 e4.
Ahluwalia N, et al. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. 2016;7(1):121–34.
Bedogni G, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33.
Long MT, Noureddin M, Lim JK. AGA clinical practice update: diagnosis and Management of Nonalcoholic Fatty Liver Disease in lean individuals: expert review. Gastroenterol. 2022;163(3):764–774.e1.
Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15(4):474–85.
Rico-Martín S, et al. Effectiveness of body roundness index in predicting metabolic syndrome: a systematic review and meta-analysis. Obes Rev. 2020;21(7):e13023.
Christakoudi S, et al. Associations of body shape index (ABSI) and hip index with liver, metabolic, and inflammatory biomarkers in the UK biobank cohort. Sci Rep. 2022;12(1):8812.
Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutr Res Rev. 2010;23(2):247–69.
Wang T, et al. The association between dietary inflammatory index and disability in older adults. Clin Nutr. 2021;40(4):2285–92.
Darbandi M, et al. Anti-inflammatory diet consumption reduced fatty liver indices. Sci Rep. 2021;11(1):22601.
Dong X, et al. Association of dietary omega-3 and omega-6 fatty acids intake with cognitive performance in older adults: National Health and nutrition examination survey (NHANES) 2011-2014. Nutr J. 2020;19(1):25.
Tu J, et al. Dietary inflammatory potential is associated with sarcopenia in patients with hypertension: national health and nutrition examination study. Front Nutr. 2023;10:1176607.
Shu Y, et al. Associations of dietary inflammatory index with prediabetes and insulin resistance. Front Endocrinol (Lausanne). 2022;13:820932.
Wu W, et al. Association between dietary inflammation index and hypertension in participants with different degrees of liver steatosis. Ann Med. 2023;55(1):2195203.
Liu Z, et al. Association between dietary inflammatory index and heart failure: results from NHANES (1999-2018). Front Cardiovasc Med. 2021;8:702489.
Mazidi M, et al. Diet with greater inflammatory potential is associated with higher prevalence of fatty liver among US adults. Eur J Clin Nutr. 2019;73(12):1653–6.
Vilarinho S, et al. Emerging role of genomic analysis in clinical evaluation of lean individuals with NAFLD. Hepatol. 2021;74(4):2241–50.
Orkin S, et al. Body composition measured by bioelectrical impedance analysis is a viable alternative to magnetic resonance imaging in children with nonalcoholic fatty liver disease. JPEN J Parenter Enteral Nutr. 2022;46(2):378–84.
Pouwels S, et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord. 2022;22(1):63.
Barbaresko J, et al. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71(8):511–27.
Schuster S, et al. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15(6):349–64.
Wang T, He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50.
Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Phys Cell Phys. 2021;320(3):C375–91.
Abdollahzad H, et al. The relationship between the dietary inflammatory index and metabolic syndrome in Ravansar cohort study. Diabetes Metab Syndr Obes. 2020;13:477–87.
Kapellos TS, et al. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front Immunol. 2019;10:2035.
Figueroa-Vega N, et al. Analysis of the percentages of monocyte subsets and ILC2s, their relationships with metabolic variables and response to hypocaloric restriction in obesity. PLoS One. 2020;15(2):e0228637.
Martins FO, Conde SV. Impact of Diet Composition on Insulin Resistance. Nutrients. 2022;14(18):3716.
Lau LHS, Wong SH. Microbiota, obesity and NAFLD. Adv Exp Med Biol. 2018;1061:111–25.
Dietrich P, Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28(4):637–53.
Polyzos SA, Kountouras J, Mantzoros CS. Adipose tissue, obesity and non-alcoholic fatty liver disease. Minerva Endocrinol. 2017;42(2):92–108.
Feldman A, et al. Clinical and metabolic characterization of lean Caucasian subjects with non-alcoholic fatty liver. Am J Gastroenterol. 2017;112(1):102–10.
Aggeletopoulou I, Kalafateli M, Tsounis EP, Triantos C. Epigenetic Regulation in Lean Nonalcoholic Fatty Liver Disease. Int J Mol Sci. 2023;24(16):12864.
Shen J, et al. PNPLA3 gene polymorphism and response to lifestyle modification in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2014;30(1):139–46.
Wong VW-S, et al. Incidence of non-alcoholic fatty liver disease in Hong Kong: a population study with paired proton-magnetic resonance spectroscopy. J Hepatol. 2015;62(1):182–9.
Bluemel S, et al. Precision medicine in alcoholic and nonalcoholic fatty liver disease via modulating the gut microbiota. Am J Physiol Gastrointest Liver Physiol. 2016;311(6):G1018–36.
Ye M, et al. Alleviation of non-alcoholic fatty liver disease by Huazhi Fugan granules is associated with suppression of TLR4/NF-κB signaling pathway. Clínica e Investigación en Arteriosclerosis (English Edition). 2021;33(5):257–66.
Wang H, Huang M, Bei W, Yang Y, Song L, Zhang D, Zhan W, Zhang Y, Chen X, Wang W, Wang L, Guo J. FTZ attenuates liver steatosis and fibrosis in the minipigs with type 2 diabetes by regulating the AMPK signaling pathway. Biomed Pharmacother. 2021;138:111532.
Yang Q-H, et al. Effects of Chaihu-Shugan-san and Shen-Ling-Bai-Zhu-san on p38 MAPK pathway in Kupffer cells of nonalcoholic steatohepatitis. Evid Based Complement Alternat Med. 2014;2014:1–8.
Zhao Q, Tan X, Su Z, Manzi HP, Su L, Tang Z, Zhang Y. The Relationship between the Dietary Inflammatory Index (DII) and Metabolic Syndrome (MetS) in Middle-Aged and Elderly Individuals in the United States. Nutrients. 2023;15(8):1857.
Sokol A, et al. Association between the dietary inflammatory index, waist-to-hip ratio and metabolic syndrome. Nutr Res. 2016;36(11):1298–303.
Meyer ML, et al. Polycystic ovary syndrome signs and metabolic syndrome in premenopausal Hispanic/Latina women: the HCHS/SOL study. J Clin Endocrinol Metab. 2020;105(3):e447–56.
Acknowledgments
The efforts of the NHANES 2003-2018 individuals in data collecting and sharing are all appreciated.
Disclosure statement
The author(s) did not disclose any potential conflicts of interest.
Funding
The Chinese National Natural Science Foundation (No. 82172983) provided support for this work. The data in this research is accessible upon request from authors.
Author information
Authors and Affiliations
Contributions
LRL and XWS developed and designed the study. LRL, YY and YD worked together to gather, manage and analyze the data. LRL, XWS,YD and YY wrote the draft, which was then revised by YC and JL. All writers contributed to the writing process and approved the final draft.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The NCHS Ethics Review Board performed and authorized the human subject’s study. To take part in the research, participants provided written consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, L., Shu, X., Yi, Y. et al. Dietary inflammatory impact on NAFLD development in obese vs. lean individuals: an analysis based on NHANES 2003–2018. Lipids Health Dis 23, 127 (2024). https://doi.org/10.1186/s12944-024-02082-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02082-4