Abstract
Background
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a key regulator of serum low-density lipoprotein (LDL) cholesterol levels. Recently, PCSK9 has additionally been related to metabolic risk factors such as the levels of triglycerides, apolipoprotein B (apoB), insulin, and glucose, as well as body mass index. The purpose of this study was to investigate correlations between serum levels of PCSK9 and apoB-containing atherogenic lipoproteins in patients with coronary artery disease (CAD).
Methods
Serum levels of PCSK9 and lipoprotein(a) [Lp(a)]; small, dense LDL; and oxidized LDL were measured in 101 patients with CAD who were not receiving lipid-lowering therapy.
Results
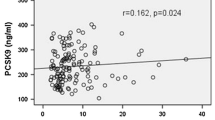
Serum hetero-dimer PCSK9 levels were positively correlated with serum levels of Lp(a) (r = 0.195, p = 0.05); small, dense LDL (r = 0.336, p = 0.0006); and oxidized LDL (r = 0.268, p = 0.008). Multivariate regression analyses showed that serum hetero-dimer PCSK9 was a significant predictor of serum levels of Lp(a) (β = 0.235, p = 0.01); small, dense LDL (β = 0.143, p = 0.03); and oxidized LDL (β = 0.268, p = 0.008).
Conclusions
Serum PCSK9 levels were positively correlated with serum levels of Lp(a); small, dense LDL; and oxidized LDL in patients with CAD. This suggests that the interaction between serum PCSK9 and apoB-containing lipoproteins plays a role in establishing the atherosclerotic status of patients.
Trial registration
UMIN Clinical Trials Registry, UMIN ID: C000000311.
Similar content being viewed by others
Background
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a key regulator of serum low-density lipoprotein (LDL) cholesterol levels [1, 2]. PCSK9, which is secreted by the liver into the circulation, binds the hepatic LDL receptors (LDLRs), causing their subsequent degradation [3, 4]. Although the mechanism underlying PCSK9-mediated degradation of LDLR is extremely complex, it bind to LDLR subsequently targeting them for intracellular destruction within the hepatocyte, resulting in an increase in LDL cholesterol levels [5–7]. Therefore, PCSK9 antibodies represent attractive candidates for lowering LDL cholesterol levels.
Elevated levels of lipoprotein(a) [Lp(a)]; small, dense LDL; and oxidized LDL are recognized as risk factors for atherosclerotic cardiovascular disease (ASCVD) [8–11]. The levels of both small, dense LDL and oxidized LDL may be lowered by statin therapy [12, 13]; however, the availability of pharmacological agents for lowering Lp(a) levels is limited. Therefore, Lp(a) levels represent a residual risk factor for cardiovascular events in this statin era [14]. Although monoclonal antibodies against PCSK9 have been reported to lower Lp(a) levels [15], the mechanisms underlying this effect are poorly understood.
Several demographic and metabolic parameters appear to correlate with serum PCSK9 levels, including plasma LDL cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, apolipoprotein B (apoB), insulin, glucose, smoking, and body mass index [16–18]. In addition, circulating PCSK9 strongly correlates with intermediate-density lipoprotein particles, suggesting a link between PCSK9 and triglyceride-rich lipoprotein metabolism [19]. However, to our knowledge, correlations between serum levels of PCSK9 and small, dense LDL or oxidized LDL have not been evaluated to date. In addition, few studies concerning the association of PCSK9 with Lp(a) have been reported. Therefore, the aim of this study was to investigate correlations between serum levels of PCSK9 and apoB-containing atherogenic lipoproteins such as Lp(a); small, dense LDL; and oxidized LDL in patients with coronary artery disease (CAD).
Methods
Patients and study design
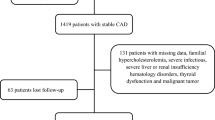
The present study is a post-hoc analysis of the Treatment With Statin on Atheroma Regression Evaluated by Intravascular Ultrasound With Virtual Histology (TRUTH) study, which was a prospective, open-labeled, randomized, multicenter trial performed at 11 Japanese centers [20]. In brief, 164 patients with angina pectoris, who were not receiving any lipid-lowering therapy, were randomly treated with either 4 mg/day of pitavastatin or 20 mg/day of pravastatin.
The patients included in the TRUTH study were considered for the present study if an adequate serum volume, before statin treatment, was available in frozen samples from these patients; a total of 101 patients met this inclusion criterion.
This study was conducted in accordance with the Declaration of Helsinki and with the approval of the ethical committees of Yokohama Sakae Kyosai Hospital. Each patient enrolled in the present study provided written informed consent.
Laboratory analysis
Serum levels of total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides were measured using standard enzymatic methods (AU2700; Beckman Coulter, CA, USA) and commercial enzymatic kits (Kyowa Medex, Tokyo, Japan). Serum levels of two forms of PCSK9, mature (hetero-dimer) and furin-cleaved, were measured at a central laboratory (BML, Kawagoe, Japan) using sandwich enzyme-linked immunosorbent assays (ELISAs) [21]. It has been reported that furin-cleaved PCSK9 has no activity to regulate LDLR and serum LDL cholesterol or less activity than mature PCSK9 [21]. Serum oxidized LDL levels were measured by an enzyme immunoassay [22]. Serum Lp(a) levels were measured by a latex agglutination turbidimetric immunoassay using the commercially available Lp(a)-LATEX (Sekisui Medical Co., Ltd., Tokyo) with an autoanalyzer (JCA-BM8040; JEOL Ltd., Tokyo). Serum small, dense LDL levels were measured by a homogeneous assay (Denka Seiken Co., Ltd., Tokyo) [23].
Statistical analysis
Statistical analysis was performed using StatView, version 5.0 (SAS Institute, Cary, North Carolina). The results are expressed as means ± SD or median values (range). Univariate and multivariate linear regression analyses were performed to assess the correlations between serum levels of apoB-containing atherogenic lipoprotein and biochemical parameters, including nominal variables (gender, hypertension, diabetes mellitus, and smoking) and numerical variables (age, body mass index, total cholesterol, LDL cholesterol, triglycerides, HDL cholesterol, apoA1, apoB, hetero-dimer PCSK9, and furin-cleaved PCSK9). The variables with a p value < 0.1 on univariate analysis were entered into multivariate models. Statistical significance was set at p < 0.05.
Results
The baseline characteristics of the subjects are listed in Table 1. Eighty-four patients were of male gender with a mean age of 67 ± 10 years. Forty-five of the patients were additionally diabetic.
Serum levels of lipid, PCSK9, and apoB-containing lipoproteins are shown in Table 2. The mean levels of LDL cholesterol and apoB were 129 ± 31 mg/dl and 103 ± 24 mg/dl, respectively. Serum hetero-dimer PCSK9 levels were 145 ± 60 ng/ml. Median levels of Lp(a) were 16 mg/dl, and mean levels of small, dense LDL and oxidized LDL were 25.8 ± 13.9 mg/dl and 11.8 ± 8.5 U/ml, respectively.
Correlations between levels of apoB-containing lipoproteins and biochemical parameters are shown in Table 3. Univariate analysis indicated that total cholesterol, apoB, and hetero-dimer PCSK9 levels (Fig. 1) positively correlated with serum levels of Lp(a). Multivariate regression analysis showed that serum apoB and PCSK9 levels were significant positive predictors of Lp(a) levels. In addition, total cholesterol, LDL cholesterol, triglycerides, apoB, and hetero-dimer PCSK9 levels (Fig. 2) positively correlated with serum small, dense LDL levels. Multivariate regression analysis showed that apoB and hetero-dimer PCSK9 levels were significant positive predictors of small, dense LDL levels. Moreover, univariate and multivariate analyses showed that serum hetero-dimer PCSK9 levels positively correlated with serum oxidized LDL levels (Fig. 3). Therefore, weak, but significant, positive correlations were observed between serum levels of hetero-dimer PCSK9 and apoB-containing lipoproteins such as Lp(a); small, dense LDL; and oxidized LDL.
Discussion
The major findings of the present study are as follows: first, serum hetero-dimer PCSK9 levels were positively correlated with serum Lp(a) levels. Second, significant positive correlations were observed between serum levels of hetero-dimer PCSK9 and small, dense LDL or oxidized LDL. Finally, the serum hetero-dimer PCSK9 level was a significant predictor of serum levels of apoB-containing atherogenic lipoproteins.
Lp(a) is a LDL-like particle synthesized by the liver, that consists of an apoB100 molecule linked to a very large glycoprotein known as the apolipoprotein(a) [8, 24]. The biological role of Lp(a) is uncertain; however, elevated levels of Lp(a) have been identified as an independent risk factor for ASCVD [8, 9]. Several recent clinical trials have reported that PCSK9 antibodies represent promising novel candidate drugs for lowering the levels of both LDL cholesterol and Lp(a) [25–27]. However, the mechanism by which PCSK9 inhibitors reduce Lp(a) levels remains unclear. Serum Lp(a) levels in familial hypercholesterolemia with LDLR mutations have been shown to be elevated, suggesting that Lp(a) is catabolized via the LDLR pathway [28]; however, statins, whose main mechanism of action involves the upregulation of LDLR, are unable to reduce Lp(a) levels effectively [29]. In addition, Tada et al. recently reported that serum Lp(a) was elevated in patients with familial hypercholesterolemia caused by PCSK9 gain-of-function mutations to the same level as that in familial hypercholesterolemia caused by LDLR mutations [30]. This suggests that the LDLR plays an important role in Lp(a) catabolism. Furthermore, Romagnuolo et al. reported that Lp(a) catabolism is regulated by PCSK9 via LDLR in HEPG2 cells and primary human fibroblasts [31]. However, a previous study reported that apoB in Lp(a) does not interact with LDLR, suggesting that LDLR does not play a role in Lp(a) kinetics [24].
In order to understand the mechanism by which PCSK9 antibodies reduce Lp(a) levels, elucidation of the correlation between serum levels of PCSK9 and Lp(a) is required. A recent study by Nekaies et al. reported that plasma PCSK9 levels correlated positively with Lp(a) concentrations [32]. However, Yang et al. reported that plasma PCSK9 levels are not associated with Lp(a) levels [33]. Therefore, the correlation between plasma PCSK9 and Lp(a) levels remains controversial. Significant positive correlations were observed between serum levels of PCSK9 and Lp(a) in the present study; furthermore, serum apoB levels were additionally found to be positively correlated with serum Lp(a) levels. Our findings suggest that both PCSK9 and apoB interact with Lp(a). Variations in terms of correlation between PCSK9 and Lp(a) observed between patients may be attributed to differences in race, as both PCSK9 and Lp(a) levels vary considerably by race and other factors [18, 24, 34].
Small, dense LDL has been proposed to enhance atherogenicity owing to its higher rate of penetration into the arterial wall, prolonged plasma half-time, and lower affinity of LDLR [35]. Numerous previous studies have examined the association of small, dense LDL with traditional cardiovascular risk factors [36, 37]. In agreement with these previous reports, univariate analysis performed in the present study indicated that serum small, dense LDL levels were correlated with the concentration of total cholesterol, LDL cholesterol, triglycerides, and apoB. However, multivariate regression analysis showed that apoB and PCSK9 levels were significant predictors of small, dense LDL levels. Recently, Kwakernaak et al. found no association between plasma PCSK9 levels and small, dense LDL in healthy subjects [19]. However, Zhang et al. reported that plasma PCSK9 levels are positively associated with plasma small, dense LDL in patients with CAD; however, this association was not observed in subjects without CAD [38]. Therefore, the levels of plasma PCSK9 and small, dense LDL may be correlated in atherosclerotic patients. Although the exact mechanisms underlying the positive correlation between PCSK9 and small, dense LDL levels remain to be elucidated, PCSK9-induced LDLR degradation, which results in lower LDLR levels, potentially affects LDL subfractions, as characterized by the increased levels of small, dense LDL.
Oxidized LDL is also highly atherogenic and elevated levels of oxidized LDL recognized as a risk factor for ASCVD [11, 39]. Oxidized LDL levels have been reported to be positively correlated with PCSK9 concentration [40]. Consistent with this finding, we observed a positive correlation between serum levels of PCSK9 and oxidized LDL. This observation may be attributed to the involvement of PCSK9 in inflammatory and oxidative processes [41, 42]. In addition, inhibition of PCSK9 suppresses the inflammatory response induced by oxidized LDL in macrophages [43]. Further, circulatory PCSK9 is not only present in its free form, but is additionally complexed with apoB-containing lipoproteins [44]. Our findings indicate that serum PCSK9 levels positively correlated with apoB-containing lipoproteins such as Lp(a); small, dense LDL; and oxidized LDL.
Several recent clinical trials have reported that PCSK9 antibodies reduce Lp(a) levels [25–27]. However, there are no reports on the effects of PCSK9 antibodies on small, dense LDL or oxidized LDL. In view of the finding that PCSK9 antibodies reduced LDL cholesterol levels and particle numbers [45], we speculate that PCSK9 antibodies reduce small, dense LDL as well as oxidized LDL levels.
Plasma PCSK9 levels have been reported to be associated with severity of CAD as well as future risk of cardiovascular disease [46, 47]. In addition, plasma PCSK9 levels were significantly higher in patients with peripheral artery disease, especially those with extensive, severe, and complicated forms of the condition [40], suggesting that PCSK9 functions as a proatherogenic molecule [48]. Furthermore, PCSK9 is expressed in atherosclerotic plaques [41, 49]. Therefore, PCSK9-targeting strategies may represent potential therapeutic options for the treatment of ASCVD, whose mechanisms of action go beyond the reduction of LDL cholesterol.
The present study has several limitations in that it provides a post-hoc analysis of the results of the TRUTH trial. Plasma PCSK9 levels have been reported to be elevated in patients with acute myocardial infarction and associated with severity of CAD [46, 50]. In the present study, all subjects were patients with CAD; no control group was studied. In addition, serum PCSK9 levels were measured using frozen samples. Further, we did not evaluate the LDLR and PCSK9 expression in hepatocytes. Finally, the small number of patients included in the study resulted in low statistical power.
Despite these limitations, to the best of our knowledge, this is the first study to examine the correlations between serum levels of PCSK9 and apoB-containing atherogenic lipoproteins. A prospective study with a larger number of patients is required to confirm our conclusions.
Conclusions
Serum PCSK9 levels were positively correlated with serum levels of Lp(a); small, dense LDL; and oxidized LDL in patients with CAD. This suggests that serum PCSK9 levels are correlated with those of apoB-containing lipoproteins in atherosclerotic patients.
Abbreviations
- apo:
-
Apolipoprotein
- ASCVD:
-
Atherosclerotic cardiovascular disease
- CAD:
-
Coronary artery disease
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- LDLR:
-
LDL receptor
- Lp(a):
-
Lipoprotein(a)
- PCSK9:
-
Proprotein convertase subtilisin/kexin type 9
- TRUTH:
-
Treatment with statin on atheroma regression evaluated by intravascular ultrasound with virtual histology
References
Seidah NG, Khatib AM, Prat A. The proprotein convertases and their implication in sterol and/or lipid metabolism. Biol Chem. 2006;387:871–7.
Lambert G, Krempf M, Costet P. PCSK9: a promising therapeutic target for dyslipidemias? Trends Endocrinol Metab. 2006;17:79–81.
Park SW, Moon YA, Horton JD. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J Biol Chem. 2004;279:50630–8.
Benjannet S, Rhainds D, Essalmani R, Mayne J, Wickham L, Jin W, Asselin MC, Hamelin J, Varret M, Allard D, Trillard M, Abifadel M, Tebon A, Attie AD, Rader DJ, Boileau C, Brissette L, Chrétien M, Prat A, Seidah NG. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem. 2004;279:48865–75.
Li J, Tumanut C, Gavigan JA, Huang WJ, Hampton EN, Tumanut R, Suen KF, Trauger JW, Spraggon G, Lesley SA, Liau G, Yowe D, Harris JL. Secreted PCSK9 promotes LDL receptor degradation independently of proteolytic activity. Biochem J. 2007;406:203–7.
McNutt MC, Lagace TA, Horton JD. Catalytic activity is not required for secreted PCSK9 to reduce low density lipoprotein receptors in HepG2 cells. J Biol Chem. 2007;282:20799–803.
Zhang DW, Lagace TA, Garuti R, Zhao Z, McDonald M, Horton JD, Cohen JC, Hobbs HH. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem. 2007;282:18602–12.
Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Reiner Z, Taskinen MR, Tokgözoglu L, Tybjærg-Hansen A, Panel EASC. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–53.
Kamstrup PR, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117:176–84.
El Harchaoui K, van der Steeg WA, Stroes ES, Kuivenhoven JA, Otvos JD, Wareham NJ, Hutten BA, Kastelein JJ, Khaw KT, Boekholdt SM. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;49:547–53.
Toshima S, Hasegawa A, Kurabayashi M, Itabe H, Takano T, Sugano J, Shimamura K, Kimura J, Michishita I, Suzuki T, Nagai R. Circulating oxidized low density lipoprotein levels. A biochemical risk marker for coronary heart disease. Arterioscler Thromb Vasc Biol. 2000;20:2243–7.
Nozue T, Michishita I, Ito Y, Hirano T. Effects of statin on small dense low-density lipoprotein cholesterol and remnant-like particle cholesterol in heterozygous familial hypercholesterolemia. J Atheroscler Thromb. 2008;15:146–53.
Sasaki S, Kuwahara N, Kunitomo K, Harada S, Yamada T, Azuma A, Takeda K, Nakagawa M. Effects of atorvastatin on oxidized low-density lipoprotein, low-density lipoprotein subfraction distribution, and remnant lipoprotein in patients with mixed hyperlipoproteinemia. Am J Cardiol. 2002;89:386–9.
Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, Mora S. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). Circulation. 2014;129:635–42.
Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, Stein EA. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408–17.
Troutt JS, Alborn WE, Cao G, Konrad RJ. Fenofibrate treatment increases human serum proprotein convertase subtilisin kexin type 9 levels. J Lipid Res. 2010;51:345–51.
Baass A, Dubuc G, Tremblay M, Delvin EE, O'Loughlin J, Levy E, Davignon J, Lambert M. Plasma PCSK9 is associated with age, sex, and multiple metabolic markers in a population based sample of children and adolescents. Clin Chem. 2009;55:1637–45.
Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab. 2009;94:2537–43.
Kwakernaak AJ, Lambert G, Dullaart RP. Plasma proprotein convertase subtilisin kexin type 9 is predominantly related to intermediate density lipoproteins. Clin Biochem. 2014;47:679–82.
Nozue T, Yamamoto S, Tohyama S, Umezawa S, Kunishima T, Sato A, Miyake S, Takeyama Y, Morino Y, Yamauchi T, Muramatsu T, Hibi K, Sozu T, Terashima M, Michishita I. Statin treatment for coronary artery plaque composition based on intravascular ultrasound radiofrequency data analysis. Am Heart J. 2012;163:191–9.
Hori M, Ishihara M, Yuasa Y, Makino H, Yanagi K, Tamanaha T, Kishimoto I, Kujiraoka T, Hattori H, Harada-Shiba M. Removal of plasma mature and furin-cleaved proprotein convertase subtilisin/kexin 9 by low-density lipoprotein-apheresis in familial hypercholesterolemia: development and application of a new assay for PCSK9. J Clin Endcrinol Metab. 2015;100:E41–9.
Kohno H, Sueshige N, Oguri K, Izumidate H, Masunari T, Kawamura M, Itabe H, Takano T, Hasegawa A, Nagai R. Simple and practical sandwich-type enzyme immunoassay for human oxidatively modified low density lipoprotein using antioxidized phosphatidylcholine monoclonal antibody and antihuman apolipoprotein-B antibody. Clin Biochem. 2000;33:243–53.
Ito Y, Fujimura M, Ohta M, Hirano T. Development of a homogeneous assay for measurement of small dense LDL cholesterol. Clin Chem. 2011;57:57–65.
Lamon-Fava S, Diffenderfer MR, Marcovina SM. Lipoprotein(a) metabolism. Curr Opin Lipidol. 2014;25:189–93.
Norata GD, Ballantyne CM, Catapano AL. New therapeutic principles in dyslipidaemia: focus on LDL and Lp(a) lowering drugs. Eur Heart J. 2013;34:1783–9.
Desai NR, Kohli P, Giugliano RP, O'Donoghue ML, Somaratne R, Zhou J, Hoffman EB, Huang F, Rogers WJ, Wasserman SM, Scott R, Sabatine MS. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C Assessment with Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined with Statin Therapy (LAPLACE)-Thrombolysis in Myocardial Infarction (TIMI) 57 trial. Circulation. 2013;128:962–9.
Raal FJ, Giugliano RP, Sabatine MS, Koren MJ, Langslet G, Bays H, Blom D, Eriksson M, Dent R, Wasserman SM, Huang F, Xue A, Albizem M, Scott R, Stein EA. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol. 2014;63:1278–88.
Kraft HG, Lingenhel A, Raal FJ, Hohenegger M, Utermann G. Lipoprotein(a) in homozygous familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2000;20:522–8.
Tziomalos K, Athyros VG, Wierzbicki AS, Mikhailidis DP. Lipoprotein a: where are we now? Curr Opin Cardiol. 2009;24:351–7.
Tada H, Kawashiri MA, Yoshida T, Teramoto R, Nohara A, Konno T, Inazu A, Mabuchi H, Yamagishi M, Hayashi K. Lipoprotein(a) in familial hypercholesterolemia with proprotein convertase subtilisin/kexin type 9 (PCSK9) gain-of-function mutations. Circ J. 2016;80:512–8.
Romagnuolo R, Scipione CA, Boffa MB, Marcovina SM, Seidah NG, Koschinsky ML. Lipoprotein(a) catabolism is regulated by proprotein convertase subtilisin/kexin type 9 through the low density lipoprotein receptor. J Biol Chem. 2015;290:11649–62.
Nekaies Y, Baudin B, Kelbousi S, Sakly M, Attia N. Plasma proprotein convertase subtilisin/kexin type 9 is associated with Lp(a) in type 2 diabetic patients. J Diabetes Complications. 2015;29:1165–70.
Yang SH, Li S, Zhang Y, Xu RX, Zhu CG, Guo YL, Wu NQ, Qing P, Gao Y, Cui CJ, Dong Q, Sun J, Li JJ. Analysis of the association between plasma PCSK9 and Lp(a) in Han Chinese. J Endocrinol Invest. 2016;39:875–83.
Cui Q, Ju X, Yang T, Zhang M, Tang W, Chen Q, Hu Y, Haas JV, Troutt JS, Pickard RT, Darling R, Konrad RJ, Zhou H, Cao G. Serum PCSK9 is associated with multiple metabolic factors in a large Han Chinese population. Atherosclerosis. 2010;213:632–6.
Diffenderfer MR, Schaefer EJ. The composition and metabolism of large and small LDL. Curr Opin Lipidol. 2014;25:221–6.
Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E, Ballantyne CM. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2014;34:1069–77.
Ai M, Otokozawa S, Asztalos BF, Ito Y, Nakajima K, White CC, Cupples LA, Wilson PW, Schaefer EJ. Small dense LDL cholesterol and coronary heart disease: results from the Framingham Offspring Study. Clin Chem. 2010;56:967–76.
Zhang Y, Xu RX, Li S, Zhu CG, Guo YL, Sun J, Li JJ. Association of plasma small dense LDL cholesterol with PCSK9 levels in patients with angiographically proven coronary artery disease. Nutr Metab Cardiovasc Dis. 2015;25:426–33.
Iwai M, Yoshino G, Matsushita M, Morita M, Matsuba K, Kazumi T, Baba S. Abnormal lipoprotein composition in normolipidemic diabetes patients. Diabetes Care. 1990;13:792–6.
Chao TH, Chen IC, Li YH, Lee PT, Tseng SY. Plasma levels of proprotein convertase subtilisin/kexin type 9 are elevated in patients with peripheral artery disease and associated with metabolic disorders and dysfunction in circulating progenitor cells. J Am Heart Assoc. 2016;5, e003497.
Urban D, Pöss J, Böhm M, Laufs U. Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J Am Coll Cardiol. 2013;62:1401–8.
Ding Z, Liu S, Wang X, Deng X, Fan Y, Shahanawaz J, Shmookler Reis RJ, Varughese KI, Sawamura T, Mehta JL. Cross-talk between LOX-1 and PCSK9 in vascular tissues. Cardiovasc Res. 2015;107:556–67.
Tang Z, Jiang L, Peng J, Ren Z, Wei D, Wu C, Pan L, Jiang Z, Liu L. PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF-kB activation in THP-1-derived macrophages. Int J Mol Med. 2012;30:931–8.
Kosenko T, Golder M, Leblond G, Weng W, Lagace TA. Low density lipoprotein binds to proprotein convertase subtilisin/kexin type-9 (PCSK9) in human plasma and inhibits PCSK9-mediated low density lipoprotein receptor degradation. J Biol Chem. 2013;288:8279–88.
Koren MJ, Kereiakes D, Pourfarzib R, Winegar D, Banerjee P, Hamon S, Hanotin C, McKenney JM. Effect of PCSK9 inhibition by alirocumab on lipoprotein particle concentrations determined by nuclear magnetic resonance spectroscopy. J Am Heart Assoc. 2015;4, e002224.
Li S, Guo YL, Xu RX, Zhang Y, Zhu CG, Sun J, Qing P, Wu NQ, Li JJ. Plasma PCSK9 levels are associated with the severity of coronary stenosis in patients with atherosclerosis. Int J Cardiol. 2014;174:863–4.
Leander K, Mälarstig A, Van't Hooft FM, Hyde C, Hellénius ML, Troutt JS, Konrad RJ, Öhrvik J, Hamsten A, de Faire U. Circulating proprotein convertase subtilisin/kexin type 9 (PCSK9) predicts future risk of cardiovascular events independently of established risk factors. Circulation. 2016;133:1230–9.
Li S, Li JJ. PCSK9: A key factor modulating atherosclerosis. J Atheroscler Thromb. 2015;22:221–30.
Ferri N, Tibolla G, Pirillo A, Cipollone F, Mezzetti A, Pacia S, Corsini A, Catapano AL. Proprotein convertase subtilisin kexin type 9 (PCSK9) secreted by cultured smooth muscle cells reduces macrophages LDLR levels. Atherosclerosis. 2012;220:381–6.
Almontashiri NA, Vilmundarson RO, Ghasemzadeh N, Dandona S, Roberts R, Quyyumi AA, Chen HH, Stewart AF. Plasma PCSK9 levels are elevated with acute myocardial infarction in two independent retrospective angiographic studies. PLoS One. 2014;9, e106294.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
TN contributed to the study design, analysis and interpretation of data, and manuscript preparation. HH, KO, TK, and TI contributed to measure serum PCSK9 levels. TH contributed to measure serum small, dense LDL levels. IM contributed to the study design and managed the study. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and with the approval of the ethical committees of Yokohama Sakae Kyosai Hospital. Each patient enrolled in the present study provided written informed consent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Nozue, T., Hattori, H., Ogawa, K. et al. Correlation between serum levels of proprotein convertase subtilisin/kexin type 9 (PCSK9) and atherogenic lipoproteins in patients with coronary artery disease. Lipids Health Dis 15, 165 (2016). https://doi.org/10.1186/s12944-016-0339-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-016-0339-8