Abstract
Tyrosine kinase inhibitors (TKIs)-treatments bring significant benefit for patients harboring epidermal growth factor receptor (EGFR) mutations, especially for those with lung cancer. Unfortunately, the majority of these patients ultimately develop to the acquired resistance after a period of treatment. Two central mechanisms are involved in the resistant process: EGFR secondary mutations and bypass signaling activations. In an EGFR-dependent manner, acquired mutations, such as T790 M, interferes the interaction between TKIs and the kinase domain of EGFR. While in an EGFR-independent manner, dysregulation of other receptor tyrosine kinases (RTKs) or abnormal activation of downstream compounds both have compensatory functions against the inhibition of EGFR through triggering phosphatidylinositol 3-kinase (PI3K)/Akt and mitogen-activated protein kinase (MAPK) signaling axes. Nowadays, many clinical trials aiming to overcome and prevent TKIs resistance in various cancers are ongoing or completed. EGFR-TKIs in accompany with the targeted agents for resistance-related factors afford a promising first-line strategy to further clinical application.
Similar content being viewed by others
Background
EGFR is a transmembrane glycoprotein belonging to the ErbB family of RTKs which includes ErbB-1 (EGFR), ErbB-2 (HER2/neu), ErbB-3 (HER3), and ErbB-4 (HER4) [1, 2]. Upon binding with ligands, EGFR is activated and leads to the excitation of subsequent intracellular signaling pathways, such as the PI3K/Akt and MAPK, which are involved in the proliferation, differentiation, migration, and apoptosis of certain cells [3,4,5]. Consequently, overactivation of EGFR signaling pathways is detected in various malignant tumors, including non-small cell lung cancer (NSCLC), breast cancer, head and neck cancer, colon cancer, ovarian cancer, and the like [6,7,8].
To attenuate the effects that EGFR pathways take on cancers, EGFR TKIs that bind the tyrosine kinase domain of EGFR specifically and inhibit its activity are widely administrated for clinical application. For instance, erlotinib and gefitinib (small molecular EGFR-TKIs) are used to treat patients with EGFR-mutant NSCLC and show significant efficacy [9]. Nevertheless, cancer cells gradually acquire resistance to these drugs, resulting in progression and relapse [10]. Besides the transformation from NSCLC into small cell lung cancer (SCLC) and the process of epithelial to mesenchymal transition (EMT) [11], there are the other two main mechanisms involving in the process of resistance. Firstly, the genetically secondary EGFR mutations could get rid of the inhibition of respective TKIs [12, 13]. Secondly, activation of bypass survival tracks via other RTKs or alternative downstream compounds also account for the acquired resistance [14] (Fig. 1 and Fig. 2). In this review, we mainly focus on the latter mechanism and summarize the existing bypass tracks contributing to TKI resistance via EGFR-independent manners.
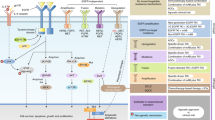
Secondary RTKs-induced EGFR-TKIs resistance. EGFR could trigger downstream PI3K/Akt and MAPK signaling axes which in turn stimulate the transcription factors to drive the associated genes expression which are related with proliferation, angiogenesis, invasion and metastasis. TKIs inhibit EGFR-drived signal transduction by interacting with the tyrosine kinase domain of EGFR. Other RTKs are involved in the development of TKIs resistance via a EGFR-indepenfent way: 1. Amplification of MET activates PI3K through transactivating ErbB3; 2. HGF overexpression; 3. ErbB2 amplification; 4. ErbB3 activation; 5. IGF1R activation by IGF binding or IGFBP reduction; 6. AXL activation; 7. FGFR1 activation
Alternative downstream compounds-induced EGFR-TKIs resistance. 1. PTEN loss: suppressed HGR1 downregulates PTEN expression which in general inhibits the PI3K/Akt activation. 2. PIK3CA mutation-drived abnormal activation of PI3K pathway. 3. BRAF mutation-drived abnormal activation of MAPK signaling axis
EGFR-triggered signaling pathways in cancers
RTKs are a kind of receptor for various growth factors, cytokines, and hormones. RTKs have a similar molecular structure: an extracellular ligand-binding region, a single hydrophobic transmembrane domain, and a cytoplasmic protein tyrosine kinase region plus additional carboxy terminal and juxtamembrane regulatory regions [3]. The RTK family mainly consists of ErbBs, fibroblast growth factor receptors (FGFRs), insulin-like growth factor receptors (IGFRs), vascular endothelial growth factor receptors (VEGFRs), and hepatocyte growth factor receptors (HGFRs) [3]. Thereinto, EGFR is a paradigm and its intracellular signaling pathways are relevant to the emergence and progression of various cancers, especially NSCLC. Binding with a specific set of ligands, such as epidermal growth factor (EGF), transforming growth factor-alpha (TGF-α), amphiregulin, betacellulin, or epiregulin, EGFR would form a homodimer by itself or form a heterodimer with other ErbB family members. Subsequently, the dimerization of EGFR would activate its cytoplasmic tyrosine kinases domain and then trigger a series of signal transduction [6, 15].
Two primary downstream signaling pathways of EGFR are the PI3K/Akt/PTEN/mTOR and the RAS/RAF/MEK/ERK (Fig. 1). Phosphorylated tyrosine kinase of EGFR acts as a docking site for PI3K which can stimulate the generation of phosphatidylinositol-3,4,5-triphosphate (PIP-3) and promote the activation of Akt [16]. Subsequently, the mammalian target of rapamycin (mTOR), a downstream target of Akt, is activated and provokes the expression of associated proteins needed for the cell cycle progression from the G1 to the S phase [17]. Accordingly, overactivation of this pathway suppresses apoptosis and stimulates tumor growth [18, 19]. Moreover, ligands-EGFR binding drives the MAPK signaling cascade. The dimerization of EGFR activates RAS leading to the phosphorylation of RAF-kinases which in turn phosphorylates MEK. And motivated MEK could impel the activation of ERK inducing to the production of subsequent cell cycle-associated transcription factors (Myc, c-Fos, CREB, NF-κB). And those functional transcription factors ultimately stimulate the cumulation of cyclin D catalyzing the division of cells [20].
EGFR-independent signaling pathways involved in TKIs resistance
Secondary RTKs-induced TKIs resistance
MET amplification
MET, belonging to the RTKs family, is amplified and relevant to the TKIs resistance in EGFR-dependent cancers, especially in lung cancer. In a gefitinib-sensitive lung cancer cell line HCC827, focal amplification of MET was found to stimulate ErbB3 phosphorylation which in turn activated downstream PI3K/Akt signaling axis compensating the inhibitory effect of gefitinib on EGFR [21]. On the contrary, MET-specific short hairpin RNA (shRNA) restrained MET expression and then recovered the ability of gefitinib to retard PI3K/Akt pathway [21]. Meanwhile, ErbB3-specific shRNA also inhibited the phosphorylation of Akt and controlled the advancement of cell cycle in resistant cells [21]. Moreover, of the 18 gefitinib/erlotinib–resistant lung cancer patients, 4 (22%) with high level of MET were detected [21]. NSCLC patients with classic EGFR-activating mutations were reported to have concomitant MET amplification leading to de novo clinical resistance [22]. Besides lung cancer, MET amplification-drived therapeutic resistance was also reported in other ErbB-dependent cancers, such as colorectal cancer, esophagogastric cancer, ovarian cancer, and so on [23,24,25].
Referring to the mechanisms of MET amplification in TKI-resistant tumors, it was acknowledged that MET amplification was pre-existed at low frequencies in untreated HCC827 cells and NSCLC patients (approximately 4%) [26], and under the subsequently drug-selective pressure, these cells appeared to be the dominant clones holding MET amplification and led to clinical gefitinib or erlotinib resistance [27]. Nevertheless, the reason why above mechanism has not been reported in other EGFR mutant cell lines and cancers is not clear so far.
Dual targeting of EGFR and MET may provide an effective approach to prevent the development of MET-amplified EGFR TKI–resistant tumors [21]. Currently, several advancing clinical trials are conducted to assess the availability of combining the MET-targeted drugs (MET-TKIs or MET-MAbs) with EGFR TKIs in the treatment of EGFR-mutant tumor with MET-amplification [28, 29].
Hepatocyte growth factor (HGF) overexpression
HGF, known as the ligand of MET, is primarily produced by lung cancer cells [30] and stromal cells [31]. The binding between HGF and MET induced various biological effects, such as mitogenic, morphogenic, and antiapoptotic activities [32]. And the complex restored the activation of PI3K/Akt pathway driving the TKI resistance and contributing to the carcinogenesis, proliferation, and metastasis in EGFR-mutant lung cancer [33]. It was reported by Yano, S et al. that unlike the MET amplification, HGF-induced MET activation, acting as a specific mechanism of gefitinib resistance in lung adenocarcinoma harboring EGFR-activating mutations, motivated the PI3K/Akt signaling in an ErbB3-independent manner [34].
HGF is not spontaneously secreted at a detectable level in two gefitinib-sensitive lung adenocarcinoma cell lines (PC-9 and HCC827 cells) [35]. By pretreatment with HGF, these two cell lines were rescued from the gefitinib-induced cell death via a dose-dependent manner that the higher concentration of HGF overcome the cell growth inhibitory effect of gefitinib [34]. Consistently, this phenomenon was also showed in H1975, A431 and HN11 cell lines [27]. In addition, a joint study recruiting 97 tumor specimens from Japanese lung cancer patients with EGFR-mutation reported that HGF overexpression was detected more frequently than other factors (T790 M and MET amplification) in both 23 tumors with acquired resistance (61%) and 45 tumors with intrinsic resistance (29%) [36]. The research implied that HGF might play a crucial role in causing both acquired and intrinsic resistance to EGFR-TKI.
Interestingly, HGF facilitated MET amplification both in vitro and in vivo through upregulating pre-existing MET-amplified clones [27, 37]. Therefore, activation of MET signaling axis, either by amplification or ligand stimulation, is a unique bypass resistance of lung cancer cells to TKI. Simultaneous blockade of the two approaches with EGFR-TKI and HGF-MET antagonists could resist the drug resistance and accelerate the successful treatment for lung cancer patients to the full extent.
ErbB2/HER2 amplification
In recent years, there are some inconsistent views concerning the influence of ErbB2 dysregulation on the susceptibility of tumor cells to EGFR-TKIs in NSCLC [38,39,40]. Traditionally, several preclinical and clinical studies focusing on EGFR-positive (including EGFR mutant, high gene copy number and overexpression) NSCLC patients suggested that increased copy number of ErbB2 gene was susceptible to gefitinib therapy and was correlated with better response rate, disease control rate, and survival preclinical studies reported that gefitinib has a prominent antiproliferative effect on tumors with ErbB2 overexpression [41,42,43]. Nevertheless, ErbB2 copy number is not the necessary and the unique factor influencing anti-tumor effect of gefitinib in NSCLC patients. A multivariate analysis certified that EGFR mutation, by contrast, is a more crucial factor for beneficial clinical outcomes in gefitinib-treated NSCLC patients than ErbB2 and EGFR copy numbers [44]. Intriguingly, in a current study, ErbB2 amplification was recognized as an unacknowledged mechanism mediating the acquired TKIs resistance of NSCLC with the absence of the EGFR T790 M mutation [45]. Of 26 EGFR-mutant lung adenocarcinoma patients with acquired resistance to gefitinib or erlotinib, 3 (12%) were detected with ErbB2 amplification by FISH analysis [45]. In order to verify the potential correlation, wild-type ErbB2 cDNAs was introduced to the TKI-sensitive cell lines (PC-9 and HCC827) and then the ErbB2 amplification (> 50-fold above baseline) resulted in the resistance to erlotinib [45]. Moreover, under the treatment with erlotinib, inhibition of ErbB2 with small interfering RNAs (siRNAs) impeded the growth of PC-9, HCC827, and H3255 cell lines without EGFR T790 M [45]. Afatinib, a TKI targeting both EGFR and ErbB2, in combined with anti-EGFR antibody could remarkably attenuate the ErbB2 signaling and in turn resumed the sensitivity of lung cancer and colorectal cancer to TKIs in vitro and in vivo [45, 46].
ErbB3/HER3 activation
It was elucidated that the resistances to EGFR- or ErbB2-TKIs during the treatment of several malignancies were initiated by ErbB3 [47,48,49,50]. ErbB3 is a unique member of ErbB family in that it was regarded as an inactive kinase. However, ErbB3 can be transactivated and transphosphorylated by forming a heterodimers with other ErbB members [51]. Functionally, ErbB3 plays a compensatory role in supplanting the TKIs-inhibited EGFR or ErbB2 to trigger and sustain the activation of typical PI3K/Akt signaling pathway in vitro and in vivo [47]. Unlike the EGFR and ErbB2 motivating the PI3K through the adaptor proteins, ErbB3 could bind the p85 subunit of PI3K to activate PI3K directly, implicating the priority and prevalence of the ErbB3-drived resistance in TKIs-treated tumors [52].
ErbB3-induced drug resistance is primarily mediated by three methods. At first, as mentioned above, MET amplification was known to endow ErbB3 signaling with persistent activation and contribute to the resistance to gefitinib in lung cancer cell lines [21]. Besides, it was demonstrated that the ErbB2-ErbB3 heterodimer was responsible for the stimulation of downstream oncogenic signaling in ErbB2+ breast cancer cells [53]. When the ErbB2 was undermined significantly by TKIs, signaling activities buffering the inhibitory effects of TKIs on ErbB2 were recovered through upregulating the production of ErbB3 and weakening the activity of ErbB3 phosphatase so that lead to the resistance to gefitinib and erlotinib [47]. Third, by binding with its ligand heregulin (HRG) or neuregulin 1 (NRG1), ErbB3 formed a heterodimer with another ErbB receptor. Consequently, the ligand-receptor complex strongly triggered PI3K/Akt axis mediating the resistance to anticancer kinase inhibitors in various cancers [54,55,56]. For example, among nine HER2-amplified breast cell lines, eight were resistant to the lapatinib by applying ErbB3 ligand NRG1 [56]. And Xia et al. suggested that acquired resistance to lapatinib in the HER2+ breast cancer can be driven by autocrine induction of HRG [57]. On account of above mechanisms, inactivating ErbB3 is identified as an encouraging approach to resist drug resistance [58].
IGF1R activation
Activation of IGF1R is another mechanism conferring the acquired resistance against gefitinib to EGFR-amplified and EGFR-mutant cancer cell lines [58]. And the signaling mediated by IGF1R participated in the early stage of TKIs-resistance [59].
In gefitinib-resistant A431 squamous cancer cells, sustained PI3K signaling in the presence of gefitinib was a result of IGF1R-induced signal transduction [60]. Concurrent inhibition of EGFR and IGF1R obstructed the initiation of resistance to gefitinib treatment and reverse the resistant phenotype both in A431 cell line and tumor xenografts [60]. The consistent phenomenon was also found in another gefitinib-resistance cell line model, the head and neck HN11 cells [60]. In the sight of the molecular mechanism, gene expression profiles of the resistant cell line models showed that IGF binding proteins-3 (IGFBP-3) and IGFBP-4, known as negative regulators interfering IGF-IGF1R binding and owning IGF-independent growth inhibition activities, were responsible to the IGF1R-triggered drug resistance [60,61,62]. The reduction of EGF caused by the EGFR-TKIs treatments downregulated the expression of IGFBP-3 and IGFBP-4. This might lead to the maintenance of IGF1R-induced PI3K/Akt signaling confronting the TKIs-mediated EGFR blockade [60]. Undoubtedly, addition of IGFBP-3 to the A431 cells resensitized the effects of gefitinib and retorted the resistance phenotype [60]. Recently, Zhou et al. pointed out that IGF1R induced acquired resistance of NSCLC cells against EGFR-TKIs mainly via stimulating EMT process triggered by upregulated Snail expression and repressed E-cadherin expression [63].
Albeit above preclinical researches showed the potent correlation between the IGF1R activation and TKIs resistance, there was insufficient study focusing on this trend in clinical patients. It has been reported that the high frequency of IGF1R (39–84%) was detected in patients with various cancers [64,65,66,67], however, further study is needed to determine the explicit proportion of high IGF1R expression patients among those having TKIs resistance. To sum up, all these findings provide potential therapeutic targets to surmount TKIs resistance in EGFR-mutant cancers and enhance the efficiency of TKIs treatments.
Other bypass RTKs
AXL, a subfamily member of RTKs, is correlated with cell survival, proliferation, metastasis, and phagocytosis [68, 69]. The increased abundance of AXL and its ligand (GAS6) was found in EGFR-TKI resistant NSCLC specimens at the frequency of 20% and 25%, respectively [70]. The aberrant activation of AXL was showed to be required for the development of erlotinib resistance in EGFR-mutant NSCLC models both in vitro and in vivo via Akt, MAPK or NF-κB downstream signaling [70]. What’s more, this process driven by AXL may be correlated with some histological changes, such as EMT [71]. Besides NSCLC, overactivation of AXL was also implicated to the emergence of acquired resistance to imatinib in gastrointestinal stromal tumors and to lapatinib in HER2 positive breast tumor [72, 73]. Inhibition or knockdown of AXL either in the A549 cell line or in a xenograft model exhibited a decreased tumor growth rate and a restored chemosensitivity [74, 75]. Collectively, synthetical treatment combining with representative TKIs and AXL inhibitors to patients with acquired resistance may be a promising strategy to enhance the therapeutic efficacy. Another RTK, FGFR1, formed an autocrine loop with its ligand FGF2 and was identified as an alternative pathway mediating the resistance to EGFR-TKI in a PC-9 cell line model [76]. Meanwhile, inhibition of FGFR1 or FGF2 retarded the growth of resistant PC-9 cells and resensitized the cells to gefitinib-treatment.
Abnormal activation of downstream compounds
Phosphatase and tensin homolog (PTEN) loss
PTEN, acting as a tumor inhibitor, negatively regulates the PI3K/Akt signaling cascade by converting PIP-3 back to PIP-2 [77, 78]. The loss of PTEN decreased erlotinib-induced apoptosis and induced erlotinib-resistance in EGFR-mutant cells via reactivation of Akt and EGFR [79, 80]. In the gefitinib-resistant PC-9 cell line model, reduced PTEN expression was relevant with increased Akt phosphorylation [81]. On the other hand, along with the high PTEN expression, the therapeutic efficacy of gefitinib and erlotinib was restored in the gefitinib-sensitive NSCLC PC-9 cell line. And knockdown of PTEN with siRNA in PC-9 cells contributed to acquired resistance to gefitinib and erlotinib [81]. Retrieval of PTEN expression also enhanced the sensitivity of prostate cancer cells to EGFR inhibition [82]. Furthermore, low expression of PTEN was detected in metastases samples from gefitinib-refractory NSCLC patients [81].
Mechanically, the transcription factor, EGR1, is responsible to the abnormal expression of PTEN. By a nuclear translocation manner, EGR1 played a positive role in regulating PTEN expression [83]. However, this manner was found to be suppressed in resistant cell models and be recovered in the revertant models [81]. It is clear that the expression of PTEN can be controlled by downregulated EGR1 at a transcriptional level.
PIK3CA and BRAF mutations
Mutational activation of the downstream signaling components, such as PI3K/Akt or MEK/ERK, which was independent on the EGFR was identified as a novel mechanism of TKIs resistance [84, 85]. PIK3CA gene encodes the catalytic subunit of PI3K and has occasionally mutation in lung cancer [84]. In a vitro study, PIK3CA mutation which led to sustained PI3K/Akt signaling conferred the resistance of EGFR-mutant HCC827 cells to gefitinib [86]. Whereafter, Sequist, LV et al. firstly demonstrated PIK3CA mutations in 5% EGFR-mutant patients with acquired resistance to EGFR-TKIs [84]. Combining TKI and PI3K inhibitor has been introduced to therapeutic intervention in cancers harboring PIK3CA mutations.
Additionally, BRAF, known as a member of RAS signaling pathway genes, was reported to be involved in pro-mitogenic activity and acquired resistance to EGFR TKIs in lung cancer and colorectal cancer through activating the MAPK signaling axis [87, 88]. BRAF mutations were generally existed in malignant melanoma (30%–40%), whereas it only accounted for approximately 1% of NSCLC [85]. Nevertheless, the small proportion of BRAF mutations resulted in negative results (poor prognosis) and provided cognition about mechanisms of acquired resistance to EGFR-TKIs in lung cancer [85].
Mechanisms of resistance to third generation EGFR-TKIs
Nowadays, the third generation EGFR-TKIs, including osimertinib, rociletinib (CO-1686), HM61713 (BI 1482694), ASP8273, EGF816, and PF-06747775, were widely introduced to replace the first generation EGFR-TKIs to overcome the status of drug resistance [89,90,91,92]. A recent clinical trial (NCT02151981) showed that AZD9291 significantly improved objective response rate (ORR) and PFS in T790 M-mutant NSCLC patients who had disease progression on first-line EGFR-TKIs [93]. Subsequently, patients were also resistant to these TKIs after 10 months of treatment, suggesting that additional mechanisms may reduce the efficacy of these inhibitors [13]. In vitro experiment identified three major mutants of EGFR (L718Q, L844 V, and C797S) in resistant cell clones. Among them, C797S mutation was a key factor conferring resistance to the third-generation inhibitors in the existence of del 19 [13].
Moreover, bypass tracts including amplifications of other tyrosine kinases or abnormal activation of downstream compound also mediated the resistance to third-generation TKIs. HER2 and MET amplifications led to poor response to CO-1686 and were detected in patients who had disease progression on CO-1686 or osimertinib treatment [94, 95]. Besides, in an AURA trial, re-biopsy tissues of 4 NSCLC patients with acquired resistance to osimertinib showed different mechanisms of resistance, including FGFR1 amplification, PTEN deletion, MAPK1 and Akt3 overexpression, and SCLC transition [96]. KRAS alteration resulting in increased RAS signaling existed in relapsed biopsy tissues and mutant KRAS transduced cells which were both less sensitive to third generation TKIs [95, 97]. Blocking alternative pathways may provide a promising strategy for improving the drug sensitivity and overcoming the resistance to third generation TKIs.
Conclusions and perspectives
Currently, the mechanism study on the resistance to EGFR-TKIs has attracted broad attention. There are two major ways involving the initiation and development of resistance to TKI. One is the secondary mutations of EGFR which alter the drug target site of EGFR so that prevent effective interaction with TKIs [9, 98]. Another is activation of bypass tracts via an EGFR-independent manner, such as motivating other RTKs or dysregulating downstream signaling components.
Based on the recognition of above resistant mechanisms, new clinical trials covering phase I-IV are emerging to provide therapeutic interventions adapting for patients with refractory or recurring cancers by inhibiting the alternative pathways [99,100,101] (Table 1). Some of these trials had favorable results and now are available for clinical application. Moreover, new generation of TKIs are on their way to evade the resistance and enhance the therapeutic efficiency. Further clinical evaluation is required to offer individualized treatments for those specific patients.
Abbreviations
- EGF:
-
Epidermal growth factor
- EGFR:
-
Epidermal growth factor receptor
- EMT:
-
Epithelial-mesenchymal transition
- FGFRs:
-
Fibroblast growth factor receptors
- HGF:
-
Hepatocyte growth factor
- HGFRs:
-
Hepatocyte growth factor receptors
- HRG:
-
Heregulin
- IGFBP-3:
-
IGF binding proteins-3
- IGFRs:
-
Insulin-like growth factor receptors
- MAPK:
-
Mitogen-activated protein kinase
- mTOR:
-
Mammalian target of rapamycin
- NRG1:
-
Neuregulin 1
- NSCLC:
-
Non-small cell lung cancer
- ORR:
-
Objective response rate
- PI3K:
-
Phosphatidylinositol 3-kinase
- PIP-3:
-
Phosphatidylinositol-3,4,5-triphosphate
- PTEN:
-
Phosphatase and tensin homolog
- RTKs:
-
Receptor tyrosine kinases
- SCLC:
-
Small cell lung cancer
- shRNA:
-
Short hairpin RNA
- siRNA:
-
Small interfering RNA
- TGF-α:
-
Transforming growth factor-alpha
- TKIs:
-
Tyrosine kinase inhibitors;
- VEGFRs:
-
Vascular endothelial growth factor receptors
References
Yu S, Li A, Liu Q, Yuan X, Xu H, Jiao D, et al. Recent advances of bispecific antibodies in solid tumors. J Hematol Oncol. 2017;10:155.
Wheeler DL, Huang S, Kruser TJ, Nechrebecki MM, Armstrong EA, Benavente S, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–56.
Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–34.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37.
Kampa-Schittenhelm KM, Heinrich MC, Akmut F, Rasp KH, Illing B, Dohner H, et al. Cell cycle-dependent activity of the novel dual PI3K-MTORC1/2 inhibitor NVP-BGT226 in acute leukemia. Mol Cancer. 2013;12:46.
Ellis LM. Epidermal growth factor receptor in tumor angiogenesis. Hematol Oncol Clin North Am. 2004;18:1007–21.
Viloria-Petit AM, Kerbel RS. Acquired resistance to EGFR inhibitors: mechanisms and prevention strategies. Int J Radiat Oncol Biol Phys. 2004;58:914–26.
Gajiwala KS, Feng J, Ferre R, Ryan K, Brodsky O, Weinrich S, et al. Insights into the aberrant activity of mutant EGFR kinase domain and drug recognition. Structure. 2013;21:209–19.
Mu XL, Li LY, Zhang XT, Wang MZ, Feng RE, Cui QC, et al. Gefitinib-sensitive mutations of the epidermal growth factor receptor tyrosine kinase domain in chinese patients with non-small cell lung cancer. Clin Cancer Res. 2005;11:4289–94.
Colabufo NA, Contino M, Niso M, Berardi F, Leopoldo M, Perrone R. EGFR tyrosine kinase inhibitors and multidrug resistance: perspectives. Front Biosci (Landmark Ed). 2011;16:1811–23.
Jakobsen KR, Demuth C, Sorensen BS, Nielsen AL. The role of epithelial to mesenchymal transition in resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Transl Lung Cancer Res. 2016;5:172–82.
Zhou Q, Yang JJ, Chen ZH, Zhang XC, Yan HH, Xu CR, et al. Serial cfDNA assessment of response and resistance to EGFR-TKI for patients with EGFR-L858R mutant lung cancer from a prospective clinical trial. J Hematol Oncol. 2016;9:86.
Wang S, Tsui ST, Liu C, Song Y, Liu D. EGFR C797S mutation mediates resistance to third-generation inhibitors in T790M-positive non-small cell lung cancer. J Hematol Oncol. 2016;9:59.
Niederst MJ, Engelman JA. Bypass mechanisms of resistance to receptor tyrosine kinase inhibition in lung cancer. Sci Signal. 2013;6:re6.
Arteaga CL. The epidermal growth factor receptor: from mutant oncogene in nonhuman cancers to therapeutic target in human neoplasia. J Clin Oncol. 2001;19(18 Suppl):32S–40S.
Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–92.
Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, et al. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016;31:61–71.
Morgensztern D, HL ML. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anti-Cancer Drugs. 2005;16:797–803.
Liu Q, Yu S, Li A, Xu H, Han X, Wu K. Targeting interlukin-6 to relieve immunosuppression in tumor microenvironment. Tumour Biol. 2017;39(6):1010428317712445.
Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22.
Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43.
Sequist LV, Martins RG, Spigel D, Grunberg SM, Spira A, Janne PA, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–9.
Bardelli A, Corso S, Bertotti A, Hobor S, Valtorta E, Siravegna G, et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov. 2013;3:658–73.
Kwak EL, Ahronian LG, Siravegna G, Mussolin B, Godfrey JT, Clark JW, et al. Molecular heterogeneity and receptor Coamplification drive resistance to targeted therapy in MET-amplified Esophagogastric cancer. Cancer Discov. 2015;5:1271–81.
Tang C, Jardim DL, Hong D. MET in ovarian cancer: metastasis and resistance? Cell Cycle. 2014;13:1220–1.
Cappuzzo F, Marchetti A, Skokan M, Rossi E, Gajapathy S, Felicioni L, et al. Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol. 2009;27:1667–74.
Turke AB, Zejnullahu K, Wu YL, Song Y, Dias-Santagata D, Lifshits E, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88.
Wu YL, Yang CH, Kim DW, Su WC, Ahn MJ, Lee DH, et al. Vansteenkiste JF, Zhang L, Felip E, Peng B, gong Y, Zhao S, Amagasaki T, et al. safety and efficacy of INC280 in combination with gefitinib (gef) in patients with EGFR-mutated (mut), MET-positive NSCLC: a single-arm phase lb/ll study. J Clin Oncol. 2014;32(5s. suppl):abstr 8017.
Ou SH, Kwak EL, Siwak-Tapp C, Dy J, Bergethon K, Clark JW, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011;6:942–6.
Harvey P, Warn A, Newman P, Perry LJ, Ball RY, Warn RM. Immunoreactivity for hepatocyte growth factor/scatter factor and its receptor, met, in human lung carcinomas and malignant mesotheliomas. J Pathol. 1996;180:389–94.
Tokunou M, Niki T, Eguchi K, Iba S, Tsuda H, Yamada T, et al. C-MET expression in myofibroblasts: role in autocrine activation and prognostic significance in lung adenocarcinoma. Am J Pathol. 2001;158:1451–63.
Yamada T, Matsumoto K, Wang W, Li Q, Nishioka Y, Sekido Y, et al. Hepatocyte growth factor reduces susceptibility to an irreversible epidermal growth factor receptor inhibitor in EGFR-T790M mutant lung cancer. Clin Cancer Res. 2010;16:174–83.
Stabile LP, Lyker JS, Land SR, Dacic S, Zamboni BA, Siegfried JM. Transgenic mice overexpressing hepatocyte growth factor in the airways show increased susceptibility to lung cancer. Carcinogenesis. 2006;27:1547–55.
Yano S, Wang W, Li Q, Matsumoto K, Sakurama H, Nakamura T, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68:9479–87.
Matsumoto K, Nakamura T. Hepatocyte growth factor and the met system as a mediator of tumor-stromal interactions. Int J Cancer. 2006;119:477–83.
Yano S, Yamada T, Takeuchi S, Tachibana K, Minami Y, Yatabe Y, et al. Hepatocyte growth factor expression in EGFR mutant lung cancer with intrinsic and acquired resistance to tyrosine kinase inhibitors in a Japanese cohort. J Thorac Oncol. 2011;6:2011–7.
Yano S, Takeuchi S, Nakagawa T, Yamada T. Ligand-triggered resistance to molecular targeted drugs in lung cancer: roles of hepatocyte growth factor and epidermal growth factor receptor ligands. Cancer Sci. 2012;103:1189–94.
Zhang J, Cao J, Li J, Zhang Y, Chen Z, Peng W, et al. A phase I study of AST1306, a novel irreversible EGFR and HER2 kinase inhibitor, in patients with advanced solid tumors. J Hematol Oncol. 2014;7:22.
Yu S, Li A, Liu Q, Li T, Yuan X, Han X, et al. Chimeric antigen receptor T cells: a novel therapy for solid tumors. J Hematol Oncol. 2017;10:78.
Cretella D, Saccani F, Quaini F, Frati C, Lagrasta C, Bonelli M, et al. Trastuzumab emtansine is active on HER-2 overexpressing NSCLC cell lines and overcomes gefitinib resistance. Mol Cancer. 2014;13:143.
Moasser MM, Basso A, Averbuch SD, Rosen N, et al. The tyrosine kinase inhibitor ZD1839 ("Iressa") inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res. 2001;61:7184–8.
Anido J, Matar P, Albanell J, Guzman M, Rojo F, Arribas J, et al. ZD1839, a specific epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, induces the formation of inactive EGFR/HER2 and EGFR/HER3 heterodimers and prevents heregulin signaling in HER2-overexpressing breast cancer cells. Clin Cancer Res. 2003;9:1274–83.
Cappuzzo F, Varella-Garcia M, Shigematsu H, Domenichini I, Bartolini S, Ceresoli GL, et al. Increased HER2 gene copy number is associated with response to gefitinib therapy in epidermal growth factor receptor-positive non-small-cell lung cancer patients. J Clin Oncol. 2005;23:5007–18.
Soh J, Toyooka S, Ichihara S, Fujiwara Y, Hotta K, Suehisa H, et al. Impact of HER2 and EGFR gene status on gefitinib-treated patients with nonsmall-cell lung cancer. Int J Cancer. 2007;121:1162–7.
Takezawa K, Pirazzoli V, Arcila ME, Nebhan CA, Song X, de Stanchina E, et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov. 2012;2:922–33.
Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med. 2011;3:99ra86.
Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–41.
Hamburger AW. The role of ErbB3 and its binding partners in breast cancer progression and resistance to hormone and tyrosine kinase directed therapies. J Mammary Gland Biol Neoplasia. 2008;13:225–33.
Liu B, Ordonez-Ercan D, Fan Z, Edgerton SM, Yang X, Thor AD. Downregulation of erbB3 abrogates erbB2-mediated tamoxifen resistance in breast cancer cells. Int J Cancer. 2007;120:1874–82.
Telesco SE, Shih AJ, Jia F, Radhakrishnan R. A multiscale modeling approach to investigate molecular mechanisms of pseudokinase activation and drug resistance in the HER3/ErbB3 receptor tyrosine kinase signaling network. Mol BioSyst. 2011;7:2066–80.
Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–9.
Prigent SA, Gullick WJ. Identification of c-erbB-3 binding sites for phosphatidylinositol 3′-kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J. 1994;13:2831–41.
Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100:8933–8.
Yarar D, Lahdenranta J, Kubasek W, Nielsen UB, MacBeath G. Heregulin-ErbB3-driven tumor growth persists in PI3 Kinase mutant cancer cells. Mol Cancer Ther. 2015;14:2072–80.
Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–87.
Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–9.
Xia W, Petricoin EF 3rd, Zhao S, Liu L, Osada T, Cheng Q, et al. An heregulin-EGFR-HER3 autocrine signaling axis can mediate acquired lapatinib resistance in HER2+ breast cancer models. Breast Cancer Res. 2013;15:R85.
Li H, Batth IS, Qu X, Xu L, Song N, Wang R, et al. IGF-IR signaling in epithelial to mesenchymal transition and targeting IGF-IR therapy: overview and new insights. Mol Cancer. 2017;16:6.
Cortot AB, Repellin CE, Shimamura T, Capelletti M, Zejnullahu K, Ercan D, et al. Resistance to irreversible EGF receptor tyrosine kinase inhibitors through a multistep mechanism involving the IGF1R pathway. Cancer Res. 2013;73:834–43.
Guix M, Faber AC, Wang SE, Olivares MG, Song Y. Qu S, et al. acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118:2609–19.
Grimberg A, Cohen P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol. 2000;183:1–9.
Torng PL, Lee YC, Huang CY, Ye JH, Lin YS, Chu YW, et al. Insulin-like growth factor binding protein-3 (IGFBP-3) acts as an invasion-metastasis suppressor in ovarian endometrioid carcinoma. Oncogene. 2008;27:2137–47.
Zhou J, Wang J, Zeng Y, Zhang X, Hu Q, Zheng J, et al. Implication of epithelial-mesenchymal transition in IGF1R-induced resistance to EGFR-TKIs in advanced non-small cell lung cancer. Oncotarget. 2015;6:44332–45.
Cappuzzo F, Toschi L, Tallini G, Ceresoli GL, Domenichini I, Bartolini S, et al. Insulin-like growth factor receptor 1 (IGFR-1) is significantly associated with longer survival in non-small-cell lung cancer patients treated with gefitinib. Ann Oncol. 2006;17:1120–7.
Browne BC, Eustace AJ, Kennedy S, O'Brien NA, Pedersen K, McDermott MS, et al. Evaluation of IGF1R and phosphorylated IGF1R as targets in HER2-positive breast cancer cell lines and tumours. Breast Cancer Res Treat. 2012;136:717–27.
Yeo CD, Park KH, Park CK, Lee SH, Kim SJ, Yoon HK, et al. Expression of insulin-like growth factor 1 receptor (IGF-1R) predicts poor responses to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in non-small cell lung cancer patients harboring activating EGFR mutations. Lung Cancer. 2015;87:311–7.
Fidler MJ, Basu S, Buckingham L, Walters K, McCormack S, Batus M, et al. Utility of insulin-like growth factor receptor-1 expression in gefitinib-treated patients with non-small cell lung cancer. Anticancer Res. 2012;32:1705–10.
Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83.
Hafizi S, Dahlback B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev. 2006;17:295–304.
Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44:852–60.
Suda K, Tomizawa K, Fujii M, Murakami H, Osada H, Maehara Y, et al. Epithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol. 2011;6:1152–61.
Mahadevan D, Cooke L, Riley C, Swart R, Simons B, Della Croce K, et al. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene. 2007;26:3909–19.
Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R, et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res. 2009;69:6871–8.
Li Y, Ye X, Tan C, Hongo JA, Zha J, Liu J, et al. Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene. 2009;28:3442–55.
Linger RM, Cohen RA, Cummings CT, Sather S, Migdall-Wilson J, Middleton DH, et al. Mer or Axl receptor tyrosine kinase inhibition promotes apoptosis, blocks growth and enhances chemosensitivity of human non-small cell lung cancer. Oncogene. 2013;32:3420–31.
Terai H, Soejima K, Yasuda H, Nakayama S, Hamamoto J, Arai D, et al. Activation of the FGF2-FGFR1 autocrine pathway: a novel mechanism of acquired resistance to gefitinib in NSCLC. Mol Cancer Res. 2013;11:759–67.
Chow LM, Baker SJ. PTEN function in normal and neoplastic growth. Cancer Lett. 2006;241:184–96.
Sastry SK, Elferink LA. Checks and balances: interplay of RTKs and PTPs in cancer progression. Biochem Pharmacol. 2011;82:435–40.
Sos ML, Koker M, Weir BA, Heynck S, Rabinovsky R, Zander T, et al. PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer Res. 2009;69:3256–61.
Yamasaki F, Johansen MJ, Zhang D, Krishnamurthy S, Felix E, Bartholomeusz C, et al. Acquired resistance to erlotinib in A-431 epidermoid cancer cells requires down-regulation of MMAC1/PTEN and up-regulation of phosphorylated Akt. Cancer Res. 2007;67:5779–88.
Yamamoto C, Basaki Y, Kawahara A, Nakashima K, Kage M, Izumi H, et al. Loss of PTEN expression by blocking nuclear translocation of EGR1 in gefitinib-resistant lung cancer cells harboring epidermal growth factor receptor-activating mutations. Cancer Res. 2010;70:8715–25.
Wu Z, Gioeli D, Conaway M, Weber MJ, Theodorescu D. Restoration of PTEN expression alters the sensitivity of prostate cancer cells to EGFR inhibitors. Prostate. 2008;68:935–44.
Okamura H, Yoshida K, Morimoto H, Haneji T. PTEN expression elicited by EGR-1 transcription factor in calyculin A-induced apoptotic cells. J Cell Biochem. 2005;94:117–25.
Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26.
Ohashi K, Sequist LV, Arcila ME, Moran T, Chmielecki J, Lin YL, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci U S A. 2012;109:E2127–33.
Engelman JA, Mukohara T, Zejnullahu K, Lifshits E, Borras AM, Gale CM, et al. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. J Clin Invest. 2006;116:2695–706.
Onitsuka T, Uramoto H, Nose N, Takenoyama M, Hanagiri T, Sugio K, et al. Acquired resistance to gefitinib: the contribution of mechanisms other than the T790M, MET, and HGF status. Lung Cancer. 2010;68:198–203.
Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227–35.
Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–61.
Sequist LV, Rolfe L, Allen AR. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2015;373:578–9.
Wang S, Cang S, Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J Hematol Oncol. 2016;9:34.
Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–99.
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-Pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–40.
Chabon JJ, Simmons AD, Lovejoy AF, Esfahani MS, Newman AM, Haringsma HJ, et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun. 2016;7:11815.
Ortiz-Cuaran S, Scheffler M, Plenker D, Dahmen L, Scheel AH, Fernandez-Cuesta L, et al. Heterogeneous mechanisms of primary and acquired resistance to third-generation EGFR inhibitors. Clin Cancer Res. 2016;22:4837–47.
Kim TM, Song A, Kim DW, Kim S, Ahn YO, Keam B, et al. Mechanisms of acquired resistance to AZD9291: a mutation-selective, irreversible EGFR inhibitor. J Thorac Oncol. 2015;10:1736–44.
Eberlein CA, Stetson D, Markovets AA, Al-Kadhimi KJ, Lai Z, Fisher PR, et al. Acquired resistance to the mutant-selective EGFR inhibitor AZD9291 is associated with increased dependence on RAS signaling in preclinical models. Cancer Res. 2015;75:2489–500.
Planchard D, Loriot Y, Andre F, Gobert A, Auger N, Lacroix L, et al. EGFR-independent mechanisms of acquired resistance to AZD9291 in EGFR T790M-positive NSCLC patients. Ann Oncol. 2015;26:2073–8.
Husain H, Scur M, Murtuza A, Bui N, Woodward B, Kurzrock R. Strategies to overcome bypass mechanisms mediating clinical resistance to EGFR tyrosine Kinase inhibition in lung cancer. Mol Cancer Ther. 2017;16:265–72.
Regad T. Targeting RTK signaling pathways in cancer. Cancers (Basel). 2015;7:1758–84.
Leung AW, de Silva T, Bally MB, Lockwood WW. Synthetic lethality in lung cancer and translation to clinical therapies. Mol Cancer. 2016;15:61.
Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–7.
Tolaney SM, Tan S, Guo H, Barry W, Van Allen E, Wagle N, et al. Phase II study of tivantinib (ARQ 197) in patients with metastatic triple-negative breast cancer. Investig New Drugs. 2015;33:1108–14.
Tarhini AA, Rafique I, Floros T, Tran P, Gooding WE, Villaruz LC, et al. Phase 1/2 study of rilotumumab (AMG 102), a hepatocyte growth factor inhibitor, and erlotinib in patients with advanced non-small cell lung cancer. Cancer. 2017;123:2936–44.
Nurwidya F, Takahashi F, Murakami A, Kobayashi I, Kato M, Shukuya T, et al. Acquired resistance of non-small cell lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Respir Investig. 2014;52:82–91.
Naidu R, Yadav M, Nair S, Expression KMK. Of c-erbB3 protein in primary breast carcinomas. Br J Cancer. 1998;78:1385–90.
Weiss J, Sos ML, Seidel D, Peifer M, Zander T, Heuckmann JM, et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med. 2010;2:62ra93.
Dutt A, Ramos AH, Hammerman PS, Mermel C, Cho J, Sharifnia T, et al. Inhibitor-sensitive FGFR1 amplification in human non-small cell lung cancer. PLoS One. 2011;6:e20351.
Zhong J, Li L, Wang Z, Bai H, Gai F, Duan J, et al. Potential resistance mechanisms revealed by targeted sequencing from lung Adenocarcinoma patients with primary resistance to epidermal growth factor receptor (EGFR) tyrosine Kinase inhibitors (TKIs). J Thorac Oncol. 2017;12(12):1766–78.
Chen G, McQuade JL, Panka DJ, Hudgens CW, Amin-Mansour A, Mu XJ, et al. Clinical, molecular, and immune analysis of Dabrafenib-Trametinib combination treatment for BRAF inhibitor-refractory metastatic melanoma: a phase 2 clinical trial. JAMA Oncol. 2016;2:1056–64.
Acknowledgements
Not applicable.
Funding
This review was supported by National Natural Science Foundation of China (Grant No. 81572608 and 81672984) and National Major Scientific Instruments and Equipments Development Project (2013YQ030923).
Availability of data and materials
The material supporting the conclusion of this review has been included within the article.
Author information
Authors and Affiliations
Contributions
QL wrote the manuscript. SY,WZ, and SQ discussed and revised the manuscript. QC and KW designed the manuscript. All authors had read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, Q., Yu, S., Zhao, W. et al. EGFR-TKIs resistance via EGFR-independent signaling pathways. Mol Cancer 17, 53 (2018). https://doi.org/10.1186/s12943-018-0793-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-018-0793-1