Abstract
Background
Transplant recipients are immunocompromised and vulnerable to developing tuberculosis. However, active tuberculosis incidence is rapidly declining in South Korea, but the trend of tuberculosis infection among transplant recipients has not been elucidated. This study aimed to evaluate the risk of active tuberculosis after transplantation, including risk factors for tuberculosis and standardized incidence ratios, compared with that in the general population.
Methods
This retrospective study was conducted based on the South Korean health insurance review and assessment database among those who underwent transplantation (62,484 recipients) between 2008 and 2020. Tuberculosis incidence was compared in recipients treated during higher- (2010–2012) and lower-disease burden (2016–2018) periods. Standardized incidence ratios were analyzed using the Korean Tuberculosis Surveillance System. The primary outcome was the number of new tuberculosis cases after transplantation.
Results
Of 57,103 recipients analyzed, the overall cumulative incidence rate 1 year after transplantation was 0.8% (95% confidence interval [CI]: 0.7–0.8), significantly higher in the higher-burden period than in the lower-burden period (1.7% vs. 1.0% 3 years after transplantation, P < 0.001). Individuals who underwent allogeneic hematopoietic stem cell transplantation had the highest tuberculosis incidence, followed by those who underwent solid organ transplantation and autologous hematopoietic stem cell transplantation (P < 0.001). The overall standardized incidence ratio was 3.9 (95% CI 3.7–4.2) and was the highest in children aged 0–19 years, at 9.0 (95% CI 5.7–13.5). Male sex, older age, tuberculosis history, liver transplantation, and allogeneic hematopoietic stem cell transplantation were risk factors for tuberculosis.
Conclusions
Transplant recipients are vulnerable to developing tuberculosis, possibly influenced by their immunocompromised status, solid organ transplant type, age, and community prevalence of tuberculosis. Tuberculosis prevalence by country, transplant type, and age should be considered to establish an appropriate tuberculosis prevention strategy for high-risk groups.
Graphical Abstract

Similar content being viewed by others
Background
Transplantation is a complex, multidisciplinary procedure that requires considerable medical resources and is used to treat various intractable diseases or end-stage organ failure. Recipients of transplants are administered immunosuppressive therapy, which exposes them to opportunistic infections such as tuberculosis. Tuberculosis remains a prevalent disease worldwide, with an estimated 10.6 million new active tuberculosis cases in 2021, making it the second leading cause of death among infectious diseases after coronavirus disease [1]. In the pathogenesis of tuberculosis, exogenous exposure to Mycobacterium tuberculosis and suppression or senescence of the host immunity are crucial endogenous factors [2, 3]. Therefore, transplant recipients in highly endemic areas, a representative immunocompromised population, are a high-risk group for opportunistic tuberculosis infection [4]. The incidence of tuberculosis in recipients of solid organ transplants (SOTs) varies from 1.2 to 6.4%, reaching 15% in highly endemic areas [5, 6]. Tuberculosis incidence in recipients of hematopoietic stem cell transplants (HSCTs) is 0.0014–16% [7]. However, the current data in the literature have been obtained from different countries with varied incidence rates of active tuberculosis. Thus, comparing such data is limited by detection bias due to differences in the actual tuberculosis surveillance systems across countries.
Tuberculosis was previously “endemic” in South Korea, according to the World Health Organization category (100–299 new and relapse cases per 100,000 population per year) [1]. Since 2011, active tuberculosis management and aggressive tracing and treatment of latent tuberculosis have been implemented through the nationwide public–private tuberculosis management project. Consequently, the number of new tuberculosis cases decreased sharply from 100.8 per 100,000 population per year in 2011 to 49.4 per 100,000 population per year in 2020 (Additional file 1: Table S1) [8]. Notably, thousands of transplants are performed yearly in South Korea (Additional file 2: Table S2), providing an excellent opportunity to observe differences in the incidence of active tuberculosis among transplant recipients in settings with different tuberculosis incidence rates using the same population-based database. This study aimed to evaluate the risk of active tuberculosis after transplantation, including the risk factors for active tuberculosis and standardized incidence ratios (SIRs), compared with that in the general population.
Methods
Study design and population
This was a population-based, retrospective cohort study. We used the nationwide claims database of the Health Insurance Review and Assessment (HIRA) in South Korea. The HIRA reviews all healthcare claims from the National Health Insurance Service (NHIS), a universal insurer covering 97% of the South Korean population [9]. The HIRA database contains the age and sex of the insured individual; healthcare provider type; diagnosis code based on the International Classification of Disease, Revision 10, Clinical Modification (ICD-10-CM); and procedures and prescriptions covered by the NHIS.
Transplant recipients were individuals with procedure codes for transplantation claimed between January 1, 2008, and December 31, 2020. The detailed procedure codes for transplantation are described in Additional file 3: Table S3. When the same procedure code persisted for HSCT within 60 days, it was considered a single transplantation case (tandem autologous HSCT). Simultaneous kidney and pancreatic transplantations were considered for kidney transplantation. Small intestine transplantation and pancreas transplantation alone were classified as other transplantation types. However, dual transplantations other than those mentioned above, re-SOT with an interval of ≥ 30 days, and corneal and scleral transplantations were not included in this study. Data from 2008 were excluded for washout. Recipients who died on the day of transplantation were excluded. Patients diagnosed with tuberculosis within the last year before transplantation were also excluded because diagnostic codes may persist after transplantation, making it difficult to distinguish between pre-existing and new infections.
Definitions
As a primary outcome, tuberculosis was defined as active tuberculosis with pulmonary or extrapulmonary tuberculosis codes and one or more prescriptions for tuberculosis (Additional file 4: Table S4). When both codes were applicable, they were classified as pulmonary tuberculosis. Tuberculosis history was defined as the presence of diagnostic codes for active tuberculosis 1 year before transplantation. Comorbidity was defined as the presence of one or more of the diagnostic codes summarized in Additional file 5: Table S5 within a year of transplantation [10]. Death was defined as death at discharge with no subsequent claims. To compare the impact of tuberculosis burden in the general population on the timing of transplantation, burden periods were selected based on the incidence rate in the general population. The highest incidence rate (96.4–98.4 per 100,000 population per year) was observed in 2010–2012, and this was defined as the higher-burden period. In contrast, the lowest incidence rate (76.8–65.9 per 100,000 population per year) was observed in 2016–2018, and this was defined as the lower-burden period [8]. Each period was limited to 3 years to ensure that the two periods were similar.
Statistical analysis
The data are presented as a number (percentage) for categorical variables and a mean [standard deviation] for continuous variables. The baseline characteristics of the study population were compared using the Student’s t-test for continuous variables and the Chi-squared test for categorical variables.
All participants were followed up from the transplantation date to the tuberculosis diagnosis date, date of death, or end of study date, December 31, 2020—whichever came first. The tuberculosis’ cumulative incidence was estimated using a Fine and Gray sub-distribution hazard model, considering death as a competing risk event [11]. The cumulative incidence curves were investigated using tuberculosis burden, transplantation type (SOT, allogeneic HSCT, autologous HSCT), SOT subtype, and age at transplantation. They were compared using Gray’s test. Multiple comparisons with Bonferroni adjustments were performed. Risk factors associated with tuberculosis were examined using the Fine and Gray sub-distribution hazard model after considering death as a competing event. We ran a univariable model for each risk factor and a multivariable model to evaluate the effect of risk factors after adjusting for the confounding factors. Age, sex, diabetes mellitus, hypertension, asthma, chronic obstructive pulmonary disease, liver cirrhosis, chronic kidney disease, and previous tuberculosis history were considered risk factors, as reported previously [12]. The relative risk of developing tuberculosis among transplant recipients compared with the tuberculosis risk of the general population in South Korea was calculated using the SIR. SIR was the ratio of the observed to the expected number of tuberculosis cases. The expected numbers of tuberculosis cases were computed using sex-specific and 5-year-age-specific incidence rates in the general population. Confidence intervals (95% CIs) were calculated assuming a Poisson distribution at the 95% level [13]. We used data from the Korean Tuberculosis Surveillance System, a mandatory reporting system for confirmed tuberculosis cases, to compare tuberculosis incidence between transplant recipients and the general population [8]. All statistical analyses were performed using SAS Enterprise Guide version 7.1 (SAS Institute, Cary, NC, USA). Two-sided P-values < 0.05 were considered significant.
Ethics
This study was reviewed and approved by the Institutional Review Board of Severance Hospital, Yonsei University College of Medicine, Seoul, Korea (Reg. No. 4-2020-0018). The requirement for informed consent was waived owing to the use of anonymous data.
Results
Characteristics of the study population
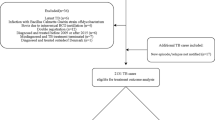
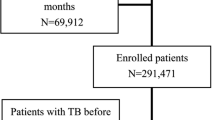
From 2008 to 2020, 62,484 insured individuals who had undergone transplantation were identified. Of these, 3,378 were excluded, and 57,103 were finally included in the analyses (Fig. 1). The male-to-female ratio was 1.6:1, and the mean follow-up duration was 5.30 [3.54] years. By age group, the 40–59-year-old group accounted for most of the participants at 55.7%, followed by the ≥ 60-year-old (18.5%), 20–39-year-old (17.3%), and < 20-year-old (8.5%) groups. According to transplant type, SOT accounted for 64.1%, allogeneic HSCT for 19.6%, and autologous HSCT for 16.2%. Among the recipients of SOTs, 57.1% had kidney transplantation (KT), 37.0% had liver transplantation (LT), 3.7% had heart transplantation (HT), 1.7% had lung transplantation, and 0.4% had others. Of these recipients, 3.0% had a tuberculosis history before transplantation. The other baseline characteristics of all study populations are presented in Table 1. The detailed baseline characteristics of SOT and HSCT recipients are presented in Additional file 6: Table S6 and Additional file 7: Table S7, respectively.
Incidence of active tuberculosis
During the follow-up period (mean [SD], 1.8 [2.0] years), tuberculosis occurred in 830 (1.5%) patients after transplantation. The overall cumulative incidence rate was 0.8% (95% CI 0.7–0.8) 1 year after transplantation, 1.1% (95% CI 1.0–1.1) at 2 years, 1.4% (95% CI 1.3–1.5) at 5 years, and 1.7% (95% CI 1.6–1.9) at 10 years. The cumulative incidence in the higher-burden period (2010–2012) was 1.7% 3 years after transplantation, significantly lower than that in the lower-burden period (2016–2018) (1.0%, P < 0.001) (Fig. 2a). Comparing tuberculosis incidence according to transplantation type, allogeneic HSCT had the highest tuberculosis incidence, followed by SOT. In contrast, autologous HSCT had the lowest rate (P < 0.001). Notably, the probability of developing tuberculosis among allogeneic HSCT recipients increased steeply up to 2 years after transplantation. Allogeneic HSCT and SOT differed in incidence up to 4 years after transplantation (1.7% for allogeneic HSCT and 1.4% for SOT, P = 0.026). However, there was almost no statistical difference thereafter, with 1.9% and 1.8% at 10 years for HSCT and SOT, respectively (P = 0.88). Tuberculosis incidence 2 years after transplantation was 1.5% in allogeneic HSCT recipients, 2.4 times higher than 0.6% in autologous HSCT recipients (P = 0.001) (Fig. 2b). By SOT, the highest cumulative incidence rate 5 years after transplantation occurred among those who had other transplantation types (2.4%), including small intestine and pancreas transplantations alone, followed by lung transplantation (2.0%), LT (1.6%), HT (1.4%), and KT (1.3%); however, the difference was not significant (Fig. 2c). Based on age group, the incidence of tuberculosis increased with age (P < 0.001). The 10-year cumulative incidence rate for those aged ≥ 60 years was 2.4%, which was 3.6 times higher than that for those aged 0–9 years (0.7%, P < 0.001) (Fig. 2d). In the SOT and allogeneic HSCT groups, tuberculosis incidence increased significantly with age, similar to the trend in the overall transplantation group. However, no difference in tuberculosis incidence by age group was observed in the autologous HSCT group. Based on sex, male recipients had a significantly higher tuberculosis risk than females, regardless of transplantation type (P < 0.001).
Among all active tuberculosis cases, pulmonary tuberculosis accounted for 64.7% (n = 537) and extrapulmonary tuberculosis accounted for 34.5% (n = 286). There was no significant difference in the incidence of pulmonary and extrapulmonary tuberculosis according to transplantation type (Additional file 8: Table S8).
Risk factors for tuberculosis and mortality
Table 2 summarizes the tuberculosis risk factors for transplant recipients. In the multivariate analysis, male sex (hazard ratio [HR]: 1.4, 95% CI 1.2–1.6), older age ≥ 60 years (HR 1.7, 95% CI 1.3–2.1; reference, 20–39-year-old group), tuberculosis history (HR 1.5, 95% CI 1.1–2.1), and LT and allogeneic HSCT (HR 1.7, 95% CI 1.04–2.8; HR 2.1, 95% CI 1.4–3.3, respectively; reference transplantation, KT) were associated with increased risk of active tuberculosis. Similar risk factors for tuberculosis were identified in the SOT (Additional file 9: Table S9) and HSCT (Additional file 10: Table S10) subgroup analyses.
Among transplant recipients with tuberculosis, the all-cause mortality rate during the study period was 27.0%, significantly higher than that of transplant recipients without tuberculosis (17.1%, P < 0.001).
Comparison with the general population
The overall age-adjusted SIR result revealed that transplant recipients had 3.9 times higher tuberculosis risk than the general Korean population (SIR: 3.9, 95% CI 3.7–4.2). The SIR of transplanted recipients aged < 20 years was 9.0 (95% CI 5.7–13.5), the highest among all age groups. Meanwhile, the SIR of the 20–39-year-old group was 5.0 (95% CI 4.2–6.00), that of the 40–59-year-old group was 4.3 (95% CI 3.9–4.7), and that of the 60-year-old group was 3.0 (95% CI 2.7–3.4). Based on the transplantation type, the SIR of LT recipients was 3.5 times (95% CI 3.1–4.0); KT, 3.4 times (95% CI 3.0–3.8); autologous HSCT, 7.4 times (95% CI 6.4–8.5); and allogeneic HSCT, 3.1 times (95% CI 2.5–3.8) more than that of the general population (Fig. 3).
Discussion
We revealed the effect of exogenous exposure on tuberculosis occurrence in high-risk transplant recipients by comparing different incidence rates in Korea. In addition, the risk of tuberculosis varied by up to 2.4 times depending on the transplant type (SOT or HSCT). Additionally, the risk differed depending on the type of SOT. Also, tuberculosis risk differed by the recipient’s age at the time of transplantation; the lowest risk was observed in the pediatric population, but their SIR was the highest.
The 10-year cumulative tuberculosis incidence among transplant recipients in this study (1.7%) was comparable to the cumulative incidence in previous studies in countries/regions with a similar tuberculosis prevalence (50–100 cases per 100,000) [14, 15]. In Taiwan (55–67 cases per 100,000 of the general population during 2006–2011), the 10-year cumulative incidence of tuberculosis was 3.5% among 2,040 adult HSCT recipients [15]. In addition, the 5-year cumulative tuberculosis incidence in adult SOT recipients in 2011–2016 in Korea was 1.9% [16]. Our results are significant because these large-scale population-based data provide a more specific range of active tuberculosis incidence among transplant recipients in countries with a moderate prevalence of endemic tuberculosis [17]. Moreover, this study’s case definitions and criteria can be applied in future population-based studies in other countries.
The most notable finding in the present study is that active tuberculosis incidence in transplant recipients decreased by almost half (58.8%; 1.7% vs. 1.0% 3 years after transplantation), as the tuberculosis incidence rate in the general Korean population decreased from approximately 100 to 70 per 100,000. In the Spanish Network of Infection in Transplantation cohort study conducted among 4,388 SOT recipients in Spain, where tuberculosis incidence was low, 95% of patients with tuberculosis were infected within 1 year after transplantation [6]. Comparatively, our data revealed a more linear pattern for active tuberculosis after transplantation. This suggests that tuberculosis exposure after transplantation is as essential as the reactivation of latent tuberculosis in high-risk populations in endemic countries/regions. Transplant recipients are at risk of tuberculosis at both early and later transplantation periods. Therefore, latent tuberculosis screening in the pre-transplantation period, as recommended in the current guidelines for transplantation-associated infection, and early suspicion and aggressive diagnostic testing for tuberculosis in the late post-transplantation period in moderate-to-highly endemic countries/regions are necessary.
Our study also directly compared allogeneic HSCT, autologous HSCT, and SOT. Allogeneic recipients of HSCT had the highest cumulative incidence of active tuberculosis, followed by recipients of SOT and autologous HSCT. In a previous study, tuberculosis incidence was 10 times higher in recipients of SOT than in those of HSCT [7, 18, 19], possibly due to long-term immunosuppressant use. However, allogeneic and autologous HSCT should be considered separately [19]. The profound and prolonged deficiency of cell-mediated immunity in allogeneic HSCT due to pre-transplant conditioning therapy, immunosuppression after transplantation, and graft-versus-host disease is a major factor in tuberculosis development after transplantation. In addition, the fact that tuberculosis generally occurs within 1 year after transplantation is a more critical factor in the degree of immunosuppression than the long-term use of immunosuppressants that have been previously proposed. Previous studies have reported that tuberculosis risk differs according to the type of transplanted organ and is particularly high in recipients of lung transplants [6, 16, 18, 20]. Our cohort also displayed similar tendencies, with more cases of tuberculosis among those who underwent lung transplantation, followed by those who underwent LT, KT, and HT. Individuals with small intestine and pancreatic transplantations—classified as other transplantations—also had a high incidence, probably because of the relatively few transplants.
Interestingly, children had the lowest tuberculosis incidence among transplant recipients, but they had the highest tuberculosis risk compared to the general population of children of the same age. Previous literature on tuberculosis in pediatric recipients of SOT and HSCT is sparse, and the underlying reason is not apparent [21]. One possible explanation could be that pediatric transplant recipients have a relatively low tuberculosis exposure risk compared to adult or elderly transplant recipients (shorter exposure during lifetime and lower tuberculosis incidence in Korea in recent years compared to the past), which could result in lower age-specific transplant tuberculosis rates. However, a higher incidence of active tuberculosis than children of the same age should be noted. Therefore, upon exposure to tuberculosis, aggressive screening and treatment for latent tuberculosis in immunocompromised children are warranted [22].
This study has few limitations. First, we could not evaluate the effects of immunosuppressive agents. Second, information on latent tuberculosis was unavailable in our dataset. National guidelines for the diagnosis and treatment of latent tuberculosis have been suggested since 2014, but there is a lack of assessment regarding its impact on transplant patients. The development and treatment of latent tuberculosis after transplantation may have affected active tuberculosis development. Third, although mortality was higher in the tuberculosis group than in the non-tuberculosis group, the Charlson comorbidity index was also higher; therefore, it was challenging to directly explain the high mortality among those with tuberculosis.
Nevertheless, our study has several strengths. First, to the best of our knowledge, this is the largest population-based study evaluating active tuberculosis incidence among transplant recipients compared with that among the age- and sex-adjusted general population. Second, by comparing various transplants according to the same definition and criteria, we could measure the relative risk according to transplant type and organ transplantation. Finally, this study is relevant because it measured tuberculosis development risk in pediatric transplant recipients previously neglected in research.
Conclusions
Tuberculosis development risk after transplantation is several times higher than that in the general population and is influenced by community prevalence. Furthermore, the prevalence by country, transplant type, and age should be considered to establish an appropriate tuberculosis prevention strategy for these high-risk groups.
Availability of data and materials
Data are available through the Health Insurance Review & Assessment Service (https://opendata.hira.or.kr).
Abbreviations
- CI:
-
Confidence interval
- HIRA:
-
Health insurance review and assessment
- HR:
-
Hazard ratio
- HSCT:
-
Hematopoietic stem cell transplant
- HT:
-
Heart transplantation
- KT:
-
Kidney transplantation
- LT:
-
Liver transplantation
- NHIS:
-
National Health Insurance Service
- SOT:
-
Solid organ transplant
- SIR:
-
Standardized incidence ratio
References
World Health Organization. Global tuberculosis report 2022. Geneva: World Health Organization; 2022.
Furin J, Cox H, Pai M. Tuberculosis. Lancet. 2019;393(10181):1642–56.
Donald PR, Marais BJ, Barry CE 3rd. Age and the epidemiology and pathogenesis of tuberculosis. Lancet. 2010;375(9729):1852–4.
Muñoz P, Rodríguez C, Bouza E. Mycobacterium tuberculosis infection in recipients of solid organ transplants. Clin Infect Dis. 2005;40(4):581–7.
Aguado JM, Herrero JA, Gavaldá J, Torre-Cisneros J, Blanes M, Rufí G, et al. Clinical presentation and outcome of tuberculosis in kidney, liver, and heart transplant recipients in Spain. Spanish Transplantation Infection Study Group, GESITRA. Transplantation. 1997;63(9):1278–86.
Torre-Cisneros J, Doblas A, Aguado JM, San Juan R, Blanes M, Montejo M, et al. Tuberculosis after solid-organ transplant: incidence, risk factors, and clinical characteristics in the RESITRA (Spanish Network of Infection in Transplantation) cohort. Clin Infect Dis. 2009;48(12):1657–65.
Russo RL, Dulley FL, Suganuma L, França IL, Yasuda MA, Costa SF. Tuberculosis in hematopoietic stem cell transplant patients: case report and review of the literature. Int J Infect Dis. 2010;14(Suppl 3):e187–91.
Korea Disease Control and Prevention Agency. Annual report on the notified tuberculosis in tuberculosis in Korea 2020. Cheongju: Korea Disease Control and Prevention Agency; 2021.
Kim L, Kim JA, Kim S. A guide for the utilization of health insurance review and assessment service national patient samples. Epidemiol Health. 2014;36: e2014008.
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9.
Bellach A, Kosorok MR, Rüschendorf L, Fine JP. Weighted NPMLE for the subdistribution of a competing risk. J Am Stat Assoc. 2019;114(525):259–70.
Narasimhan P, Wood J, MacIntyre CR, Mathai D. Risk factors for tuberculosis. Pulm Med. 2013;2013: 828939.
Boyle P, Parkin DM. Cancer registration: principles and methods. Statistical methods for registries. IARC Sci Publ. 1991;95:126–58.
Chen CH, Shu KH, Ho HC, Cheng SB, Lin CC, Wei HJ, et al. A nationwide population-based study of the risk of tuberculosis in different solid organ transplantations in Taiwan. Transplant Proc. 2014;46(4):1032–5.
Fan WC, Liu CJ, Hong YC, Feng JY, Su WJ, Chien SH, et al. Long-term risk of tuberculosis in haematopoietic stem cell transplant recipients: a 10-year nationwide study. Int J Tuberc Lung Dis. 2015;19(1):58–64.
Kwon DE, Han SH, Han KD, La Y, Lee KH. Incidence rate of active tuberculosis in solid organ transplant recipients: Data from a nationwide population cohort in a high-endemic country. Transpl Infect Dis. 2021;23(6): e13729.
Sorohan BM, Ismail G, Tacu D, Obrișcă B, Ciolan G, Gîngu C, et al. Mycobacterium tuberculosis infection after kidney transplantation: a comprehensive review. Pathogens. 2022;11(9):1041.
Aguado JM, Silva JT, Samanta P, Singh N. Tuberculosis and transplantation. Microbiol Spectr. 2016. https://doi.org/10.1128/microbiolspec.TNMI7-0005-2016.
Al-Anazi KA, Al-Jasser AM, Alsaleh K. Infections caused by mycobacterium tuberculosis in recipients of hematopoietic stem cell transplantation. Front Oncol. 2014;4:231.
de Lopez Castilla D, Schluger NW. Tuberculosis following solid organ transplantation. Transpl Infect Dis. 2010;12(2):106–12.
Vecino R, Santiago B, Baquero-Artigao F, López GL, García C, Muñoz G, et al. Tuberculosis in pediatric solid organ and hematopoietic stem cell transplant recipients. Pediatr Infect Dis J. 2012;31(7):774–7.
Nolt D, Starke JR. Tuberculosis infection in children and adolescents: testing and treatment. Pediatrics. 2021;148(6):e2021054663. https://doi.org/10.1542/peds.2021-054663.
Acknowledgements
The authors thank Medical Illustration & Design, part of the Medical Research Support Services of Yonsei University College of Medicine, for all the artistic support related to this work.
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education [grant number 2019R1A6A1A03032869] and by the Ministry of Science and ICT [grant number NRF-2020R1G1A1005010]. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
JHH, ML, and JMK: integrity of the data, study supervision, and access and verification of the underlying data. ML, IJ, and EHK: conceptualization, methodology, and funding acquisition. JHH, SMH, YRK, SL, KI, MYK, JGA, and JSY: investigation, data curation, and project administration. All authors: formal analysis, visualization, and validation. JHH and ML: writing—original draft. IJ, SJJ, and JMK writing—review and editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Institutional Review Board of Severance Hospital, Yonsei University College of Medicine, Seoul, Korea (Reg. No. 4–2020-0018). The requirement for informed consent was waived owing to the use of anonymous data.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
The annual reported number and incidence of tuberculosis in Korea, 2008-2020.
Additional file 2: Table S2.
The case number of transplantations by year in Korea, 2008-2020.
Additional file 3: Table S3.
HIRA code for Transplantation.
Additional file 4: Table S4.
Drug ATC codes used to define tuberculosis.
Additional file 5: Table S5.
ICD-10 Code for tuberculosis and co-variables.
Additional file 6: Table S6.
Baseline characteristics of patients with SOT.
Additional file 7: Table S7.
Baseline characteristics of patients with HSCT.
Additional file 8: Table S8.
Pulmonary and extrapulmonary tuberculosis according to transplant type.
Additional file 9: Table S9.
Risk factors associated with the development of TB after SOT.
Additional file 10: Table S10.
Risk factors associated with the development of TB after HCST.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hyun, J., Lee, M., Jung, I. et al. Changes in tuberculosis risk after transplantation in the setting of decreased community tuberculosis incidence: a national population-based study, 2008–2020. Ann Clin Microbiol Antimicrob 23, 1 (2024). https://doi.org/10.1186/s12941-023-00661-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-023-00661-4