Abstract
Background
Coronary heart disease (CHD), the leading cause of death globally, might be developed or exacerbated by air pollution, resulting high burden to patients. To date, limited studies have estimated the relations between short-term exposure to air pollution and CHD disease burden in China, with inconsistent results. Hence, we aimed to estimate the short-term impact and burden of ambient PM pollutants on hospitalizations of CHD and specific CHD.
Methods
PM10 and PM2.5 were measured at 82 monitoring stations in 9 cities in Sichuan Province, China during 2017-2018. Based on the time-stratified case-crossover design, the effects of short-term exposure to particle matter (PM) pollution on coronary heart disease (CHD) hospital admissions were estimated. Meanwhile, the linked burden of CHD owing to ambient PM pollution were estimated.
Results
A total of 104,779 CHD records were derived from 153 hospitals from these 9 cities. There were significant effects of PM pollution on hospital admissions (HAs) for CHD and specific CHD in Sichuan Province. A 10 μg/m3 increase of PM10 and PM2.5 was linked with a 0.46% (95% CI: 0.08, 0.84%), and 0.57% (95% CI: 0.05, 1.09%) increments in HAs for CHD at lag7, respectively. The health effects of air pollutants were comparable modified by age, season and gender, showing old (≥ 65 years) and in cold season being more vulnerable to the effects of ambient air pollution, while gender-specific effects is positive but not conclusive. Involving the WHO’s air quality guidelines as the reference, 1784 and 2847 total cases of HAs for CHD could be attributable to PM10 and PM2.5, separately. The total medical cost that could be attributable to exceeding PM10 and PM2.5 were 42.04 and 67.25 million CNY from 2017 to 2018, respectively.
Conclusions
This study suggested that the short-term exposure to air pollutants were associated with increased HAs for CHD in Sichuan Province, which could be implications for local environment improvement and policy reference.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Coronary heart disease (CHD), also named ischemic heart disease, is the leading cause of death globally [1]. According to the American Heart Association, the cases of CHD would increase nearly 100% by 2030 [2]. The prevalence of CHD is second leading cause of death worldwide, resulting in escalating death for decades like in China, in which the average annual growth rate being 9.85% from 1980 to 2016 [3, 4].
To date, diverse and growing data have discerned that growing risks and burden of CHD are linked with short-term exposure to particle matter (PM) with aerodynamic diameter ≤ 2.5 (PM2.5) μm or ≤ 10 μm (PM10) [5,6,7,8,9]. PM2.5 pollution would cause around the 2.0% loss of China’s gross domestic product (GDP) by 2030 without necessary measure being taken [10]. For instance, a study revealed that PM2.5 accounted for 26.8% CHD deaths in China [11]. The Global Burden of Disease study identified that ambient PM pollution induced around 11.1% of all deaths in China [12]. A study conducted in Chengdu estimated that a 10 μg/m3 increase in PM2.5 was involved with a 1.2% (95% CI: 0.3, 2.2%) increase in hospital admissions (HAs) for CHD [9]. Primarily, the relationships of HAs for CHD were involved [13, 14], only limited studies associated with specific CHD, like chronic coronary heart disease (CCHD) [15], acute myocardial infarction (AMI) [16, 17], and unstable angina (UA) [15], which could also link with large quantity of HAs regarding the exposure to PM pollution.
Interestingly, from these epidemiology studies, various primary questions were still remained, since these studies mainly confined in limited regions in developed countries. As exposure to PM pollution being ubiquitous, especially for residents in developing countries exposing to high levels of contaminants, PM pollution contributes comparably to public health crisis. Particularly, like the areas of China which with high PM pollution levels, this would contribute to increase the considerable health and economic burden [18, 19]. Like Sichuan Province, it ranked as the fourth of heavily air polluted regions in China, with high annual mean PM levels [20]. The mean levels of PM2.5 and PM10 levels reached up to 77.4 and 106.4 μg/m3 during January 2015 and February 2017 in Sichuan, resulting in about fifteen and seven times higher than the annual guidelines (5 μg/m3 for PM2.5 and 15 μg/m3 for PM10) proposed by World Health Organization [21]. Owing to the high population densities and rapid development of industrialization, Sichuan Province is characterized by the intensive consumption of energy and anthropogenic emissions, resulting in severely regional pollution. Besides, the rank of death cause for CHD is increasing during the last decade, which had become the third death cause in Sichuan Province and brought heavy economic burden to their family [10]. Nevertheless, available studies evaluated the relationship of PM pollution and CHD only regarding to Chengdu, which is the provincial capital city of Sichuan Province [9]. While no studies systematically revealed the short-term effect of PM levels on HAs for CHD and economic burden, in addition to the specific CHD involved in Sichuan Province, which is gaining comparable public attention for serious PM pollution and special topography locating in the Sichuan Basin [22].
To fill this data gap, this study involved a time-stratified case-crossover analysis to estimate the impact of ambient PM pollutants on hospitalizations of CHD and specific CHD in 9 cities of Sichuan Basin between January 1, 2017 and December 31, 2018. Besides, the linked burden of CHD owing to ambient PM pollution were estimated.
Materials and methods
Study area
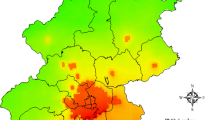
This study was implemented in the urban regions of 9 cities in Sichuan Province (Fig. 1). Nine cities, Chengdu (CD), Mianyang (MY), Nanchong (NC), Guangan (GA), Meishan (MS), Zigong (ZG), Liangshanzhou (LSZ), Yibin (YB), and Luzhou (LZ) are involved to discern the effects of air pollution on CHD hospitalizations in these cities. Owing to the lower data quality or limit availability of health data, some cities are exclusive, such as Ganzizhou (GZZ), Abazhou (ABZ) and Guangyuan (GY).
Data
Hospitalization data
The records of CHD were collected from hospital electronic health records (EHRs) of 153 hospitals in 9 cities of Sichuan Province, which contains information on gender, age, date of hospitalization, detailed residence of the patient, primary diagnoses, hospitalization cost, and disease code according to the International Classification of Disease, 10 the Revision (ICD-10). The Daily hospitalization data of CHD (ICD-10: I20-I25) during the period of January 1, 2017 and December 31, 2018 were involved. Besides, three specific subtypes of CHD were involved, including CCHD (ICD-10: I25) [23], AMI (ICD-10: I21-I22) [17, 24], and UA (ICD-10: I20.0) [25], which were all involving comparable quantity of patients, leading cause of mortality, and available evidence on the relations between them and air pollution [24, 26, 27]. The category of these three subtypes was based on the first diagnose as the central factor of hospitalization.
Environmental data
The daily mean nitrogen dioxide (NO2), sulfur dioxide (SO2), ozone (O3), PM10 and PM2.5 were obtained from Sichuan Environmental Monitoring Station between January 1, 2017 and December 31, 2018. There were 82 air monitoring stations in the 9 cities: 19 in CD, 9 MY, 9 in NC, 6 in GA, 6 in MS, 6 in ZG, 17 LSZ and 10 YB. Meteorological data of daily average temperature and relative humidity were available from Sichuan Meteorological Bureau. To assess air pollution exposure, inverse distance weighting (IDW) method were involved [28]. More specifically, the locations of all CHD cases and monitoring stations were geocode using AutoNavi Maps API (https://lbs.amap.com/). Then, for each hospitalization of CHD and measuring station, the exposure of air pollutant on the hospitalized day (lag 0) was estimated by the inverse distance (1/d2) weighted average of concentrations at all monitoring stations. The single-day lag exposures (lag 1 to lag 7), moving average day exposure (lag 0-1 to lag 0-7) were confirmed. Moreover, lag 0-1, lag 2-7, lag 0-7 were adopted as time window to estimate immediate, delayed or prolonged effects [17]. To deduce liable data, the validation of exposure for each air pollution was involved [28]. The predicted and measured daily levels of air pollutants at all measuring stations were adopted to estimate statistical indicators, like the mean absolute error (MAE). More details could be found at S1 of Supporting Information (SI).
Modeling
Statistical methods
Based on the time-stratified case-crossover design, the exposure to air pollution and daily CHD hospitalizations were estimated. Time-series analysis are associated to observe the relationship between PM pollution and HAs for CHD [29,30,31,32]. Case-crossover study as a self-matched case-control proposed by Maclure [33], it would compare the exposure in the case period and cases with exposure in nearby referent periods as reference, to confirm the differences of exposure which might influence the daily count of records [34]. The reference days were selected from the same day of the week in the same month of the same year, when HAs of CHD were recorded, to adjust the influence of long-term tendency, seasonality effect, and day of week from the design framework [35]. Daily cases of CHD approximately followed poisson distribution [36]. A linear model of conditional poisson regression with time-stratified case-crossover design was involved to estimate the short-term exposure of air pollutants and daily CHD hospitalizations in 9 cities of Sichuan Province in China, applying temperature, relative humidity and holidays (assigned a value of 1 on national holidays and 0 on the other days) as potential confounders. Relied on the per 1 μg/m3 increase of air levels, the Relative Risk (RR) and 95% confidence intervals (CIs) were estimated. In the exploratory analysis, the natural cubic splines with three degrees of freedom for temperature, relative humidity and holidays was introducing to new models, to examine the nonlinear effect. Then the linear model was better than nonlinear model, with smaller values of the Akaike’s Information Criterion.
Relative risk increase (RRI) was estimated by RR-1. The RRI in HAs for CHD per 10 μg/m3 increase of PM levels were calculated as follows:
where β is the exposure-response coefficient of PM-HAs association from conditional poisson regression combined under the time-stratified case-crossover design, which refers to a unit increase in PM pollutants [37].
Single-pollutant models were implemented to discern the effects of air pollution. As aforementioned, to analyze the temporal effect of air levels with different lag structures, from lag 0 to lag 7, from lag 0-1 to lag 0-7, and lag 2-7 were involved. Single day lag refers to the pollution levels on the current day, while cumulative day lag corresponds to moving average of pollution concentrations for the current and previous days. The cumulative day lag influence was unconstrained distributed lag model, which could present unbiased results from the estimation of the overall effect [38].
When estimating the effects of some potential effect modifiers, stratified time-stratified analyses have been involved linking with various subgroups by age group (< 45 years, 45-64 years and ≥ 65 years), gender (male and female) and season (warm season: April-September, and cold season: October-March), applying the above analyses for these subgroups. The statistic differences from stratified analyses (e.g., the difference between male and female) were estimated by Z-test [39].
All analysis were conducted using R version 4.0.4 with gnm package for conditional poisson regression combined under the time-stratified case-crossover design. All statistical tests with p values of < 0.05 were considered as statistically significant. The availability of geocode were obtained from AutoNavi via amapGeocode package.
Calculating the number of HAs for CHD due to PM pollution
Based on the coefficients from conditional poisson regression combined under the time-stratified case-crossover design, the attributable number of HAs for CHD was calculated. WHO’s air quality guidelines (24 h mean: 45 for μg/m3 PM10 and 15 μg/m3 for PM2.5) [21] were involved as reference levels. The equation was shown as follows:
where ANi is the number of HAs which could be attributable to exceeding PM exposures on day i; xi (μg/m) is the exposure level of PM pollution on day i; x0 is reference concentration from air quality guideline of WHO; Ni is the number of HAs on day i; AN is the sum of overall ANi during the study period. x0, as the base case from the air quality guideline of WHO, is the theoretical minimum threshold levels, below which PM pollution has no effect on HAs [39]. The largest effect in the single pollutant models is involved to estimate the attributable to exceeding PM pollution [19].
Evaluating the corresponding hospitalization economic cost
The economic cost of HAs for CHD due to PM exposure were estimated, which involved with total hospital admission expenses and out-of-pocket cost. The equations were shown as follows:
where ACytotal and ACypocket indicate the total hospital admission cost and out-of-pocket cost which could be attributable to exceeding PM exposure in year y; ANy is the sum of overall ANy during year y; Costytotal and Costypocket represent the case-average total hospital admission expenses and out-of-pocket cost in year y; CPIy is the product of customer price indexs from year y + 1 to 2018; ACtotal is the sum of ACytotal, and ACypocket is the sum of ACypocket during the study period.
Sensitivity analysis
The two-pollutant models were adopted to estimate the effect after adjusting for co-pollutants. To confirm the robustness of association results, the sensitivity analysis was estimated by evaluating the cases within circular areas of 50 km surrounding air monitoring stations, which could deduce the potential effect of distance between the air pollution monitory site and the address of patient [28].
Results
Data description
The descriptive results were displayed in Tables 1 and 2, with data from 153 hospitals located in the 9 cities of Sichuan Province. Between 2017 and 2018 (Table 1), a total of 104,779 CHD hospital admissions (55,891 males and 48,888 females) were recorded, including 83,471 HAs for CCHD, 12817 HAs for AMI, and 3946 HAs for UA. Population age < 45, 45-64, and age ≥ 65 accounted for 4, 24.4 and 71.6% of the total CHD hospitalizations, separately. The mean daily HAs for CHD were slightly higher in cold season than warm season. The overall medical expensed were 1997.6 million China Yuan (CNY), and 781.3 million CNY were self-paid, while the case-average total medical expenses and the out-of-pocket cost were 1.4 and 0.6 thousand CNY, respectively. Daily average concentrations of NO2, O3, SO2, PM10, and PM2.5 were 30.0 μg/m3, 81.8 μg/m3, 12.5 μg/m3, 71.7 μg/m3, and 46.0 μg/m3, while the temperature and relative humidity were 17.4 °C and 77.2%, respectively. Detailed summary statistics of specific characteristics for each city can be found in Tables S1, S2 and S3. Person correlation coefficients among these variables was displayed in Fig. S2 of SI.
Health effects of PM exposure in overall and subgroup population
The associations between PM pollutants and HAs in CHD at various lag days adopting single-pollutant models were displayed in Fig. 2. Overall, there were obvious associations of PM pollutants with HAs for CHD, and general significance at lag 4, lag 6 and lag 7 with the largest association at lag 7. A 10 μg/m3 increase of PM10 and PM2.5 corresponded to a RRI of 0.46% (95% CI: 0.08, 0.84%), and 0.57% (95% CI: 0.05, 1.09%) increases in HAs for CHD at lag 7, respectively.
To figure the influence of PM10, and PM2.5 levels on CHD inpatient visits for various subgroups, the stratified analyses involving age, season, and sex lag 7 were illustrated in Fig. 3, after adjustment for temperature, relative humidity and holidays. Owing to the largest effects in single-pollutant model on lag 7, the stratified analyses on lag 7 were performed. In age-specific analysis, the old were more susceptible to PM pollution, with an increase of 10 μg/m3 for PM10, and PM2.5 linking with a RRI of 0.62% (95% CI: 0.33, 0.91%), and 0.98% (95% CI: 0.59, 1.37%) of HAs for CHD, respectively. In gender-specific analysis, the impacts of PM pollution on HAs for CHD were positive and significant. Noteworthily, the difference between male and female for PM10 was statistically significant (p = 0.02), but the difference between male and female for PM2.5 did not reach statistical significance (p > 0.05). As for the differences of season groups, the exposure to PM10 and PM2.5 in cold season was higher, associated with a RRI of 1.16% (95% CI: 0.73, 1.59%) and 1.55% (95% CI: 1.02, 2.09%) in HAs for CHD, based on the increase of 10 μg/m3 of PM10 and PM2.5, separately. However, the differences of stratified season analyses for PM pollution were not statistically significant (p > 0.05).
The effects of PM exposure on HAs for specific CHD were displayed in Fig. 4. For CCHD, the significant effects of PM10 were found at lag 4-7, lag 0-5 to lag 0-7, and lag 2-7 days, while at lag4-7 for PM2.5. As for AMI, the effects of PM10 were significant at lag 6, and at 5-7 for PM2.5. However, for UA, the PM pollution displayed no adverse effect.
Sensitivity analysis
A two-pollutant model was involved to discern the coupled effects of various pollutants on the number of CHD inpatients (Table S4), excluding the associated effects between PM2.5 and PM10 owing to the high correlation (r = 0.95). In the co-pollutant models, the increases of 10 μg/m3 for PM10 and PM2.5 at lag 7 were linked with RRI of 1.06% (95% CI: 0.59, 1.55%) and 1.63% (95% CI: 0.92, 2.35%) increments in CHD hospitalizations after the adjustment for NO2, respectively. After adjustment for SO2, the effects of PM pollution on CHD were still significant and robust. Percentage (95% CI) in HAs for CHD associated with an increase of 10 μg/m3 of PM10, and PM2.5 at different lag days within the circular areas of 50 km surrounding air monitoring stations were shown in Fig. S2. When excluding the cases living beyond 50 km from any monitoring station, it suggested that the main models were generally robust.
Attributable health risks and economic costs due to PM pollution
Table 3 listed the attributable number of HAs and the related economic cost due to exceeding PM exposure involving WHO air quality standard in Sichuan Province, 2017-2018. Based on the reference concentrations, 1784 and 2847 total cases of HAs for CHD could be attributable to PM10 and PM2.5, separately. As for the total medical expenses and out-of-pocket cost, 42.04 and 16.42 million CNY, and 67.25 and 26.27 million CNY HAs for CHD could be attributable to exceeding PM10 and PM2.5 in 2017 and 2018, respectively.
Discussion
In this study, based on the case-crossover design, the significant associations between short-term exposure to ambient PM pollution and HAs for CHD in 9 cities in Sichuan Province during 2017-2018 had been discerned. As aforementioned, PM pollution was linked with increased daily HAs for CHD, when controlling the influences from confounders which including daily temperature, relative humidity and holidays. Stratified analysis discerned that the old patients and in cold period might more easily engage in the effects of outdoor air pollutants, being consistent with prior studies [31, 40, 41]. As previous studies found, a significant effect of PM pollution was found and varied greatly with various subtypes of CHD [42]. Furthermore, involving the WHO’s air quality as the reference, PM pollution entailed comparable economic burden to HAs for CHD, which was 132.33 million Yuan in total during the study period, 2017-2018.
Increasing studies reported the positive associations between PM pollution and HAs for CHD, since U.S. Environmental Protection Agency (EPA) suggested to discern the short-term relationship between PM pollution and cardiovascular [43]. These epidemiology studies investigated the effect of PM pollution on HAs for CHD, which is in line with the results of this study. A study in Shanghai reported that a 10 μg/m3 increase in PM10 and PM2.5 was coincided with an increase in HAs for CHD by 0.25% (95% CI: 0.10, 0.39%) and 0.57% (95% CI:0.46, 0.68%), separately [13]. A study conducted in Chengdu estimated that a 10 μg/m3 increase in PM2.5 was involved with a 1.2% (95% CI: 0.3, 2.2%) increase in HAs for CHD [9]. The results presented the effects of PM pollutions on HAs for CHD on different regional scale, contributing to explaining the PM effects all over the world. Besides, this study in Sichuan Province improved the understand of adverse effects of PM pollutants on HAs for CHD.
The mechanism of induced CHD, including specific CHD, from PM pollution is plausible. The increasing PM levels may trigger myocardial infarction, owing to the relationship between hemodynamic and hemostatic alterations with PM pollutions [44], which could be involved with increased plasma viscosity [45], acceleration of heart rates [46, 47] and diminished heart rate variability and ventricular fibrillation and increased number of therapeutic interventions in patients with implanted cardioverter-defibrillators [48]. Besides, different size-specific PM pollution would cause discrepant health effects, with various physical properties, chemical constituents and biological activities [49, 50]. Primarily, PM10 is generated by road traffic, while PM2.5 is from photochemical reactions or combustion. Since the differences of composition, source and deposition in body, PM10 and PM2.5 would induce different effects on health [51]. Toxicological studies found PM10 and PM2.5 both could contribute to pulmonary inflammation, cellular toxicity and oxidative stress, resulting in cardiovascular harm, morbidity and even mortality [52,53,54]. Some studies found that PM10 evoked a more extensive interstitial inflammation [55, 56], whereas others reported PM2.5 would link with higher cytotoxicity than PM10 [57]. Uniformly, different particle size fractions would impact coagulation, autonomic tone, inflammation parameters and systemic cytotoxicity in various ways.
Owing the differences of regions, population, and social factors and so on, there were various lag associations between air levels and exposure, which would differ according the change of lag time [58]. As Haley et al. [59] founded that different lag time would indicate different results, even contrary data, when applying the effect of PM2.5 on cardiovascular disease admissions. Buadong et al. [60] reported the lag 1 of PM10 would increase most visits of emergency cardiovascular disease. Xu et al. [61] also observed that hospitalizations of CHD showing strong relation with PM10 and PM2.5 under short-term exposure. As the result of this study, we found a 10 μg/m3 increase of PM10 would correspond to a 0.46% (95% CI: 0.08, 0.84%), and 0.57% (95% CI: 0.05, 1.09%) increment in CHD admissions at lag 7. Significant and positive associations for PM levels and HAs for CHD, which reached the peak at lag 7 day and produced the largest effect estimates, being considerable indications to prevent or remedy CHD [62].
The significant linear exposure-response relationship between PM2.5 and CHD hospital admissions was not found, which was, however, consistent with some previous studies [63, 64]. Also, at present there were reports found the increase CHD hospital admissions linking with short-time elevated levels of inhalable and/or fine particle matters pollution by various time-series and case-crossover studies [65, 66]. As aforementioned, PM2.5 concentrations were comparably lower when compared with other pollutants for these 9 cities in Sichuan Province, which might shrink the effect on health exposure.
The increased risks from PM pollutions were not equally distributed within the subgroups. Compared with people aged < 65 years, the old (age ≥ 65 years) were more susceptive to the effects of ambient air pollution. This was consistent with other studies [66, 67], owing to the old are more vulnerable to air pollution. The effect modification by gender of PM pollution on HAs for CHD were positive but not uniform on the statistical significance. Interestingly, results of gender-specific effects from prior studies were not conclusive [68], while reasons possibly were associated with difference in exposure and physiology. According to Exposure Factors Handbook of Chinese Population, the outdoor time of male and female is different, spending 258 min/day and 210 min/day for males and female, respectively [69]. While females are more susceptive to air pollution with frail physique, sensitive air responsiveness, easily deposition in lung and likely infaust socioeconomic status [70, 71]. Cold season was linking with higher effect of air pollution. As previous studies found higher association in cold season [66, 72]. Likely, owing to the association with low temperature and enhanced blood pressure and viscosity during cold season, this link would deduce heart attacks and strokes with growing winter morbidity and mortality [73]. Likewise, age, temperature and period of the year had shown a role as effect modifiers [74]. The potential factors of stratified variations of effects should be further discerned.
Primary prevention may effectively link with the type shifts of coronary heart events, from AMI to less worsening UA and from acute forms (AMI or UA) to CCHD [75]. In terms of these three specific CHD, CCHD associates with prior myocardial infarction, prior coronary revascularization or multi-vessel CHD without revascularization [76]. Previous studies have discerned the adverse effect of PM2.5 concentrations on CCHD [15, 27, 77, 78]. Xie et al. [78] reported that the significantly stronger effects of PM2.5 for CCHD compared with AMI at lag 3 days, which is accordant with this study. Ban et al. [15] found that significant effects of PM2.5 on HAs for CCHD, with an increase of 10 μg/m3 associated with 0.53% (95%CI: 0.39, 0.66%) on lag 0-1. The results indicated that the associated between PM pollution and CCHD could be acute and cumulative. Available evidence revealed that the regions with higher levels of PM2.5 displayed high rates of CCHD mortality. PM2.5, as fine aerosol particles, would penetrate to the gas-exchange regions of lung, then these ultrafine particles could pass through the lungs into blood circulation and harm other organs [79].
AMI, an event of myocardial necrosis caused by unstable ischemic syndrome, a severe subtype of CHD [80]. Abundant evidence supported the association of PM pollution with AMI. A systematic meta-analysis and review collected the effect of PM pollution on AMI [81]. For the patients with confirmed myocardial infarctions in Boston, the elevated levels of PM2.5 were linked with increased risks of AMI only within a few hour or a day after PM exposure [82]. The study conducted in Shanghai reported that 2.30% (95%CI: 1.41, 3.18%) increase in daily emergency visits for AMI involved with an 10 μg/m3 increase in PM10 [42]. A study in Tuscany (central Italy) found that a 1.30% (95%CI: 0.4, 4.1%) increase of HAs for AMI would link with a 10 μg/m3 increase in PM10 [16]. AMI is a major death cause and adult disability globally, affecting comparable quantity of older adults [83]. While available studies were limited, more related studies should be involved. Based on the data of this study, 63.1% of AMI patients were over 65 years, which would increase the substantial burden to the society with the elderly being growing.
Contrary to existing data, there was no adverse relationship found between PM pollution and HAs for UA. The study in Beijing estimated that significant association between PM2.5 and HAs for UA, involving an estimated risk increase of 0.66% (95%CI: 0.58, 0.73%) with each increment of 10 μg/m3 PM2.5 [15]. A study conducted in Taiyuan found that a μg/m3 increase in PM10 and PM2.5 would associate with 1.00% (95CI: 0.60-1.30%) and 1.50% (95%CI: 0.90-2.00%), respectively [84]. So far, there were limited investigations concerning the effects of PM pollution on UA, which was urgent for more studies to further discern the potential pattern. Owing to the different distinct study design and modeling strategies, the deviations of this study and these results could be derived. Besides, available studies mainly associated with short-term effects of PM pollution on HAs for UA, and long-term effects should also take into consideration. Further studies with large study range and population are needed to elucidate the heterogeneous results.
The burden of HAs for CHD attributable to PM exposure was estimated, which is central for cost-effective policy-making and CHD prevention. Both AN and AC are useful assessment for corresponding economic burden of exceeding PM exposures, providing more data to discern the potential association between air pollution and health [85, 86]. Wu et al. [86] adopted such method to assess the economic cost of HAs for mental disorders. A study of Wuhan calculated that attributable hospitalizations and economic costs to PM10 and PM2.5 were 249 and 340, and 4.82 and 6.57 million RMB from 2015 to 2017, respectively [14, 19, 62]. Yu et al. [87] demonstrated that 27.31% of CHD burden were attributable exposure to PM2.5 in Hubei. These studies presented positive association between burden and PM pollutions. Meanwhile, we found attributable number of HAs, total corresponding medical costs, and out-of-pocket cost were 1784 and 2847, 49.88 and 84.37 million CNY, and 16.42 and 26.27 million CNY, respectively, attributed to PM10 and PM2.5 from 2017 to 2018. More hospitalizations and economic lost could be avoided if recorded PM levels is at lower levels. The results of burden from PM pollution in this study were inconsistent with previous study, PM2.5 contributing more to economic burden [14]. The fraction of health and economic burden fell from 2017 to 2018, which elucidated the contribution of efficient measures for controlling PM pollutions by Chinese government since 2015 [88]. To date, this is the first study involving the estimation on burden of HAs for CHD due to PM exposure in Sichuan Province. The primary public health implications of our study might be the straightforward evidence of economic burden were quantified.
In addition, there were several characteristics of this study. First, the data on CHD hospitalizations were collected from the tertiary and secondary hospitals in 9 cities and involved 104,779 cases across the Sichuan Province. The large size of records enabled to elucidate the small adverse coronary effects with high precision, and link with regional variations within the province. Second, to our knowledge, this is the first investigation to confirm the effect of outdoor air pollution to HAs for CHD across the Sichuan Province associating with case-crossover design, which is a credible analysis to evaluate the short-term effects of air pollution on health [89]. Third, IDW interpolation method was used to constructed high spatial resolution for exposure estimation, to improve the spatial accessibility of pollutant levels and facilitate the confirmation of exposure effects. Besides, this study is also the first one to simultaneously estimate and compare the short-term associations of PM pollution on specific subtypes of CHD across Sichuan Province.
There were some limitations of this study. First, this study was associated with 9 urban districts of Sichuan Province, without involving other cities owing to the low data quality and unavailability to the data. Then, monitoring data from limited stations, which would be involved as the agent for personal exposure. This process was linking with ineluctable error of measurement, which was an inherent limitation of epidemiology studies relating to environment and disease [90]. We hope some sophisticated and precise methods, like satellite-based spatiotemporal models [17, 91] could be involved, to deduce various evidence. Third, this study might understate the real economic burden of CHD owing to PM pollutions, since the economic cost only involved the total medical expenses and out-of-pocket cost, without the outpatient expenses and indirect medical cost. Generally, all limitations of study should be mentioned, then further researched.
Conclusions
The results of this study estimated the associations between air levels and CHD hospitalizations in 9 cities Sichuan Province, China, involving the time-stratified case-crossover analysis. Based on the effects and burden of PM pollution on HAs for CHD and specific CHD, the event rate and burden of CHD would decrease with lower pollution levels, shedding light on the strategies of public health prevention. In summary, our study can supplement limited evidence of PM health effects in China. Further efforts are needed to understand the socioeconomic and demographic factors that might modify the effects of air pollution.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–128.
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528.
Liu J, Liu Y, Wang L, Yin P, Liu S, You J, et al. The disease burden of cardiovascular and circulatory diseases in China, 1990 and 2010. Zhonghua Yu Fang Yi Xue Za Zhi. 2015;49(4):315–20.
Disease NCfC. Annual report on cardiovascular health and diseases in China (2019). Beijing: Science Press; 2020.
Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;394(10204):1145–58.
Li G, Zeng Q, Pan X. Disease burden of ischaemic heart disease from short-term outdoor air pollution exposure in Tianjin, 2002–2006. Eur J Prev Cardiol. 2016;23(16):1774–82.
Wang L, Wu X, Du J, Cao W, Sun S. Global burden of ischemic heart disease attributable to ambient PM2. 5 pollution from 1990 to 2017. Chemosphere. 2021;263:128134.
Carugno M, Consonni D, Randi G, Catelan D, Grisotto L, Bertazzi PA, et al. Air pollution exposure, cause-specific deaths and hospitalizations in a highly polluted Italian region. Environ Res. 2016;147:415–24.
Liu S, Wang L, Zhou L, Li W, Pu X, Jiang J, et al. Differential effects of fine and coarse particulate matter on hospitalizations for ischemic heart disease: a population-based time-series analysis in southwestern China. Atmos Environ. 2020;224:117366.
Xie Y, Dai H, Dong H, Hanaoka T, Masui T. Economic impacts from PM2.5 pollution-related health effects in China: a provincial-level analysis. Environ Sci Technol. 2016;50(9):4836–43.
Song C, He J, Wu L, Jin T, Chen X, Li R, et al. Health burden attributable to ambient PM2. 5 in China. Environ Pollut. 2017;223:575–86.
Collaborators GRF. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet (London, England). 2016;388(10053):1659.
Xu A, Mu Z, Jiang B, Wang W, Yu H, Zhang L, et al. Acute effects of particulate air pollution on ischemic heart disease hospitalizations in Shanghai, China. Int J Environ Res Public Health. 2017;14(2):168.
Wang X, Yu Y, Yu C, Shi F, Zhang Y. Associations between acute exposure to ambient air pollution and length of stay for inpatients with ischemic heart disease: a multi-city analysis in central China. Environ Sci Pollut Res. 2020;27(35):43743–54.
Ban J, Ma R, Zhang Y, Li T. PM2.5-associated risk for cardiovascular hospital admission and related economic burdens in Beijing, China. Sci Total Environ. 2021;799:149445.
Nuvolone D, Balzi D, Chini M, Scala D, Giovannini F, Barchielli A. Short-term association between ambient air pollution and risk of hospitalization for acute myocardial infarction: results of the cardiovascular risk and air pollution in Tuscany (RISCAT) study. Am J Epidemiol. 2011;174(1):63–71.
Stafoggia M, Renzi M, Forastiere F, Ljungman P, Davoli M, de’Donato F, et al. Short-term effects of particulate matter on cardiovascular morbidity in Italy: a national analysis. Eur J Prev Cardiol. 2021. https://doi.org/10.1093/eurjpc/zwaa08.
Ma Z, Hu X, Sayer AM, Levy R, Zhang Q, Xue Y, et al. Satellite-based spatiotemporal trends in PM2. 5 concentrations: China, 2004–2013. Environ Health Perspect. 2016;124(2):184–92.
Wang X, Yu C, Zhang Y, Shi F, Meng R, Yu Y. Attributable risk and economic cost of cardiovascular hospital admissions due to ambient particulate matter in Wuhan, China. Int J Environ Res Public Health. 2020;17(15):5453.
Zhang X, Wang Y, Niu T, Zhang X, Gong S, Zhang Y, et al. Atmospheric aerosol compositions in China: spatial/temporal variability, chemical signature, regional haze distribution and comparisons with global aerosols. Atmos Chem Phys. 2012;12(2):779–99.
WHO. WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. Geneva: World Health Organization; 2021.
Ning G, Wang S, Ma M, Ni C, Shang Z, Wang J, et al. Characteristics of air pollution in different zones of Sichuan Basin, China. Sci Total Environ. 2018;612:975–84.
Emery C, Torreton E, Briere J-B, Evers T, Fagnani F. Economic burden of coronary artery disease or peripheral artery disease in patients at high risk of ischemic events in the French setting: a claims database analysis. J Med Econ. 2020;23(5):513–20.
Liu H, Tian Y, Xiang X, Sun K, Juan J, Song J, et al. Air pollution and hospitalization for acute myocardial infarction in China. Am J Cardiol. 2017;120(5):753–8.
Kerr AJ, Wang TKM, Jiang Y, Grey C, Wells S, Poppe KK. The importance of considering both primary and secondary diagnostic codes when using administrative health data to study acute coronary syndrome epidemiology (ANZACS-QI 47). Eur Heart J Qual Care Clin Outcomes. 2020.
Kuźma Ł, Pogorzelski S, Struniawski K, Dobrzycki S, Bachórzewska-Gajewska H. Effect of air pollution on the number of hospital admissions for acute coronary syndrome in elderly patients. Pol Arch Intern Med. 2019;130(1):38–46.
Hu Z. Spatial analysis of MODIS aerosol optical depth, PM 2.5, and chronic coronary heart disease. Int J Health Geogr. 2009;8(1):1–10.
Liu Y, Pan J, Zhang H, Shi C, Li G, Peng Z, et al. Short-term exposure to ambient air pollution and asthma mortality. Am J Respir Crit Care Med. 2019;200(1):24–32.
Luo L, Zhang Y, Jiang J, Luan H, Yu C, Nan P, et al. Short-term effects of ambient air pollution on hospitalization for respiratory disease in Taiyuan, China: a time-series analysis. Int J Environ Res Public Health. 2018;15(10):2160.
Tian Y, Liu H, Liang T, Xiang X, Li M, Juan J, et al. Fine particulate air pollution and adult hospital admissions in 200 Chinese cities: a time-series analysis. Int J Epidemiol. 2019;48(4):1142–51.
Tian Y, Liu H, Zhao Z, Xiang X, Li M, Juan J, et al. Association between ambient air pollution and daily hospital admissions for ischemic stroke: a nationwide time-series analysis. PLoS Med. 2018;15(10):e1002668.
Gu J, Shi Y, Zhu Y, Chen N, Wang H, Zhang Z, et al. Ambient air pollution and cause-specific risk of hospital admission in China: a nationwide time-series study. PLoS Med. 2020;17(8):e1003188.
Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133(2):144–53.
Janes H, Sheppard L, Lumley T. Case-crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology. 2005;16(6):717–26.
Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect. 2009;117(6):898–903.
Ho AFW, Zheng H, Earnest A, Cheong KH, Pek PP, Seok JY, et al. Time-stratified case crossover study of the association of outdoor ambient air pollution with the risk of acute myocardial infarction in the context of seasonal exposure to the southeast Asian haze problem. J Am Heart Assoc. 2019;8(6):e011272.
Gasparrini A, Leone M. Attributable risk from distributed lag models. BMC Med Res Methodol. 2014;14(1):1–8.
Zanobetti A, Schwartz J, Samoli E, Gryparis A, Touloumi G, Atkinson R, et al. The temporal pattern of mortality responses to air pollution: a multicity assessment of mortality displacement. Epidemiology. 2002;13(1):87–93.
Evans J, van Donkelaar A, Martin RV, Burnett R, Rainham DG, Birkett NJ, et al. Estimates of global mortality attributable to particulate air pollution using satellite imagery. Environ Res. 2013;120:33–42.
Panagiotakos DB, Pitsavos C, Chrysohoou C, Skoumas I, Stefanadis C. Prevalence and five-year incidence (2001-2006) of cardiovascular disease risk factors in a Greek sample: the ATTICA study. Hell J Cardiol. 2009;50(5):388–95.
Howard BV, Comuzzie A, Devereux RB, Ebbesson SO, Fabsitz RR, Howard WJ, et al. Cardiovascular disease prevalence and its relation to risk factors in Alaska Eskimos. Nutr Metab Cardiovasc Dis. 2010;20(5):350–8.
Xie J, He M, Zhu W: Acute effects of outdoor air pollution on emergency department visits due to five clinical subtypes of coronary heart diseases in Shanghai, China. 2014. (1349-9092 (Electronic)).
EPA US. Integrated Science Assessment (ISA) for particulate matter (final report, Dec 2009). Washington, DC: U.S. Environmental Protection Agency; 2009. EPA/600/R-08/139F
Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103(23):2810-5.
Peters A, Döring A, Wichmann H-E, Koenig W. Increased plasma viscosity during an air pollution episode: a link to mortality? Lancet. 1997;349(9065):1582–7.
Liao D, Creason J, Shy C, Williams R, Watts R, Zweidinger R. Daily variation of particulate air pollution and poor cardiac autonomic control in the elderly. Environ Health Perspect. 1999;107(7):521–5.
Gold D, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B. Ambient pollution and heart rate variability. Circulation. 2000;101(11):1267–73.
Peters A, Liu E, Verrier RL, Schwartz J, Gold DR, Mittleman M, et al. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11(1):11–7.
Faiz Y, Siddique N, He H, Sun C, Waheed S. Occurrence and profile of organophosphorus compounds in fine and coarse particulate matter from two urban areas of China and Pakistan. Environ Pollut. 2018;233:26–34.
Huang YC, Karoly ED, Dailey LA, Schmitt MT, Silbajoris R, Graff DW, et al. Comparison of gene expression profiles induced by coarse, fine, and ultrafine particulate matter. J Toxicol Environ Health A. 2011;74(5):296–312.
Lippmann M, Yeates DB, Albert RE. Deposition, retention, and clearance of inhaled particles. Br J Ind Med. 1980;37(4):337.
Tong H, Cheng W-Y, Samet JM, Gilmour MI, Devlin RB. Differential cardiopulmonary effects of size-fractionated ambient particulate matter in mice. Cardiovasc Toxicol. 2010;10(4):259–67.
Becker S, Soukup JM, Sioutas C, Cassee FR. Response of human alveolar macrophages to ultrafine, fine, and coarse urban air pollution particles. Exp Lung Res. 2003;29(1):29–44.
Schins RPF, Lightbody JH, Borm PJA, Shi T, Donaldson K, Stone V. Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol Appl Pharmacol. 2004;195(1):1–11.
Happo MS, Salonen RO, Hälinen AI, Jalava PI, Pennanen AS, Dormans JAMA, et al. Inflammation and tissue damage in mouse lung by single and repeated dosing of urban air coarse and fine particles collected from six European cities. Inhal Toxicol. 2010;22(5):402–16.
Gerlofs-Nijland ME, Rummelhard M, Boere AJF, Leseman DLAC, Duffin R, Schins RPF, et al. Particle induced toxicity in relation to transition metal and polycyclic aromatic hydrocarbon contents. Environ Sci Technol. 2009;43(13):4729–36.
Choi JH, Kim JS, Kim YC, Kim YS, Chung NH, Cho MH. Comparative study of PM2.5 - and PM10 - induced oxidative stress in rat lung epithelial cells. J Vet Sci. 2019;5(1):11–8.
Schwartz J. The distributed lag between air pollution and daily deaths. Epidemiology. 2000;11(3):320–6.
Haley VB, Talbot TO, Felton HD. Surveillance of the short-term impact of fine particle air pollution on cardiovascular disease hospitalizations in New York State. Environ Health. 2009;8(1):1–10.
Buadong D, Jinsart W, Funatagawa I, Karita K, Yano E. Association between PM10 and O3 levels and hospital visits for cardiovascular diseases in Bangkok, Thailand. J Epidemiol. 2009;19(4):182–8.
Xu M, Guo Y, Zhang Y, Westerdahl D, Mo Y, Liang F, et al. Spatiotemporal analysis of particulate air pollution and ischemic heart disease mortality in Beijing, China. Environ Health. 2014;13(1):1–12.
Wang Z, Peng J, Liu P, Duan Y, Huang S, Wen Y, et al. Association between short-term exposure to air pollution and ischemic stroke onset: a time-stratified case-crossover analysis using a distributed lag nonlinear model in Shenzhen, China. Environ Health. 2020;19(1):1–12.
Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356(5):447–58.
Pope Iii CA, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–41.
Dominici F, Burnett R. Risk models for particulate air pollution. J Toxicol Environ Health A. 2003;66(16-19):1883–90.
Ye X, Peng L, Kan H, Wang W, Geng F, Mu Z, et al. Acute effects of particulate air pollution on the incidence of coronary heart disease in Shanghai, China. PLoS One. 2016;11(3):11.
Han C, Lim YH, Lee KS, Hong YC. Acute effects of ambient temperature on hypotension hospital visits: a time-series analysis in seven metropolitan cities of Korea from 2011 to 2015. Environ Int. 2019;131:7.
Clougherty JE. A growing role for gender analysis in air pollution epidemiology. Environ Health Perspect. 2010;118(2):11.
China MoEPotPsRo. Exposure factors handbook of chinese population. Beijing: China Environmtal Science Press; 2013. p. 366.
Clougherty JE. A growing role for gender analysis in air pollution epidemiology. Environ Health Perspect. 2010;118(2):167–76.
Kan H, London SJ, Chen G, Zhang Y, Song G, Zhao N, et al. Season, sex, age, and education as modifiers of the effects of outdoor air pollution on daily mortality in Shanghai, China: the Public Health and Air Pollution in Asia (PAPA) study. Environ Health Perspect. 2008;116(9):1183–8.
Qiu H, Yu IT-S, Wang X, Tian L, Tse LA, Wong TW. Cool and dry weather enhances the effects of air pollution on emergency IHD hospital admissions. Int J Cardiol. 2013;168(1):500–5.
Li M, Huang X, Zhu L, Li J, Song Y, Cai X, et al. Analysis of the transport pathways and potential sources of PM10 in Shanghai based on three methods. Sci Total Environ. 2012;414:525–34.
Nawrot TS, Torfs R, Fierens F, De Henauw S, Hoet PH, Van Kersschaever G, et al. Stronger associations between daily mortality and fine particulate air pollution in summer than in winter: evidence from a heavily polluted region in western Europe. J Epidemiol Community Health. 2007;61(2):4.
Koopman C, Bots ML, van Dis I, Vaartjes I. Shifts in the age distribution and from acute to chronic coronary heart disease hospitalizations. Eur J Prev Cardiol. 2016;23(2):170–7.
Vedin O, Hagström E, Gallup D, Neely ML, Stewart R, Koenig W, et al. Periodontal disease in patients with chronic coronary heart disease: prevalence and association with cardiovascular risk factors. Eur J Prev Cardiol. 2015;22(6):771–8.
Hu Z, Rao KR. Particulate air pollution and chronic ischemic heart disease in the eastern United States: a county level ecological study using satellite aerosol data. Environ Health. 2009;8(1):26.
Xie W, Li G, Zhao D, Xie X, Wei Z, Wang W, et al. Relationship between fine particulate air pollution and ischaemic heart disease morbidity and mortality. Heart. 2015;101(4):257–63.
Nemmar A, Hoet PM, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts M, et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105(4):411–4.
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231–64.
Link MS, Dockery DW. Air pollution and the triggering of cardiac arrhythmias. Curr Opin Cardiol. 2010;25(1):16.
Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103(23):2810–5.
Abubakar I, Tillmann T, Banerjee A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385(9963):117–71.
Lu D, Jiang H, Zhang J, Qiao X, Shang J, He J, et al. Correlation between atmospheric particulate matter pollution and number of patients admitted to hospital due to unstable angina in Taiyuan, Shanxi. 疾病监测. 2019;34(10):944–7.
Lin H, Liu T, Xiao J, Zeng W, Li X, Guo L, et al. Quantifying short-term and long-term health benefits of attaining ambient fine particulate pollution standards in Guangzhou, China. Atmos Environ. 2016;137:38–44.
Wu Z, Chen X, Li G, Tian L, Wang Z, Xiong X, et al. Attributable risk and economic cost of hospital admissions for mental disorders due to PM2.5 in Beijing. Sci Total Environ. 2020;718:137274.
Yu W, Liu S, Jiang J, Chen G, Luo H, Fu Y, et al. Burden of ischemic heart disease and stroke attributable to exposure to atmospheric PM2.5 in Hubei province, China. Atmos Environ. 2020;221:117079.
Council CS: Chinese State Council (Ed.), Atmospheric pollution prevention and control action plan Beijing. 2013. http://english.mee.gov.cn/News_service/infocus/201309/t20130924_260707.shtml.
Jaakkola J. Case-crossover design in air pollution epidemiology. Eur Respir J. 2003;21(40 suppl):81s–5s.
Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108(5):419–26.
Fasola S, Maio S, Baldacci S, La Grutta S, Ferrante G, Forastiere F, et al. Short-term effects of air pollution on cardiovascular hospitalizations in the Pisan longitudinal study. Int J Environ Res Public Health. 2021;18(3):1164.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China through grants 72174032 and 82004251. The research projects of “Xinglin Scholars” Nursery Talent in 2021 (No. MPRC2021013) and Discipline Talent in 2020 (No. 030055071). Research Plan of Chengdu University of Traditional Chinese Medicine. Department of science and technology of Sichuan Province Key R & D projects through grants 2021YFS0260.
Author information
Authors and Affiliations
Contributions
Conceptualization: Lian Yang, Wanyanhan Jiang, Han Chen; Data curation: Wanyanhan Jiang, Jiaqiang Liao, Xi Yang, Biao Yang, Yuqin Zhang, Xiaoqi Pan, Lulu Lian; Formal analysis: Wanyanhan Jiang, Han Chen, Jiaqiang Liao, Xi Yang, Biao Yang, Yuqin Zhang; Investigation: Wanyanhan Jiang, Han Chen, Xiaoqi Pan, Lulu Lian; Methodology: Wanyanhan Jiang, Han Chen, Jiaqiang Liao; Project administration: Lian Yang; Resources: Lian Yang; Software: Han Chen, Wanyanhan Jiang; Supervision: Lian Yang, Wangyanhan Jiang, Han Chen; Validation: Lian Yang; Visualization: Wangyanhan Jiang, Han Chen; Roles/Writing - original draft: Wanyanhan Jiang, Han Chen; Writing - review & editing: Lian Yang. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, W., Chen, H., Liao, J. et al. The short-term effects and burden of particle air pollution on hospitalization for coronary heart disease: a time-stratified case-crossover study in Sichuan, China. Environ Health 21, 19 (2022). https://doi.org/10.1186/s12940-022-00832-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12940-022-00832-4