Abstract
Background
We aimed to probe the association of serum 25-hydroxyvitamin D [25(OH)D] concentrations with all-cause and cause-specific mortality among patients with gout and hyperuricemia (HUA).
Methods
The study included 1169 gout patients and 7029 HUA patients from the National Health and Nutrition Examination Survey (NHANES) 2007–2018 and 2001–2018, respectively. The association between serum 25(OH)D and mortality was evaluated by Cox proportional hazard and restricted cubic spline models.
Results
Among participants with gout and HUA, the weighted mean concentrations of serum 25(OH)D were 71.49 ± 30.09 nmol/L and 64.81 ± 26.92 nmol/L, respectively. Vitamin D deficiency occurred in 29.68% of gout patients and 37.83% of HUA patients. During 6783 person-years of follow-up among gout patients, 248 all-cause deaths occurred, among which 76 died from cardiovascular disease (CVD) and 49 died from cancer. 1375 HUA patients were recorded for all-cause mortality during 59,859 person-years of follow-up, including 427 CVD deaths and 232 cancer deaths. After multifactorial adjustment, per one-unit increment in natural log-transformed 25(OH)D was associated with lower risk of 55% all-cause mortality and 61% CVD mortality among gout patients, and a 45% reduced risk of cancer mortality among HUA patients. Restricted cubic splines showed a U-shaped relationship with all-cause and CVD mortality among HUA patients, with inflection points of 72.7 nmol/L and 38.0 nmol/L, respectively. The results were robust in subgroup and sensitivity analyses.
Conclusions
Serum 25(OH)D was negatively linearly correlated with mortality among gout patients, whereas U-shaped correlated with mortality in HUA patients. These results indicate that adequate vitamin D status could prevent premature death.
Similar content being viewed by others
Background
Gout is a typical inflammatory arthritis caused by deposits of urate crystals in joints, affecting approximately 5.1% of U.S. adults according to the latest data [1, 2]. Globally, the number of gout patients worldwide increased to 53 million in 2019 and is projected to increase to over 120 million cases in 2035, making it the most common inflammatory arthritis in adults in the western world [3]. Elevated serum uric acid (SUA) levels and gout have been identified as independent risk factors for diabetes, cardiovascular diseases, and chronic kidney diseases [4]. Though there are several major advances in gout treatment, including anti-inflammatory drugs, synthetic adrenocorticotropic hormone, oral colchicine [5], studies have shown that gout patients have a 17% increased risk of mortality than general population [6], thus determining the controllable causes is vitally important to reduce complications and mortality among patients with gout and HUA.
Nutritional intake and dietary pattern have been confirmed to be closely related to the risk of gout and HUA, which is also an important part of the lifestyle management among patients with gout and HUA [7]. For example, low vitamin D levels have been reported to be significantly associated with increased risk of gout and HUA [8, 9]. Vitamin D is one of the familiar fat-soluble vitamins, playing a significant role in cell differentiation and the immune system as well as the regulation of the growth and development of skeleton [10]. As the main circulating vitamin D metabolite, serum 25-hydroxyvitamin D [25(OH)D] is widely used to assess vitamin D condition [11]. Vitamin D deficiency is associated with increased risk of multiple health outcomes, including preeclampsia, cardiovascular disease (CVD), diabetes and so on [12]. Epidemiological studies have found that low serum 25(OH)D concentration can increase the risk of death among the general population, as well as patients with hypertension, diabetes, CVD and metabolic syndrome [13]. Although most prospective studies suggest that serum 25(OH)D level and mortality exist moderate to strong negative association [14], there is no article focus on the associations between 25(OH)D concentration and mortality in patients with gout and HUA, which need further research to sort these out.
In our research, we prospectively surveyed the association of serum 25(OH)D concentrations with all-cause and cause-specific mortality in adults with gout and HUA in United States (US), and further determined optimal serum 25(OH) D concentrations in patients with gout or HUA.
Methods
Data source and study population
The National Health and Nutrition Examination Survey (NHANES) was a program of nationally representative studies designed to assess the health and nutritional status of the non-institutionalized US population with the combination of interviews and physical examinations. Details of survey content and data collection methods have been described [15]. NHANES is a biannual cross-sectional study conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC), with all participants providing written informed consent [16]. The protocols of NHANES were approved by the Research Ethics Review Board of NCHS.
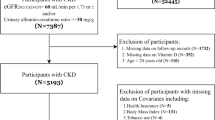
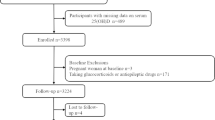
In this study, we obtained data from six NHANES cycles during 2007–2018 on gout patients and nine NHANES cycles during 2001–2018 on HUA patients. Patients identified with gout were obtained from self-reported personal interview data on a diversity of health conditions (n = 1656, aged over 20 years). After excluding 187 patients with missing serum 25(OH)D concentrations, 298 patients with cancer at baseline, and 2 patients with missing all-cause mortality data (Figs. 1), 1169 gout patients were ultimately involved in the final analysis. For HUA patients, HUA was defined as the SUA levels ≥ 420 umol/L in men and ≥ 360 umol/L in women [17]. Based on the participants with HUA aged over 20 years (n = 8167), we eliminated 128 patients with missing serum 25(OH)D concentrations, 997 patients with cancer at baseline, and 13 patients missing all-cause mortality data (Figs. 1), 7029 HUA patients were finally included.
Measurement of serum 25(OH)D concentrations
Serum 25(OH)D concentrations were measured by DiaSorin radioimmunoassay kit (Stillwater, MN, USA) in NHANES 2001–2006 and a standardized liquid chromatography-tandem mass spectrometry (LC-MS/MS) in NHANES 2007–2018. Serum 25(OH)D data from NHANES 2001–2006 has been converted using regression to equivalent 25(OH)D measurements in the standardized LC-MS/MS method [18]. According to the recommendations of CDC, we used the LC-MS/MS–equivalent data for all analyses.
Ascertainment of mortality
To determine mortality status in the follow-up population, we used the 2001–2018 NHANES public-use linked mortality file, which has linked data from NCHS with death certificate records from the National Death Index (NDI). We collected information on mortality status and follow-up time (in months) from the date of survey participation to the end of the follow-up period (December 31, 2019). Furthermore, specific causes of death were determined by the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10), including diseases of the heart (I00-I09, I11, I13, I20-I51), malignant neoplasms (C00-C97).
Assessment of covariates
We obtained the data on demographic information (age, gender, race/ethnicity, education level, family income), smoking status, alcohol consumption and disease status (hypertension, diabetes) through the standardized questionnaires from in-home interviews. Body weight and height were measured when people participated in the physical examinations at a mobile examination center. Body mass index (BMI) was calculated as body weight (kg) divided by the square of height (m2).
Specifically, race/ethnicity was categorized as Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, other Hispanic or other. Education levels were classified as less than high school, high school or equivalent, college or above. Family poverty income ratio (PIR) was defined as ratio of family income to poverty threshold, and was divided into three categories: 1, 1–3, and > 3. Normal weight (< 25 kg/m2), overweight (25 to < 30 kg/m2), and obesity (≥ 30 kg/m2) BMI categories were established. Smoking status was categorized as smoker and non-smoker. Alcohol intake was defined by the daily alcohol consumption in the past 12 months and classified by whether ≥ 4 drinks/day.
Statistical analyses
Given the complex survey designs adopted by NHANES, all estimates accounted for sample weights, clustering, and stratification [19]. Characteristics of the study population are presented as mean ± standard deviation (SD) for continuous variables and percentages (%) for discontinuous variables. Based on the Endocrine Society Clinical Practice guidelines [20], serum 25(OH)D levels were classified into four groups, i.e., severe vitamin D deficiency (< 25.00 nmol/L), vitamin D deficiency (25.00-49.99 nmol/L), vitamin D insufficiency (50.00-74.99 nmol/L), and vitamin D sufficiency (≥ 75.00 nmol/L). To calculate the differences in baseline characteristics across the four groups of vitamin D status, continuous variables were compared using analysis of variance (ANOVA) test and categorical variables were compared using the chi-square (χ2) test. The association between serum 25(OH)D concentrations and mortality were investigated by Cox regression models. Model 1 was unadjusted, and Model 2 adjusted for age, gender, race/ethnicity, and survey cycle. In Model 3, we further adjusted for education level, PIR, BMI, smoking status, alcohol consumption, hypertension, diabetes and uric acid. The linear trend was tested according to the statistical significance of the median value for categorical variables for serum 25(OH)D. In addition, serum 25(OH)D concentrations were also analyzed as continuous variable after natural log-transformed.
To explore the dose-response relationship between 25(OH)D concentrations and mortality, we established restricted cubic spline models fitted for Cox proportional hazards models, and the number of knots was determined based on the lowest value of the Akaike information criterion (AIC) [21]. If within two of each other for different knots, the lowest number of knots was uniformly chosen to balance best fit and overfitting, with 3 knots at the 10th, 50th, and 90th percentiles of serum 25(OH)D concentrations. If nonlinearity was detected, we conducted a recursion algorithm to calculate the inflection point of the association between 25(OH)D and mortality. Stratified analyses were performed by age (< 60 years old or ≥ 60 years old), gender (male, female), race/ethnicity (Whites or non-Whites), hypertension (yes or no), diabetes (yes or no) and BMI (< 30.00 kg/m2 or ≥ 30.00 kg/m2) in the fully adjusted model. Interaction on the multiplicative scale was assessed by conducting likelihood ratio tests.
Moreover, we conducted several sensitivity analyses to test the robustness of our results. First, participants who died within the first 2 years of follow-up were excluded to minimize the potential reverse causation bias. Second, repeated analyses were conducted based on quartiles of serum 25(OH)D. Third, given that some dietary factors might influence the association of interest [22], intake of total fat (in quartiles), sugars (in quartiles), carbohydrate (in quartiles), fiber (in quartiles) and vitamin C (in quartiles) were further adjusted. Forth, the observed relationships were likely to be influenced due to suggestive biological links [23], lipid profiles (the ratio of total cholesterol to HDL) were further adjusted. Fifth, as renal dysfunction could influence circulating vitamin D levels and cardiovascular events [22], kidney function assessed by estimated glomerular filtration rate (calculated by using the improved modification of diet in renal disease formula) was further adjusted.
All statistical analyses were conducted with R 4.2.3, and a two-tailed P < 0.05 was considered statistically significant.
Results
Baseline characteristics of study participants
Based on serum 25(OH)D status, the baseline characteristics of involved participants were revealed in the Table 1, respectively. There were 1169 gout patients [62.55 (13.07) years old; 30.20% female] and 7029 HUA patients [53.57 (17.79) years old; 43.80% female] enrolled in the study. The weighted mean concentrations of serum 25(OH)D were 71.49 ± 30.09 nmol/L for gout patients and 64.81 ± 26.92 nmol/L for HUA patients, respectively. 29.68% of gout participants had deficient vitamin D, and 62.53% had insufficient vitamin D. There were 37.83% HUA participants with deficient vitamin D, and 74.08% with insufficient vitamin D. Gout and HUA patients with higher 25(OH)D levels tended to be older, non-Hispanic white, less likely to be obese, had lower tobacco and alcohol consumption and higher household income.
Relationships of 25(OH)D concentration with mortality
During 6784 person-years of follow-up, 248 deaths among gout patients were documented, among which were 76 CVD deaths and 49 cancer deaths. Participants with HUA were followed up for a total of 59,859 person-years with 427 deaths from CVD and 232 deaths from cancer. After multivariate adjustments, including age, sex, race/ethnicity, survey cycle, education level, PIR, BMI, smoking status, alcohol consumption, hypertension, diabetes and uric acid, the multivariate-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) from lowest to highest serum 25(OH)D categories (< 25.00, 25.00-49.99, 50.00-74.99, and ≥ 75.00 nmol/L) were 1.00 (reference), 0.67 (0.42, 1.09), 0.46 (0.30, 0.72), and 0.30 (0.19, 0.45), respectively, for all-cause mortality (P trend < 0.001); 1.00 (reference), 0.58 (0.19, 1.73), 0.32 (0.12, 0.81), and 0.21 (0.08, 0.57), respectively, for CVD mortality (P trend = 0.003); and 1.00 (reference), 0.85 (0.20, 3.66), 0.47 (0.09, 2.34), and 0.60 (0.21, 1.77), respectively, for cancer mortality (P trend = 0.550) (Table 2).
Using multivariate adjustments aforementioned, we observed parallel results in participants with HUA (Table 3). Compared with the reference, the risk of mortality among the other three comparison groups decreased to 0.71 (0.53, 0.94), 0.50 (0.37, 0.67), and 0.43 (0.30, 0.60), respectively, for all-cause mortality (P trend < 0.001); 0.63 (0.41, 0.98), 0.45 (0.28, 0.72), and 0.49 (0.30, 0.79), respectively, for CVD mortality (P trend = 0.014); and 0.78 (0.40, 1.52), 0.63 (0.32, 1.22), and 0.51 (0.27, 0.96), respectively, for cancer mortality (P trend = 0.020).
The detection of dose–response relationships
As demonstrated in Figs. 2 and 3, with full adjustment for confounders, serum 25(OH)D concentration was linearly and negatively associated with all-cause and CVD mortality in gout patients, and with cancer mortality in the HUA patients (both P for linearity < 0.05). Per one-unit increment in natural log-transformed 25(OH)D levels was associated with 55% and 61% reduced risk of all-cause and CVD mortality among gout patients, respectively, and associated with 45% decreased risk of cancer mortality among HUA patients, respectively (Tables 2 and 3). Differently, we found U-shaped associations between serum 25(OH)D concentrations and all-cause and CVD mortality in participants with HUA, with inflection points of 72.7 nmol/L and 38.0 nmol/L for 25(OH)D, respectively.
Stratified analyses and sensitivity analyses
Consistent results were observed across a wide range of subgroups stratified by age, gender, race/ethnicity, hypertension, diabetes and BMI (Supplementary Tables 1–3, 7–9). When the analysis was stratified by gender, we observed a significant interaction between serum 25(OH)D levels and gender for all-cause and cancer mortality in participants with gout (P interaction < 0.05, Supplementary Tables 1 and 3). For participants with HUA, there were pronounced interactions between serum 25(OH)D concentrations and age for all-cause and CVD mortality (P interaction < 0.05, Supplementary Tables 7, 8). No significant interactions were detected between serum 25(OH)D levels and other stratifying variables (all P for interaction > 0.05, Supplementary Tables 1–3, Supplementary Tables 7–9).
In the sensitivity analyses, the protective effect of serum 25(OH)D on mortality remained steady after excluding deaths within the first 2 years of follow-up (Supplementary Table 4, Supplementary Table 10). Consistent results were found in the sensitivity analyses based on quartiles of serum 25(OH)D (Supplementary Table 5, Supplementary Table 11). Similar results were observed when we further adjusted for intake of total fat, sugars, carbohydrate, fiber and vitamin C; or lipid profiles; or estimated glomerular filtration rate (Supplementary Table 6, Supplementary Table 12). Regarding CVD mortality in gout patients, high 25(OH)D concentration still exhibited a directionally protective impact with wide confidence intervals, although these results did not reach statistical significance, which could be largely influenced due to reduced power.
Discussion
As far as we know, this prospective cohort study with a relatively large sample size was the first to examine the association between serum 25(OH)D concentrations and mortality among participants with gout and HUA. We observed negative linear associations between serum 25(OH)D and all-cause and CVD mortality among gout patients, and cancer mortality in HUA patients. Serum 25(OH)D was U-shaped in relation to all-cause and CVD mortality among HUA patients, with inflection points of 72.7 nmol/L and 38.0 nmol/L, respectively. Furthermore, various sensitivity analyses and stratified analyses demonstrated the robustness of these findings.
In line with previous studies concentrating on the general population and chronic disease patients [22, 24, 25], we observed that low 25(OH)D levels were associated with increased all-cause mortality. Additionally, different from the linear associations among gout patients observed in restricted cubic spline regression, we found that U-shaped relationships between serum 25(OH)D and all-cause and CVD mortality among HUA patients, which have also been reported in studies of low muscle mass, gestational diabetes mellitus and fracture risk [26,27,28]. Furthermore, we found that from the U-shaped curve, we identified the inflection point for serum 25(OH)D was 72.7 nmol/L for all-cause and 38.0 nmol/L for CVD mortality. Nevertheless, there is still a debate on the threshold for optimal 25(OH)D concentrations. The Endocrine Society has proposed that the optimal serum concentration of 25(OH)D in general adults should be at least 75.00 nmol/L for better health [20]. A meta-analysis of 62,548 people in the general population showed that individuals with serum concentrations of 25(OH)D between 75 and 87.5 nmol/L had the lowest risk of death [29]. However, in a large cohort study from the UK Biobank, the 25(OH)D concentration associated with the lowest risk of all-cause mortality was 60 nmol/L among the general population [30]. In addition, it has also been proposed that the serum 25(OH)D threshold for all-cause and CVD mortality in osteoarthritis patients of US was 27.70 nmol/L and 54.40 nmol/L, respectively [31]. The reasons for the above controversy may be due to differences in the target population, sample size, and underlying health status. Given that HUA is a chronic metabolic disease caused by purine metabolism disorders, the management of serum 25(OH)D should receive more attention. Therefore, our findings can help us to accurately identify the mortality risk of patients with gout and HUA in clinical practice, and is conducive to the development of personalized treatment plans. and further confirmation was still needed in large clinical trials.
However, the relationship between serum vitamin D and cancer mortality varied in our study, in which high vitamin D levels had a protective effect in reducing cancer mortality among HUA patients, but such inverse association did not reach statistical significance in gout patients, which may due to the reduced power caused by insufficient sample. Our observation of the inverse association is consistent with previous meta-analyses of observational studies on general populations, which drew a conclusion that people with lower baseline serum 25(OH)D levels were more likely to die from cancer by summarizing 12 prospective cohorts [32]. Similarly, meta-analysis of randomized controlled trials (RCTs) also found that vitamin D supplementation resulted in a decrease in cancer mortality [33, 34]. By making rough comparisons, we found that the HRs reported in our analysis were smaller than those among general population or other patients, which indicated that HUA patients might benefit more from higher vitamin D levels, thus highlighting the significance of sufficient vitamin D intake is crucial in HUA patients. For restricted cubic spline regression, although our study showed a linear negative relationship between serum vitamin D and cancer mortality in participants with HUA, a few studies have explored the threshold for 25(OH)D in relation to cancer mortality. For example, a German population-based cohort study reported optimal 25(OH)D concentrations for cancer mortality at around 75 nmol/L [35]. A subsequent study involved 365,530 participants from UK Biobank revealed a decrease in cancer mortality risk appearing to level off at 45 nmol/L [30]. The inconsistency might be partly explained by the differences in the target participants, number of cancer deaths and the association magnitude for different cancers with 25(OH)D.
Despite the associations in several subgroups did not reach statistical significance due to limited sample size, particularly among gout patients, in general, the results of stratified analyses were in line with that of our main analysis. Taking into account racial differences in 25(OH)D levels, we performed a stratified analysis by race/ethnicity (white or non-white), showing that non-white patients with gout and HUA benefitted more from high 25(OH)D concentrations for all-cause mortality. Compared with white people, non-white people have darker skin pigment, less ability to synthesize vitamin D using limited ultraviolet radiation B (UVB), and have lower serum 25(OH)D levels [36]. Studies have shown that blacks are more likely to reduce the risk of infection and further reduce mortality than whites after vitamin D supplementation [37]. Additionally, serum 25(OH)D concentrations and age had pronounced interactions on all-cause mortality in HUA patients, which is consistent with previous studies among other disease-specific patients based on NHAENS [38]. Higher serum 25(OH)D levels had a more evident protective effect on HUA patients aged < 60 years than those aged ≥ 60 years. One possible reason for this difference may be that the elder tend to have more chronic comorbidities than the younger, leading to impaired liver and kidney function and affecting the absorption and conversion of vitamin D [39]. Thus, older people need more vitamin D to maintain health and the sensitivity to changes in vitamin D concentrations is relatively poor [40]. For the observed interaction between vitamin D and gender on mortality among gout patients, further studies are warranted to confirm this finding given the limited sample size. Notably, previous synthesized evidence suggested that gout increases the risk of all-cause mortality, particularly among blacks, men, the elderly, and that gout patients have high risk of comorbidities of diabetes and hypertension [41, 42]. Admittedly, the relationship between vitamin D and gout mortality may be driven by patient characteristics and comorbidities given that gout mortality is multifactorial. Therefore, future studies are warranted to validate our findings across a broader spectrum of risk factors related to mortality among gout patients.
The mechanisms underlying the observed association between vitamin D and mortality among gout or HUA patients remain to be elucidated. Accumulating evidence showed that vitamin D deficiency can promote insulin resistance [43], which is inversely associated with the renal clearance of SUA and lead to HUA in turn [44]. Low vitamin D level can cause secondary hyperparathyroidism, which can lead to an elevated level of parathyroid hormone (PTH) [45]. Subsequently, increased PTH concentration can influence the absorption, secretion and transport of uric acid and cause HUA [46, 47]. In a meta-analysis of seven cross-sectional studies, both individuals with vitamin D deficiency and insufficiency have shown significantly higher level of serum uric acid compared with vitamin D-sufficient individuals [8]. Monosodium urate is the prerequisite for uric acid crystal formation [48], the occult deposition of which may induce inflammation, mechanical damage of the joint, and even systemic consequences, considered as a critical risk factor for gout [49]. In addition, previous studies have showed that lower serum 25(OH)D level was more common in patients with gout and may be involved to the development or deterioration of the disease [50].
There are some limitations to this study. First, due to incomplete data, we cannot rule out other confoundings, such as genetic constitution, metabolic syndrome, the use of medications such as diureticum, ciclosporin and so on [1]. Second, we screened out gout patients only based on a simple self-reported question, “Doctors ever told you had gout?“, without further available participants’ medical records, and the SUA level was measured only once, which may induce diagnostic ascertainment bias. In addition, given the small number of cancer or CVD deaths, the statistical power of our study to detect the association between serum vitamin D and cancer-specific mortality was limited. Finally, the patients included in the study were all residents of US, and the conclusions may not be applicable to other populations with different socioeconomic characteristics.
Conclusion
Serum 25(OH)D concentrations was linearly correlated with decreased risk of mortality among gout patients, and U-shaped correlated with mortality in HUA patients plateaued at 72.7 nmol/L for all-cause mortality. These results provide related clues for the health management and highlight the potential advantages of monitoring vitamin D concentrations to reduce mortality risk in adult patients with gout and HUA.
Data availability
The datasets used and/or analyzed during the current study are publicly available and accessible.
Abbreviations
- HUA:
-
Hyperuricemia
- NHANES:
-
National Health and Nutrition Examination Survey
- SUA:
-
serum uric acid
- 25(OH)D:
-
25-hydroxyvitamin D
- CVD:
-
cardiovascular disease
- US:
-
United States
- NCHS:
-
National Center for Health Statistics
- CDC:
-
Centers for Disease Control and Prevention
- LC-MS:
-
liquid chromatography-tandem mass spectrometry
- BMI:
-
Body mass index
- PIR:
-
Poverty income ratio
- SD:
-
standard deviation
- PTH:
-
parathyroid hormone
References
Dalbeth N, Gosling AL, Gaffo A, Abhishek A. Gout. Lancet. 2021;397(10287):1843–55.
Yokose C, McCormick N, Lu N, Tanikella S, Lin K, Joshi AD, Raffield LM, Warner E, Merriman T, Hsu J, et al. Trends in prevalence of gout among US Asian adults, 2011–2018. JAMA Netw Open. 2023;6(4):e239501.
He Q, Mok TN, Sin TH, Yin J, Li S, Yin Y, Ming WK, Feng B. Global, Regional, and national prevalence of gout from 1990 to 2019: age-period-cohort analysis with Future Burden Prediction. JMIR Public Health Surveill. 2023;9:e45943.
Bardin T, Richette P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med. 2017;15(1):123.
Terkeltaub R. Gout. Novel therapies for treatment of gout and hyperuricemia. Arthritis Res Ther. 2009;11(4):236.
Vargas-Santos AB, Neogi T, da, Castelar-Pinheiro R, Kapetanovic G, Turkiewicz MC. A: Cause-Specific Mortality in Gout: Novel Findings of Elevated Risk of Non-Cardiovascular-Related Deaths. Arthritis Rheumatol 2019, 71(11):1935–1942.
Yokose C, McCormick N, Choi HK. The role of diet in hyperuricemia and gout. Curr Opin Rheumatol. 2021;33(2):135–44.
Charoenngam N, Ponvilawan B, Ungprasert P. Vitamin D insufficiency and deficiency are associated with a higher level of serum uric acid: a systematic review and meta-analysis. Mod Rheumatol. 2020;30(2):385–90.
Zhang YY, Qiu HB, Tian JW. Association between Vitamin D and hyperuricemia among adults in the United States. Front Nutr. 2020;7:592777.
Wang R, Wang W, Hu P, Zhang R, Dong X, Zhang D. Association of Dietary Vitamin D Intake, Serum 25(OH)D(3), 25(OH)D(2) with Cognitive Performance in the Elderly. Nutrients 2021, 13(9).
Pludowski P, Takacs I, Boyanov M, Belaya Z, Diaconu CC, Mokhort T, Zherdova N, Rasa I, Payer J, Pilz S. Clinical practice in the Prevention, diagnosis and treatment of vitamin D Deficiency: a Central and Eastern European Expert Consensus Statement. Nutrients 2022, 14(7).
Liu D, Meng X, Tian Q, Cao W, Fan X, Wu L, Song M, Meng Q, Wang W, Wang Y. Vitamin D and multiple Health outcomes: an Umbrella Review of Observational studies, randomized controlled trials, and mendelian randomization studies. Adv Nutr. 2022;13(4):1044–62.
Cao M, He C, Gong M, Wu S, He J. The effects of vitamin D on all-cause mortality in different diseases: an evidence-map and umbrella review of 116 randomized controlled trials. Front Nutr. 2023;10:1132528.
Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2(1):76–89.
About the National Health. and Nutrition Examination Survey [www.cdc.gov/nchs/nhanes/about_nhanes.htm]
Centers for Disease Control and Prevention NCHS research ethics review board (ERB). approval [https://www.cdc.gov/nchs/nhanes/irba98.htm. ].
Han Y, Cao Y, Han X, Di H, Yin Y, Wu J, Zhang Y, Zeng X. Hyperuricemia and gout increased the risk of long-term mortality in patients with heart failure: insights from the National Health and Nutrition Examination Survey. J Transl Med. 2023;21(1):463.
Analytical Note for 25-Hydroxyvitamin D Data Analysis Using NHANES III. (1988–1994), NHANES 2001–2006, and NHANES 2007–2010(October 2015) [https://wwwn.cdc.gov/nchs/nhanes/vitamind/analyticalnote.aspx]
Inoue K, Ritz B, Brent GA, Ebrahimi R, Rhee CM, Leung AM. Association of Subclinical Hypothyroidism and Cardiovascular Disease with Mortality. JAMA Netw Open. 2020;3(2):e1920745.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30.
Cao Y, Li P, Zhang Y, Qiu M, Li J, Ma S, Yan Y, Li Y, Han Y. Association of systemic immune inflammatory index with all-cause and cause-specific mortality in hypertensive individuals: results from NHANES. Front Immunol. 2023;14:1087345.
Wan Z, Guo J, Pan A, Chen C, Liu L, Liu G. Association of serum 25-Hydroxyvitamin D concentrations with all-cause and cause-specific mortality among individuals with diabetes. Diabetes Care. 2021;44(2):350–7.
Wang J, Fan J, Yang Y, Moazzen S, Chen D, Sun L, He F, Li Y. Vitamin D Status and Risk of All-Cause and Cause-Specific Mortality in Osteoarthritis Patients: Results from NHANES III and NHANES 2001–2018. Nutrients 2022, 14(21).
Nanri A, Mizoue T, Goto A, Noda M, Sawada N, Tsugane S. Vitamin D intake and all-cause and cause-specific mortality in Japanese men and women: the Japan Public Health Center-based prospective study. Eur J Epidemiol. 2023;38(3):291–300.
Cai B, Zhou M, Xiao Q, Zou H, Zhu X. L-shaped association between serum 25-hydroxyvitamin D and all-cause mortality of individuals with rheumatoid arthritis. Rheumatology (Oxford). 2023;62(2):575–82.
Zhang G, Wang X, Tong M, Chen J, Ji Q. U-Shaped Association of Standardized Serum 25-Hydroxyvitamin D with risk of low muscle Mass: a Population-based cross-sectional study. J Multidiscip Healthc. 2023;16:2167–77.
Milajerdi A, Abbasi F, Mousavi SM, Esmaillzadeh A. Maternal vitamin D status and risk of gestational diabetes mellitus: a systematic review and meta-analysis of prospective cohort studies. Clin Nutr. 2021;40(5):2576–86.
Bleicher K, Cumming RG, Naganathan V, Blyth FM, Le Couteur DG, Handelsman DJ, Waite LM, Seibel MJ. U-shaped association between serum 25-hydroxyvitamin D and fracture risk in older men: results from the prospective population-based CHAMP study. J Bone Min Res. 2014;29(9):2024–31.
Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95(1):91–100.
Fan X, Wang J, Song M, Giovannucci EL, Ma H, Jin G, Hu Z, Shen H, Hang D. Vitamin D status and risk of all-cause and cause-specific mortality in a large cohort: results from the UK Biobank. J Clin Endocrinol Metab 2020, 105(10).
Xiao Q, Cai B, Yin A, Huo H, Lan K, Zhou G, Shen L, He B. L-shaped association of serum 25-hydroxyvitamin D concentrations with cardiovascular and all-cause mortality in individuals with osteoarthritis: results from the NHANES database prospective cohort study. BMC Med. 2022;20(1):308.
Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, Khan H, Baena CP, Prabhakaran D, Hoshen MB, et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348:g1903.
Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev 2014(1):Cd007470.
Zhang Y, Fang F, Tang J, Jia L, Feng Y, Xu P, Faramand A. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673.
Schöttker B, Haug U, Schomburg L, Köhrle J, Perna L, Müller H, Holleczek B, Brenner H. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am J Clin Nutr. 2013;97(4):782–93.
Hagenau T, Vest R, Gissel TN, Poulsen CS, Erlandsen M, Mosekilde L, Vestergaard P. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis. Osteoporos Int. 2009;20(1):133–40.
Gibbons JB, Norton EC, McCullough JS, Meltzer DO, Lavigne J, Fiedler VC, Gibbons RD. Association between vitamin D supplementation and COVID-19 infection and mortality. Sci Rep. 2022;12(1):19397.
Zhang JJ, Yu HC, Li Y, Zhang YB, Geng TT, Lu Q, Liao YF, Guo KQ, Du L, Ruan HL, et al. Association between serum 25-hydroxy vitamin D concentrations and mortality among individuals with metabolic dysfunction-associated fatty liver disease: a prospective cohort study. Am J Clin Nutr. 2022;116(5):1409–17.
Wang H, Chen W, Li D, Yin X, Zhang X, Olsen N, Zheng SG. Vitamin D and chronic diseases. Aging Dis. 2017;8(3):346–53.
Whiting SJ, Calvo MS. Correcting poor vitamin D status: do older adults need higher repletion doses of vitamin D3 than younger adults? Mol Nutr Food Res. 2010;54(8):1077–84.
Wang X, Li X, Wang H, Chen M, Wen C, Huang L, Zhou M. All-cause and specific mortality in patients with gout: a systematic review and meta-analysis. Semin Arthritis Rheum. 2023;63:152273.
Lottmann K, Chen X, Schadlich PK. Association between gout and all-cause as well as cardiovascular mortality: a systematic review. Curr Rheumatol Rep. 2012;14(2):195–203.
Szymczak-Pajor I, Śliwińska A. Analysis of Association between Vitamin D Deficiency and insulin resistance. Nutrients 2019, 11(4).
Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol. 2013;25(2):210–6.
Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22(4):477–501.
Hui JY, Choi JW, Mount DB, Zhu Y, Zhang Y, Choi HK. The independent association between parathyroid hormone levels and hyperuricemia: a national population study. Arthritis Res Ther. 2012;14(2):R56.
Chin KY, Nirwana SI, Ngah WZ. Significant association between parathyroid hormone and uric acid level in men. Clin Interv Aging. 2015;10:1377–80.
Narang RK, Dalbeth N. Pathophysiology of gout. Semin Nephrol. 2020;40(6):550–63.
Han Y, Zhang Y, Zeng X. Assessment of causal associations between uric acid and 25-hydroxyvitamin D levels. Front Endocrinol (Lausanne). 2022;13:1024675.
Liu SG, Li YY, Sun RX, Wang JL, Li XD, Han L, Chu N, Li CG. Polymorphisms in the vitamin D receptor and risk of gout in Chinese Han male population. Rheumatol Int. 2015;35(6):963–71.
Acknowledgements
The authors sincerely thank the researchers and participants of the original articles for their collection and management of data resources.
Funding
This work was supported by grants from National Natural Science Foundation of China (82204843), Zhejiang Province Traditional Chinese Medicine Science and Technology Plan Project (2023ZR084), Science and Technology Project of Zhejiang Provincial Health Commission (2023KY845), Natural Science Youth Exploration Program of Zhejiang Chinese Medicine University (2023JKZKTS14) and Department of Science and Technology of the State Administration of Traditional Chinese Medicine-Zhejiang Province Joint Construction Project (GZY-ZJ-KJ-24012).
Author information
Authors and Affiliations
Contributions
Ke Liu: Conceptualization, Methodology, Formal analysis, Writing-original draft, Visualization. Xuanni Lu: Formal analysis, Data curation, Writing-original draft. Anqi Wang: Formal analysis, Data curation, Writing-original draft. Wei-Wei Chen: Conceptualization. Jiayu Li, Supervision, Project administration. Xiao-Hui Sun: Supervision, Project administration. Lin Huang, Supervision, Project administration, Funding acquisition. Zhixing He, Project administration. Chengping Wen, Project administration. Ying-Ying Mao: Supervision, Project administration, Funding acquisition. Ding Ye: Conceptualization, Writing-review & editing, Methodology, Supervision, Project administration, Funding acquisition. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The NHANES was approved by the National Center for Health Statistics Research Ethics Review Board.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, K., Lu, X., Wang, A. et al. Association of serum 25-hydroxyvitamin D concentrations with all-cause and cause-specific mortality among individuals with gout and hyperuricemia. Nutr J 23, 89 (2024). https://doi.org/10.1186/s12937-024-00992-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-024-00992-8