Abstract
Background
Malaria, a treatable disease mainly caused by Plasmodium falciparum has remained a health challenge in Africa, a continent that accounted for 96% of total global cases and deaths in 2021. Uganda, a malaria endemic country is experiencing malaria parasite resistance to some of the drugs used in the artemisinin-based combination therapy (ACT). In an effort to prioritize herbal medicines for new product development, this review synthesized the available safety and efficacy literature on the Ugandan anti-malarial plants to suggest most effective herbal plants.
Methods
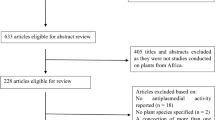
Literature was exhaustively searched using engines and databases, such as Google scholar, Pubmed, and Scopus-indexed journals during the period of June 2020–December 2021. In the first phase, information on ethnobotanical uses of anti-malarial plants in Uganda was gathered and synthetized to generate a list of plants, followed by data on anti-malarial efficacy (both in vitro and in vivo) on each listed plant. Minimum inhibitory concentrations (µg/ml), and % parasite suppression for every plant were scored using The Research Initiative on Traditional and Antimalarial Methods (RITAM) scoring system. The best twenty (20) plants were evaluated for acute safety (LD50) data in rat model, plant parts used, ease of cultivation, presence of clinical studies and other relevant factors for suggesting the best three (3) plants for future anti-malarial product development.
Results
Over one hundred twenty-six (126) plant species are used in Uganda for treatment of malaria in local communities. Out of these, about 33% (41) have been studied for efficacy and safety, with Artemisia annua and Vernonia amygdalina being the most extensively studied and among the best twenty (20) anti-malarial plants in Uganda. Both are limited by parasite recrudescence in clinical studies. Microglossa pyrifolia, a very potent plant (IC50 = 0.03 – 0.05 µg/ml has potential to penetrate the liver and could ameliorate the challenge of recrudescence if combined with A. annua and V. amygdalina in a polyherbal formulation.
Conclusion
There are many plants with promising potential for malaria treatment in Uganda and a herbal combination of A. annua, V. amydalina and M. pyrifolia could offer the next herbal ACT if carefully studied and developed.
Similar content being viewed by others
Background
Malaria is a treatable disease but life threatening, causing acute febrile illness [1]. The endemic has continued to cripple the global population with children under five years and the African continent carrying the highest disease burden over the years. The World Health Organization (WHO) reported increasing malaria cases from 2019 to 2021 (227, 241, and 247 million cases in 2019, 2020 and 2021, respectively) in eighty-five (85) endemic countries [2,3,4]. The WHO African region accounted for 94% of the cases in 2019, 95% in both 2020 and 2021, and four African countries (Nigeria—31.3%, Democratic Republic of Congo—12.6%, United Republic of Tanzania—4.1%, and Niger—3.9%) were responsible for half of the total global cases in 2021 [2]. Even though the rise in malaria infections has been attributed to disruptions in the health care system in 2019 by Covid-19 pandemic (by 6% in 2020) [5], the African region has remained off-track from Global Technical Strategy (GTS) 2020 targets for reduction of malaria cases by 45%.
Uganda is among the six (6) African countries that accounted for 55% of the global malaria cases, a disease now termed essentially as an African problem since the continent accounts for over 95% of total global cases and 96% of all deaths [6] According to the Uganda Ministry of Health malaria report 2017–2018, Uganda remains one of the ten (10) countries in sub-Saharan Africa, accounting for 70% of the global malaria cases and deaths [7]. The country also ranked 5th in terms of global malaria morbidity and 9th for malaria mortality in 2018 according to World Health Organization [8]. Uganda also accounted for 5% and 5.1% of the global malaria cases in 2020 and 2021 respectively [5]. In addition, a malaria incidence rate of 42.4% was also reported in 2020 in Apac district (Uganda) from children between the ages of 1 to 4 years [9]. Based on these statistics, malaria is still a serious health challenge in Uganda and other African countries, and requires more efforts to realize GTS target of reducing its global incidence and mortality rates by at least 90% by 2030.
Artemisinin-based combination therapy (ACT) remains the gold standard of malaria treatment in Uganda. ACT was adopted as first-line treatment for malaria in Uganda in 2004 and this policy change has proven to be very vital in the reduction of malaria death tolls in the country [10, 11]. Currently, reports of resistance of the malaria parasite Plasmodium falciparum against Artemisinin-based combinations have surfaced in East Africa, mainly Uganda and Rwanda [12]. There was a decreased susceptibility to artemether–lumefantrine in Northern and Eastern Uganda associated with multiple polymorphisms, notably Pfkelch13 469Y mutations against lumefantrine [12,13,14]. Though these mutations are associated with delayed parasite clearance by ACT, the researchers did not find any association between Pfkelch13 nonsynonymous mutations and delayed parasite clearance, with exception of one patient. In addition to the mutation against artemisinin, deletions in pfhrp2 and pfhrp3 (pfhrp2/3) genes that render the parasite undetectable by RDT have also been reported [3]. Another R622I mutation occurred independently in Africa, having been found in Eritrea, Ethiopia, Somalia and Sudan, and with increasing frequency in the Horn of Africa (Somali Peninsula). These findings are reaffirming the continued evolution in the phenotypes and genotypes of the Plasmodium parasite in Africa, thus raising alarm of increase such cases of resistance in near future. Therefore, there is the need for more research into the development of new therapeutics and other innovative approaches as alternatives to combat this African health problem.
In Uganda, the use of herbal medicines among local communities (60–80%) for management of malaria and other disease conditions continues to offer reliable alternatives for new product development [15]. These ethnobotanical uses have also been supported by several preclinical (in vitro and in vivo animal studies) and clinical efficacy studies. Safety data on most of these plants have also been generated over the years. With the complexity of phytochemical compounds in a single crude extract, plant-based therapies have minimal susceptibility to microbial resistance always associated with synthetic drugs, including ACT, which are based on individual chemical compounds. In addition, positive synergistic and/or clinically beneficial interactions among different chemicals in one extract have also been reported [16]. An example of such is increased absorption of artemisinin by other components of Artemisinia annua tea [17]. Therefore, selection of the most efficacious anti-malarial plant phyto-extracts for dosage form standardization using latest and advanced formulation principles/technologies is key for addressing the treatment challenges presented by this endemic disease.
Previous reviews on anti-malarial plants in Uganda [18], mainly focused on ethnobotanical surveys which usually capture frequency of use and a summary of existing literature on the phyto-compounds and efficacy, without objective analysis of these information to select the most efficacious. The Research Initiative on Traditional and Antimalarial Methods (RITAM) founded in 1999 designed a standard score criteria that is useful for analysing literature on anti-malarial plants [19]. Based on the RITAM score system, each plant (or herbal remedy) is given a numerical value (score) based on frequency of ethnobotanical citations, laboratory efficacy in vitro, in vivo, and safety. This method is objective and can rigorously guide in selection of the most efficacious but safe plants for further anti-malarial product development.
Therefore, this review synthesized the available safety and efficacy literature on the Ugandan anti-malarial plants to reliably suggest the highly ranking ones for subsequent herbal product innovations based on scientifically validated criteria.
Methods
A comprehensive literature search was conducted using available databases including Pubmed, Google scholar, and Scopus-indexed journals. Objective keywords such as ethnobotanical surveys, anti-malarial plants in Uganda, and others were used to get peer-reviewed articles on the herbal remedies mentioned for malaria treatment. Only ethnobotanical studies on anti-malarial plants in Uganda were included in the review. For each survey included in the study, at least 5–10 plants with the highest frequency of use were considered to generate a list of potential anti-malarial plants.
In the second phase, further searches were exhaustively done on each listed plant with specificity on plant name, anti-plasmodial activity, in vitro anti-malarial efficacy, in vivo anti-malarial efficacy, safety profile, LD50, and acute toxicity. Priority was given to the studies conducted on plants collected from Uganda that have met the minimal quality requirements of data rigour, methods and scientific validity. Only safety studies that reported the minimum lethal dose (LD50 in mg/kg) in animals (rat model) were included. RITAM score system was adapted with modifications [19]. The scores were awarded to each plant employed in studies that have demonstrated the best efficacy from in vitro to in vivo with considerations of the solvent and the parts of the plant used for extraction, as summarized in Table 1. Plants with no scientific data on efficacy were excluded at this stage. The total score for each plant were calculated and they were ranked based on values from the highest to the lowest.
In the third phase, the twenty best plants selected from phase two were further assessed for information on the plant parts used, extraction solvent system, ease of plant cultivation, safety level, confirmation of preclinical efficacy in clinical trials and exclusiveness of the potency reported. These assessments were interpreted and summarized for the selection of the best three plants as crude actives for possible development of efficacious, cost effective and commercially sustainable anti-malarial products.
Results and discussion
Ugandan anti-malarial plants
Various ethnobotanical survey studies have been conducted in Uganda on the plants locally used for malaria. Extensive review of ethnobotanical surveys in the country with special consideration for the different geographical regions (Eastern, Western, Central and Northern) [18] revealed fifteen plant species as the most commonly used in Uganda. These plants included Bidens pilosa, Tithonia diversifolia, Vernonia amygdalina, Vernonia lasiopus, Carica papaya, Hoslundia opposita, Mangifera indica, Cymbopogon citratus, Justicia betonica, Markhamia lutea, Moringa oleifera, Aristolochia elegans, Cajanus cajan, Toddalia asiatica, and Azadirachta indica.
An ethnobotanical survey conducted on the plants used for treatment of malaria in Mpigi district documented eighty-six plant species [20]. Among these, the most commonly reported with Fr (Frequency of report) values from 38 to 17 included (in descending order) V. amygdalina, B. pilosa, J. betonica, Microglossa pyrifolia, Clerodendrum rotundifolium, V. lasiopus, Aloe dawei, Leonotis nepetofolia.
In yet another similar study conducted in Butebo County, Eastern Uganda [21], thirty-three plant species were documented, but the eight most common ones with PPK (percentage of people who have knowledge about the use of a species in the treatment of malaria) values ranging from 90 to 70% in the descending order include Chamaecrista nigricans, Zanthoxylum chalybeum, Schkuhria pinnata, Ocimum basilicum, Euclea latideus, Erythrina abyssinica, A. indica, and Ocoba spinosa.
Another ethnobotanical survey was also carried out in Budondo sub-county located in Jinja district north-east of Kampala [22]. From the study, a total of thirty-seven plant species were documented for treatment of malaria. Among these, the most common with percentage of mention ranging from 64.8 to 15.4% included V. amygdalina, Aloe vera, Callistermon citrinus, Mormodica foetida, Cyphostemma adenocaule, and Eucalyptus globulus.
In Kamuli district, Eastern Uganda [23], twenty-seven plant species for treatment of malaria were reported. V. amygdalina, M. foetida, Z. chalybeum, Lantana camara, M. indica, and Chenopodium ambrosioïdes, were the most frequently mentioned species. Another ethnobotanical survey was also conducted in Mbarara district, western Uganda [24]. From this study, a total of twenty plant species were documented and eight considered to be the most commonly used with frequency of mention ranging from 102 to 24 included (in descending order): V. amygdalina, Pseudarthria hookeri, C. rotundifolium, Lantana trifolia, T. asiatica, V. lasiopus, and Erlangea cordifolia.
In Cegere sub-county, Apac district, northern Uganda, a total of 20 plant species were documented for preventing and treating malaria in the area [25], and seven most commonly used with citation frequency ranging from 69 to 7 (in descending order) include S. pinnata, Baccharoides adoensis, A. indica, Crotalaria ochroleuca, A. vera, M. oleifera, and Curcuma longa.
Anti-malarial plants used in the areas of Abukamola, Angeta, Oculokori, and Omarari of Alebtong district (Northern Uganda) were also documented [26]. A total of forty-three plant species were reported and the most common with PRK values ranging from 23.5 to 9.9% included Clerodendrum umbellatum, Canthium lactescens, Crotolaria laburnifolia, Chasmanthera dependens, Chamaecrista hildbrandtii, and Securidaca longipenduculata.
Another study which captured ethnomedicinal use, preference for species and ecological viability of plants used for treatment of malaria was earlier conducted among the communities living around the Sango Bay Forest Reserve in southern Uganda [27]. Sixteen plant species were unveiled and the five most common ranked basing on the importance index (which focuses on the level of relevance attached to each plant by the respondents for management of the disease under investigation) included Hallea rubrostipulata, V. amygdalina, Warburgia ugandensis, Syzygium guineense, and Z. chalybeum.
Tugume et al. [28] also documented all the medicinal plants in Mabira Central Forest Reserve (CFR) in Central Uganda. According to the researchers, the thirteen most important medicinal plants for treatment of malaria included V. amygdalina, M. feotida, Indigofera congesta, Solanum nigrum, A. vera, Hoslundia opposita, Markhamia lutea, V. lasiopus, Melanthera scandens, Aristolochia elegans, Alstonia boonei, and J. betonica.
Furthermore, a total of fifty-six plant species were reported to be used for treatment of malaria in Nyakayojo sub-county in south western Uganda [29]. Among them, the fourteen most commonly reported included V. amygdalina, Aloe sp., wild sp., J. betonica, Vernonia adoensis, T. diversifolia, A. indica, Clutia abyssinica, V. lasiopus, Solanecio mannii, M. pyrifolia, Bothriocline longipes, Conyza bonariensis, Guizotia scabra, and Gynura scandens.
In a comprehensive literature review on the plants used for treatment of malaria in Uganda, approximately 182 plant species were documented. Among these, 112 plant species, including Artemisia annua, were reported to have been investigated for anti-malarial activities, with 96% showing positive results. These tested plants were compared and sorted with the plants reported above in the various ethnobotanical surveys from different parts of the country to generate a list of about 126 plant species [30]. This implies that there are over 126 plant species currently used by local communities in Uganda for management of malaria and the majority of them have been tested.
Efficacy of selected anti-malarial studies
The standard RITAM criterion for selection of the most efficacious and safe anti-malarial plants which consists of numerical values allocated for in vitro anti-plasmodial IC50 (mg/ml), percentage chemosuppression (%) in mice model and acute toxicity (LD50) in the rat model was adapted. Out of the 126 Ugandan anti-malarial plants, only 41 plant species were selected for the RITAM score and their rankings are as indicated in Table 2. Four (4) different rankings were created based on: (1) total score of efficacy (both MIC and % parasite suppression) and safety (LD50); (2) total efficacy score only; (3) in vitro score (MIC) only; and (4) in vivo score only. The second ranking is the most suitable for precise rating of the plants based exclusively on efficacy. A summary of the subsequent analysis of the best 20 plants selected is presented in Table 3.
Selection of plants for possible polyherbal anti-malarial therapy
Based exclusively on the RITAM score and considering the available data on efficacy (both in vitro and in vivo), the twenty plants (Table 3) form the list of the best 20 potential anti-malarial plants in Uganda which could be enrolled for further studies in antimalarial herbal product developments. Further review of the literature on these plants revealed that only five (5) plants including A. annua, V. amygdalina, C. longa, Artemisia afra, and Aspilia africana have been studied up to clinical level. Among these, A. annua and V. amygdalina are the most extensively studied and their active compounds are known, isolated, and some already synthetized [31, 32]. In a relatively similar analysis which adhered to all the aspects of RITAM score including clinical correlations, A. annua, and V. amygdalina ranked as the best two anti-malarial plants [19]. The available clinical study on C. longa focused on pharmacokinetics (mainly bioavailability) and it showed that, there was limited bioavailability of the plant’s active anti-malarial compound curcumin [33, 34]. The clinical study of A. afra was later retracted due to data irregularities [35]. Aspilia africana demonstrated 70% effectiveness in malaria treatment [36], but there is no information on the active compounds responsible for this efficacy to guide product formulation standardization (no chemical markers known). Though extensively studied, the clinical efficacy of A. annua and V. amydalina are both limited by parasite recrudescence which hampers complete remission of the parasite from the body [37, 38]. Studies have also been done on the combination of two plants, but parasite recrudescence persisted [39]. Therefore, there is a need to conduct more clinical studies on the other plants in the best 20 list (Table 3), or search for other combinations that can eliminate the parasite recrudescence, which is associated with the inability of actives to penetrate the liver (the organ harbouring the parasites) after blood parasite clearance and reduced drug concentrations in blood [40, 41].
Interestingly, additional literature searches on the other plants in the best 20 list (Table 3), indicated that M. pyrifolia (aqueous extract) is one of the plants with most potent anti-plasmodial activity. It obtained maximum RITAM score for in vitro findings (Table 2, Rank 3). It also ranked third best among known anti-malarial plants in the Africa based on in vitro anti-plasmodial potency in a systemic review [42]. The plant also causes liver toxicity, indicative of the ability of the actives to penetrate the liver [43]. It is, therefore, possible that addition of this plant in lower doses to a combination of A. annua, and V. amygdalina could address the challenge of parasite recrudescence without causing liver toxicity since polyherbalism has advantages of minimizing toxic effects of certain active ingredients [16].
As much as RITAM score is important for prioritizing plants for further anti-malarial studies, it cannot be used alone since it does not adequately capture other factors, such as plant parts used, extraction solvent (aqueous preferred), and ease of cultivation. These are important in natural product development to foresee the cost of production and the possibility of commercialization. Therefore, based on current literature of the efficacy (at in vitro, mice and clinical levels), safety profile and other factors indicated in Table 3, A. annua, V. amygdalina and M. pyrifolia could offer a promising alternative of natural and herbal combination therapy, but needs anti-malarial activity optimization study. However, this selection does not rule out the relevance of the other plants in our list of 20 as equally potential plants for further anti-malarial studies.
Description of the selected plants for development of herbal drug delivery systems
Artemisia annua
Artemisia annua (Fig. 1) is known as sweet wormwood, sweet annie or annual wormwood in English. Botanically, it has been classified as Family—Asteraceae, Genus—Artemisia, Species: annua [30, 31].
As shown in Fig. 1, the plant is a large vigorous weedy annual shrub often reaching more than 2 m tall, usually ribbed single-stemmed with alternate branches and stem covered with fine, silky grey-green hairs [46]. It naturally grows to 30–100 cm high but cultivated plants may reach 200 cm high, and is widely distributed in the temperate, cool temperate and subtropical zones (mainly in Asia) of the world [47]. It originated from China and grows mainly in the middle, eastern and southern parts of Europe and in the northern, middle and eastern parts of Asia. However, a few countries are currently cultivating A. annua on both large and small scale, such as China, Kenya, the United Republic of Tanzania, and other countries in Africa (including Uganda), and altitudes ranging 1000–1500 m are favourable for its growth [48].
Artemisia annua (called Qinghao—Chinese) has long been used in China as a herbal remedy with its first documentation dating 168 BC [48]. Its first record for malaria was made by Ge Hong in 341 AD. Li Shizen also wrote in his Pharmacopoeia in 1596 that qinghao cures cold and hot fevers. Based on this, the Chinese scientists led by Zhenxing Wei and You-Tou Li developed extraction methods to finally isolate its active compound, artemisinin (Fig. 2) in 1972 [45].
Since the isolation of the active ingredient, artemisinin has influenced the current treatment of malaria. In most African countries, ACT was introduced right on time when the parasite had already developed resistance against the previous drugs, including chloroquine, quinine and others. The trends over the years on this novel plant is fascinatingly filled with disagreements among various researchers on some issues and agreements on other issues, especially between researchers from herbal regimen and those from synthetic regimen. The anti-malarial literature on the plant ranges from in vitro, in vivo in animals to several human clinical trials.
The earliest clinical study conducted on A. annua leaf infusion was performed 20 years ago as a pilot trial in a rural primary health care scheme, involving a district hospital and three health centres, in the eastern Democratic Republic of the Congo (DRC) from February to December 2001 [49]. In the study, extract groups (2) received 5 g (A5) and 9 g (A9) of the leaf powder prepared in 1 L per day and the control group were on quinine and later changed to chloroquine. A 77% cure rate was reported in A5 group and 70% in A9 while the positive control led to 91% by day 7. Parasite recrudescence was observed in the infusion groups after day 7 though there was no clear method used to distinguish recrudescence from new infections. However, there was a rapid improvement of malaria symptoms in artemisia groups, leading to a conclusion that, “malaria monotherapy with tea preparation cannot be recommended for treatment because of recrudescence as well as concerns of possible artemisinin resistance. A similar study was done in Tanzania in 2002—2003 with same doses of A. annua infusion but sulfadoxine/pyrimethamine used as control [50]. Compared to the previous trial [49], the number of participants available by day 7 was lower (4–8, compared to 39–43). However, the cure rate reported in this work (70% & 77.7% for 5 g and 9 g doses, respectively) was similar to the study from DRC. Another similarity with both studies is that the plant materials were all collected from Germany and a high rate of recrudescence.
Contrary to the findings in the two clinical studies above, a cure rate of 91.8% was reported in an Ethiopian survey that assessed the use of the plant for malaria treatment among locals with no major adverse effects [51]. The researchers recommended that a policy and regulatory mechanisms to integrate herbal medicines to modern health care system should be established and adopted. But these findings were only based on experiences of people, with no scientific methods to prove the cure and may not be applicable for informing policy. Interestingly, these findings were corroborated by a pharmacovigilance study in Kenya and Uganda which revealed that, over 3000 cases of presumed malaria (250 children and 54 pregnant women in 1st trimester) were treated using A. annua [52]. However, the latter study reported poor compliance by children to the infusion because of bitterness and vomiting. Two miscarriages were reported among the pregnant patients. In a randomized Ugandan clinical trial [53], consumption of the A. annua tea infusion (5 g) once a week significantly reduced the risk of suffering more than one episode of malaria in 9 months by 55%. No serious side effects were reported except the bitter taste associated with the plant. Another study [54] supported the efficacy reported of A. annua tea infusion for malaria treatment. The researchers reported a potent activity demonstrated by the infusion in vitro against both chloroquine resistant (W2) and sensitive (D10) strains (IC50 (ug/ml): 1.11 ± 0.21 for D10 and 0.88 ± 0.35 for W2). With the minimal concentration of artemisinin in the infusion (0.18 ± 0.02% of the leaf powder), which was too low to be responsible for the observed activity, they postulated the possibility of artemisinin acting synergistically with other ingredients in the extract to give such an impressive effect.
Despite the efficacy of the crude plant extracts reported above, the WHO in 2012 ruled out the use of A. annua plant material in any form including capsules and tea for malaria treatment or prevention [55]. The international regulatory body decision was based exclusively on two trials described previously [35, 36], stating that the reported clinical outcomes were unsatisfactory with malaria recrudescence, low dose of the infusions compared to the recommended ACT dose may promote anti-malarial resistance, and reported interactions between artemisinin and other compounds in the infusions are unsatisfactory. The WHO recommended that an extensive fundamental and clinical research be done to demonstrate that the non-pharmaceutical dosage forms of the plant are safe and effective for malaria, and that their use would not lead to development of artemisinin-resistant parasite. A follow-up study on the possibility of synergism among various compounds in the plant was conducted [56], different varieties of the plant were tested in comparison with pure artemisinin. It was observed that the IC50 of all the A. annua tea infusions were not significantly different from that of pure artemisinin, and thus in agreement with the WHO analysis. The researchers, therefore, concluded that, “artemisinin seems to be the only active anti-malarial agent in A. annua, but they did not comprehensively explain the similarity in the IC50’s between the extract and the pure drug since the extract obviously has lower quantity of the active and would be expected to show lower activity if their conclusion is to be justified.
In a comprehensive literature review on the use of A. annua for malaria management [37], reviewers highlighted discrepancies in the studies used for informing the above WHO decision. They also presented several studies that provided evidence of efficacy of the plant infusion for malaria especially those that proved biopharmaceutical interaction viz., a number of compounds from flavonoids, terpenes polysaccharides and others that improved absorption of artemisinin and some exhibiting anti-plasmodial activities. They suggested that the tea could play a critical role in malaria prophylaxis to reduce incidence of malaria in different communities or in temporary relief of malaria as the patient buys time to access a hospital or a clinic stocked with ACT. More scientific evidences have also emerged recently all supporting the synergism between artemisinin and other compounds. For example, in a combination of artemisinin with three other components in high contents in the A. annua (arteannuin B, arteannuic acid, and scopoletin), a sharper reduction in parasitaemia (93%) compared to the pure artemisinin (30%) in animal model was reported [17]. This indicated clear synergism among the different compounds. The pharmacokinetic studies showed increased absorption in the combination groups. The findings imply that specific components in the plant might offer a possibility to develop new artemisinin-based natural combination therapy for malaria treatment. In a similar study, arteannuin B was reported to inhibit biotransformation of artemisinin through inhibition of CYP3A [57]. This observed effect of arteannuin B explains the enhanced anti-plasmodial potency of the plant extract, but the synergism did not reduce the rate of recrudescence. Therefore, there is still the need to combine the extract with other anti-malarial agents to prolong activity and alleviate recrudescence.
The limitation of A. annua tea infusion in malaria treatment by parasite recrudescence was further evidenced in another randomized controlled trial, which used artemether-lumefantrine as control. The study reported negative parasitaemia in the tea infusion within few days but recrudescence surfaced on day 14 and 28. The researchers also attributed the observed activity to interaction between artemisinin and other components (possibly flavonoids), and recommended combination of the extract with other agents to extend therapeutic action [31]. In contrast to a large-scale randomized, double blind and controlled study showing 91% and 100% cure rates in children and adults, respectively, compared to 50 and 30% (children and adults respectively) for the standard control (artesunate-amodiaquine – ASAQ) [58], no recrudescence was reported after 28 days in the extract group. Due to strong criticism of the study findings [35], the publication was retracted. Despite continued challenges in acceptability of A. annua extract for malaria prophylaxis or treatment, more evidence on its effectiveness continues to surface. Another study in 2020 [59] reported that, the malaria prophylaxis provided by the plant infusion that was thought to last for few weeks actually extends for months and years. This was explained by an observation that the IgE induced Artemisia consumption remained for months on the skin. Another researcher described therapeutics based on isolated molecules such as quinine or its derivative chloroquine, artemisinin or its derivatives (artesunate), as having higher tendencies to cause drug resistance than crude plant extracts having complex chemical composition [60]. Therefore, standardization of crude extract formulations will not only lead to introduction of phytopharmaceuticals into the conventional health care mainstream, but also prevent any possible drug resistance which is common with synthetic drugs.
Vernonia amygdalina
Vernonia amygdalina (Fig. 3) is a popular African vegetable that grows as a shrub or small tree indigenous to Central and East African including Uganda [61]. Ecologically and botanically, the plant has been previously described as follows [51]. It grows up to 10 m tall along rivers and lakes, in forests margins, woodland and grassland up to 2800 m altitude, in regions where mean annual rainfall is 750- 2000 mm The bark is light grey or brown; fissured, brittle branches. Leaves lanceolate oblong; up to 28 × 0 cm, but usually 10–15 × 4–5 cm. Flower heads thistle like, small, creamy white, 10 mm long, grouped in dense heads, axillary and terminal, forming large flat clusters, 15 cm in diameter, sweetly scented.
V. amygdalina [62]
The plant is commonly referred to as bitter leaf but in Uganda, it has various local names; Luganda—Mululuuza, Lunyankole—Mubirizi, Acholi—Labori, Lugishu—Mululisi, Madi—Okelo-okelo, Iteso—etutum, and Lugbara—Echero [63]. It is used for treatment of multiple diseases in Uganda including schistosomiasis, amoebic dysentery, and gastrointestinal problems. Masaba reported reported the chewing of the pith of the plant by Chimpanzees for treating parasitic infections and determined the in vitro anti-plasmodial activity of aqueous and acetone–water extract obtaining IC50 of 76.7 µg/ml and 25.5 µg/ml respectively [64]. Another in vitro activity was tested in fresh isolated P. falciparum parasites from patients in Nigeria [65]. The researchers reported IC50 of 11.2 µg/ml for ethanolic leaf extract and 13.6 µg/ml for aqueous extract. These anti-plasmodial activities were higher than the study by Masaba [64]. The difference could be attributed to geographical locations (Nigeria vs Uganda) and parasite strains (isolates from Nigeria vs school child chloroquine sensitive strain—Uganda).
Several in vivo animal anti-malarial studies have also been conducted to validate the above in vitro findings (Table 4). The variations in the activities from different studies may emanate from many factors including geographical locations, route of administration, different extraction methods, and antimalarial method (prophylactic, curative and suppressive). The highest activity reported so far with the standard Peter’s four-day suppressive test was from the V. amygdalina collected from Uganda, that is, 75.15% at 400 mg/kg [66]. A previous study in Uganda [61], gave a similar activity at an even lower dose but the extract was administered by IP (intraperitoneal) route.
In addition to the in vivo animal studies validating the anti-malarial efficacy of the plant, two clinical studies have also been conducted in Uganda [56, 57]. The first was done in Bushenyi at Rukararwe Partnership Workshop for Rural Development (RPWRD), and the product was coded AM—which refers to antimalaria V. amygdalina leaf powder [67]. Complete parasite clearance was achieved only in one case, but the geometric mean of parasite count declined significantly by day 7 (5540/µl day 0 to 511/ µl day 7). There was also marked symptomatic improvement in 17/19 patients. No severe side effects were observed, and the most common minor ones reported included vomiting, abdominal pain, nausea, and bitter taste. These side effects affected the compliance of patients leading to six (6) dropouts. However, the study recommended a larger randomized controlled trial to determine whether the symptomatic improvement was the result of AM treatment or of natural immunity to malaria. The second trial [32] conducted in Kasese district (Uganda) reported adequate clinical response (ACR) on day 14 in 67% of the cases. However, complete parasite clearance occurred in only 32% of those with ACR, and of these, parasite recrudescence occurred in 71%. Just like the previous study, no severe side effects were reported except nocturia, insomnia and cough. According to the researchers, V. amygdalina is moderately effective and further work is necessary to establish the optimum dosage regimen, possibly in combination with other anti-malarial agents. A follow up study was conducted to validate this claim [68], and it reported 80.71% chemosuppression from a combination of V. amygdalina (125 mg/kg) and chloroquine (5 mg/kg), in mice against chloroquine resistant clones of Plasmodium berghei strain ANKA. The study concluded that, V. amygdalina leaf extract dose – dependently restored the efficacy of CQ against CQ resistant P. berghei malaria in mice. In another study in Uganda, a combination of V. amygdalina with A. annua achieved 100% parasite clearance in mice model [39]. There was still a challenge of shorter survival time (10.67 ± 1.09 days) compared to more than 30.0 ± 0.0 days for the ACT, P = 0.000. The study concluded that, “the V. amygdalina – A. annua petroleum ether extract combination shows promise for use as an herbal artemisinin combination against malaria, however the survival times need improvement to match that of the ACT”.
The phytochemicals responsible for above-described activities of V. amygdalina have been identified, isolated and their anti-plasmodial effect determined. They include (with their IC50) sesquiterpene lactones: vernodalin (4.0 µg/ml), vernolide (8.4 µg/ml), vernodalol (4.2 µg/ml), and hydroxyvernolide (11.4 µg/ml); and also steroid glycoside: vernonioside B1 (46.1 µg/ml) [38, 70]. The bitter taste of the plant leaf decoction is attributed to the steroid glycosides (vernoniosides A1 – A4 and B1 – B4). The LD50 of the leaf decoction has been reported to be > 2000 mg/kg [61, 62].
Microglossa pyrifolia
Microglossa pyrifolia (Fig. 4) is an erect or scandent shrub that grows up to 5 m high, occurring throughout tropical Africa and Asia [71]. The plant is locally known as Kafugakande in Uganda – Luganda [72], Nyabungodide in Kenya [73]. Diola in Senegal and Gambia, and Bulom in Sierra Leon [71]. The leaves are simple alternate, carried by a short petiole (10–15 mm long). The leaf blade is oval, 5 to 10 cm long and 2.5 to 4 cm wide.
M. pyrifolia [78]
The plant leaf is used locally in Uganda for treatment of malaria and several in vitro anti-plasmodial studies have provided evidence supporting this use (Table 5). The plant leaves have demonstrated very potent (IC50 < 2 µg/ml) anti-plasmodial activity according to the RITAM ranking of in vitro efficacy across different Plasmodium strains and countries. Interestingly in Uganda, the aqueous leaf extract also showed a very potent activity [72]. A similar finding was reported earlier in a Kenyan study, though the extract used in the latter was methanol [73]. The promising anti-plasmodial activity of this plant has been attributed to presence of several terpenoids, such as E-phytol, 1,3- hydroxyoctadeca-9Z, 11E, 15-trien-oic-acid and 6E-geranylgeraniol-19-oic-acid, which exhibited IC50 values between 2.5 and 13.7 µg/ml [74]. The higher IC50 values shown by these active compounds compared to the crude extracts is pointing to possible combined effects (synergism or additive) among the compounds in the extract against the malaria parasite. In a recent mini-review on the anti-plasmodial activities of various plants around the world, M. pyrifolia leaf extracts (ethyl acetate and aqueous solvents) ranked among the best three in terms of their lower IC50 indicating higher potency [75]. The researchers attributed the potency of the plant to 6E-geranylgeraniol-19-oic-acid, tannins and other polar compounds. The 6E-geranylgeraniol-19-oic-acid is also known to be present in aqueous extracts despite its lipophilic character [74].
In terms of safety, the plant leaf methanolic extract showed a higher cytotoxicity antiplamodial ratio (CAR) of 1578.0 (chloroquine sensitive strain, D-6) and 946.8 (chloroquine resistant strain, W-2), which are indicative of weak toxicities [73]. In a similar study, 89.7% reduction of ATP levels have been reported with the methanolic extract of the plant indicating a hepatotoxic effect [76]. This finding also agreed with the study [43], that listed the plant among those associated with liver fibrosis in Rakai—Uganda. The study mentioned that plants of Microglossa family contain diterpenoids known to cause liver toxicity. In addition, herbs in Asteraceae family contain pyrrolizidine alkaloids which are associated with veno-occlusive liver disease [77]. Unfortunately, there are no in vivo toxicity studies on the plant to determine its LD50 for guiding dose selection for efficacy studies.
Most of the anti-malarial studies on the plant stopped at in vitro despite its extremely potent anti-plasmodial activity. Therefore, there is a need to conduct in vivo anti-malarial activities for this plant since its anti-plasmodial potency has already been demonstrated in different countries and strains of the malaria parasite. It is also possible that the plant (in combination) may successfully improve anti-malarial efficacy of A. annua and/or V. amygdalina, especially the challenge of parasite recrudescence. Addition of the plant extract in small doses in such a combination may also control its potential liver toxicity.
Conclusion
There are many plants used in Uganda for management of malaria and ~ 33% have been either studied for efficacy and safety locally or outside the country with the plant materials collected from within the country. Most of these efficacy studies cover in vitro anti-plasmodial and in vivo chemosuppressive tests, but some plants such as M. pyrifolia that have demonstrated very potent anti-plasmodial effects have not been studied in vivo for both efficacy and safety. Artemisia annua, V. amygdalina, C. longa, A. africana, and A. afra are among the few plants broadly studied for malarial treatment up to clinical trials. However, the clinical data on C. longa stops at bioavailability and the study on A. afra was retracted due to alleged inaccuracies in the study. Aspilia africana possesses good clinical outcomes but no active compounds have been reportedly detected or isolated for easy standardization of its extracts if considered for product formulation.
This leaves A. annua and V. amydalina with known active anti-malarial compounds as the main plants that can be considered now for product developments utilizing the crude extracts. They are already being cultivated, the leaves can be harvested sustainably and extracted by aqueous solvent making it cost effective for large scale product manufacturing. But their clinical outcomes are limited by malaria parasite recrudescence. It is envisaged that addition of potent aq. extracts of M. pyrifolia to a combination of the two plants, A. annua and V. amydalina in development of polyherbal anti-malarial formulations may result in complete clearance of the parasite from the body. However, this needs to be explored and extensively studied.
Availability of data and materials
Nil.
References
UNITAID. Malaria disease narrative. Geneva: World Health Organization; 2019. https://unitaid.org/assets/Malaria-Disease-narrative.pdf.
WHO. World malaria report. regional data and trends. Geneva, World Health Organization, 2022. WHO/UCN/GMP/2022.08
WHO. World malaria report. Geneva: World Health Organization; 2020.
WHO. The “World malaria report 2019” at a glance. Geneva: World Health Organization; 2019.
WHO. Word malaria report 2021. Geneva: World Health Organization; 2021.
Rosenthal PJ. Malaria in 2022: challenges and progress. Am J Trop Med Hyg. 2022;106:1565–7.
Uganda Ministry of Health. National Malaria Annual Report 2017–2018. Kampala, Uganda, 2019.
USAID, CDC U.S. President’s malaria initiative: malaria operational plan FY 2020. 2020.
Mpimbaza A, Walemwa R, Kapisi J, Sserwanga A, Namuganga JF, Kisambira Y, et al. The age-specific incidence of hospitalized paediatric malaria in Uganda. BMC Infect Dis. 2020;20:503.
Nanyunja M, Nabyonga Orem J, Kato F, Kaggwa M, Katureebe C, Saweka J. Malaria treatment policy change and implementation: the case of Uganda. Malar Res Treat. 2011;2011: 683167.
Orem JN, Ssengooba F, Macq J, Criel B. Malaria treatment policy change in Uganda: what role did evidence play ? Malar J. 2014;13:345.
Uwimana A, Legrand E, Stokes BH, Ndikumana J-LM, Warsame M, Umulisa N, et al. Emergence and clonal expansion of in vitro kelch13 R561H mutant parasites in Rwanda. Nat Med. 2020;26:1602–8.
Tumwebaze PK, Katairo T, Okitwi M, Byaruhanga O, Orena S, Asua V, et al. Drug susceptibility of Plasmodium falciparum in eastern Uganda: a longitudinal phenotypic and genotypic study. Lancet Microbe. 2021;2:e441–9.
Tumwebaze P, Conrad M, Okitwi M, Orena S, Byaruhanga O, Katairo T, et al. Decreased susceptibility of Plasmodium falciparum to both dihydroartemisinin and lumefantrine in northern Uganda. Nat Commun. 2022;13:6353.
Tabuti JRS, Kukunda CB, Kaweesi D, Kasilo OMJ. Herbal medicine use in the districts of Nakapiripirit, Pallisa, Kanungu, and Mukono in Uganda. J Ethnobiol Ethnomed. 2012;8:35.
Rasoanaivo P, Wright CW, Willcox ML, Gilbert B. Whole plant extracts versus single compounds for the treatment of malaria : synergy and positive interactions. Malar J. 2011;10(Suppl 1):S4.
Li J, Chao Z, Gong M, Wang M. Combination of artemisinin-based natural compounds from Artemisia annua L. for the treatment of malaria : pharmacodynamic and pharmacokinetic studies. Phyther Res. 2018;32:1415–20.
Ajayi CO, Elujoba AA, Kasali FM, Tenywa MG, Okella H, Weisheit A, et al. A review for selecting medicinal plants commonly used for malaria in Uganda. Afr J Pharm Pharmacol. 2020;14:347–61.
Willcox M, Benoit-vical F, Fowler D, Bourdy G, Burford G, Giani S, et al. Do ethnobotanical and laboratory data predict clinical safety and efficacy of anti-malarial plants ? Malar J. 2011;10(Suppl 1):S7.
Adia MM, Anywar G, Byamukama R, Kamatenesi-mugisha M, Sekagya Y, Kakudidi EK, et al. Medicinal plants used in malaria treatment by Prometra herbalists in Uganda. J Ethnopharmacol. 2014;155:580–8.
Philip K, Elizabeth MM, Cheplogoi PK, Samuel KT. Ethnobotanical survey of antimalarial medicinal plants used in Butebo County. Eastern Uganda Eur J Med Plants. 2017;21:1–22.
Malinga GM, Baana K, Rutaro K, Atube F, Opoke R, Opika-Opoka H, et al. An ethnobotanical study of plants used for the treatment of malaria in Eastern Uganda. Ethnobot Res Appl. 2020;19:1–15.
Tabuti JRS. Herbal medicines used in the treatment of malaria in Uganda: a case study of Budiope county. J Ethnopharmacol. 2008;116:33–42.
Katuura E, Waako P, Ogwal-Okeng J, Bukenya-Ziraba R. Traditional treatment of malaria in Mbarara District, western Uganda. Afr J Ecol. 2007;45:48–51.
Anywar G, Van KCIEA, Byamukama R, Willcox M, Nalumansi PA, de Jong J, et al. Medicinal plants used in the treatment and prevention of malaria in Cegere sub-county, Northern Uganda. Ethnobot Res Appl. 2016;14:505–16.
Opio DR, Andama E, Kureh GT. Ethnobotanical survey of antimalarial plants in areas of : Abukamola, Angeta, Oculokori and Omarari of Alebtong District in Northern Uganda. Eur J Med Plants. 2018;21:1–14.
Ssegawa A, Kasenene JM. Plants for malaria treatment in Southern Uganda: traditional use, preference and ecological viability. J Ethnobiol. 2007;27:110–31.
Tugume P, Kakudidi EK, Buyinza M, Namaalwa J, Kamatenesi M, Mucunguzi P, et al. Ethnobotanical survey of medicinal plant species used by communities around Mabira Central forest reserve, Uganda. J Ethnobiol Ethnomed. 2016;12:5.
Stangeland T, Alele PE, Katuura E, Lye KA. Plants used to treat malaria in Nyakayojo sub-county, western Uganda. J Ethnopharmacol. 2011;137:154–66.
Okello D, Kang Y. Exploring antimalarial herbal plants across communities in Uganda based on electronic data. Evid Based Complement Altern Med. 2019;2019:3057180.
de Magalhães PM, Figueira GM, de Souza JM, Ventura AMR, Ohnishi MDO, da Silva DA, et al. A. annua: a new version of a traditional tea under randomized, controlled clinical trial for the treatment of malaria. Adv Biosci Biotechnol. 2016;7:545–63.
Challand S, Willcox M. A clinical trial of the traditional medicine V. amygdalina in the treatment of uncomplicated malaria. J Altern Complement Med. 2009;15:1231–7.
Andromeda SE, Berbudi A. The role of curcumin as an antimalarial agent. Syst Rev Pharm. 2020;11:18–25.
Coma-Cros EM, Biosca A, Lantero E, Manca ML, Caddeo C, Gutiérrez L, et al. Anti-malarial activity of orally administered curcumin incorporated in eudragit®-containing liposomes. Int J Mol Sci. 2018;19:1361.
Gillibert A, Jauréguiberry S, Hansmann Y, Argemi X, Landier J, Caumes, E, et al. Comment on “A. annua and A. afra infusions vs. artesunate-amodiaquine (ASAQ) in treating Plasmodium falciparum malaria in a large scale, double blind, randomized clinical trial” Munyangi et al., 2019. Phytomedicine. 2022;96:152981.
Okokon JE, Nwidu LL, Essiet GA. Evaluation of in vivo anti-plasmodial activity of Aspilia africana. Int J Pharmacol. 2006;2:348–51.
Weathers PJ, Towler M, Hassanali A, Engeu PO. Dried-leaf Artemisia annua: a practical malaria therapeutic for developing countries. World J Pharmacol. 2014;3:39–55.
Willcox M. A clinical trial of the traditional medicine V. amygdalina in the treatment of uncomplicated malaria. J Altern Complement Med. 2009;15:1231–7.
Nambejja C, Ogwang PE, Berna O, Anyama N, Matu E. Artemisia annua L.—Vernonia amygdalina Del: a potential herbal artemisinin combination treatment against malaria. Br J Pharm Res. 2016;14:1–7.
WorldWide Antimalarial Resistance Network Methodology Study Group. Temporal distribution of Plasmodium falciparum recrudescence following artemisinin-based combination therapy: an individual participant data meta-analysis. Malar J. 2022;21:106.
Vaughan AM, Kappe SHI. Malaria parasite liver infection and exoerythrocytic biology. Cold Spring Harb Perspect Med. 2017;7: a025486.
Tajbakhsh E, Kwenti TE, Kheyri P, Nezaratizade S, Lindsay DS, Khamesipour F. Anti-plasmodial, anti-malarial activities and toxicity of African medicinal plants: a systematic review of literature. Malar J. 2021;20:349.
Auerbach BJ, Reynolds SJ, Lamorde M. Traditional herbal medicine use associated with liver fibrosis in rural Rakai, Uganda. PLoS ONE. 2012;7: e41737.
Siddiqui MF, Waghmare SP, Hajare SW, Ingole RS, Deshmukh SG, Chepte SD, et al. Phytochemical analysis and acute toxicity studies of Artemisia annua in Swiss albino mice. J Pharmacogn Phytochem. 2018;7:1893–5.
Septembre-Malaterre A, Rakoto ML, Marodon C, Bedoui Y, Nakab J, Simon E, et al. Artemisia annua, a traditional plant brought to light. Int J Mol Sci. 2020;21:4986.
Schneck J. Artemisia annua, L. Botanical Gaz. 1881;6:238–9.
WHO. Monograph on good agricultural and collection practices (GACP) for Artemisia annua L. Geneva: World Health Organization; 2006.
Dalrymple DG. Artemisia annua, artemisinin, ACTs and malaria control in Africa: the interplay of tradition, science and public policy. Washington DC: Politics and Prose; 2012.
Mueller MS, Runyambo N, Wagner I, Borrmann S, Dietz K, Heide L. Randomized controlled trial of a traditional preparation of Artemisia annua L. (Annual Wormwood) in the treatment of malaria. Trans R Soc Trop Med Hyg. 2004;98:318–21.
Blanke CH, Naisabha GB, Balema MB, Mbaruku GM, Heide L, Müller MS. Herba Artemisiae annuae tea preparation compared to sulfadoxine- pyrimethamine in the treatment of uncomplicated falciparum malaria in adults: a randomized double-blind clinical trial. Trop Doct. 2008;38:113–6.
Tiruneh G, Kebede Y, Tegbar Y. Use of the plant Artemisia annua as a natural anti-malarial herb in Arbaminch Town. Ethiop J Health Biomed Sci. 2010;2:75–82.
Willcox ML, Burton S, Oyweka R, Namyalo R, Challand S, Lindsey K. Evaluation and pharmacovigilance of projects promoting cultivation and local use of Artemisia annua for malaria. Malar J. 2011;10:84.
Ogwang PE, Ogwal JO, Kasasa S, Olila D, Ejobi F, Kabasa D, et al. Artemisia annua L. infusion consumed once a week reduces risk of multiple episodes of malaria: a randomised trial in a Ugandan community. Trop J Pharm Res. 2012;11:445–53.
De Donno A, Grassi T, Idolo A, Guido M, Papadia P, Caccioppola A, et al. First-time comparison of the in vitro antimalarial activity of Artemisia annua herbal tea and artemisinin. Trans R Soc Trop Med Hyg. 2012;106:696–700.
WHO. Position statement: effectiveness of non-pharmaceutical forms of Artemisia annua L . against malaria. Geneva, World Health Organization, 2012. WHO/HTM/GMP/2012.07
Mouton J, Jansen O, Frédérich M, van der Kooy F. Is artemisinin the only antiplasmodial compound in the Artemisia annua tea infusion? An in vitro study. Planta Med. 2013;8:468–70.
Cai T, Zhang Y, Ji J, Xing J. Investigation of the component in Artemisia annua L. leading to enhanced antiplasmodial potency of artemisinin via regulation of its metabolism. J Ethnopharmacol. 2017;207:86–91.
Munyangi J, Cornet-Vernet L, Idumbo M, Lu C, Lutgen P, Perronne C, et al. Artemisia annua and Artemisia afra tea infusions vs. artesunate-amodiaquine (ASAQ) in treating Plasmodium falciparum malaria in a large scale, double blind, randomized clinical trial. Phytomedicine. 2019;57:49–56.
Munyangi J, Gisenya P, Ogwang P, Lutgen P. An unexpected, revolutionary property of Artemisia infusions: immunoglobulins in the skin lead to a long-lasting prophylaxis. Pharm Pharmacol Int J. 2020;8:46–62.
Avitabile E, Senes N, Avino CD, Tsamesidis I, Pinna A, Medici S, et al. The potential antimalarial efficacy of hemocompatible silver nanoparticles from Artemisia species against P. falciparum parasite. PLoS ONE. 2020;15:e0238532.
Njan AA, Adzu B, Agaba AG, Byarugaba D, Díaz-llera S, Bangsberg DR. The analgesic and anti-plasmodial activities and toxicology of Vernonia amygdalina. J Med Food. 2008;11:574–81.
Makumbi OH, Nyonyintono RM. Use of Vernonia plant for home-based malaria treatment in rural Uganda: the case of Nyimbwa sub-county. Luweero District. Kampala: Uganda, Ndejje University; 2008.
Katende AB, Birne A, Tengnas B. Useful trees and shrubs for Uganda: identification, propagation and management for agricultural and pastoral communities. Regional Land Management Unit, RELMA/Sida ICRAF House, Gigir, Nairobi, 1995.
Masaba SC. The antimalarial activity of Vernonia amygdalina Del (Compositae). Trans R Soc Trop Med Hyg. 2000;94:694–5.
Sha’a Kk, Oguche S, Watila IM, Ikpa TF. In vitro antimalarial activity of the extracts of Vernonia amygdalina commonly used in traditional medicine in Nigeria. Sci World J. 2011;13:113–9.
Ajayi CO, Elujoba AA, Okella H, Oloro J, Raymond A, Weisheit A, et al. In vivo antimalarial activities of five Ugandan medicinal plants on Plasmodium berghei in mice. Eur J Med Plants. 2020;31:1–13.
Willcox ML. A clinical trial of ‘AM’, a Ugandan herbal remedy for malaria. J Public Health Med. 1999;21:318–24.
Iwalokun B. Enhanced antimalarial effects of chloroquine by aqueous Vernonia amygdalina leaf extract in mice infected with chloroquine resistant and sensitive Plasmodium berghei strains. Afr Health Sci. 2008;8:25–35.
Bekele T. In vivo anti-malarial evaluation of leaf extract of Vernonia amygdalina Del. (Asteraceae) against Plasmodium berghei. MSc Thesis, Addis Ababa University, Ethiopia, 2015.
Zakaria Y, Azlan NZ, Hassan NFN, Muhammad H. Phytochemicals and acute oral toxicity studies of the aqueous extract of Vernonia amygdalina from state of Malaysia. J Med Plants Stud. 2016;4:1–5.
Burkill HM. The useful plants of West Tropical Africa. Royal Botanical Gardens Kew. Vol. 4. 1997
Adia MA, Emami SN, Byamukama R, Faye I, Borg-Karlson A-K. Antiplasmodial activity and phytochemical analysis of extracts from selected Ugandan medicinal plants. J Ethnopharmacol. 2016;186:14–9.
Ayuko AT, Njau RN, Cornelius W, Leah N, Ndiege IO. In vitro anti-plasmodial activity and toxicity assessment of plant extracts used in traditional malaria therapy in the Lake Victoria region. Mem Inst Oswaldo Cruz. 2009;104:689–94.
Köhler I, Jenett-siems K, Kraft C, Siems K, Abbiw D, Bienzle U, et al. Herbal remedies traditionally used against malaria in Ghana: bioassay-guided fractionation of Microglossa pyrifolia (Asteraceae). Z Naturforsch C J Biosci. 2002;57:1022–7.
Azman NS, Mahboob T, Tan TC, Samudi C, Nissapatorn V, Wiart C. Plant-based therapy—how does it work on parasites ? Walailak J Sci Tech. 2018;15:551–9.
Muganga R, Angenot L, Tits M, Frédérich M. Anti-plasmodial and cytotoxic activities of Rwandan medicinal plants used in the treatment of malaria. J Ethnopharmacol. 2010;128:52–7.
Chen Z, Huo JR. Hepatic veno-occlusive disease associated with toxicity of pyrrolizidine alkaloids in herbal preparations. Neth J Med. 2010;68:252–60.
Steven J. West African plants—a photo guide—Microglossa pyrifolia (Lam.) Kuntze. http://www.westafricanplants.senckenberg.de/root/index.php?page_id=14&id=3823
Alshawsh MA, Mothana RA, Al-shamahy HA, Alsllami SF, Lindequist U. Assessment of anti-malarial activity against Plasmodium falciparum and phytochemical screening of some Yemeni medicinal plants. Evid Based Complement Altern Med. 2009;6:453–6.
Olaniyi AM, Oshiobugie MJ, Raphael AO. Experimental and mathematical model for the anti-malarial activity of methanolic root extract of Azadirachta indica (Dongoyaro) in mice infected with Plasmodium berghei NK65. J Adv Math Comput Sci. 2020;35:68–82.
Kanagasanthosh K, Shanmugapriyan S, Kavirajan V. Evaluation of acute toxicity, anti-inflammatory activity and phytochemical screening of ethanolic extract of Azadirachta indica leaves. Int J Res Dev Pharm Life Sci. 2015;4:1737–42.
Lwin KM, Mon HM, Myint KH. Evaluation of the anti-malarial activity of Curcuma longa singly and in combination with Eupatorium odoratum Linn. J Ayurvedic Herb Med. 2017;3:11–4.
Kamsu GT, Fodouop SP, Tagne RS, Kodjio N, Fakam ALN, Gatsing D. Evaluation of the acute and sub-chronic toxicity of the ethanolic extract of Curcuma longa (Zingiberaceae) in Wistar albino rats. Modern Chem Appl. 2019;7:267.
Teng WC, Chan W, Suwanarusk R, Ong A, Ho HK, Russell B, et al. In vitro antimalarial evaluations and cytotoxicity investigations of Carica papaya leaves and carpaine. Nat Product Commun. 2019;14:33–6.
Airaodion AI, Airaodion EO, Ekenjoku JA, Ogbuagu EO, Ogbuagu U. Antiplasmodial potency of ethanolic leaf extract of Carica papaya against Plasmodium berghei in infected Swiss albino mice. Asian J Med Princ Clin Pract. 2019;2:1–8.
Ismail Z, Halim SZ, Abdullah NR, Afzan A, Abdul Rashid BA, Jantan I. Safety evaluation of oral toxicity of Carica papaya Linn. leaves: a subchronic toxicity study in Sprague Dawley rats. Evid Based Complement Altern Med. 2014;2014:741470.
Daskum AM, Godly C, Qadeer MA. Anti-plasmodial activities of crude Moringa oleifera leaves extracts on chloroquine sensitive Plasmodium falciparum (3D7). Bayero J Pure Appl Sci. 2019;12:20.
Olasehinde GI, Ayanda OI, Egwari LO, Ajayi AA, Awofeso T. In vivo anti-plasmodial activity of crude ethanolic and n-hexane extracts of Moringa oleifera leaves. Int J Agric Biol. 2016;18:906–10.
Osman H, Shayoub M, Babiker E, Faiza A, Munzir M, Bashier O, et al. Assessment of acute toxicity and LD50 of Moringa oleifera ethanolic leave extract in albino rats and rabbits. J Med Biol Sci Res. 2015;1:38–43.
Cudjoe E, Donu D, Okonu RE, Amponsah JA, Amoah LE. The in vitro anti-plasmodial activities of aqueous extracts of selected Ghanaian herbal plants. J Parasitol Res. 2020;2020:5041919.
Nnamdi A, Ettebong E, Davis K. Anti-plasmodial and antioxidant activities of methanolic leaf extract and fractions of Alchornea cordifolia. J Herb Med Pharmacol. 2017;6:171–7.
Waako PJ, Gumede B, Smith P, Folb PI. The in vitro and in vivo anti-malarial activity of Cardiospermum halicacabum and Momordica foetida. J Ethnopharmacol. 2005;99:137–43.
Gathirwa JW, Rukunga GM, Njagi ENM, Omar SA, Mwitari PG, Guantai AN, et al. The in vitro anti-plasmodial and in vivo anti-malarial efficacy of combinations of some medicinal plants used traditionally for treatment of malaria by the Meru community in Kenya. J Ethnopharmacol. 2008;115:223–31.
Fatou Kane N, Cleophas Kyama M, Kangethe Nganga J, Hassanali A, Diallo M, Thuo KF. Acute toxicity effect of Artemisia afra plant extracts on the liver, kidney, spleen and in vivo anti-malarial assay on Swiss albino mice. Adv Biosci Bioeng. 2019;7:64–71.
Brandão MGL, Krettli AU, Soares LSR, Nery CGC, Marinuzzi HC. Anti-malarial activity of extracts and fractions from Bidens pilosa and other Bidens species correlated with the presence of acetylene and flavonoid compounds. J Ethnopharmacol. 1997;57:131–8.
Noumedem AC, Yamssi C, Simeni NS, Ngongang OC, Mounvera AA, Guangueu DC, et al. Anti-malarial activity of ethyl acetate extract and fraction of Bidens pilosa against Plasmodium berghei (ANKA). J Parasitol Res. 2020;2020:8832724.
Oteyo MB. Anti-bacterial activity and toxicity of non-aqeuous extract of Bidens pilosa against Escherichia coli in female Balb/c mice. Thesis: Maseno University, Kenya; 2019.
Melariri P, Campbell W, Etusim P, Smith P. In vitro and in vivo anti-plasmodial activities of extracts of Cymbopogon citratus and Vernonia amygdalina leaves. J Nat Prod. 2011;4:164–72.
Uraku A, Okaka A, Ibiam U, Agbafor K, Obasi N, Ajah P, et al. Anti-plasmodial activity of ethanolic leaf extracts of Spilanthes uliginosa, Ocimum basilicum, Hyptis spicigera and Cymbopogon citratus on mice exposed to Plasmodium berghei NK 65. Int J Biochem Res Rev. 2015;6:28–36.
Tarkang PA, Agbor GA, Tsabang N, Tchokouaha LRY, Kemeta D, Mengue YSN, et al. Effect of long-term oral administration of the aqueous and ethanol leaf extracts of Cymbopogon citratus. Ann Biol Res. 2012;3:5561–70.
Orwa JA, Ngeny L, Mwikwabe NM, Ondicho J, Jondiko IJO. Anti-malarial and safety evaluation of extracts from Toddalia asiatica. J Ethnopharmacol. 2013;145:587–90.
Waako PJ, Katuura E, Smith P, Folb P. East African medicinal plants as a source of lead compounds for the development of new anti-malarial drugs. Afr J Ecol. 2007;45(Suppl 1):102–6.
Taziebou LC, Etoa FX, Nkegoum B, Pieme CA, Dzeufiet DP. Acute and sub acute toxicity of Aspilia africana leaves. Afr J Trad Complement Altern Med. 2021;4:127–34.
Elufioye TO, Alatise OI, Fakoya FA, Agbedahunsi JM, Houghton PJ. Toxicity studies of Tithonia diversifolia in rats. J Ethnopharmacol. 2009;122:410–5.
Were PS, Kinyanjui P, Gicheru MM, Mwangi E, Ozwara HS. Prophylactic and curative activities of extracts from Warburgia ugandensis and Zanthoxylum usambarense against Plasmodium knowlesi and P. berghei. J Ethnopharmacol. 2010;130:158–62.
Karani LW, Tolo FM, Karanja SM, Khayeka-Wandabwa C. Safety of Prunus africana and Warburgia ugandensis in asthma treatment. South African J Bot. 2013;88:183–90.
Nardos A, Makonnen E. In vivo anti-plasmodial activity and toxicological assessment of hydroethanolic crude extract of Ajuga remota. Malar J. 2017;16:25.
Moustapha K, Karim T, Offianan T, Beourou S, Attemene D, Gnondjui A, et al. Assessment of antiplasmodial and anti-anaemic activities of Hoslundia opposita, an Ivorian medicinal plant. J Adv Microbiol. 2018;11:1–11.
Onwuka NA, Ezike AC, Ettebong ED, Tologbonse AA, Onyeukwu NJ. Evaluation of the immunomodulatory activity of Hoslundia opposita Vahl (Lamiaceae) leaf extract. Int J Pharmacogn Phytochem Res. 2016;8:1–7.
Feiz Haddad MH, Mahbodfar H, Zamani Z, Ramazani A. Anti-malarial evaluation of selected medicinal plant extracts used in Iranian traditional medicine. Iran J Basic Med Sci. 2017;20:415–22.
Rumiyati R, Muna LN, Hidayati DN, Jenie RI. Acute toxicity and genotoxic activity of leunca (Solanum nigrum) herb ethanolic extract. Indonesian J Cancer Chemoprevent. 2017;6:30–4.
Sadiq MB, Tharaphan P, Chotivanich K, Tarning J, Anal AK. In vitro anti-oxidant and anti-malarial activities of leaves, pods and bark extracts of Acacia nilotica. BMC Complement Altern Med. 2017;17:372.
Ifijen IH, Maliki M, Ogbeide OK, Okonko RO, Omorogbe SO, Ikhuoria EU. Chemical substances and in-vivo anti-plasmodial activity of Ageratum conyzoides in P. berghei infected mice. J Appl Sci Environ Manage. 2019;23:1813–7.
Ukwe VC, Epueke EA, Ekwunife OI, Okoye C, Akudor GC, Ubaka CM. Anti-malarial activity of aqueous extract and fractions of leaves of Ageratum conyzoides in mice infected with Plasmodium berghei. Int J Pharm Sci. 2010;2:33–8.
Ndjakou Lenta B, Vonthron-Sénécheau C, Fongang Soh R, Tantangmo F, Ngouela S, Kaiser M, et al. In vitro antiprotozoal activities and cytotoxicity of some selected Cameroonian medicinal plants. J Ethnopharmacol. 2007;111:8–12.
Okpo SO, Igwealor CO, Eze GI. Sub acute toxicity study on the aqueous extract of Albizia zygia stem bark. J Pharm Bioresour. 2016;13:32–41.
Stangeland T, Wangensteen H, Katuura E, Lye KA, Paulsen BS. Antioxidant and anti-plasmodial activity of extracts from three ugandan medicinal plants. J Med Plants Res. 2010;4:1916–23.
Ajayi AM, Dunde WO, Abba S, Dare SS, Opkanachi OA, Tanayen JK, et al. Phytochemical, acute toxicity and anti-inflammatory studies on aqueous extract of Hallea rubrostipulata stem bark. Int J Pharm Biomed Sci. 2012;3:203–6.
Inbaneson SJ, Sundaram R, Suganthi P. In vitro anti-plasmodial effect of ethanolic extracts of traditional medicinal plant ocimum species against Plasmodium falciparum. Asian Pac J Trop Med. 2012;5:103–6.
Bbosa G, Mwebaza N, Lubega A, Musisi N, Kyegombe DB, Ntale M. Anti-plasmodial activity of leaf extracts of Zanthoxylum chalybeum. Br J Pharm Res. 2014;4:705–7.
Musila MF, Dossaji SF, Nguta JM, Lukhoba CW, Munyao JM. In vivo anti-malarial activity, toxicity and phytochemical screening of selected anti-malarial plants. J Ethnopharmacol. 2013;146:557–61.
Njenga D, Irungu B, Mbaria J, Mutai C, Nguta J. Anti-plasmodial activity, cytotoxicity and acute toxicity of Zanthoxylum chalybeum. World J Pharm Pharm Sci. 2016;5:208–17.
Wresdiyati T, Stephany S, Handharyani E, Sa’diah S, Astawan M. Acute toxicity test of pigeon pea leaves extract (Cajanus cajan) in rats. E3S Web Conf. 2020;151: 01043.
Lacroix D, Prado S, Kamoga D, Kasenene J, Namukobe J, Krief S, et al. Anti-plasmodial and cytotoxic activities of medicinal plants traditionally used in the village of Kiohima. Uganda J Ethnopharmacol. 2011;133:850–5.
Nyangacha CRM, Gathirwa JW, Muthaura CN, Mungai GM, Mwikwabe N, Ondicho JM, et al. Anti-malarial activity and toxicity evaluation of Kenyan Hugonia castaneifolia, Teclea nobilis and Turraea mombassana. Afr J Health Sci. 2012;23:305–15.
Onyango DW, Midiwo JO. In vivo evaluation of anti-malarial activity of stem and root extracts of Erythrina abyssinica. European J Med Plants. 2019;27:1–5.
Katuura E, Waako P, Tabuti JRS, Bukenya-Ziraba R, Bukenya-Ziraba R, Ogwal-Okeng J. Anti-plasmodial activity of extracts of selected medicinal plants used by local communities in Western Uganda for treatment of malaria. Afr J Ecol. 2007;45(Suppl. 3):94–8.
Muregi FW, Ishih A, Miyase T, Suzuki T, Kino H, Amano T, et al. Anti-malarial activity of methanolic extracts from plants used in Kenyan ethnomedicine and their interactions with chloroquine (CQ) against a CQ-tolerant rodent parasite in mice. J Ethnopharmacol. 2007;111:190–5.
Musila MN, Muthoni BG, Koech SC, Ngugi MP, Mbinda WM. Evaluation of in vivo toxicity of methanolic leaf extract of Vernonia lasiopus. J Pharmacogn Nat Prod. 2017;3:133.
Wele M, Kirkman L, Diarra N, Goita Y, Doumbia M, Traore K, et al. Anti-plasmodial potential and phytochemical screening of ten plants used as anti-malarial in Mali. Eur J Med Plants. 2017;19:1–9.
Jigam AA, Musa R, Abdullahi A, Lawal B. Characterization of anti-plasmodial, analgesic and anti-inflammatory fraction of Maytenus senegalensis leaf extract in mice. Clin Phytosci. 2020;6:56.
Malebo HM, Wiketye V, Katani SJ, Kitufe NA, Nyigo VA, Imeda CP. In vivo anti-plasmodial and toxicological effect of M. senegalensis traditionally used in the treatment of malaria in Tanzania. Malar J. 2015;14:79.
Mante PK, Adongo DW, Kukuia KKE, Ameyaw EO, Woode E. Neuropharmacological assessment of an aqueous bark extract of Antiaris toxicaria in rodents. Am J Pharmacol Toxicol. 2012;7:123–4.
Irungu BN, Rukunga GM, Mungai GM, Muthaura CN. In vitro antiplasmodial and cytotoxicity activities of 14 medicinal plants from Kenya. South African J Bot. 2007;73:204–7.
de Oliveira D, da Silva M, dos Santos LG, Orlandi L, Paiva A. Evaluation of acute toxicity, antioxidant activity, flavonoid quantification and total phenols from the hydroethanolic extract from leaves of Leonotis nepetaefalia. Rev Eletrônica Farmácia. 2012;9:1.
Obbo CJD, Kariuki ST, Gathirwa JW, Olaho-Mukani W, Cheplogoi PK, Mwangi EM. In vitro antiplasmodial, anti-trypanosomal and anti-leishmanial activities of selected medicinal plants from Ugandan flora: refocusing into multi-component potentials. J Ethnopharmacol. 2019;229:127–36.
Al-Musayeib NM, Mothana RA, Al-Massarani S, Matheeussen A, Cos P, Maes L. Study of the in vitro anti-plasmodial, anti-leishmanial and anti-trypanosomal activities of medicinal plants from Saudi Arabia. Molecules. 2012;17:11379–90.
Kigondu EVM, Rukunga GM, Keriko JM, Tonui WK, Gathirwa JW, Kirira PG, et al. Anti-parasitic activity and cytotoxicity of selected medicinal plants from Kenya. J Ethnopharmacol. 2009;123:504–9.
Bbosa S, Kyegombe DB, Lubega A, Musisi N, Ogwal-Okeng J, Odyek O. Anti-Plasmodium falciparum activity of Aloe dawei and Justicia betonica. Afr J Pharm Pharmacol. 2013;7:2258–63.
Muregi FW, Chhabra SC, Njagi ENM, Lang’at-Thoruwa CC, Njue WM, Orago ASS, et al. In vitro anti-plasmodial activity of some plants used in Kisii, Kenya against malaria and their chloroquine potentiation effects. J Ethnopharmacol. 2003;84:235–9.
Bihonegn T, Giday M, Yimer G, Animut A, Sisay M. Anti-malarial activity of hydromethanolic extract and its solvent fractions of Vernonia amygdalina leaves in mice infected with Plasmodium berghei. SAGE Open Med. 2019;7:1–10.
Abosi AO, Raseroka BH. In vivo anti-malarial activity of Vernonia amygdalina. Br J Biomed Sci. 2003;6:89–91.
Omollo CO. The anti-malarial and biochemical studies of Microglossa pyrifolia and Trimeria grandifolia from Lake Victoria Basin, Kenya. MSc Thesis, Jomo Kenyatta University of Agriculture and Technology, Kenya, 2011.
Zirihi GN, Mambu L, Guede-Guina F, Bodo B, Grellier P. In vitro anti-plasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. J Ethnopharmacol. 2005;98:281–5.
Acknowledgements
We are grateful to Pharm-Biotechnology and Traditional Medicine Centre (PHARMBIOTRAC), Mbarara University of Science and Technology (MUST) for offering conducive working environment with reliable internet services and access to various journal databases which rapidly facilitated the literature search process.
Funding
No funding being received to support the review process.
Author information
Authors and Affiliations
Contributions
JRA conceived the idea and concept of this review, searched for literature, synthesized the retrieved information from the articles, and drafted the manuscript. JT and NCN provided technical guidance throughout the process of the review. All the authors reviewed and approved the final copy of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publications
Not applicable.
Competing interests
The authors declare no conflict of interest whether personal or financial that would have influenced the outcomes of this review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Angupale, J.R., Tusiimire, J. & Ngwuluka, N.C. A review of efficacy and safety of Ugandan anti-malarial plants with application of RITAM score. Malar J 22, 97 (2023). https://doi.org/10.1186/s12936-023-04486-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-023-04486-6