Abstract
Background
Understanding the relationship between malaria infection risk and disease outcomes represents a fundamental component of morbidity and mortality burden estimations. Contemporary data on severe malaria risks among populations of different parasite exposures are scarce. Using surveillance data, we compared rates of paediatric malaria hospitalisation in areas of varying parasite exposure levels.
Methods
Surveillance data at five public hospitals; Jinja, Mubende, Kabale, Tororo, and Apac were assembled among admissions aged 1 month to 14 years between 2017 and 2018. The address of each admission was used to define a local catchment population where national census data was used to define person-year-exposure to risk. Within each catchment, historical infection prevalence was assembled from previously published data and current infection prevalence defined using 33 population-based school surveys among 3400 children. Poisson regression was used to compute the overall and site-specific incidences with 95% confidence intervals.
Results
Both current and historical Plasmodium falciparum prevalence varied across the five sites. Current prevalence ranged from < 1% in Kabale to 54% in Apac. Overall, the malaria admission incidence rate (IR) was 7.3 per 1000 person years among children aged 1 month to 14 years of age (95% CI: 7.0, 7.7). The lowest rate was described at Kabale (IR = 0.3; 95 CI: 0.1, 0.6) and highest at Apac (IR = 20.3; 95 CI: 18.9, 21.8). There was a correlation between IR across the five sites and the current parasite prevalence in school children, though findings were not statistically significant. Across all sites, except Kabale, malaria admissions were concentrated among young children, 74% were under 5 years. The median age of malaria admissions at Kabale hospital was 40 months (IQR 20, 72), and at Apac hospital was 36 months (IQR 18, 69). Overall, severe anaemia (7.6%) was the most common presentation and unconsciousness (1.8%) the least common.
Conclusion
Malaria hospitalisation rates remain high in Uganda particularly among young children. The incidence of hospitalized malaria in different locations in Uganda appears to be influenced by past parasite exposure, immune acquisition, and current risks of infection. Interruption of transmission through vector control could influence age-specific severe malaria risk.
Similar content being viewed by others
Background
Despite reductions in the risk of malaria infection, morbidity and mortality across sub-Saharan Africa (SSA) over the last two decades, gains have not been uniform in the region [1, 2]. Six countries account for nearly half of the world’s malaria disease burden including Nigeria (25%), Democratic Republic of the Congo (12%), Uganda (5%), and Cote d’Ivoire, Mozambique and Niger (4% each) [2]. The latest figures for Africa suggest that life-threatening morbidity leading to death from malaria across Africa remains unacceptably high [2].
During the 1990s and early 2000s the relationship between the frequency of malaria parasite exposure and severe disease incidence was the subject of intense investigation across different transmission settings in African [3,4,5,6,7,8,9,10,11,12]. These studies formed the basis of provisional modelling of the relationship between infection exposure and severe disease outcomes [13,14,15,16]. The likelihood of a new malaria infection resulting in life-threatening disease is principally determined by the ability of the host to mount an immune response; a function of previous parasite exposure, age and prompt treatment of mild disease with effective medicines. The relationship between parasite exposure and disease outcome represents the fundamental basis of the determination of the malaria disease burden in time and place [17,18,19]. Over the last 15 years there have been few investigations on the clinical epidemiology of severe malaria, notably during a time of changing parasite transmission intensity and treatment access across SSA, with research into severe malaria becoming a neglected topic [20].
In Uganda, despite a reduction in national under-five malaria prevalence from 30.4% in 2016 [21] to 16.9% in 2018–2019 [22], modelled projections, show that the country experienced approximately 12.3 million malaria cases and 13,203 malaria deaths in 2018, with little evidence of change since 2016 [2]. Malaria continues to contribute to 20–30% of all paediatric admissions to hospitals across the country [23], overwhelming emergency clinical services. The last comparisons of the rates and clinical phenotypes of hospitalized malaria, at three sites in Uganda, was undertaken in 2002–2003 [7]. Almost 20 years on, the link between a history of infection and severe disease is poorly defined, limiting our ability to understand the effects of infection prevention through vector control on disease profile in Uganda. Here we present a comparison of the rates of malaria hospitalisation and the clinical spectrum of severe disease across five catchment areas close to district hospitals where the historical and current malaria parasite exposure has been characterized.
Methods
Hospital surveillance
In 2010, the Uganda Malaria Surveillance Project (UMSP), in collaboration with the National Malaria Control Programme (NMCP), established an inpatient malaria surveillance program in the children’s wards at six public hospitals [24,25,26,27]. These six hospitals reflected the diversity of malaria transmission across Uganda. A structured Medical Record Form (MRF) was developed, enabling a standardized information record system for all admissions at participating sites. The MRF facilitated capture of detailed individual level information including patient demographics, residential address, presenting symptoms and clinical signs, laboratory test results, treatment prescribed, diagnosis and outcome upon discharge. Attending nurses, clinical officers, and physicians completed the MRF. UMSP supplied MRFs and basic malaria laboratory supplies. Health workers were trained on the use of the MRF, importance of good medical record keeping, use of data to improve the quality of services, and proper case management of uncomplicated and severe malaria. Emphasis was placed on ensuring all children were screened for malaria parasites using microscopy and/or malaria Rapid Diagnostic Tests (mRDT) on admission or during admission. Haemoglobin concentrations were only performed when requested by clinicians.
In 2016, the inpatient malaria surveillance program was expanded to provide a platform for investigation of aetiologies of Acute Febrile Illnesses (AFI) [27]. Improvements were made to the coverage of routine malaria testing and the documentation of patient’s physical address. The MRF was adapted to incorporate additional variables relevant to expanded bacterial and viral diagnostic capacity, described elsewhere [27]. Under the AFI program, the data management system was upgraded to a web-based data management system hosted on the District Health Information System (DHIS-2), allowing for real time data entry.
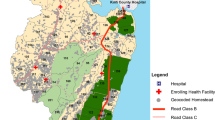
The present study focused on a retrospective analysis of admissions to five hospitals from 2017 to 2018 among children aged 1 month to 14 years from communities located close to five hospitals shown in Fig. 1 and Table 1. Arua hospital was not included in this analysis because it served a large refugee community hence might not have represented a stable population subject to local malaria exposure.
Case definitions
Patient diagnoses were made at admission and at discharge. A check list of most common diagnoses was incorporated in the MRF. Upon evaluation of patients, based on their judgment informed by history, examination findings and available test results, clinicians checked one or more diagnosis. It was not unusual for children to have more than one diagnosis; occasionally complications of illness were listed as diagnoses. A free text field was included for recording diagnosis not included in the list of available diagnosis. Patients who had any record under the possible diagnoses, or notes, of AIDS, TB, Sickle Cell Disease, epilepsy, congenital malformations, poisoning, burns, trauma, snake or animal bites, elective surgery or measles were immediately excluded from the data series, irrespective of whether they had a diagnosis of malaria. Here we presumed the underlying conditions to be the primary cause for admission and malaria a coincidental diagnosis. Patients who had any record of malaria were then identified and checked against malaria test (microscopy or mRDT) results, those who had a negative malaria test results were reclassified as unknown or replaced by other diagnoses if reported.
Malaria admissions were further classified according to the criteria of severe malaria approximating to those defined by the WHO [30] including, cerebral malaria, severe malaria anaemia, respiratory distress and haemoglobinuria, based on admission symptoms, signs, and laboratory findings. Consciousness was defined through observation of the child at admission. Unconsciousness was not formally evaluated using a Blantyre or Glasgow coma score, rather an APVU (Alert, responds to Pain or Voice or Unconscious) score was used [31], where U, approximates to a Blantyre Coma Score of 3 or less. Given the incomplete nature of haemoglobin results on all malaria admissions, it was not possible to use the strict definition of severe malaria anaemia (SMA) [30]. Therefore, SMA was classified if a) available haemoglobin results at, or during, admission was< 5 g/dl; b) for those without a haemoglobin result < 5.0 g/dl, if the child received blood transfusion during admission; and then c) if not classified by a) and b) whether they presented on admission with clinical signs of severe pallor. Respiratory distress was classified as deep breathing recorded through observation at admission. Haemoglobinuria was classified as present based on a reported history of passing ‘tea’ coloured urine by caretakers of the admitted child. Other features of severe malaria [30] could not be classified from information available on the MRFs, including prostration (age relevant inability to sit, stand or breastfeed not standardized between sites), hyper-parasitaemia (parasite counts per microlitre not recorded) or multiple convulsions within 24 h. Whether a child had had a seizure during the illness episode was documented, however, the number, type and duration of seizures was not recorded. The outcome of admission was incomplete across all hospitals, including large numbers having absconded prior to formal discharge, making case-fatality rates impossible to compute overall or for specific disease phenotypes. All relevant data were abstracted from the electronic MRF databases. All the subsequently selected patients aged 1 month to 14 years from the catchment areas with missing data in the electronic database were crosschecked against original hard copy clinical and laboratory registers for completeness. A set of unique identifiers were used to match electronic records to entries in the register.
Defining residences and denominators
A variety of information on each patient’s residential address was available on the MRF. This information was used to locate residential parishes (third level census administration areas). Lists of village names per parish, Google Earth and other digital place name gazetteers [32] were used to provide a parish code for all the malaria admissions. Rural, or peri-urban, parishes were then selected that were closest, within 20 km, to the hospital. Urban residences were excluded, as anecdotal evidence suggests that they may have been provided by visitors from other areas rather than local residents. Twenty kilometers is within the distances used to define optimized hospital catchments in Tanzania [33], Uganda [34, 35] and Kenya [36,37,38], accounting for most acute febrile admissions. Furthermore, the selection of parishes close to the sentinel hospital excluded communities that may have had better access to “competing” hospitals elsewhere in respective regions.
The selected parishes were then used to extract total population counts from the 2014 national census [39], projected to 2018 using reported district intercensal growth rates. Total population counts were corrected to single year age group (0-, 1-, 2- … 14 years) denominators using regional rural, household census data from the 2016 Demographic and Health Survey in each respective region [40]. Age-specific populations at risk were finally corrected to the number of person-years-of observation depending on the surveillance period, with the first year of life adjusted for 11 months.
Defining transmission intensity
To understand the role of parasite exposure on the epidemiology of severe malaria risks both historical and current infection prevalence were defined. It was not possible to identify specific survey data on community-based malaria prevalence for each of the catchment areas selected for the present study. However, district-wide survey data undertaken between 2010 and 2016 among children aged less than 15 years, where at least 15 children had been examined with microscopy were identified from previous datasets [1, 29] updated with other sub-national survey data [41]. Data were aggregated as mean estimates of age-corrected (2–10 years) prevalence and crude estimates of prevalence (total positives/total examined) for all surveys 2010–2016 pre-hospital surveillance period.
To represent contemporary infection prevalence, school-based surveys within the catchment parishes selected for the 2017–2018 surveillance period were undertaken at the end of the hospital recruitment period in 2019. School children provide a simple, reliable source of community-level malaria prevalence [42, 43]. Catchment parishes were used to define sample size. An estimate of expected district prevalence was used from previously analysed malaria prevalence risk undertaken by the NMCP [44]. A precision for the estimate was set at 5% and the confidence interval set at 95%, and assuming a non-response rate of 10%. Based on these assumptions, a total of 3400 children in 33 schools participated in the survey. For each area, schools were randomly selected from district inventories of public, day schools, 100 to 150 children were randomly selected to participate from classes Primary One to Primary Six from each school in the region. Children of consenting parents were interviewed to collect information on demographics, mosquito net usage during the night prior to the survey, use of indoor residual house-spraying in their homesteads and recent history of illness including fever. A finger prick blood sample was collected for thick and thin blood smear and a malaria rapid diagnostic test (mRDT) (CareStart™) for detection of malaria parasites. Thick and thin blood smears were stained with 2% Giemsa for 30 min. 100 high power fields were used to detect and quantify infection using experienced UMSP microscopists. mRDT results were read as per manufacturer’s instructions. Children with positive mRDT results were treated with artemether-lumefantrine as per recommended national guidelines [45]. Slide results were considered final if the first and second expert readings agreed on parasites densities (< 25% level of disagreement). If the first and second expert readings were discordant, a third expert-reader was used to arbitrate.
Analysis
All clinical data were stored on the DHIS-2 platform and school data were double entered using EpiData (v3.1). Data analysis was performed in STATA (version 14; STATA Corp., College Station, TX, USA) and R version 3.6.1 (R Core Team (2019), Vienna, Austria). Patient’s demographics were summarised as proportions and median ages with interquartile range (IQR). Kruskal Wallis was used to test for differences in median age. The incidence rate (IR) of hospitalised malaria was defined as the number of cases with a primary diagnosis of malaria (confirmed by microscopy or mRDT) divided by person-years of observation expressed per 1000 people. Since re-admissions were not documented all malaria admissions were included in the IR calculation, and therefore strictly best referred to as period prevalence. The prevalence of each severe malaria phenotype was defined as the number of hospitalised children with a specified severe malaria phenotype divided by the number of all hospitalised children without missing information required to classify by that phenotype. The age specific, site specific, and syndrome specific IR with 95% confidence intervals were computed using the exact Poisson distribution. Poisson regression was used to compute the incidence rate ratios (IRR) for the age groups < 1 year, 1–4 years, 5–9 years and 10–14 years, adjusted for site. The association between parasite prevalence and IR of malaria hospitalisation was determined using a Spearman rank correlation coefficient (rho) and confidence intervals constructed using Fisher’s z transformation.
Results
Characteristics of study sites
The five rural, catchment parishes used to define hospital admission rates are shown in Table 1 and Fig. 1. Surveillance at each of the five hospitals started at different times in 2017 and observation periods ranged between 21 and 24 months, ending in December 2018 (Table 1). The projected population years of observation for children aged 1 month to less than 15 years within each catchment area is shown in Table 1. Catchment areas were between 2 and 19 km from respective hospitals.
The five selected hospital sites (Fig. 1) and the districts where they are located, represent historically stable, endemic malaria ecologies as demonstrated by the high frequencies of the heterozygote state of the sickle cell gene (haemoglobin AS) and high average annual rainfall. The exception being the high-altitude district of Kabale; a low transmission setting (Table 1). Between 2010 and 2016, prior to the hospital surveillance, community-based childhood parasite prevalence in the five districts ranged from 4.9% in Kabale to 56.3% in Tororo. During this period the natural endemicity in Apac was altered using biannual rounds of indoor residual spraying (IRS) with Bendiocarb from 2010 to 2014 [46] (Table 1). IRS was suspended in Apac in May 2014 [47], however one round of IRS with Actellic® was conducted in May 2017 [48]. In Tororo, biannual IRS with Bendiocarb began in December 2014 through 2015, in 2016 IRS involved annual spray rounds with Actellic® [49] and continued throughout the hospital surveillance period 2017–2018 (Table 1). Vector control in Mubende, Kabale and Jinja has relied only on Long-Lasting Insecticide treated Net (LLIN) distribution.
The school-based surveys among children aged 6–16 years, undertaken at the end of the hospital surveillance period, demonstrated a diverse pattern of malaria endemicity ranging from < 1% Plasmodium falciparum slide positivity in Kabale to 54% in Apac (Table 1). These estimates of community-based infection prevalence reflect historical transmission ecology (Kabale) and recent suspension (Apac) or introduction (Tororo) of IRS. Furthermore, reported LLIN use the night prior to the survey by school children was lowest in Apac (24%), and between 40 and 90% in all other catchment areas (Table 1).
Age and malaria admissions from selected parishes
One thousand seven hundred eight admissions aged 1 month to 14 years of age with a primary diagnosis of malaria, confirmed by microscopy and/or mRDT, without underlying causes for admission were identified with a residence within selected catchment areas across the five hospitals. The overall hospitalisation incidence rate (IR) across the combined sites was 7.3 (95% CI 7.0, 7.7) per 1000 children 1 month to 14 years per annum. The annualized rates of malaria hospitalisation varied significantly between hospital catchments, with the lowest rates observed at the very low transmission site at Kabale (IR = 0.3; 95% CI: 0.1, 0.6) and the highest at Apac (IR = 20.3; 95% CI: 18.9, 21.8) (Table 2).
Among all admissions the median age of malaria hospitalisation was 30 months (IQR: 17.5, 60), 1257 (73.6%) of all admissions were aged less than 5 years. Children presenting to Kabale hospital had the highest median age (40 months; IQR: 20,72), followed by Apac (36 months; IQR: 18, 69) and the remaining sites the median age of the malaria admissions was between 27 and 30 months (Table 2). Except for Kabale, rates of admission at each of the other four sites increased after the first birthday, peaking during the second year of life, and declining thereafter (Fig. 2). Rates of malaria hospitalisation were lower among children aged 5–9 years (IRR = 0.28; 95% CI: 0.25, 0.32; p < 0.001) and among those aged 10–14 years (IRR = 0.09; 95% CI: 0.07, 0.11) as compared to children aged 1–4 years after adjusting for site (Supplement S1).
There was correlation between current catchment-specific school-based infection parasite prevalence and the overall malaria admission incidence rate in each site (rho = 0.70; 95% CI: − 0.48, 0.98) (Fig. 3), although this did not reach statistical significance (p = 0.19). The association remained unchanged even when we restricted the rates of admission to children aged 1–23 months of age (rho = 0.70; 95% CI: − 0.48, 0.98; p = 0.19), a period most likely representative of current transmission intensity (Supplement S2).
Prevalence of severe malaria phenotypes
Overall, unconsciousness (a surrogate marker of cerebral malaria, 30/1674; 1.8%) was the least common severe disease phenotype among all admissions. However, unconsciousness was present in 1/6 of the admissions at Kabale and was more common in Mubende (19/253; 7.5%) compared to other sites (Table 3). The composite definition of severe malaria anaemia (SMA) depended on the availability of information, a total of 129 (7.5%) SMA events were recorded, 37 based on haemoglobin, 63 based on a history of blood transfusion and 29 based on a presentation of severe pallor. Of all admissions, 34 (2%) and 10 (0.6%) patients could not be classified for unconsciousness and SMA, respectively, for lack of relevant information. Overall, the composite indicator of SMA (129/1690; 7.6%) was considerably more common than unconsciousness and most prevalent among malaria admissions at Mubende (51/253; 20.2%) and least prevalent in Apac (15/762; 2.0%) (Table 3). No children with SMA were noted in Kabale hospital. Evidence of respiratory distress was present among 55/169 (3.2%) malaria admissions, a history of passing ‘tea’ coloured urine (haemoglobinuria) was reported as present in 84/1691 (5%), and highest at Mubende 21/253 (8.3%) (Table 3). Overall, children who were unconscious (median age 60 months, IQR: 26, 84) were older than those who had severe anaemia (median age 36 months, IQR: 18, 72), respiratory distress (median age 19 months, IQR: 13, 42), and haematuria (median age 48 months, IQR: 23, 72), (p < 0.001) (Table 3).
Discussion
We have presented an ecological comparison of the rates of malaria hospitalisation against estimates of historical and current levels of malaria transmission from five communities in Uganda. Unsurprisingly, in areas of historical and contemporary very low transmission, paediatric malaria hospitalisation is uncommon (Kabale). Consistent with earlier observations in high transmission settings in Africa during the 1990s-early 2000s [3,4,5,6,7,8,9,10,11,12], Apac exhibited an extremely high incidence of hospitalized malaria among children aged under 24 months of age (Fig. 2). However, unlike previous reported age-prevalence and incidence estimates from areas with hyper-endemic transmission studied in the 1990s [3,4,5,6,7,8,9,10,11,12], Apac demonstrated an age-profile of hospitalized malaria incidence that included a much higher incidence among older children aged 5–10 years and 10–15 years (Table 2; Fig. 2). The unexpectedly high rate of malaria admissions among older (> 5 years) children in Apac, traditionally an area of intense transmission, may have resulted from the rapid reduction in malaria parasite exposure following the IRS campaigns in 2010–2014 [46]. The historical reduction in transmission may have facilitated the emergence of an ‘atypically’ non-immune cohort of older children in 2017–18 unusually susceptible to malaria and its complications. Periods of sustained malaria control, followed by relaxation or withdrawal of effective interventions, has been shown to result in rebound [50], which was also documented in Apac [47].
Since 2014, the traditionally high transmission (Table 1) region in Tororo, continues to be under intensive IRS (Table 1) [49], sustaining protection against infection among children aged 1 month to 4 years of age at-risk of hospitalisation with malaria during the 2017–2018 surveillance period. In Tororo children older than 5 years would have been exposed to high transmission intensity from birth (Table 1). Contemporary estimates of infection prevalence are 7.5% compared to an estimated historical prevalence above 50% (Table 1). The age pattern and broad overall rate of malaria hospitalisation were similar in Tororo compared to Jinja and Mubende, with current transmission between 10 and 32% respectively, with 74% of all paediatric admissions occurring before the 5th birthday and a peak in the second year of life (Table 2; Fig. 2). Despite a significant reduction in parasite exposure in Tororo, rates of malaria hospitalisation remain high compared to other sites of sustained high transmission (Mubende) or in transition without the use of IRS (Jinja). Infection risks have not been eliminated in Tororo and some premunition might still be occurring among young children. However, cessation of IRS might result in a changing age-profile of disease as witnessed in Apac.
Overall, cerebral malaria was comparatively uncommon as a severe complication of malaria admissions and when observed had a higher mean age compared to other severe disease phenotypes (Table 3). This has been recently confirmed in Kenya [31]. Severe malaria anaemia (SMA), as defined in our series based on available information on haemoglobin concentrations, transfusion history or severe pallor at admission, was a more common presentation, and consistent with earlier observations in Uganda [7] and recently reported in Kenya [31]. Those with SMA, while younger than patients presenting with cerebral malaria, include older children and this severe disease phenotype is not restricted to very young children, with 37.5% of those defined as SMA being 5–14 years of age. The observation of higher rates of SMA among older children requires further investigation and how to improve case-management in all pediatric age groups [51, 52]. Haemoglobinuria was reported as the presence of “tea colored urine” by caretakers among 4.8% of all malaria admissions (Table 3). The reported haemoglobinuria may be a part of the Blackwater fever syndrome, an increasingly common feature of severe malaria in parts of Uganda [53, 54], but interestingly might be less common outside Uganda [53].
We have described malaria admission among communities with relatively easy access to emergency care services in Uganda. The overall incidence of hospitalisation with malaria was 7 per 1000 children aged 1 month to 14 years of age per annum, ranging from very low levels in areas with very little transmission to over 20 per 1000 per annum in high transmission settings. These rates of hospitalized care for paediatric malaria are high and provide confirmatory evidence of the continued importance of malaria as a contributor to poor child survival in Uganda. Our estimates do not capture those with severe malaria who seek care elsewhere or die before reaching hospital. Treatment seeking for severe malaria remains poorly defined and requires further investigation, not just for the interpretation of hospitalised disease incidence but to also define points of improved pre-referral care [55,56,57]. Our results therefore represent approximate estimates of community acquired malaria requiring in-patient care. However, selecting catchments with relatively easy access to emergency care allows for a more reliable estimate of severe, potentially life-threatening disease incidence, compared to incidence defined among communities with limited access. A more comprehensive understanding of the epidemiology of severe, life-threatening disease and death requires prospective surveillance, however there are very few studies that have examined the long-term parasite exposure versus changing severe diseases profile in Africa [58].
The electronic AFI surveillance system operated at the sentinel hospitals introduced a standardized paediatric admission record system including universal malaria testing. This system provided opportunities to examine linked spatial and clinical data. Standardized admission record systems have been introduced in other settings, as part of routine health systems improvement tools [59,60,61]. Inevitably, the completeness and reliability of information available within hospital data systems depends upon resources and the intended objectives of surveillance programs. Hospital surveillance provides unique opportunities to monitor the changing clinical epidemiology of severe malaria and other febrile illnesses and with minimal investment could be implemented in a sustained form at national sentinel sites [62]. Investments are required for admission and data clerks, information and data systems and ensuring a basic minimum supply of laboratory supplies. These surveillance systems of severe disease we contend would be valuable resources to understand the changing landscape of severe disease epidemiology across Africa.
Conclusion
Our results suggest that the epidemiology of severe, life-threatening malaria is consistent with early and frequent parasite exposure, and subsequent acquisition of immunity. In high transmission settings, under conditions of intense malaria control and reduced transmission for several years, the risk of severe malaria shifts to older children. This shift manifests in later years, as an increase in risk of severe malaria in older children, otherwise unexpected if transmission had not been interrupted. However, despite this increase, severe disease remains a rare event in children aged 10 years and above. The present analysis highlights the importance of both historical and current malaria exposure upon patterns of severe disease in a community. Current disease risks are determined by present exposure, but in the context of historical exposure and acquired immunity, complicating interpretation of observations based on ecological comparisons between sites over time. However, linkage of well-defined communities with easy access to hospital care provides better insight into the changing disease epidemiology resulting from changing transmission intensity. Indeed, longer-term hospital surveillance linked to the geography of hospital access, malaria control interventions, and parasite exposure offers future possibilities for better understanding of the epidemiology of severe malaria in Africa.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AFI:
-
Acute Febrile Illness
- CI:
-
Confidence interval
- DHIS-2:
-
District Health Information System-2
- IQR:
-
Inter-quartile range
- IR:
-
Incidence rate
- IRS:
-
Indoor residual spraying
- mRDT:
-
Malaria Rapid Diagnostic Test
- LLIN:
-
Long-lasting insecticide treated nets
- MRF:
-
Medical Record Form
- NMCP:
-
National Malaria Control Programme
- SMA:
-
Severe malaria anaemia
- UMSP:
-
Uganda Malaria Surveillance Project
References
Snow RW, Sartorius B, Kyalo D, Maina J, Amratia P, Mundia CW, et al. The prevalence of Plasmodium falciparum in sub Saharan Africa since 1900. Nature. 2017;550:515–8.
World malaria report. Geneva: World Health Organization; 2018. License: CC BY-NC-SA 3.0 IGO. 2019.
Snow RW, Bastos de Azevedo I, Lowe BS, Kabiru EW, Nevill CG, Mwankusye S, et al. Severe childhood malaria in two areas of markedly different falciparum transmission in East Africa. Acta Trop. 1994;57:289–300.
Snow RW, Omumbo JA, Lowe B, Molyneux SM, Obiero JO, Palmer A, et al. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–4.
Slutsker L, Taylor TE, Wirima JJ, Steketee RW. In-hospital morbidity and mortality due to malaria-associated severe anaemia in two areas of Malawi with different patterns of malaria infection. Trans R Soc Trop Med Hyg. 1994;88:548–51.
Modiano D, Sirima BS, Sawadogo A, Sanou I, Pare J, Konate A, et al. Severe malaria in Burkina Faso: influence of age and transmission level on clinical presentation. Am J Trop Med Hyg. 1998;59:539–42.
Idro R, Aloyo J, Mayende L, Bitarakwate E, John CC, Kivumbi GW. Severe malaria in children in areas with low, moderate and high transmission intensity in Uganda. Tropical Med Int Health. 2006;11:115–24.
Issifou S, Kendjo E, Missinou MA, Matsiegui PB, Dzeing-Ella A, Dissanami FA, et al. Differences in presentation of severe malaria in urban and rural Gabon. Am J Trop Med Hyg. 2007;77:1015–9.
Reyburn H, Mbatia R, Drakeley C, Bruce J, Carneiro I, Olomi R, et al. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA. 2005;293:1461–9.
Okiro EA, Al-Taiar A, Reyburn H, Idro R, Berkley JA, Snow RW. Age patterns of severe paediatric malaria and their relationship to Plasmodium falciparum transmission intensity. Malar J. 2009;8:4.
Carneiro I, Roca-Feltrer A, Griffin JT, Smith L, Tanner M, Armstrong Schellenberg J, et al. Age-patterns of malaria vary with severity, transmission intensity and seasonality in sub-Saharan Africa: a systematic review and pooled analysis. PLoS One. 2010;5:e8988.
Roca-Feltrer A, Carneiro I, Smith L, Armstrong Schellenberg JRM, Greenwood BM, Schellenberg D. The age patterns of severe malaria syndromes in sub-Saharan Africa across a range of transmission intensities and seasonality settings. Malar J. 2010;9:282.
Gupta S, Snow RW, Donnelly C, Marsh K, Newbold C. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med. 1999;5:340–3.
Gupta S, Snow RW, Donnelly CA, Newbold C. Acquired immunity and postnatal clinical protection in childhood cerebral malaria. P Roy Soc B Biol Sci. 1999;266:33–8.
Ghani AC, Sutherland CJ, Riley EM, Drakeley CJ, Griffin JT, Gosling RD, et al. Loss of population levels of immunity to malaria as a result of exposure-reducing interventions: consequences for interpretation of disease trends. PLoS One. 2009;4:4383.
Griffin JT, Hollingsworth TD, Reyburn H, Drakeley CJ, Riley EM, Ghani AC. Gradual acquisition of immunity to severe malaria with increasing exposure. P Roy Soc B Biol Sci. 2015;282:20142657.
Greenwood BM, Marsh K, Snow RW. Why do some children develop severe malaria? Parasitol Today. 1991;7:277–81.
Snow RW, Marsh K. The consequences of reducing transmission of Plasmodium falciparum in Africa. Adv Parasitol. 2002;52:235–64.
Rodriguez-Barraquer I, Arinaitwe E, Jagannathan P, Kamya MR, Rosenthal PJ, Rek J, et al. Quantification of anti-parasite and anti-disease immunity to malaria as a function of age and exposure. eLife. 2018;7:e35832.
Maitland K. Severe malaria in African children—the need for continuing investment. New Engl J Med. 2016;375:2416–7.
Uganda Bureau of Statistics (UBOS), ICF International. Uganda malaria indicator survey 2014–15. Kampala and Rockville: UBOS and ICF International; 2015.
Uganda National Malaria Control Division (NMCD), Uganda Bureau of Statistics (UBOS), and ICF. Uganda malaria indicator survey 2018–19. Kampala and Rockville: NMCD, UBOS, and ICF; 2019.
Presidents Malaria Initiative. Uganda operational plan FY 2019. 2019. https://www.pmi.gov/docs/default-source/default-document-library/malaria-operational-plans/fy19/fy-2019-uganda-malaria-operational-plan.pdf?sfvrsn=3. Accessed Feb 2020.
Mpimbaza A, Sears D, Sserwanga A, Kigozi R, Rubahika D, Nadler A, et al. Admission risk score to predict inpatient pediatric mortality at four public hospitals in Uganda. PLoS One. 2015;10:e0133950.
Mpimbaza A, Miles M, Sserwanga A, Kigozi R, Wanzira H, Rubahika D, et al. Short report: comparison of routine health management information system versus enhanced inpatient malaria surveillance for estimating the burden of malaria among children admitted to four hospitals in Uganda. Am J Trop Med Hyg. 2015;92:18–21.
Sserwanga A, Sears D, Kapella BK, Kigozi R, Rubahika D, Staedke SG, et al. Anti-malarial prescription practices among children admitted to six public hospitals in Uganda from 2011 to 2013. Malar J. 2015;14:331.
Lamorde M, Mpimbaza A, Walwema R, Kamya M, Kapisi J, Kajumbula H, et al. A cross-cutting approach to surveillance and laboratory capacity as a platform to improve health security in Uganda. Health Secur. 2018;16:S76–86.
Ndeezi G, Kiyaga C, Hernandez AG, Munube D, Howard TA, Ssewanyana I, et al. Burden of sickle cell trait and disease in the Uganda Sickle Surveillance Study (US3): a cross-sectional study. Lancet Glob Health. 2016;4:195–200.
Snow RW. The prevalence of Plasmodium falciparum in sub Saharan Africa since 1900. Harvard Dataverse, V1, under a CC-BY 4.0 license; 2017. https://doi.org/10.7910/DVN/Z29FR0.
World Health Organization. Severe malaria. Tropical Med Int Health. 2014;19(s1):7–131.
Akech S, Chepkirui M, Ogero M, Agweyu A, Irimu G, English M, et al. The clinical profile of severe paediatric malaria in an area targeted for routine RTS,S/AS01 malaria vaccination in Western Kenya. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciz844.
Maina J, Ouma PO, Macharia PM, Alegana VA, Mitto B, Soce-Fall I, et al. A spatial database of health facilities managed by the public health sector in sub Saharan Africa. Sci Data. 2019;6:134.
Manongi R, Mtei F, Mtove G, Nadjm B, Muro F, Alegana V, et al. Inpatient child mortality by travel time to hospital in a rural area of Tanzania. Tropical Med Int Health. 2014;19:555–62.
Okiro EA, Bitira D, Mbabazi G, Mpimbaza A, Alegana VA, Talisuna A, et al. Increasing malaria hospital admissions in Uganda between 1999 and 2009. BMC Med. 2011;9:e37.
Zinszer K, Charland K, Kigozi R, Dorsey G, Kamya MR, Buckeridge DL. Determining health-care facility catchment areas in Uganda using data on malaria-related visits. Bull World Health Organ. 2014;92:178–86.
Okiro EA, Alegana VA, Noor AM, Snow RW. Changing malaria intervention coverage, transmission and hospitalization in Kenya. Malar J. 2010;9:285.
Moisi JC, Nokes DJ, Gatakaa H, Williams TN, Bauni E, Levine OS, et al. Sensitivity of hospital-based surveillance for severe disease: a geographic information system analysis of access to care in Kilifi district, Kenya. Bull World Health Organ. 2011;89:102–11.
Alegana VA, Khazeni C, Akech S, Snow RW. Investigating hospital catchments from in-patient malaria admissions records: a spatial statistical approach. Sci Rep. 2020;10:1324.
Uganda Bureau of Statistics (UBOS). Kampala: The National Population and Housing Census 2014 – Main Report; 2016.
Uganda Bureau of Statistics (UBOS), ICF. Uganda demographic and health survey 2016. Kampala and Rockville: UBOS and ICF; 2018.
Maziarz M, Kinyera T, Otim I, Kagwa P, Nabalende H, Legason ID, Ogwang MD, et al. Age and geographic patterns of Plasmodium falciparum malaria infection in a representative sample of children living in Burkitt lymphoma-endemic areas of northern Uganda. Malar J. 2017;16:124.
Brooker S, Kolaczinski JH, Gitonga CW, Noor AM, Snow RW. The use of schools for malaria surveillance and programme evaluation in Africa. Malar J. 2009;8:231.
Chacky F, Runge M, Rumisha SF, Machafuko P, Chaki P, Massaga J, et al. Nationwide school malaria parasitaemia survey in public primary schools, the United Republic of Tanzania. Malar J. 2018;17:452.
Talisuna AO, Noor AM, Mundia CW, Otieno V, Mitto B, Amratia P, et al. An epidemiological profile of malaria and its control in Uganda. Analysis and report funded by Roll Back Malaria and Department for International Development, UK. 2013. http://www.inform-malaria.org/wp-content/uploads/2014/05/Uganda-Epi-Report-060214.pdf.
Uganda Ministry of Health. Uganda clinical guidelines. National guidelines for management of common conditions, vol. Chapter 2; 2016. p. 195.
Kigozi R, Baxi SM, Gasasira A, Sserwanga A, Kakeeto S, Nasr S, et al. Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PLoS One. 2012;7:e42857.
Raouf S, Mpimbaza A, Kigozi R, Sserwanga A, Rubahika D, Katamba H, et al. Resurgence of malaria following discontinuation of indoor residual spraying of insecticide in an area of Uganda with previously high-transmission intensity. Clin Infect Dis. 2017;65:453–60.
Uganda Ministry of Heath. National malaria control division. Annual report. July 2017 to June 2018. 2016. https://health.go.ug/content/national-malaria-control-division-july-2017-%E2%80%93-june-2018-annual-report. Accessed Feb 2020.
Zinszer K, Charland K, Vahey S, Jahagirdar D, Rek JC, Arinaitwe E, et al. The impact of multiple rounds of indoor residual spraying on malaria incidence and hemoglobin levels in a high-transmission setting. J Infect Dis. 2020;221:304–12.
Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, Moonen B. Malaria resurgence: a systematic review and assessment of its causes. Malar J. 2012;11:122.
Opoka RO, Ssemata AS, Oyang W, Nambuya H, Chandy CJ, Karamagi C, et al. Adherence to clinical guidelines is associated with reduced inpatient mortality among children with severe anemia in Ugandan hospitals. PLoS One. 2019;14:e0210982.
Maitland K, Kiguli S, Olupot-Olupot P, Engoru C, Mallewa M, Saramago Goncalves P, et al. Immediate transfusion in African children with uncomplicated severe anemia. New Engl J Med. 2019;381:407–19.
Olupot-Olupot P, Engoru C, Uyoga S, Muhindo R, Macharia A, Kiguli S, et al. High frequency of blackwater fever among children presenting to hospital with severe febrile illnesses in eastern Uganda. Clin Infect Dis. 2017;64:939–46.
Conroy AL, Hawkes M, Elphinstone RE, Morgan C, Hermann L, Barker KR, et al. Acute kidney injury is common in pediatric severe malaria and is associated with increased mortality. Open Forum Infect Dis. 2016;3:ofw046.
Byakika-Kibwika P, Ndeezi G, Kamya MR. Health care related factors associated with severe malaria in children in Kampala, Uganda. Afri Health Sci. 2009;9:206–21.
Ilunga-Ilunga F, Leveque A, Ngongo LO, Laokri S, Dramaix M. Treatment-seeking paths in the management of severe malaria in children under 15 years of age treated in reference hospitals of Kinshasa, Democratic Republic of Congo. Tropical Med Int Health. 2015;43:11–9.
Mpimbaza A, Ndeezi G, Katahoire A, Rosenthal PJ, Karamagi C. Demographic, socioeconomic, and geographic factors leading to severe malaria and delayed care seeking in Ugandan children: a case–control study. Am J Trop Med Hyg. 2017;97:1513–23.
Njuguna P, Maitland K, Nyaguara A, Mwanga D, Mogeni P, Mturi N, et al. Observational study: 27 years of severe malaria surveillance in Kilifi, Kenya. BMC Med. 2019;17:124.
Ayieko P, Ntoburi S, Wagai J, Opondo C, Opiyo N, Migiro S, et al. A multifaceted intervention to implement guidelines and improve admission paediatric care in Kenyan District Hospitals: a cluster randomised trial. PLoS Med. 2011;8:e01001018.
Gachau S, Ayieko P, Gathara D, Mwaniki P, Ogero M, Akech S, et al. Does audit and feedback improve the adoption of recommended practices? Evidence from a longitudinal observational study of an emerging clinical network in Kenya. BMJ Glob Health. 2017;2:e000468.
Irimu G, Ogero M, Mbevi G, Agweyu A, Akech S, Julius T, et al. Approaching quality improvement at scale: a learning health system approach in Kenya. BMJ. 2018;103:1013–9.
English M, Mwaniki P, Julius T, Chepkirui M, Ouma PO, Cherutich P, et al. Hospital mortality – a neglected but rich source of information supporting the transition to high quality health systems in low and middle-income countries. BMC Med. 2018;16:32.
Acknowledgments
The authors are grateful to the clinical and nursing staff at each of the five hospitals for their continued support for the surveillance. We are also indebted to the Uganda Malaria Surveillance data officers; Jospeh Wadamba, William Wanyi, Praise Tusasirwe, Mercy Issali for their commitment to the project, and Proper Bihumuza and the HISP team for development of the web-based data management system. Ronald Bampiga and Sally Opus are acknowledged for their technical support to the school-based malaria surveys in 2019. We are grateful to Sam Mbulaiteye for providing unpublished historical survey data from the five districts, Ronald Mulebeke for help with developing the malaria schools survey and David Kyalo and Victor Alegana for their help with identification of admission addresses, age-structured household survey census data and mapped graphics. We would also like to thank Professor Moses Kamya, Drs Mohammed Lamorde and Adoke Yeka for their continued support for the surveillance program.
Funding
This work was supported through cooperative agreement number 5NU2GGH001744–02-00, funded by the US Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services. RWS is funded by Wellcome Trust Principal Fellowship (number212176), that also provided support for the school surveys. AK is supported by the DELTAS Africa Initiative [DEL-15-003]. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency with funding from the Wellcome Trust [107769] and the UK government. AK and RWS acknowledge the support of the Wellcome Trust to the Kenya Major Overseas Programme (number 203077).
Author information
Authors and Affiliations
Contributions
AM and RWS conceived the study; AM, RWS and AK analysed the data. SGS and GD provided critical input to the data interpterion. AM and RWS drafted the first version of the manuscript. RW, JK, AS, JFN, YK, AT, JFN, DR and JO participated in setting up the surveillance program and collection of data. All authors reviewed the final manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The surveillance included the collection of routine hospital data approved as public health surveillance/non-research by CDC (NCEZID #031416), and approved by the Director General of Health Services, Ministry of Health, Uganda. All data were anonymised without patients or guardian’s names prior to analysis. The school survey was approved by the Vector Control Division Research and Ethics Committee (VCDREC/117) and the Uganda National Council of Science and Technology (SS5115). Written informed consent was obtained from each child’s parent/guardian before any study-specific procedures were done, and consent documents were available in the local language. For children aged 8 years and above assent was sought in addition to the consent of the child’s parent/guardian.
Consent for publication
“Not applicable” in this section.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Supplement S1.
The incidence rate ratio (IRR) of age group adjusted for site obtained from a Poisson regression model.
Additional file 2: Supplement S2.
Malaria admission rates for children aged 1 month-23 months per 1000 person-years of observation versus school-based malaria parasite prevalence at the end of the surveillance period.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mpimbaza, A., Walemwa, R., Kapisi, J. et al. The age-specific incidence of hospitalized paediatric malaria in Uganda. BMC Infect Dis 20, 503 (2020). https://doi.org/10.1186/s12879-020-05215-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-020-05215-z