Abstract
Background
Artemisinin-based combination therapy (ACT) has been recommended as the first-line treatment by the World Health Organization to treat uncomplicated Plasmodium falciparum malaria. However, the emergence and spread of P. falciparum resistant to artemisinins and their partner drugs is a significant risk for the global effort to reduce disease burden facing the world. Currently, dihydroartemisinin-piperaquine (DHA-PPQ) is the most common drug used to treat P. falciparum, but little evidence about the resistance status targeting DHA (ACT drug) and its partner drug (PPQ) has been reported in Shandong Province, China.
Methods
A retrospective study was conducted to explore the prevalence and spatial distribution of Pfk13 and Pfcrt polymorphisms (sites of 72–76, and 93–356) among imported P. falciparum isolates between years 2015–2019 in Shandong Province in eastern China. Individual epidemiological information was collected from a web-based reporting system were reviewed and analysed.
Results
A total of 425 P. falciparum blood samples in 2015–2019 were included and 7.3% (31/425) carried Pfk13 mutations. Out of the isolates that carried Pfk13 mutations, 54.8% (17/31) were nonsynonymous polymorphisms. The mutant alleles A578S, Q613H, C469C, and S549S in Pfk13 were the more frequently detected allele, the mutation rate was the same as 9.7% (3/31). Another allele Pfk13 C580Y, closely associated with artemisinin (ART) resistance, was found as 3.2% (2/31), which was found in Cambodia. A total of 14 mutant isolates were identified in Western Africa countries (45.2%, 14/31). For the Pfcrt gene, the mutation rate was 18.1% (77/425). T76T356 and T76 were more frequent in all 13 different haplotypes with 26.0% (20/77) and 23.4% (18/77). The CVIET and CVIKT mutant at loci 72–76 have exhibited a prevalence of 19.5% (15/77) and 3.9% (3/77), respectively. The CVIET was mainly observed in samples from Congo (26.7%, 4/15) and Mozambique (26.7%, 4/15). No mutations were found at loci 97, 101 and 145. For polymorphisms at locus 356, a total of 24 isolates were identified and mainly from Congo (29.2%, 7/24).
Conclusion
These findings indicate a low prevalence of Pfk13 in the African isolates. However, the emergence and increase in the new alleles Pfcrt I356T, reveals a potential risk of drug pressure in PPQ among migrant workers returned from Africa. Therefore, continuous molecular surveillance of Pfcrt mutations and in vitro susceptibility tests related to PPQ are necessary.
Similar content being viewed by others
Background
Imported Plasmodium falciparum malaria from Africa, has become a great threat to malaria elimination in China [1]. In 2015–2019, 9702 cases of imported P. falciparum malaria have been reported in China, the top five countries of origin are Nigeria, Angola, Ghana, Cameroon and Equatorial Guinea, accounting for 60.4% (5863/9702) [2]. Artemisinin-based combinations are the first-line drugs used to treat uncomplicated P. falciparum as recommended by the World Health Organization (WHO) [3]. However, the emergence and spread of P. falciparum resistant to artemisinin and partner drugs used in ACT is a significant risk for the global effort to reduce disease burden facing the world, especially for the Greater Mekong Subregion (GMS) and Africa [4, 5]. Molecular marker studies identify and track the prevalence of key molecular mutations [6]. For example, the P. falciparum kelch-13 (Pfk13) gene, served as a molecular marker for artemisinin resistance (ART-R) isolates in a laboratory-based in vitro evolution study which was first observed at the Thai–Cambodia border in 2014, has spread to five countries in the GMS [4, 7]. So far, a total of 10 of the Pfk13 single nucleotide polymorphisms (SNPs) have been validated in vitro and in vivo as associated with delayed clearance following ACT [8]. Surveillance of Pfk13 polymorphisms associated with ART-R has also been undertaken in Africa. Recent publications indicated that the clonal expansions of R561H, which was associated with delayed parasite clearance among patients treated with ACT, have been detected in Rwanda [9]. In Uganda, C469Y and A675V, the candidate markers of ART-R, were also found in more than 15% of samples from 2018 to 2019 [10]. Similarity, the R561H was also identified as the main mutation site in Zhejiang Province among migrant workers from Rwanda [11]. In 2017, a patient infected with P. falciparum who had returned from Equatorial Guinea reported in Jiangsu Province, was found to harbour the Pfk13 M579I site, which was confirmed to be linked to ART-R with a 2.29% in vitro survival rate by ring-stage survival assay [12]. In addition, P. falciparum resistant to chloroquine (CQ), amodiaquine (AQ), or piperaquine (PPQ) harbour mutations in the P. falciparum chloroquine resistance transporter (Pfcrt), a transporter resident on the digestive vacuole membrane that can transport those weak-base 4-aminoquinoline drugs out of this acidic organelle. The Pfcrt gene K76T mutation, has been confirmed to be closely associated with CQ resistance [13, 14]. However, several novel mutations in Pfcrt were found to be associated with PPQ reduced susceptibility [15,16,17].
In Cambodia, Agrawal et al. [18] identified the locus F145I associated with a decrease in PPQ susceptibility. In a context of dihydroartemisinin-piperaquine (DHA-PPQ) resistance in Cambodia, novel Pfcrt mutations such as H97Y, M343L, and G353V were revealed to induce in vitro PPQ resistance [15]. Also treatment failures with DHA-PPQ were associated with T93S, H97Y, F145I and I218F mutations in Pfcrt and with plasmepsin 2/3 amplification in Cambodia, Thailand and Vietnam [16, 17]. Besides, the mutation I356T/L is often found both on Asian or South-American parasites [18,19,20].
Malaria was once endemic in the whole province, though no indigenous case reported in Shandong Province since 2012, whereas the imported P. falciparum has increased gradually. For example, the number of imported P. falciparum were 857 cases reported in Shandong in 2015–2019, accounting for nearly 8.8% of the total imported P. falciparum cases (n = 9702) nationwide.
The national drug policy of China was updated in 2006, since then ACT has been used as the first-line treatment for uncomplicated falciparum malaria, including DHA-PPQ, artesunate-amodiaquine (AS-AQ), artemisinin-naphthoquine phosphate (ART-NQ), and artemisinin-piperaquine (ART-PPQ). Currently, DHA-PPQ is the most common drugs used to treat P. falciparum and the resistance status targeting DHA and its partner drug (PPQ) needs to be understood. Therefore, in this study, Pfk13 and Pfcrt mutations in imported Africa and Southeast Asia patients reported in Shandong Province in eastern China from 2015 to 2019 were characterized. This study aimed to investigate and analyse the prevalence of these two gene biomarkers, of which mutant alleles confer to DHA-PPQ resistance.
Methods
Study sites and samples
The P. falciparum positive samples in this study were collected from symptomatic patients prior to anti-malarial treatment in Shandong Province from 2015 to 2019. The imported cases refer to the malaria cases or infections in which the infection was acquired outside the area in which it was diagnosed. In this study, it refers to the patient who acquired the illness from a known malaria-prevalent region outside China [21]. Individual epidemiological information was also collected from a web-based reporting system (China Information System for Diseases Control and Prevention) and analysed in this study.
A total of 425 P. falciparum blood samples from the migrant people who returned from Africa and Southeast Asia to Shandong in 2015–2019 were obtained and examined at enrollment. All the samples were microscopic and PCR positive for P. falciparum and were obtained from each patient. Demographic data of all cases including age, sex, occupation, the date of onset, interval from onset to confirmed diagnosis, interval from confirmed diagnosis to report, and source countries were recorded. For each specimen, approximately 200 µl of blood was collected from a finger prick and spotted on a piece of 3MM Whatman filter paper (GE Healthcare, Boston, MA, USA), which was allowed to air-dry. Each of the samples was labeled with a study number and stored at − 20 °C until extraction. The P. falciparum genomic DNA from approximately 20 µl of each dried blood sample was then extracted with a QIAamp DNA blood kit (QIAGEN, Valencia, CA) according to the manufacturer’s instructions.
Treatment and follow-up
Patients were treated by dihydroartemisinin and piperaquine according to the national anti-malarials regulation with a total adult dose of 2.5 mg/kg DHA and 20 mg/kg PPQ for 3 days. For follow-up, the thick and thin blood smear of the patients stayed in this hospital for treatment on Day 3 was collected. Giemsa-stained blood slides were prepared for Plasmodium speciation. Slides were examined and read by an expert microscopist certified as Level 1 by the WHO.
Molecular marker polymorphisms
To investigate polymorphisms in the Pfk13 (PF3D7_1343700), the Pfk13 gene were determined by nested PCR amplification of an 849-bp fragment (from amino acids 427–709) as described previously [4]. As for the Pfcrt (PF3D7_0709000), the primers for 72–76 sites of the Pfcrt gene were subjected to nested PCR amplification [22]. For the 93–356 sites of the Pfcrt gene, after 1 round of PCR amplification, the expected PCR product were obtained by 1.5% agarose gel electrophoresis as reported previously [23] and sent for Sanger sequencing (Shanghai BioTechnologies Co., Ltd., Shanghai, China).
Data analysis
Multiple nucleotide sequence alignments and analysis were performed using the DNAMAN software editor (https://www.lynnon.com/pc/framepc.html). Sequences were analyzed with the Blast online program (http://blast.ncbi.nlm.nih.gov/) using P. falciparum 3D7 strain as the reference control. R (Version 4.0.2) statistical software (R Foundation for Statistical Computing, Vienna, Austria) was adopted to conduct statistical analyses.
Ethical considerations
This study was reviewed and approved by the ethical committee of the National Institute of Parasitic Diseases, Chinese Centre for Disease Control and Prevention (NIPD, China CDC, No. 2019008).
Results
Demographics of P. falciparum infection
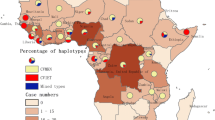
A total of 425 P. falciparum blood samples were involved in this study. Among all the participants, 418 (98.4%) were male. The patients were mainly found in the age group of 40–50 years old (n = 182, 42.8%) and the median age was 42 years old. The samples were mainly collected in Huancui (n = 21, 4.9%), Feicheng (n = 20, 4.7%), and Zhifu (n = 20, 4.7%; Fig. 1). The most common occupation was farmers (n = 196, 46.1%; Table 1). The imported P. falciparum cases were mainly reported in January (n = 52, 12.2%) and October (n = 50, 11.8%). The median (range) cases reported per month was 35 (20–52). The median interval from onset to confirmed diagnosis was 3.4 days, and the median interval from confirmed diagnosis to report was 1 days, respectively. A total of five deaths attributing to the P. falciparum were reported in the timeframe but no mutation alleles were detected in Pfk13 and Pfcrt. The P. falciparum cases were mainly imported from Nigeria (n = 88, 20.7%), Equatorial Guinea (n = 63, 14.8%), and the Democratic Republic of the Congo (Congo DRC) (n = 61, 14.4%).
Molecular analysis of Pfk13 polymorphisms
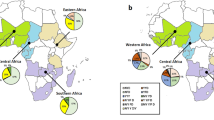
Out of all successful sequenced 425 P. falciparum isolates, a total of 31 (7.3%) isolates harboring the Pfk13 polymorphisms were identified in the returners. Among them, 17 (54.8%) were nonsynonymous polymorphisms, whereas the other 14 (45.2%) were synonymous polymorphisms, the ratio of nonsynonymous isolates to synonymous isolates was 1.2. The mutant allele A578S, Q613H, C469C, and S549S were the more frequently detected alleles, and the prevalence was all the same as 9.7% (3/31). The prevalence of mutant allele A578S and Q613H was the same as 17.6% (3/17) in nonsynonymous polymorphisms (Table 2). The Pfk13 mutation rate was found as 8.9% (4/45), 8.1% (5/62), 10.6% (10/94), 6.3% (7/111), and 4.4% (5/113) in 2015–2019, respectively (Fig. 2). Refer to the geographical distribution of Pfk13 alleles, Western Africa was the most reported region (n = 14) with Nigeria (n = 8), Guinea (n = 4) and Côte d’Ivoire (n = 2; Fig. 2). In addition, 2 isolates of C580Y were identified among the returners from Cambodia back to Shandong (Fig. 3).
Mutation prevalence of Pfcrt polymorphisms
A total of 13 different haplotypes were identified in 77 isolates (18.1%), including T76T356 found in 20 isolates (4.7%), and T76 in 18 isolates (4.2%) (Table 3). Refer to the codon 72–76, the mutation alleles with CVIET and CVIKT were identified, exhibiting a prevalence of 3.5% and 0.7%, respectively (Table 3). The CVIET were mainly distributed in Congo (5.2%, 4/77) and Mozambique (5.2%, 4/77). In addition, one isolate carrying S93 was identified in Cambodia in 2015, and no mutations was found at loci 97, 101 and 145. For the mutation alleles at 323–355, we have identified one isolate harbouring S323R334 in Ethiopia, and 2 isolates carrying T355 and T76T355, all were from Nigeria. For polymorphisms at locus 356, a total of 24 isolates were identified and most of them were from Congo ( 29.2%, 7/24). The geographical distribution of Pfcrt haplotypes showed that 30 isolates and 26 isolates harbouring Pfcrt haplotypes were found in Central Africa (39.0%, 30/77) and Western Africa (33.8%, 26/77) (Fig. 4). Interestingly, two P. falciparum isolates with k13 + crt polymorphisms were found in the patients returned from Cambodia (C580Y in Pfk13 and K76TT93SI356T in Pfcrt) and Congo (G690G in Pfk13 and K76I356T in Pfcrt).
Discussion
The artemisinin-resistant P. falciparum became a great challenge to the countries facing malaria control and elimination. China has eliminated indigenous malaria in 2021 [24], but the imported malaria, especially from Africa and Southeast Asia, has increased significantly [25,26,27]. Therefore, knowledge of the molecular markers associated with ART-R is crucial for its elimination and post-elimination surveillance. The previous work indicated that low prevalence of Pfk13 mutations found in the migrant workers from Africa, and A578S was the most common mutation site but showed no relationship with clinical or in vitro ART-R [22, 28, 29]. In this study, the migrant workers with P. falciparum infection abroad back to Shandong Province were genetically characterized and the genetic signature at the Pfk13 and Pfcrt candidate drug-resistance marker loci for DHA-PPQ was investigated.
The prevalence refers to SNPs in Pfk13 was 7.3% in this study, which was slightly higher than that reported in the previous work [22, 28,29,30,31]. Except for the A578S, which was considered as the most common Pfk13 mutant alleles [32, 33], other three alleles including Q613H, C469C, and S549S were still found. The all 3 Q613H were detected in the migrant workers from Nigeria, which was similar as reported before [34]. The synonymous mutant allele C469C, which was common in Africa, was identified in this study and the proportion was similar as reported in Burkina Faso and Senegal [35,36,37]. The Pfk13 mutant allele S549S, to our knowledge, was firstly identified in the migrant workers from Guinea, Equatorial Guinea, and Congo DRC. This was not surprising as C580Y was firstly found in the Thai–Cambodia border and now had spread into several counties including Myanmar, Laos, Vietnam and Guyana [38,39,40,41,42]. Therefore, further studies are needed to determine the extent of the spread of the Pfk13 polymorphisms in Africa, and to investigate any relationships between these mutations and changes to parasite clearance time and in vitro ART-R.
Despite of the high prevalence of Pfcrt K76T observed in GMS [43], as well as the China-Myanmar border [44, 45], the low prevalence rate (4.2%) of Pfcrt K76T mutation in African countries reported by the present study reflects high susceptibility of P. falciparum to the CQ. The codons at 72–76 with CVIET was the dominant mutant haplotypes in Pfcrt genotype and it was commonly found in African isolates [46,47,48]. Similarity, CVIET was mainly distributed in Central Africa (7.8%) and Eastern Africa (7.8%). SVMNT was mainly identified in Southeast Asia but not found in the current study. In addition, DHA + PPQ was once highly effective and adopted as the first-line anti-malarial in Cambodia, Vietnam, and Thailand, but due to the multidrug-resistant PfPailin lineage came to dominate in the GMS [49], high rates of treatment failure occurred. Studies indicated that treatment failure increased with the acquisition of mutations in Pfcrt mutation of S93, 97Y, 101 F, 145I, 343 L, 353 V, and 356T, which reduced piperaquine susceptibility [15, 50,51,52]. The 356T mutation was found with a high prevalence of 36.5% in Congo in the year of 2011–2012 [53]. However, the lower mutation result of T76T356 mixed type was 4.7%, which was reported in a few studies [23, 50]. Therefore, continuous molecular surveillance of Pfcrt mutations and in vitro susceptibility tests related to PPQ are necessary, especially for the migrant workers returning from African countries.
There are two limitations in this study. Firstly, no clinical data were included in this study, which may not provide the entire information for the molecular epidemiological analysis. Secondly, the molecular investigations on Pfk13 and Pfcrt were only conducted, but lack data on Pfmdr1 and Pfplasmepsin 2 and 3 genes due to the financial limitations. In the next phase of this study, those two genes SNP will be looked at.
Conclusion
This study identified the prevalence and spatial distribution of molecular markers Pfk13 and Pfcrt from imported P. falciparum isolates in Shandong Province. The findings suggest that a low mutation rate of Pfk13 was observed and mainly clustered in the Western and Central Africa. In addition, the low prevalence Pfcrt K76T point mutation in African countries reflects high susceptibility of P. falciparum to the CQ. However, the increase in the new alleles Pfcrt I356T, reveals a potential risk of drug pressure in PPQ. Therefore, it is imperative to carry out continuous surveillance of molecular markers among those migrant workers and explore in vitro relationship of ART-R with the clinical trials.
Availability of data and materials
The datasets analysed in this study are available from the corresponding author on reasonable request.
References
Feng J, Xia ZG, Vong S, Yang WZ, Zhou SS, Xiao N. Preparedness for malaria resurgence in China: case study on imported cases in 2000–2012. Adv Parasitol. 2014;86:231–65.
National Health Commission. Chinese report on Malaria Elimination Programme. Beijing, People’s Medical Publishing House; 2022.
Eastman RT, Fidock DA. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat Rev Microbiol. 2009;7:864–74.
Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–5.
Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–23.
Diakite SAS, Traore K, Sanogo I, Clark TG, Campino S, Sangare M, et al. A comprehensive analysis of drug resistance molecular markers and Plasmodium falciparum genetic diversity in two malaria endemic sites in Mali. Malar J. 2019;18:361.
Menard D, Khim N, Beghain J, Adegnika AA, Shafiul-Alam M, Amodu O. R, et al. A Worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med. 2016;374:2453–64.
WHO. World Malaria Report. Geneva, World Health Organization; 2021.
Uwimana A, Legrand E, Stokes BH, Ndikumana JM, Warsame M, Umulisa N, et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med. 2020;26:1602–8.
Asua V, Conrad MD, Aydemir O, Duvalsaint M, Legac J, Duarte E. T et al. Changing prevalence of potential mediators of aminoquinoline, antifolate, and artemisinin resistance across Uganda. J Infect Dis. 2021;223:985–94.
Wang X, Ruan W, Zhou S, Huang F, Lu Q, Feng X, et al. Molecular surveillance of Pfcrt and k13 propeller polymorphisms of imported Plasmodium falciparum cases to Zhejiang Province, China between 2016 and 2018. Malar J. 2020;19:59.
Lu F, Culleton R, Zhang M, Ramaprasad A, von Seidlein L, Zhou H, et al. Emergence of indigenous artemisinin-resistant Plasmodium falciparum in Africa. N Engl J Med. 2017;376:991–3.
Sa JM, Twu O. Protecting the malaria drug arsenal: halting the rise and spread of amodiaquine resistance by monitoring the PfCRT SVMNT type. Malar J. 2010;9:374.
Mulenga MC, Sitali L, Ciubotariu II, Hawela MB, Hamainza B, Chipeta J, et al. Decreased prevalence of the Plasmodium falciparum Pfcrt K76T and Pfmdr1 and N86Y mutations post-chloroquine treatment withdrawal in Katete District, Eastern Zambia. Malar J. 2021;20:329.
Ross LS, Dhingra SK, Mok S, Yeo T, Wicht KJ, Kumpornsin K, et al. Emerging southeast asian PfCRT mutations confer Plasmodium falciparum resistance to the first-line antimalarial piperaquine. Nat Commun. 2018;9:3314.
Boonyalai N, Vesely BA, Thamnurak C, Praditpol C, Fagnark W, Kirativanich K, et al. Piperaquine resistant cambodian Plasmodium falciparum clinical isolates: in vitro genotypic and phenotypic characterization. Malar J. 2020;19:269.
Ye R, Zhang Y, Zhang D. Evaluations of candidate markers of dihydroartemisinin-piperaquine resistance in Plasmodium falciparum isolates from the China-Myanmar, Thailand-Myanmar, and Thailand-Cambodia borders. Parasit Vectors. 2022;15:130.
Agrawal S, Moser KA, Morton L, Cummings MP, Parihar A, Dwivedi A, et al. Association of a novel mutation in the Plasmodium falciparum chloroquine resistance transporter with decreased piperaquine sensitivity. J Infect Dis. 2017;216:468–76.
Chenet SM, Okoth SA, Kelley J, Lucchi N, Huber CS, Vreden S, et al. Molecular profile of malaria drug resistance markers of Plasmodium falciparum in Suriname. Antimicrob Agents Chemother. 2017;61:e02655-16.
Rasmussen C, Alonso P, Ringwald P. Current and emerging strategies to combat antimalarial resistance. Expert Rev Anti Infect Ther. 2022;20:353–72.
Feng J, Tu H, Zhang L, Zhang S, Jiang S, Xia Z, Zhou S. Mapping transmission foci to eliminate malaria in the People’s Republic of China, 2010–2015: a retrospective analysis. BMC Infect Dis. 2018;18:115.
Feng J, Xu D, Kong X, Lin K, Yan H, Feng X, Tu H, et al. Characterization of pfmdr1, pfcrt, pfK13, pfubp1, and pfap2mu in travelers returning from Africa with Plasmodium falciparum infections reported in China from 2014 to 2018. Antimicrob Agents Chemother. 2021;65:e0271720.
Cheng W, Song X, Tan H, Wu K, Li J. Molecular surveillance of anti-malarial resistance pfcrt, pfmdr1, and pfk13 polymorphisms in african Plasmodium falciparum imported parasites to Wuhan, China. Malar J. 2021;20:209.
Feng J, Zhang L, Xia ZG, Zhou SS, Xiao N. Malaria-free certification in China: achievements and lessons learned from the national malaria elimination programme. Zoonoses. 2021;1:2.
Feng J, Zhang L, Huang F, Yin JH, Tu H, Xia ZG, Zhou SS, Xiao N, Zhou XN. Ready for malaria elimination: zero indigenous case reported in the People’s Republic of China. Malar J. 2018;17:315.
Feng J, Xiao H, Xia Z, Zhang L, Xiao N. Analysis of malaria epidemiological characteristics in the People’s Republic of China, 2004–2013. Am J Trop Med Hyg. 2015;93:293–9.
Feng JZL, Xia ZG, Zhou SS, Xiao N. Malaria-free certification in China: achievements and lessons learned from the national malaria elimination programme. Zoonoses. 2021;1:3–6.
Feng J, Kong X, Xu D, Yan H, Zhou H, Tu H, Lin K. Investigation and evaluation of genetic diversity of Plasmodium falciparum Kelch 13 polymorphisms imported from Southeast Asia and Africa in Southern China. Front Public Health. 2019;7:95.
Yan H, Kong X, Zhang T, Xiao H, Feng X, Tu H, et al. Prevalence of Plasmodium falciparum Kelch 13 (PfK13) and ubiquitin-specific protease 1 (pfubp1) gene polymorphisms in returning travelers from Africa reported in Eastern China. Antimicrob Agents Chemother. 2020;64:e00981-20.
Li J, Shi Y, Zhang W, Yan H, Lin K, Wei S, et al. K13-propeller gene polymorphisms of Plasmodium falciparum and the therapeutic effect of artesunate among migrant workers returning to Guangxi, China (2014–2017). Malar J. 2019;18:349.
Jin X, Zhu S, Xu W, Chen J, Ruan W, Wang X. Limited polymorphism in k13 gene of Plasmodium falciparum and k12 of Plasmodium vivax isolates imported from african and asian countries between 2014 and 2019 in Hangzhou city, China. BMC Infect Dis. 2021;21:853.
Maiga-Ascofare O, May J. Is the A578S single-nucleotide polymorphism in K13-propeller a marker of emerging resistance to artemisinin among Plasmodium falciparum in Africa? J Infect Dis. 2016;213:165–6.
Yobi DM, Kayiba NK, Mvumbi DM, Boreux R, Kabututu PZ, Akilimali PZ, et al. Biennial surveillance of Plasmodium falciparum anti-malarial drug resistance markers in Democratic Republic of Congo, 2017 and 2019. BMC Infect Dis. 2022;22:145.
Igbasi U, Oyibo W, Omilabu S, Quan H, Chen SB, Shen HM, et al. Kelch 13 propeller gene polymorphism among Plasmodium falciparum isolates in Lagos, Nigeria: molecular epidemiologic study. Trop Med Int Health. 2019;4:1011–7.
Some AF, Sorgho H, Zongo I, Bazie T, Nikiema F, Sawadogo A, Zongo M, et al. Polymorphisms in K13, pfcrt, pfmdr1, pfdhfr, and pfdhps in parasites isolated from symptomatic malaria patients in Burkina Faso. Parasite. 2016;23:60.
Madamet M, Kounta MB, Wade KA, Lo G, Diawara S, Fall M, et al. Absence of association between polymorphisms in the K13 gene and the presence of Plasmodium falciparum parasites at day 3 after treatment with artemisinin derivatives in Senegal. Int J Antimicrob Agents. 2017;49:754–6.
Ajogbasile FV, Oluniyi PE, Kayode AT, Akano KO, Adegboyega BB, Philip C, et al. Molecular profiling of the artemisinin resistance Kelch 13 gene in Plasmodium falciparum from Nigeria. PLoS ONE. 2022;17:e0264548.
Bonnington CA, Phyo AP, Ashley EA, Imwong M, Sriprawat K, Parker DM, et al. Plasmodium falciparum Kelch 13 mutations and treatment response in patients in Hpa-Pun District, Northern Kayin State, Myanmar. Malar J. 2017;16:480.
Iwagami M, Nakatsu M, Khattignavong P, Soundala P, Keomalaphet S, Lorpachan L, et al. Heterogeneous distribution of k13 mutations in Plasmodium falciparum in Laos. Malar J. 2018;17:483.
Pau MC, Pantaleo A, Tsamesidis I, Hoang H, Tuan Tran A, Hanh Nguyen TL, et al. Clinical impact of the two ART resistance markers, K13 gene mutations and DPC3 in Vietnam. PLoS ONE. 2019;14:e0214667.
Chenet SM, Akinyi Okoth S, Huber CS, Chandrabose J, Lucchi NW, Talundzic E, et al. Independent emergence of the Plasmodium falciparum Kelch propeller domain mutant allele C580Y in Guyana. J Infect Dis. 2016;213:1472–5.
Mathieu LC, Cox H, Early AM, Mok S, Lazrek Y, Paquet JC. Aet al. Local emergence in Amazonia of Plasmodium falciparum k13 C580Y mutants associated with in vitro artemisinin resistance. Elife. 2020;9:e51015.
Imwong M, Dhorda M, Myo Tun K, Thu AM, Phyo AP, Proux S, et al. Molecular epidemiology of resistance to antimalarial drugs in the Greater Mekong subregion: an observational study. Lancet Infect Dis. 2020;20:1470–80.
Feng J, Zhou D, Lin Y, Xiao H, Yan H, Xia Z. Amplification of pfmdr1, pfcrt, pvmdr1, and K13 propeller polymorphisms associated with Plasmodium falciparum and Plasmodium vivax isolates from the China-Myanmar border. Antimicrob Agents Chemother. 2015;59:2554–9.
Tang T, Xu Y, Cao L, Tian P, Shao J, Deng Y, et al. Ten-year molecular surveillance of drug-resistant Plasmodium spp. isolated from the China-Myanmar border. Front Cell Infect Microbiol. 2021;11:733788.
Awasthi G, Satya Prasad GB, Das A. Pfcrt haplotypes and the evolutionary history of chloroquine-resistant Plasmodium falciparum. Mem Inst Oswaldo Cruz. 2012;107:129–34.
Kassaza K, Long AC, McDaniels JM, Andre M, Fredrickson W, Nyehangane D, et al. Surveillance of Plasmodium falciparum pfcrt haplotypes in Southwestern Uganda by high-resolution melt analysis. Malar J. 2021;20:114.
Deutsch-Feldman M, Aydemir O, Carrel M, Brazeau NF, Bhatt S, Bailey JA, et al. The changing landscape of Plasmodium falciparum drug resistance in the Democratic Republic of Congo. BMC Infect Dis. 2019;19:872.
van der Pluijm RW, Imwong M, Chau NH, Hoa NT, Thuy-Nhien NT, Thanh NV, et al. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect Dis. 2019;19:952–61.
Foguim FT, Bogreau H, Gendrot M, Mosnier J, Fonta I, Benoit N, et al. Prevalence of mutations in the Plasmodium falciparum chloroquine resistance transporter, PfCRT, and association with ex vivo susceptibility to common anti-malarial drugs against african Plasmodium falciparum isolates. Malar J. 2020;19:201.
Eastman RT, Dharia NV, Winzeler EA, Fidock DA. Piperaquine resistance is associated with a copy number variation on chromosome 5 in drug-pressured Plasmodium falciparum parasites. Antimicrob Agents Chemother. 2011;55:3908–16.
Dhingra SK, Redhi D, Combrinck JM, Yeo T, Okombo J, Henrich PP, et al. A variant PfCRT isoform can contribute to Plasmodium falciparum resistance to the first-line partner drug piperaquine. mBio. 2017;8:e00303-17.
Dhingra SK, Gabryszewski SJ, Small-Saunders JL, Yeo T, Henrich PP, Mok S, et al. Global spread of mutant PfCRT and its pleiotropic impact on Plasmodium falciparum multidrug resistance and fitness. mBio. 2019;10:e02731-18.
Funding
The work was supported by the major vector-borne pathogens in the Belt and Road (2018ZX10101002-002), the National Natural Science Foundation of China (Grant No. 81602904) and the Nature Science Foundation of Shandong Province (ZR2019PH118).
Author information
Authors and Affiliations
Contributions
XLK and JF carried out the molecular studies and drafted the manuscript. SSZ conceived the study. YX and GY performed the sample collection and analysed the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the ethical committee of the National Institute of Parasitic Diseases, Chinese Centre for Disease Control and Prevention (NIPD, China CDC, No. 2019008).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kong, X., Feng, J., Xu, Y. et al. Molecular surveillance of artemisinin resistance-related Pfk13 and pfcrt polymorphisms in imported Plasmodium falciparum isolates reported in eastern China from 2015 to 2019. Malar J 21, 369 (2022). https://doi.org/10.1186/s12936-022-04398-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-022-04398-x