Abstract
Background
Ethiopia is among countries with a high malaria burden. There are several studies that assessed the efficacy of anti-malarial agents in the country and this systematic review and meta-analysis was performed to obtain stronger evidence on treatment outcomes of malaria from the existing literature in Ethiopia.
Methods
A systematic literature search using the preferred reporting items for systematic review and meta-analysis (PRISMA) statement was conducted on studies from Pubmed, Google Scholar, and ScienceDirect databases to identify published and unpublished literature. Comprehensive meta-analysis software was used to perform all meta-analyses. The Cochrane Q and the I 2 were used to evaluate heterogeneity of studies. Random effects model was used to combine studies showing heterogeneity of Cochrane Q p < 0.10 and I 2 > 50.
Results
Twenty-one studies were included in the final analysis with a total number of 3123 study participants. Treatment outcomes were assessed clinically and parasitologically using World Health Organization guidelines. Adequate clinical and parasitological response was used to assess treatment success at the 28th day. Overall, a significant high treatment success of 92.9% (95% CI 89.1–96.6), p < 0.001, I 2 = 98.39% was noticed. However, treatment success was higher in falciparum malaria patients treated with artemether–lumefantrine than chloroquine for Plasmodium vivax patients [98.1% (97.0–99.2), p < 0.001, I 2 = 72.55 vs 94.7% (92.6–96.2), p < 0.001, I 2 = 53.62%]. Seven studies reported the adverse drug reactions to anti-malarial treatment; of 822 participants, 344 of them were exposed to adverse drug reactions with a pooled event rate of 39.8% (14.1–65.5), p = 0.002.
Conclusions
On the basis of this review, anti-malarial treatment success was high (92.9%) and standard regimens showed good efficacy against Plasmodium falciparum (98.1%) and P. vivax (94.7%) infections in Ethiopia, but associated with high rates of adverse drug reactions (ADRs). However, these ADRs were not serious enough to discontinue anti-malarial treatment. The results of this study suggest that the current anti-malarial medications are effective and safe; however, greater priority should be placed on the discovery of new anti-malarial drugs to achieve successful outcomes as resistance seems inevitable since cases of anti-malarial drug resistance have been reported from other areas of the world.
Similar content being viewed by others
Background
Malaria is a vector-borne life-threatening disease caused by Plasmodium species that are transmitted to people through the bites of infected female Anopheles mosquitoes. It is characterized by fever, headache, chills, and vomiting and if not treated can progress to severe illness, often leading to death [1]. In 2015, an estimated 212 million cases and 429,000 deaths of malaria occurred globally [2]. Of these, around 90% of malaria cases and 92% of malaria deaths occurred in sub-Saharan Africa (SSA) [1, 2]. Ethiopia is also among countries with a high malaria burden. In 2015, an estimated 2.8 million cases and 4900 deaths due to malaria occurred in the country [2, 3].
Five Plasmodium species are responsible for malaria (Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale, and Plasmodium knowlesi), but P. falciparum and P. vivax account for the largest threat [1,2,3,4]. Plasmodium falciparum is the most common (64%) cause of malaria in Ethiopia while P. vivax accounts for the remaining cases (34%) [3]. Plasmodium falciparum causes the most severe form of malaria, however, contrary to popular belief, P. vivax can also cause severe malaria and even death [5] and Ethiopia is home to the second highest number of cases [12% (n ~1020)] and mortality [12% (n ~372)] due to P. vivax (next only to India) in the world [2]. Malaria due to P. vivax and P. ovale are also characterized by relapses [6].
Reduced efficacy of chloroquine (CQ) [7, 8] has forced a change in the selection of anti-malarials in the management of falciparum malaria. Since 2004, Ethiopia has adopted artemether–lumefantrine (AL) and CQ as first-line treatment for infection with P. falciparum and P. vivax, respectively. In cases of treatment failure of P. falciparum, quinine (QN) is the treatment of choice and in cases of severe malaria, artemether, artesunate, or QN can be used [3, 9]. Globally, artemisinin resistance in P. falciparum has emerged, especially in Southeast Asia, slowing therapeutic response and increased rates of treatment failures [10, 11]. Similarly, artemisinin resistance has been reported from Africa although there is no evidence that it has taken hold currently [12]. Many studies assessing the efficacy of anti-malarial agents against P. falciparum and P. vivax have been published. However, no systematic review on this topic was identified from Ethiopia. Hence, this systematic review and meta-analysis was performed to obtain stronger evidence on treatment outcomes of malaria from the existing literature in Ethiopia.
Methods
Search strategies
The Cochrane guidelines to conduct the meta-analysis following the preferred reporting items for systematic review and meta-analysis (PRISMA) statement [13] was used to conduct a computerized systematic search of the PubMed, Google Scholar, and Science Direct databases. Prospective observational and interventional (randomized and single-arm) studies were included in the review using the following MeSH terms: malaria AND ethiopia AND (treatment OR management) AND (artemether–lumefantrine OR artesunate OR chloroquine OR mefloquine OR primaquine OR pyrimethamine) OR resistance. Only studies conducted in Ethiopia were included in the study. In addition to published researches, unpublished postgraduate thesis reports were also assessed to be included in the study. Publication dates were not used as inclusion or exclusion criteria and research papers published before 30 January, 2017 were included. Only those articles written in English language were considered for this review.
Inclusion criteria
Papers fulfilling the following criteria were included in the study: studies presented as original articles; studies that examined malaria treatment outcomes; studies conducted in Ethiopia; studies written in English.
Exclusion criteria
The following papers were excluded from the study: studies that used CQ for the treatment of falciparum malaria; studies that assessed treatment outcomes at times other than 28 days; studies where full articles were no longer available online (Additional file 1).
Review process
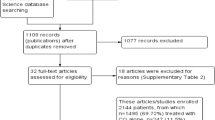
All of the research articles that were identified from searches of the electronic databases were imported into the ENDNOTE software version X5 (Thomson Reuters, USA) and duplicates were removed. Before data extraction had begun, full-length articles of the selected studies were read to confirm for fulfilling the inclusion criteria. Three reviewers (EAG, MAS, HGT) independently screened the titles and abstracts to identify potentially eligible studies. Then data were extracted from full-length articles that fulfilled the inclusion criteria (Fig. 1). Discrepancies were resolved by mutual consent after discussion and independent review from the fourth researcher (ASB).
Data extraction
Data on the types of study design (observational vs interventional), year the studies were conducted, length of study, and geographic location of the study area was first extracted. Participants’ age ranges, and gender of study participants were then extracted. Finally, data regarding the types of anti-malarial treatments, treatment outcome measures (including treatment success rates, treatment failure rates, and case fatality rates), treatment duration, and adverse drug reactions (ADRs) were extracted to be included in the systematic review and meta-analysis.
Quality assessment and sensitivity analysis
Two reviewers (EAG and MAS) independently assessed methodological quality of studies using modified Jadad scale for randomized controlled trials (RCTs) [14] and Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for observational studies [15]. The scores for the modified Jadad scale can range from 0 to 8 (low to high quality). Scores of 4–8 represent good to excellent, whereas 0–3 represents low or poor quality. The observational studies were categorized as high quality (over 75% of the STROBE checklist) and low quality (under 75% of the STROBE checklist). To address the issue of heterogeneity of the studies, a sensitivity analysis was considered stratifying the studies into high quality and low quality.
Statistical analysis
Comprehensive meta-analysis (CMA) software [16] was used to perform meta-analyses of proportions of success. The Cochrane Q and the I 2 were used to evaluate heterogeneity of studies. Random effects model was used to combine studies showing heterogeneity of Cochrane Q p < 0.10 and I2 > 50 [17]. A sub-group analysis was performed by comparing the treatment outcomes of patients with P. falciparum infection treated with AL and patients infected with P. vivax and treated with CQ. Rates of ADRs were also calculated for the different anti-malarial medications.
Ethical consideration
This study was carried out in strict accordance with the recommendations in the PRISMA guidelines [13]. Since it is a systematic review and meta-analysis, ethics committee or institutional review board permission was not required.
Results
An updated search of available literature identified 231 titles, of which two RCTs) [18, 19], 15 one-arm in vivo drug efficacy studies [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34], and four prospective observational studies [35,36,37,38] were deemed eligible (Additional file 2). The 21 studies [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38] included in this analysis came from individual treatment programmes across Ethiopia and reported treatment outcomes for a range of 69 [35] to 487 patients [26]. One unpublished Master’s thesis dissertation [36] was included and the rest were published in scientific journals [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35, 37, 38]. Some 1868 patients with P. falciparum [21, 23,24,25,26,27, 30, 31, 34,35,36,37,38] and 1255 patients with P. vivax [18,19,20, 22, 28, 29, 32,33,34,35] were analysed with an age range between 6 months and 91 years.
The most commonly reported regimens were AL [18, 21, 23,24,25, 27, 30,31,32, 34,35,36,37,38] and CQ [18,19,20, 22, 28, 29, 32,33,34,35], and treatment outcomes were assessed using clinical and parasitological criteria according to four different World Health Organization (WHO) guidelines [39,40,41,42]. Adequate clinical and parasitological response (ACPR) was taken as treatment success. ACPR was defined as absence of parasitaemia by the end of treatment (day 28) irrespective of axillary temperature without previously meeting any of the criteria of early treatment failure or late clinical failure or late parasitological failure [40,41,42]. All of the studies included in the analysis assessed outcomes at 28 days.
Malaria treatment success in Ethiopia
Twenty-one studies reported the treatment success in malaria patients with P. falciparum [21, 23,24,25,26,27, 30, 31, 34,35,36,37,38] and P. vivax [18,19,20, 22, 28, 29, 32,33,34,35] in Ethiopia. Only six studies used polymerase chain reaction (PCR) genotyping approach to differentiate recrudescence from new infections [18, 21, 23, 24, 30, 34]. For these six studies, the PCR-adjusted success and failure rates were used while PCR-unadjusted rates were used for the remaining studies. Overall, a significant high treatment success of 92.9% (95% CI 89.1–96.6), p < 0.001, I 2 = 98.39% (Fig. 2) was noticed. However, treatment success was higher in falciparum malaria patients treated with AL than CQ for P. vivax (98.1% (97.0–99.2), p < 0.001, I 2 = 72.55 vs 94.7% (92.6–96.2), p < 0.001, I 2 = 53.62%) (Figs. 3, 4).
Malaria treatment failures in Ethiopia
Treatment failures were lower in patients with falciparum malaria treated with sulfadoxine–pyrimethamine (SP), AL with a pooled rate of 7.8% (2.1–13.6), p = 0.008. Nine studies reported the treatment failures in vivax malaria patients with a pooled rate of 7.9% (4.1–11.8), p < 0.001 (Table 1). Of all the study participants, only one patient died during the course of treatment [21].
Adverse drug reactions during anti-malarial treatment in Ethiopia
Seven studies [13, 24, 27, 31, 35, 37, 38] reported ADRs to anti-malarial treatment; of 822 participants, 344 exposure to ADRs with a pooled event rate of 39.8% (14.1–65.5), p = 0.002. When examined the pooled events rates of anti-malarial drugs AL and CQ, AL has higher adverse events of 41.2% (10.1–72.4), p = 0.009 compared to CQ 35.6% (5.7–65.5), p = 0.020 (Table 1). Table 2 shows the most commonly reported ADRs.
Quality assessment and sensitivity analysis
Seventeen interventional studies [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] were assessed using the modified Jadad scale [14] while the remaining four studies [35,36,37,38] were assessed with the STROBE statement [15]. Eleven interventional and two observational studies were judged to have high quality (Additional file 3).
Meta-analysis was stratified based on the quality of the studies (high and low quality) to investigate the overall treatment outcomes across the included studies. It revealed significant differences between the low-quality studies (86.1%, 95% CI 69.7–102.6, p = <0.001, I2 = 99.35%) [20, 26, 28, 30, 32, 33, 35, 38] and high quality studies (96.9%, 95% CI 95.4–98.3, p < 0.001; I2 = 81.7%) [18, 19, 21,22,23,24,25, 27, 29, 31, 36, 37]. Publication bias was also assessed using a funnel plot (Additional file 4).
Discussion
This systematic review and meta-analysis was conducted mainly to estimate treatment outcomes of different anti-malarial medications used to treat both P. falciparum and P. vivax in Ethiopia. This systematic review identified 21 studies (from 2005 to 2016) that assessed the treatment outcomes of uncomplicated falciparum and vivax malaria in Ethiopia. These studies were conducted in the following provinces: Tigray, Amhara, Oromia, Benishangul-Gumuz, and the Southern Nations, Nationalities, and Peoples Region (SNNPR). Since poor efficacy of CQ in P. falciparum is well documented, AL replaced CQ as a treatment option for P. falciparum and CQ is no longer used in the management of falciparum malaria in Ethiopia. For this reason, studies that assessed treatment efficacy of CQ in P. falciparum were excluded from the analysis (Additional file 1). All of the studies used one or two of four WHO guidelines [39,40,41,42] to assess treatment outcomes of the study participants. Treatment outcomes were confirmed both clinically and parasitologically at 28 days for all studies.
This investigation suggested that a high proportion of treatment success of different anti-malarial drugs was seen in Ethiopia with an overall treatment success rate of 92.9%. However, the effectiveness varies between the species and the different anti-malarial drugs and most of the studies focused on AL and CQ for the management of P. falciparum and P. vivax infections, respectively. A pharmaceutical company-sponsored, pooled analysis of AL, which included seven studies, showed a 97.1% (95.2–98.3%) treatment success in patients with uncomplicated malaria [43]. This suggests that the results of treatment success with AL in uncomplicated malaria patients in Ethiopia are evenly distributed with other high malaria-endemic countries.
CQ has been found to have high treatment success rate (94.7%) for infection with P. vivax. Its superior efficacy over AL was demonstrated by two studies [18, 32] that compared the efficacy of both drugs against P. vivax. These studies showed that CQ has a better efficacy (90.9%) for the management of P. vivax than AL (75.1%). This superior efficacy of CQ over AL might be attributed to its longer elimination half-life with extended periods of post-treatment prophylaxis. Better efficacy of dihydroartemisinin–piperaquine, an artemisinin combination therapy with longer elimination half-life than AL, might support this justification [44]. Therefore, CQ is the preferred treatment option for the management of P. vivax infection in Ethiopia. However, despite the poorer efficacy of AL for P. vivax compared to CQ, it can be used in cases of mixed infections with P. vivax and P. falciparum. On the other hand, one study [19] compared the efficacy of CQ alone or in combination with PQ against P. vivax. Accordingly, the combination showed superior efficacy (99.2%) over CQ alone (96.3%). Likewise, a better parasitaemia clearance with combination of CQ and primaquine (PQ) was also seen in one study. Naing et al. [45] reported a higher rate of parasitaemia in patients treated with CQ alone when compared to patients who received PQ in combination with CQ (7.7 vs 4.9%). The standard treatment guideline for Ethiopia [9] recommends a 14-day therapy of PQ along with CQ as a first-line treatment in patients with P. vivax infections. However, despite lack of published reports, routine use of PQ suffers from availability issues.
In the current review, only one study participant died [21]. This lower rate of mortality is not surprising since the studies in this review included patients with uncomplicated malaria. However, high treatment failure rates of SP against P. falciparum were reported by Jima et al. [26]. Resistance of P. falciparum to SP has been well documented [46,47,48,49]. As a result, this combination is no longer included in the standard treatment guideline of the country [9]. A higher incidence of ADRs was noticed with AL than CQ (41.2 vs 35.6%). Detailed data of ADRs were absent from some studies, and these were not systematically recorded in most studies. Further, most studies reported ADRs that are similar to malaria symptoms, which might overlap. Nevertheless, more than one-third of adult patients on malaria treatment had experienced ADRs with AL and CQ.
Previous studies reported a higher incidence of ADRs to AL in adults with uncomplicated P. falciparum with serious adverse events rate of 1.4% [43]. This study recorded adverse event rates ranging from 72.8 to 86.1%. On the other hand, the current study identified that the adverse events to CQ in patients with P. vivax was more than 2.5 times (35.6 vs 13.3%) higher than the Naing et al. review within the same series [45]. Many of the adverse events reported are more frequent and consistent with other studies most of which are similar to well-recognized symptoms of malaria. Due to the similarities, under-reporting or over-reporting of ADRs might account for the observed differences. The higher rates of ADRs reported might be caused by an increased tendency to report symptoms during the course of anti-malarial treatment in Ethiopia. Apart from two infants who were unable to tolerate oral medications due to repeated vomiting [21], none of the studies reported serious ADRs that forced an individual to discontinue anti-malarial drug treatment. The infants were switched to intravenous drugs, but one of them died. However, there was no evidence whether the repeated vomiting in the two infants was due to the anti-malarial treatment.
This study has important implications for high malaria burden countries with high prevalence of P. falciparum and P. vivax. In view of WHO’s Global Technical Strategy for Malaria 2016–2030 to reduce global malaria incidence and mortality by at least 90% by 2030 [50], efficacy of artemisinin-combination anti-malarial drugs, particularly AL, showed promising results in treating patients with falciparum malaria and CQ for P. vivax in Ethiopia. However, resistance to anti-malarial drugs poses a major threat globally. On the basis of the results of this review, malaria treatment in Ethiopia is successful, but if the parasites develop resistance to these anti-malarial regimens, it is inevitable that treatment would be more difficult, unsuccessful and high rates of relapse could be due to multidrug-resistant malaria. Other implications are that as the previously used anti-malarial regimens were associated with higher rates of treatment failures in P. falciparum patients [7, 8], there is no guarantee how long the currently used anti-malarial drugs will remain effective. This supports calls for novel alternative anti-malarial drugs to treat malaria in the near future. Lastly, higher ADRs suggest the need for active surveillance and prospective follow-up are essential to determine the incidence of adverse events in malaria patients in Ethiopia.
This study has several strengths. A total of 21 studies were included that allowed results from a total of 3123 study participants. In addition to published research articles, unpublished researches were assessed for eligibility and one thesis dissertation was included in the analysis. The study included infection with both species, P. falciparum and P. vivax, and study outcomes were measured both clinically and parasitologically. The study assessed efficacy of commonly used anti-malarial drugs: AL, SP, CQ, and CQ–PQ. However, the current study is not without limitations. Only a few of the studies [18, 19, 32] evaluated the comparative efficacy of different anti-malarial medications and the current review relied mainly on one-arm studies, and only a few studies used PCR genotyping. Not all studies reported ADRs to anti-malarial drugs, which rendered the current study depend on fewer studies to assess the safety profiles of these drugs. None of the studies clearly reported how severity of ADRs was assessed and reporting on the severity of ADRs depended on their statement. Only studies written in English were included in the analysis as result studies written in other languages might be missed. Trials representing results from Ethiopia only were included, and a high heterogeneity was detected in the studies, which forced the random effect model to be applied reducing credibility and increasing the imprecision of the results. Therefore, interpretation of the findings of this study should be in light of these limitations.
Conclusions
On the basis of this review, anti-malarial treatment success was high (92.9%) and standard regimens showed good efficacy against P. falciparum (98.1%) and P. vivax (94.7%) infections in Ethiopia, but was associated with high rates of ADRs. However, the ADRs were not serious enough to discontinue anti-malarial treatment. The results of the current study suggest that current anti-malarial medications are effective and safe, however, greater priority should be placed on the discovery of new anti-malarial drugs to achieve successful outcomes as resistance seems inevitable since cases of anti-malarial drug resistance have been reported from other areas of the world.
References
WHO. Malaria fact sheet. 2016. http://www.who.int/mediacentre/factsheets/fs094/en/. Accessed 26 Feb 2017.
WHO. World malaria report 2016. Geneva: World Health Organization; 2016.
WHO. Malaria country profiles 2016: Ethiopia. Geneva: World Health Organization; 2016. http://www.who.int/malaria/publications/country-profiles/profile_eth_en.pdf. Accessed 26 Feb 2017.
Collins WE. Plasmodium knowlesi: a malaria parasite of monkeys and humans. Ann Rev Entomol. 2012;57:107–21.
Baird JK. Evidence and implications of mortality associated with acute Plasmodium vivax malaria. Clin Microbiol Rev. 2013;26:36–57.
Nadjm B, Behrens RH. Malaria: an update for physicians. Infect Dis Clin North Am. 2012;26:243–59.
Alene GD, Bennett S. Chloroquine resistance of Plasmodium falciparum malaria in Ethiopia and Eritrea. Trop Med Int Health. 1996;1:810–5.
Teklehaimanot A. Chloroquine-resistant Plasmodium falciparum malaria in Ethiopia. Lancet. 1986;328:127–9.
Food Medicine and Healthcare Administration and Control Authority of Ethiopia. Ethiopia standard treatment guidelines for general hospital. 2014. p. 147–50.
Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–23.
Tun KM, Imwong M, Lwin KM, Win AA, Hlaing TM, Hlaing T, et al. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. Lancet Infect Dis. 2015;15:415–21.
Lu F, Culleton R, Zhang M, Ramaprasad A, von Seidlein L, Zhou H, et al. Emergence of indigenous artemisinin-resistant Plasmodium falciparum in Africa. N Engl J Med. 2017;376:991–3.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9.
Hu Y, Wang C, Pang X, Li F, Chen W, Tan W. Antibiotics are not beneficial in the management of category III prostatitis: a meta-analysis. Urol J. 2014;11:1377.
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med. 2007;45:247–51.
Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive meta-analysis version. 2nd ed. Englewood: Biostat; 2005.
Sedgwick P. Meta-analyses: heterogeneity and subgroup analysis. BMJ. 2013;346:f4040.
Hwang J, Alemayehu BH, Reithinger R, Tekleyohannes SG, Teshi T, Birhanu SG, et al. In vivo efficacy of artemether–lumefantrine and chloroquine against Plasmodium vivax: a randomized open label trial in central Ethiopia. PLoS ONE. 2013;8:e63433.
Yeshiwondim AK, Tekle AH, Dengela DO, Yohannes AM, Teklehaimanot A. Therapeutic efficacy of chloroquine and chloroquine plus primaquine for the treatment of Plasmodium vivax in Ethiopia. Acta Trop. 2010;113:105–13.
Assefa M, Eshetu T, Biruksew A. Therapeutic efficacy of chloroquine for the treatment of Plasmodium vivax malaria among outpatients at Hossana Health Care Centre, southern Ethiopia. Malar J. 2015;14:458.
Eshetu T, Abdo N, Bedru KH, Fekadu S, Wieser A, Pritsch M, et al. Open-label trial with artemether–lumefantrine against uncomplicated Plasmodium falciparum malaria three years after its broad introduction in Jimma Zone, Ethiopia. Malar J. 2012;11:240.
Getachew S, Thriemer K, Auburn S, Abera A, Gadisa E, Aseffa A, et al. Chloroquine efficacy for Plasmodium vivax malaria treatment in southern Ethiopia. Malar J. 2015;14:525.
Getnet G, Fola AA, Alemu A, Getie S, Fuehrer H-P, Noedl H. Therapeutic efficacy of artemether–lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Enfranze, north-west Ethiopia. Malar J. 2015;14:258.
Hwang J, Alemayehu BH, Hoos D, Melaku Z, Tekleyohannes SG, Teshi T, et al. In vivo efficacy of artemether–lumefantrine against uncomplicated Plasmodium falciparum malaria in Central Ethiopia. Malar J. 2011;10:209.
Jima D, Tesfaye G, Medhin A, Kebede A, Argaw D, Babaniyi O. Safety and efficacy of artemether–lumefantrine in the treatment of uncomplicated falciparum malaria in Ethiopia. East Afr Med J. 2005;82:387–90.
Jima D, Tesfaye G, Medhin A, Kebede A, Argaw D, Babaniyi O. Efficacy of sulfadoxine–pyrimethamine for the treatment of uncomplicated falciparum malaria in Ethiopia. East Afr Med J. 2005;82:391–5.
Kefyalew T, Animut A, Tamene T, Jima D, Hailemariam A, Legesse M. Efficacy of six-dose regimen of artemether–lumefantrine for the treatment of uncomplicated falciparum malaria, three years after its introduction into Ethiopia. Parasite. 2009;16:129–34.
Ketema T, Bacha K, Birhanu T, Petros B. Chloroquine-resistant Plasmodium vivax malaria in Serbo town, Jimma zone, south-west Ethiopia. Malar J. 2009;8:177.
Ketema T, Getahun K, Bacha K. Therapeutic efficacy of chloroquine for treatment of Plasmodium vivax malaria cases in Halaba district, South Ethiopia. Parasit Vectors. 2011;4:46.
Mekonnen SK, Medhin G, Berhe N, Clouse RM, Aseffa A. Efficacy of artemether–lumefantrine therapy for the treatment of uncomplicated Plasmodium falciparum malaria in Southwestern Ethiopia. Malar J. 2015;14:317.
Wudneh F, Assefa A, Nega D, Mohammed H, Solomon H, Kebede T, et al. Open-label trial on efficacy of artemether/lumefantrine against the uncomplicated Plasmodium falciparum malaria in Metema district, Northwestern Ethiopia. Ther Clin Risk Manag. 2016;12:1293–300.
Yohannes AM, Teklehaimanot A, Bergqvist Y, Ringwald P. Confirmed vivax resistance to chloroquine and effectiveness of artemether–lumefantrine for the treatment of vivax malaria in Ethiopia. Am J Trop Med Hyg. 2011;84:137–40.
Teka H, Petros B, Yamuah L, Tesfaye G, Elhassan I, Muchohi S, et al. Chloroquine-resistant Plasmodium vivax malaria in Debre Zeit, Ethiopia. Malar J. 2008;7:220.
Nega D, Assefa A, Mohamed H, Solomon H, Woyessa A, Assefa Y, Kebede A, Kassa M. Therapeutic efficacy of artemether–lumefantrine (Coartem®) in treating uncomplicated P. falciparum malaria in Metehara, Eastern Ethiopia: regulatory clinical study. PLoS ONE. 2016;11:e0154618.
Beyene HB, Beyene MB, Ebstie YA, Desalegn Z. Efficacy of chloroquine for the treatment of vivax malaria in Northwest Ethiopia. PLoS ONE. 2016;11:e0161483.
Dessie W. Therapeutic efficacy and safety of artemether–lumefantrine (Coartem®) for the treatment of uncomplicated Plasmodium falciparum malaria in Felege Selam Health Center, Pawe, Benishangul Gumuz, Ethiopia. Thesis, Addis Ababa University; 2014.
Ebstie YA, Zeynudin A, Belachew T, Desalegn Z, Suleman S. Assessment of therapeutic efficacy and safety of artemether–lumefantrine (Coartem®) in the treatment of uncomplicated Plasmodium falciparum malaria patients in Bahir Dar district, Northwest Ethiopia: an observational cohort study. Malar J. 2015;14:236.
Kanche ZZ, Woticha EW, Gidebo KD. Therapeutic efficacy and safety of artemether–lumefantrine (Coartem®) in uncomplicated P. falciparum malaria in Wolaita Zone, Southern Ethiopia. J Biol Agric Healthc. 2016;6:42–8.
WHO. Assessment of therapeutic efficacy of antimalarial drugs: for uncomplicated falciparum malaria in areas with intense transmission. Geneva: World Health Organization; 1996.
WHO. Monitoring antimalarial drug resistance: report of a WHO consultation Geneva, Switzerland 3–5 December 2001. Geneva: World Health Organization; 2002.
WHO. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. Geneva: World Health Organization; 2003.
WHO. Methods for surveillance of antimalarial drug efficacy. Geneva: World Health Organization; 2009.
Makanga M, Bassat Q, Falade CO, Premji ZG, Krudsood S, Hunt P, et al. Efficacy and safety of artemether–lumefantrine in the treatment of acute, uncomplicated Plasmodium falciparum malaria: a pooled analysis. Am J Trop Med Hyg. 2011;85:793–804.
Gogtay N, Kannan S, Thatte UM, Olliaro PL, Sinclair D. Artemisinin-based combination therapy for treating uncomplicated Plasmodium vivax malaria. Cochrane Database Syst Rev. 2013;10:CD008492.
Naing C, Aung K, Win D-K, Wah MJ. Efficacy and safety of chloroquine for treatment in patients with uncomplicated Plasmodium vivax infections in endemic countries. Trans R Soc Trop Med Hyg. 2010;104:695–705.
Ndounga M, Mayengue PI, Tahar R, Casimiro PN, Maya DW, Miakassissa-Mpassi V, et al. Efficacy of sulfadoxine–pyrimethamine, amodiaquine, and sulfadoxine–pyrimethamine–amodiaquine combination for the treatment of uncomplicated falciparum malaria in the urban and suburban areas of Brazzaville (Congo). Acta Trop. 2007;103:163–71.
Adam I, Ibrahim MH, Alelbasit IA, Elbashir MI. Efficacy of sulfadoxin pyrimethamine for uncomplicated Plasmodium falciparum malaria in a small sample of Sudanese children. East Mediterr Health J. 2004;10:309–14.
Dahlström S, Veiga MI, Mårtensson A, Björkman A, Gil JP. Polymorphism in PfMRP1 (Plasmodium falciparum multidrug resistance protein 1) amino acid 1466 associated with resistance to sulfadoxine–pyrimethamine treatment. Antimicrob Agents Chemother. 2009;53:2553–6.
Nguyen-Dinh P, Spencer H, Chemangey-Masaba S, Churchill F. Susceptibility of Plasmodium falciparum to pyrimethamine and sulfadoxine/pyrimethamine in Kisumu, Kenya. Lancet. 1982;319:823–5.
WHO. Global technical strategy for malaria 2016–2030. Geneva: World Health Organization; 2015.
Authors’ contributions
EAG and ASB conceived the research, developed the protocol, conducted the literature search, assessed potentially relevant studies for inclusion into the review, assessed the methodological quality of the included studies, independently extracted the data, performed the statistical analysis, and drafted the manuscript, critically reviewed the manuscript, and wrote the final manuscript. MAS and HGT assessed potentially relevant studies for inclusion into the review, assessed the methodological quality of the included studies, independently extracted the data, and critically reviewed the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data and materials used for the analysis of this review are included in this and the additional documents.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gebreyohannes, E.A., Bhagavathula, A.S., Seid, M.A. et al. Anti-malarial treatment outcomes in Ethiopia: a systematic review and meta-analysis. Malar J 16, 269 (2017). https://doi.org/10.1186/s12936-017-1922-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-017-1922-9