Abstract
Colorectal neoplasms are one of the deadliest diseases among all cancers worldwide. Thymoquinone (TQ) is a natural compound of Nigella sativa that has been used in traditional medicine against a variety of acute/chronic diseases such as asthma, bronchitis, rheumatism, headache, back pain, anorexia, amenorrhea, paralysis, inflammation, mental disability, eczema, obesity, infections, depression, dysentery, hypertension, gastrointestinal, cardiovascular, hepatic, and renal disorders. This review aims to present a detailed report on the studies conducted on the anti-cancer properties of TQ against colorectal cancer, both in vitro and in vivo. TQ stands as a promising natural therapeutic agent that can enhance the efficacy of existing cancer treatments while minimizing the associated adverse effects. The combination of TQ with other anti-neoplastic agents promoted the efficacy of existing cancer treatments. Further research is needed to acquire a more comprehensive understanding of its exact molecular targets and pathways and maximize its clinical usefulness. These investigations may potentially aid in the development of novel techniques to combat drug resistance and surmount the obstacles presented by chemotherapy and radiotherapy.
Graphical Abstract

Similar content being viewed by others
Introduction
According to the GLOBOCAN report, colorectal cancer is the third most common cancer and the second leading cause of death among all cancers globally, affecting both genders [1]. In the year 2020, the estimated quantity of fresh instances of colorectal cancer was 1.9 million, leading to 930,000 fatalities. These statistics are estimated to increase to 3.2 million fresh instances and 1.6 million fatalities by the year 2040 [2]. The endangerment variables correlated with colorectal cancer involve an inactive way of life (obesity), ingestion of a diet high in crimson flesh, alcohol, and low fiber foods, smoking, and chronic inflammation. Furthermore, genetic predisposition has been determined to be a probable cause for the emergence of colorectal cancer [3, 4]. Aging is another risk factor for colorectal cancer (CRC) since 90 percent of new incidences occurs in 50 years old or later [4]. Moreover, men have higher risk (1.3-fold) for CRC than women [5]. The significance of screening for CRC cannot be emphasized enough since it has the potential to identify precancerous polyps as well as early-stage cancers at the most opportune time for treatment. Colonoscopy, fecal occult blood testing, and stool DNA testing are some of the existing screening methods available [6]. According to the American Cancer Society, individuals who are at average risk of developing CRC should start screening at age 45 with promptness.
Preventing CRC onset can be achieved by inducing lifestyle changes such as maintaining a nutrient-rich diet, keeping excess weight off, participating in activities that encourage physical movement, as well as avoiding the use of tobacco products and excess alcohol intake. Moreover, using nonsteroidal anti-inflammatory drugs (NSAIDs) for chemoprevention purposes has been known to effectively lower the risk of developing CRC [7].
5-fluorouracil, capecitabine, oxaliplatin, irinotecan, and monoclonal antibodies such as bevacizumab, panitumumab, and cetuximab are well-known chemotherapy agents administered as monotherapy or combination therapy [8,9,10]. Despite the positive effects of the aforementioned agents in CRC control and treatment, most patients complain of neutropenia, vomiting, diarrhea, neurotoxicity, and mucositis which are attributed to especially higher doses of these drugs [11].
Nigella sativa, also known as black seed or black cumin, is an annual herb in the Ranunculacea family with numerous medicinal properties [12]. It contains a variety of active ingredients, including TQ, alkaloids, saponins, flavonoids, proteins, and fatty acids and these components have been shown to have positive effects in treating various diseases [13]. Preclinical studies have demonstrated its potential for use as an anti-cancer, antimicrobial, analgesic, antipyretic, contraceptive and anti-fertility, anti-oxytocic, anti-tussive, anti-inflammatory and antioxidant agent [13]. This review aims to present a detailed report on the studies conducted on the anti-cancer properties of thymoquinone (TQ) against CRC, both in vitro and in vivo.
Colorectal cancer pathogenesis
The development of colorectal cancer is a complex process that is influenced by a combination of genetic and environmental factors and involves the accumulation of genetic alterations in normal colonic mucosa. These alterations can include mutations in genes such as adenomatous polyposis coli (APC), KRAS, TP53, and SMAD4 [14]. APC mutations, which are found in up to 80% of sporadic colorectal cancer cases, are considered an early event in the pathogenesis of the disease [14]. APC is a tumor suppressor gene that regulates cell proliferation and differentiation. When mutated, it can lead to the activation of the Wnt signaling pathway, promoting cell proliferation and inhibiting apoptosis [7]. KRAS mutations, found in approximately 40% of colorectal cancer cases, are associated with a poor prognosis. KRAS is a proto-oncogene that regulates cell growth and differentiation. When mutated, it can lead to the activation of downstream signaling pathways that promote cell proliferation and survival [7, 14]. TP53 mutations, found in approximately 50% of colorectal cancer cases, are also associated with a poor prognosis. TP53 is a tumor suppressor gene that regulates cell cycle arrest, DNA repair, and apoptosis. When mutated, it can lose its tumor suppressor function, promoting cell proliferation and inhibiting apoptosis. SMAD4 mutations, found in approximately 10% of colorectal cancer cases, are associated with a poor prognosis as well. SMAD4 is a tumor suppressor gene that regulates TGF-β signaling. When mutated, it can lead to the activation of downstream signaling pathways that promote cell proliferation and survival [7, 14].
Epigenetic alterations, which are changes in gene expression that do not involve changes to the DNA sequence, play a critical role in the development of colorectal cancer. The two most common epigenetic alterations in this disease are DNA methylation and histone modification. DNA methylation involves the addition of methyl groups to cytosine residues in CpG dinucleotides. When CpG islands located in the promoter regions of tumor suppressor genes become hypermethylated, it can lead to the silencing of these genes and the loss of their tumor suppressor function. This can promote cell proliferation and inhibit apoptosis [15]. Histone modification refers to changes in the structure of chromatin that can affect gene expression. Histones can be modified through processes such as acetylation, methylation, phosphorylation, or ubiquitination. These modifications can either activate or repress gene expression depending on their location within the chromatin structure [15].
The escalation from colorectal adenoma to carcinoma is caused by three cardinal pathways: microsatellite instability (MSI), chromosomal instability (CIN), and CpG island methylator phenotype (CIMP) [16]. When there is a malfunction in the DNA mismatch repair genes, MSI occurs, leading to an amassment of transformations in microsatellite areas throughout the genome. MSI is perceived in approximately 15% of sporadic CRC cases and is correlated with a superior prognosis [16]. On the other hand, CIN occurs due to aneuploidy or chromosomal rearrangements, leading to a buildup of genetic changes throughout the genome. CIN is identified in approximately 85% of sporadic CRC cases and is related to an adverse prognosis [16]. Finally, CIMP is identified upon hypermethylation of CpG islands situated in promoter regions of tumor suppressor genes, leading to their suppression. CIMP is identified in approximately 20% of sporadic CRC cases and is correlated with an unfavorable prognosis [16].
Anticancer effects of TQ
Nigella sativa L. (also known as black cumin, black seed, and black caraway) is a spice that also has been used in traditional medicine against a variety of acute/chronic diseases such as asthma, bronchitis, rheumatism, headache, back pain, anorexia, amenorrhea, paralysis, inflammation, mental disability, eczema, obesity, infections, depression, dysentery, hypertension, gastrointestinal, cardiovascular, hepatic, and renal disorders based on antimicrobial/viral, antioxidative, anti-diabetic, anti-inflammatory, and immunomodulatory properties of its components specifically TQ [17,18,19,20,21,22,23,24].
TQ is the main bioactive component of the volatile oil of N. sativa [18, 25,26,27]. Other components of N. sativa L. are thymohydroquinone, thymol, carvacrol, nigellidine, nigellicine, and α-hederin [28, 29]. Thanks to chemo preventive and anti-tumor properties, TQ and N. sativa has been used against various neoplasms including lung, hepatobiliary, liver, breast, pancreas, hematopoietic/leukemia, kidney, bladder, cervix, skin, ovary, prostate, osteosarcoma, fibrosarcoma, and colorectal cancers in-vivo and in-vitro with less cytotoxicity against normal cells [18, 20, 22, 25, 30]. Different studies showed that TQ could target various mechanisms involved in cancer progression including proliferation [21, 22, 24, 30,31,32,33,34,35,36,37,38], migration [21, 30, 32, 34,35,36, 39,40,41] invasion/metastasis [22, 30, 32, 35, 41, 42], angiogenesis [22, 31, 41], colony formation [35, 36], tubulogenesis [43, 44], epithelial-mesenchymal transition (EMT) [32, 34, 42, 45], autophagy [45,46,47], and cancer stemness [43].
Furthermore, TQ enhanced tumor cell cytotoxicity [21, 43, 48], cell cycle arrest at G2/M [49,50,51,52,53,54,55], G1/S [19, 31, 56, 57], S [58], and G0/G1 [30, 38, 42, 48, 50, 59], apoptosis [19, 22, 30, 31, 35, 37, 38, 48, 56, 60,61,62,63,64,65], and necroptosis [66].
A vast variety of proteins and signaling pathways that play key roles in cancer pathogenesis are reported to be modulated by TQ including inhibition of E2F-1 [67], eEF-2 K [21], microphthalmia‑associated transcription factor (MITF) [33], Rac1 [40], Notch1 [68], Src/FAK [21, 31, 69,70,71], PI3K/Akt/mTOR [21, 31, 34, 36, 48, 72, 73], TGF‑β/Smad2/3 [32], Wnt/β-catenin [33, 42], tubulin α/β [74], NF-κB and p65 [21, 22, 31], TNF-α [75], anti-apoptotic proteins (IAP1/2, XIAP, Bcl-2, Bcl-xL Mcl-1, c-FLIP and survivin) [22, 30, 31, 48, 50, 62, 63, 76], PD-L1 [45, 64], HIF-1α [77], MUC4 [69, 78], ENA-78 and Gro [79], androgen receptor [67], Plk1 [80], IRAK1 [81], proliferative proteins (cyclin A, cyclin B1, cyclin D1/2/3, cyclin E, CDK2/4, c-Myc, Ki-67, PCNA) [22, 31, 42, 48, 50, 57, 65, 67, 73, 82, 83], CXCR4 [84, 85], Integrin-β1 [47], Beclin-1 and LC3 [47], COX-2 [22, 84], HSP70 [58], u-PA [86], MMP-2/9 [22, 39, 70], MMP-3/7 [42, 87], Trx1 [88], VEGF [22, 31, 39], ERK1/2 [41, 70, 89], JNK [71, 90], NLRP3 [91], BCR-ABL [37], JAK2/STAT3 [31, 37, 65, 72, 73], FLT3-ITD [38], IL-8 [54], STAT5a/b [37, 72, 73], EP2 [92], vimentin, TWIST1, SLUG, SNAIL1, ZEB1, and N-cadherin [32, 34, 45], and promoting expression of SH-PTP2 [31], SHP-1 [38, 93], SOCS-1/3 [38, 93], p16 [50], p21 (CIP1/WAF1) [51, 67], p27 (Kip1) [48, 67], activated p38/MAPK [61, 69], p53 [40, 48, 50], p62 [46], p73α/β [59, 74], PPAR-γ [30], PTEN [52, 72], PKM2 [94], Bax [48, 62, 63, 95], LKB1/AMPK [96], Par-4 [97], Bad [48], LC3-II [46], E-cadherin [32, 34, 45, 98], Tristetraprolin (TTP) [78], gelsolin [99], TIMP3 [39], HSPA6 [100], Nrf2 [64], IL17RD [101], cleavage of caspases-3/7/8/9, and PARP [30, 31, 36, 48, 61, 63, 95], TRAIL [102], DR5 (TRAIL-R2) [54, 102, 103], cytochrome C release [48, 95] and ROS production/oxidative stress [54, 59, 65, 104].
Hypermethylation of tumor suppressor genes which leads to the downregulation of these TSGs contributes to several malignancies [38] Epigenetic modulation is another mechanism of action of TQ to combat cancers, in this regard, expression of SHP-1 and SOCS-3, two TSGs inhibiting JAK/STAT pathway, increased through TQ-induced hypomethylation of CpG island of these genes’ promoters [38, 93]. This modulation was considered to be related with TQ-induced upregulation of TET2 and WT1 and downregulation of DNA methyltransferases 1/3A/3B, UHRF1, HDAC1/2 [38, 59, 88, 105].
Several studies indicated that TQ exerted its anti-cancer characteristics via non-coding RNAs such as upregulation of miR-603 [21] and miR-1-3p [39], miR-34a [40, 106, 107], miR-125a-5p [108], miR-16 [106, 109], miR-877-5p [45], and miR-375 [109].
In-vitro studies also mentioned the combination of TQ with anti-neoplastic agents or flavonoids such as thalidomide [31], temozolomide [110], bortezomib [31], doxorubicin [30], 5-fluorouracil [30, 111], paclitaxel [30], methotrexate [62, 63], cisplatin [79, 112], tamoxifen [113], topotecan [114], gemcitabine [68, 94], curcumin [36], quercetin [115], and emodin [116] augmented cytotoxic effects of these chemicals against cancer cells.
Interestingly, since TQ like other flavonoids and polyphenolic compounds are sensitive to light and pH, incorporation of TQ into various nanoparticles increased its solubility, stability, and improved its efficacy against tumor cells [117,118,119]. Various nanoparticles have been used in this manner including chitosan encapsulating poly D,L-lactic-co-glycolic acid (PLGA) [118], lipid [120], liposome [60, 121, 122], PEG [40], and PLGA [20], zinc oxide [123], mesoporous silica [124], and lipid polymer hybrid nanoparticles [125].
TQ and colorectal cancer
A multitude of preclinical studies have been conducted in the realm of colorectal cancer research (Fig. 1). As previously discussed, TQ demonstrates a notable cytotoxic effect against cancer cells, while sparing normal cells [126]. In the study conducted by Eftekhar et al., it was observed that TQ significantly enhances the Area Under the Curve (AUC), Maximum Concentration (Cmax), and Time to Reach Maximum Concentration (Tmax) of 5-FU, thereby augmenting its pharmacokinetic profile. Interestingly, even trace amounts of TQ were observed to potentiate the growth-inhibitory effects of 5-FU on colorectal cancer cells. Furthermore, TQ was found to reduce the viability of HT-29 cells in a dose-dependent manner, with an IC50 value of 0.284 mM. Notably, the combined administration of TQ and 5-FU resulted in an enhanced cytotoxic effect compared to the individual suppressive impact of 5-FU. This combination demonstrated a significant suppressive effect at 5-FU concentrations of 0.027 and 0.055 mM, suggesting that TQ could potentially amplify the growth-suppressive effects of 5-FU on cancer cells [127]. The concurrent administration of 30 μM imatinib, a tyrosine kinase inhibitor, and 10 μM TQ resulted in the suppression of ABCB1, ABCG2, and hOCT1, thereby enhancing the uptake of imatinib in HCT-116 cells [128]. The combination of 20 and 40 µM TQ with 0.2 µM cisplatin amplified the cytotoxicity of cisplatin in HCT-116 and COLO205 cells. This data suggests that TQ potentiates cisplatin-induced cell death in a dose-dependent manner, indicating a potential role for TQ in augmenting the chemosensitivity of colon cancer cells [129]. Research conducted by Osorio-Pérez demonstrated a significant reduction in the expression levels of miR-21-5p in HCT-15 cells following TQ treatment [130]. Interestingly, the combination of TQ with ionizing radiation (IR) enhanced cytotoxicity against HT-29 and HCT-116 cells. The combination of a low dose of TQ (3 µM) with IR (2 Gy) resulted in complete inhibition of sphere formation by the fifth generation. This outcome was linked to the suppression of stemness and DNA repair mechanisms [131]. An in vivo study was conducted to evaluate the protective role of TQ against DMH-induced CRC in adult male Wistar rats. Both pre and post treatment with TQ significantly inhibited CRC initiation and progression. Notably, pre-treatment with TQ was more effective than post-treatment. The protective effects of TQ include reduced ROS production and lipid peroxidation (MDA) [132]. Moreover, TQ alone or in combination with vitamin D showed favorable outcomes in Azoxymethane-induced CRC rats [133] (see Table 1).
TQ, acting via multiple targets, may serve as a potential natural therapeutic agent against CRC. This is achieved through the augmentation of apoptosis and oxidative stress, coupled with the attenuation of cell cycle progression/proliferation, inflammation, CRC-associated signaling pathways, angiogenesis, and metastasis
TQ-loaded polymeric nanocapsules were synthesized by Ramzy et al., utilizing the nanoprecipitation technique, with Eudragit S100 serving as the polymeric shell. Anisamide was conjugated as a targeting ligand for sigma receptors, which are overexpressed by colon cancer cells. The anisamide-targeted nanocapsules exhibited superior cytotoxicity compared to non-conjugated nanocapsules and free TQ against HT-29 cells following 48 h of incubation. This increased cytotoxicity can be attributed to the high level of sigma receptor expression on HT-29 cells, leading to enhanced uptake of nanocapsules [134]. One study suggested that TQ has the potential to enhance replication fidelity and that the chemo preventive effects of TQ in Lynch syndrome are due to this property. TQ has been observed to decrease the incidence and multiplicity of intestinal tumors in Msh2 loxP/loxP Villin-Cre mice as well as MSI in Msh2-deficient epithelium [135]. Nano formulations of TQ with PLGA and PVA also enhanced the efficiency of TQ in HCT-116 xenograft models and also showed protective effects against doxorubicin-induced cardiotoxicity [136]. Encapsulated TQ in lipid nanocapsules (LNCs) enhanced its specificity and cellular absorption. In vivo studies revealed that intratumoral administration of TQ-LNCs led to a reduction in tumor size in mice with colorectal cancer, compared to the control group. Interestingly, TQ-LNCs proved to be more effective than free TQ in inducing tumor cell death [137].
TQ targets signaling pathways
In a study conducted by El-Baba et al., an experiment was performed on colorectal HCT116wt cells using the PepChip Kinomics v2 peptide array. Following treatment with 40 μM TQ for a duration of 24 h, a significant increase in phosphorylation was observed in 104 proteins. Out of these proteins, 50 proteins and kinases exhibited an upregulation of ≥ twofold (out of 1152 kinase substrate peptides). Further analysis revealed that among the top 50 candidate proteins, 24 were classified into the cancer-related networks “cytoskeleton”, “PI3K/AKT”, and “Wnt signaling”. Upon the introduction of TQ, significant structural alterations were observed in P21-Activated Kinase 1 (PAK1), which disrupted its scaffold function in the pro-survival PAK1/MEK/ERK1/2 signaling pathway. This led to the modification of several signaling mechanisms: The binding affinity between ERK1/2 and PAK1 is enhanced, which inhibits the phosphorylation of pPAK1Thr212 by ERK1/2. This results in an increase in phosphorylation at the Thr423 site, which interferes with the catalytic domain of PAK1 and prevents PAK1 activation. Ultimately, this cascade of events leads to the induction of apoptosis [138].
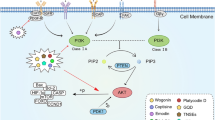
The PI3K/Akt signaling pathway is frequently implicated in the progression of CRC [139]. TQ has been observed to inhibit PI3K/Akt activation in CRC cell lines, including HCT-116 and SW480 probably through enhancing PTEN tumor suppressor [61, 139, 140]. This inhibition could potentially alter metabolic reprogramming in CRC cells, as evidenced by the suppression of factors and enzymes related to glycolysis and the Warburg effect, including HIF1α, hexokinase2, PDHK1, and LDHA. Conversely, TQ was found to enhance the expression of the PDH enzyme [61, 139]. The suppression of the PI3K-AKT/HK2 pathway is associated with a reduction in the tumorigenic capabilities of CRC cells, including wound healing and invasiveness [139].
Previous research has suggested that PGE2 enhances COX-2 expression by activating the EP4/β-catenin pathway, implying that PGE2 regulates p-PI3K, p-AKT, and p-GSK-3β expression in LoVo cells. It has been noted that PGE2 initiates downstream signaling through EP2 and EP4 to induce a variety of biological reactions. The administration of TQ was observed to diminish the increase in COX-2 expression induced by PGE2, and β-catenin significantly influenced the modulation of EP2 and EP4 by PGE2. Hsu et al. reported that TQ successfully inhibited PGE2/EP2/EP4-induced activation of p-Akt/p-PI3K/p-GSK3β/β-catenin/LEF-1/TCF-4 in LoVo cells [141]. Results showed that TQ decreased nuclear translocation of β-catenin/LEF-1/TCF-4 in a concentration-dependent manner which led to downregulation of COX-2. Researchers concluded that COX-2 inhibition led to suppressing cell migration as well as metastasis in vivo [141]. Within the nucleus, β–catenin operates as a transcription factor, forming a complex with TCF/LEF that binds to DNA enhancer sequences. This interaction results in the upregulation of certain genes, including the proto-oncogene c-myc [142]. Research conducted by Lang et al., revealed that TQ translocated β–catenin to the membrane, thereby suppressing c-myc expression in APCMin mice. TQ was found to inhibit the phosphorylation of GSK-3β, likely through the suppression of MEK1/2 rather than PI3K. In untreated colorectal cells, GSK-3β undergoes phosphorylation at the Ser9 position via several pathways (such as Ras-Raf-MEK, PI3K-AKT1, and WNT), rendering it inactive. However, following TQ administration, there was a decrease in GSK-3β Ser9 phosphorylation (which is downstream of Ras, Raf, MEK). This results in β-catenin being relocated to the membrane and a reduction in nuclear c-myc (due to phosphorylation, ubiquitination, and eventual degradation) [143].
TQ has been reported to increase activated (phosphorylated) forms of JNK1/2 and ERK1/2 in DLD-1 cells, likely through ROS production. However, this effect was abolished after 24 h treatment. No alterations were observed in the expression of p-p38 and total p38 protein as well as total JNK and ERK protein in response to TQ [126]. STAT3 is perpetually active in colon cancer and plays a crucial role in cell proliferation by transcriptionally activating pro-survival genes [144]. The treatment of cells with TQ obstructed the continuous phosphorylation of STAT3 at the tyrosine-705 residue and reduced the nuclear localization of p-STAT3. Furthermore, TQ could target EGFR, Src kinase and JAK2. Incubation of 50 μM TQ in HCT-116 cells decreased activated form of JAK2 (p-JAK2) followed by a suppression in p-STAT3 [145]. TQ has been identified as a potent inhibitor of NF-κB, a key cellular transcription factor. At a concentration of 60 µM, TQ was found to suppress the phosphorylation of NF-κB p65 subunit, resulting in the inhibition of its downstream genes including VEGF, c-Myc, and Bcl-2 in COLO205 cells [129].
The effects of TQ on cell proliferation/cycle
El-Najjar reported that TQ inhibited the proliferation of a panel of CRC cell lines, including HT-29, HCT-116, DLD-1, Lovo, and Caco-2, in a time and concentration-dependent manner. Among these cell lines, Caco-2 was the most sensitive and HT-29 was considered the most resistant to TQ according to their IC50 [126]. Gali-Muhtasib et al. [146] found that TQ arrested the cell cycle at G1/S within 24 h/48 h of 60 µM TQ treatment. At elevated doses of TQ (100 µM) and with extended incubation periods, there was a noticeable accumulation of a sub-G1 peak of hypodiploid cells to the left of the G1 peak, along with a corresponding decrease in the S population. The G1/S cell cycle arrest is attributed to p21/WAF1 expression, which prevents transition to the S phase and is enhanced by TQ-induced p53 expression [146].
DLD-1 cells were subjected to a treatment of 40 μM TQ for either 24 or 48 h, and then collected for flow cytometric analysis of DNA content via PI staining. TQ induced a significant rise in the proportion of cells in the preG1 phase of the cell cycle in a time-dependent manner: at 40 μM TQ for 24 h, it increased from 2.5% to 18.8%, and for 48 h, it escalated from 4.0% to 31.2% [126]. Another study used 10 μM and 20 μM TQ and evaluated the effect of TQ following 24 and 72 h. In HCT-116 cells, a 24-h exposure to 20 μM TQ led to a notable build-up of pre-G1 events with a reduction in G1, S, and G2/M events, while no alteration was detected with 10 μM TQ. After 72 h, 10 μM TQ triggered a significant G1/S halt with diminished G2/M events; at 20 μM, the most pronounced disturbance (albeit less than after a 24-h treatment) was the build-up of pre-G1 events [147]. TQ was observed to increase the proportion of HT29 cells in the G2/M phase, while simultaneously reducing the count in the S-phase when compared to untreated cells. Conversely, TQ resulted in a halt at the G0/G1-phase in SW480 cells. This was accompanied by a decline in CCND1 and CCND3 mRNA/protein levels, while an increment was observed in p21 and p27 levels [61] In addition, TQ lessened the expression of STAT3 target gene products, such as survivin, c-Myc, cyclin-D1, -D2, and elevated the expression levels of the cell cycle regulatory proteins p27 and p21 [145]. The growth of HCT-15 cells was observed to be suppressed by TQ in a dose-dependent manner (IC50: 82.59 µM). Furthermore, the growth of these cells was adversely affected even when exposed to higher doses of TQ [130]. TQ with ionizing radiation caused G2/M arrest in HT-29 and HCT-116 cells. While radiation (2 Gy) alone led to a minor increase in the proportion of HCT116 cells in the G2/M phase, the combination of radiation with TQ (10 µM and 30 µM) resulted in a significant accumulation of cells in the G2/M phase. Furthermore, there was a notable decrease in the percentage of cells at G0/G1 in HCT116 cells treated with 30 µM TQ and radiation. In HT29 cells, cell cycle arrest was observed in irradiated cells and in cells treated with 60 µM TQ. This effect was more pronounced in cells treated with a combination of TQ and radiation compared to either treatment alone. Interestingly, when combined with radiation, TQ led to a significant increase in the G2/M population from 21% in the control to 26% and 31% at TQ concentrations of 10 µM and 60 µM, respectively [131].
The effect of TQ on cell death and induction of apoptosis
Treatment with 100 µM of TQ after 24 h reduced Bcl-2 and increased apoptosis in a p53-dependent manner in HCT-116 cells. The suppression of p53 expression curtailed the over-expression of p53 induced by TQ and significantly reduced apoptosis, indicating that p53 is the primary regulator of apoptosis induced by TQ [146]. TQ diminished the expression of the anti-apoptotic proteins Bcl-2 and Bcl-xl, while it amplified the expression of the pro-apoptotic protein Bax in HCT116 cells following treatment with 25 or 50 µM TQ after 24/48/72 h. The administration of TQ to HCT116 cells triggered the cleavage of caspase-9, -7, and -3, and PARP, and heightened the activity of caspase-3. The pre-treatment of cells with a pan-caspase inhibitor z-VAD-fmk nullified the TQ-induced caspase-3 activity, as well as the cleavage of caspase-3 and PARP. Furthermore, obstructing the activation of caspase-3 led to the cessation of TQ-induced apoptosis [145]. TQ instigated a significant increase in apoptosis that was dependent on the concentration after an exposure of 24 h, with most apoptotic events taking place in the late-apoptotic quadrant (A+ /PI+), reaching 10% for 10 µM and 23% for 20 µM. However, after 72 h, there were diminished and non-significant escalations in apoptotic cells observed in these cells, even with a treatment of 20 µM in HCT-116 cells [147].
Treatment with 100 µM of TQ in HT-29 cells led to an increase in the necrosis rate, exceeding 90% after 24 h [148]. TQ was found to increase reactive oxygen species (ROS), specifically the superoxide radical O2− , which subsequently led to DNA damage. This was confirmed by the high expression of γH2AX, a marker of DNA damage [149]. ROS production occurred in both p53+/+ and p53−/− HCT-116 cells, but was higher in p53+/+ cells. Gali-Muhtasib et al. indicated that p53-induced CHEK1 reduction contributed to apoptosis. Also, CRC clinical samples verified that CHEK1 inhibition was observed in p53 expressing patients rather than p53 null patients [149]. To confirm that p53-induced CHEK1 inhibition is related to caspase-3 dependent apoptosis, HCT-166 p53+/+ and p53−/− xenograft mouse models were established, and similar results were obtained [149]. TQ was observed to induce the apoptotic cleavage of PARP, resulting in an 89 kDa fragment at 24 h. However, in the presence of IPA-3, a PAK1 inhibitor, this cleavage was more pronounced and occurred earlier, at 6 h. Intriguingly, the combination of TQ (40 μM) and IPA-3 (10 μM) led to a significant increase in cell death and reduced cell viability by 70% in HCT-116 wt cells [138]. Among CRC cell lines, HT-29 is reported to be the most resistant to TQ-induced apoptosis, while DLD-1 showed TQ-induced early apoptosis. The rise in apoptosis over time due to TQ was further validated by the M30 immunofluorescent pictures, which displayed distinct cytoplasmic indications for the M30 antibody following TQ administration. The M30 cytodeath antibody, which identifies a specific caspase cleavage site within cytokeratin 18, is a characteristic indicator of early apoptosis initiation. Moreover, a 2.5-fold and fourfold surge in caspase-3/7 activity was noted at 24 h and 48 h respectively, following the administration of 40 μM TQ [126]. El-Najjar et al. have stated that oxidative stress is the mechanism through which TQ exerts its anti-cancer and pro-apoptotic effects [126].
Intraperitoneal (i.p.) injection of TQ was found to decrease DMH-induced CRC in female bulb/c mice by promoting caspase-3 and apoptosis. Similar results were obtained in HCT-116 xenograft of this model. Injection of TQ significantly decreased both the count and size of Aberrant Crypt Foci (ACF) at the 10-week mark, with ACF numbers dropping by 86%. Tumor multiplicity was also reduced at the 20-week mark, decreasing from 17.8 in the DMH group to 4.2 in mice injected with TQ. This suppression was observed at the 30-week mark and was long-term; tumors did not re-grow even when TQ injection was discontinued for 10 weeks [150].
The effect of TQ against cancers based on human studies
A phase 1 randomized, double-blind, placebo-controlled trial was conducted to assess the safety of a black cumin oil formulation containing 5% TQ, administered at a dose of 200 mg per adult per day for a period of 90 days, on healthy participants. The study did not report any serious adverse side effects or significant changes in hematological parameters. Similarly, no significant changes were observed in biochemical parameters related to liver function (ALT, AST, ALP) and renal function (serum creatinine and urea). However, the lipid profile analysis showed a significant reduction in total cholesterol, LDL, VLDL, and triglycerides, albeit within the normal range [151]. A clinical trial was conducted in a randomized, double-blind, placebo-controlled manner to assess the potential benefits of Nigella sativa seeds oil as an adjunctive therapy for hypertension, blood sugar regulation, and lipid metabolism. The intervention group were given 2.5 ml of N. sativa seeds oil twice daily for 8 weeks. There was a notable reduction in blood pressure, total cholesterol, low-density lipoprotein, MDA, and FBS levels, along with a significant rise in high-density lipoprotein and Glutathione Reductase levels [152]. In a separate study, it was found that when administered in conjunction with a daily dose of 1000 mg Metformin, both 50 and 100 mg doses of TQ showed a decrease in HbA1c and blood glucose levels. This combination therapy proved to be more effective than the standard treatment of diabetes, which involves administering Metformin alone [153]. A study conducted by Soleymani and colleagues revealed that a hydrogel made from N. sativa had a significant impact on alleviating the symptoms of acne vulgaris. The treatment was also found to be well-tolerated by the patients [154]. Ammar and others concluded that supplementing with black cumin oil, taken as 500 mg soft gel capsules three times a day for a duration of 6 months, has been found to provide additional benefits when used alongside metformin in improving conditions related to Polycystic Ovary Syndrome (PCOS). These benefits include the resumption of regular menstrual cycles, weight loss, alteration in body fat distribution, and the restoration of oxidative balance [155].
According to https://clinicaltrials.gov/, a phase II clinical trial conducted by Nabil investigated the chemopreventive effects of N. sativa. This randomized, controlled study (NCT03208790) enrolled 48 patients with premalignant oral lesions1. The participants were given either a 10 mg N. sativa tablet to the buccal mucosa, a 5 mg buccal N. sativa tablet, or a placebo. The primary outcome measure was the size of the lesion at 3 months post-treatment compared to the initial dimensions. Although the study was completed in 2020, the results have not yet been published [156, 157].
To the best of our current understanding, there have been no clinical studies that specifically investigate the impact of TQ on colorectal cancer.
Conclusion
Despite notable progress in surgical and chemotherapy procedures, the survival rates of patients with life-threatening diseases such as cancers are still affected by drug resistance and adverse side effects experienced under chemotherapy or radiotherapy [166]. Hence, it is imperative to fortify exploration and development endeavors to enhance the efficacy of prevalent remedial protocols while concurrently curtailing their adverse influence on patient health and standard of living. In this regard, natural agents like TQ have shown immense promise in advancing cancer treatment outcomes. Various studies have illustrated that TQ, through its capability to modulate different signaling pathways, can provide potent anti-cancer properties. The anti-inflammatory and antioxidant features of TQ have been well documented as capable of suppressing colorectal malignancies. Additionally, TQ may affect cellular processes such as apoptosis, angiogenesis, cell cycle, and proliferation, as well as metastasis, thereby enhancing its anticancer effects.
In summary, TQ stands as a promising natural therapeutic agent that can enhance the efficacy of existing cancer treatments while minimizing the associated adverse effects. However, further research is of vital importance in order to acquire a more comprehensive understanding of its exact molecular targets and pathways and maximize its clinical usefulness. These investigations may potentially aid in the development of novel techniques to combat drug resistance and surmount the obstacles presented by chemotherapy and radiotherapy.
Availability of data and materials
Not applicable.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020 GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag C, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72(2):338–44.
Hossain MS, Karuniawati H, Jairoun AA, Urbi Z, Ooi J, John A, et al. Colorectal cancer: a review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers. 2022;14(7):1732.
Sawicki T, Ruszkowska M, Danielewicz A, Niedźwiedzka E, Arłukowicz T, Przybyłowicz KE. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers. 2021;13(9):2025.
Lansdorp-Vogelaar I, Meester R, de Jonge L, Buron A, Haug U, Senore C. Risk-stratified strategies in population screening for colorectal cancer. Int J Cancer. 2022;150(3):397–405.
Janz T, Lu K, Povlow MR, Urso B, Lu KB, Urso BA. A review of colorectal cancer detection modalities, stool DNA, and fecal immunochemistry testing in adults over the age of 50. Cureus. 2016;8(12):e931.
Grady WM, Markowitz SD. The molecular pathogenesis of colorectal cancer and its potential application to colorectal cancer screening. Dig Dis Sci. 2015;60:762–72.
Gustavsson B, Carlsson G, Machover D, Petrelli N, Roth A, Schmoll H-J, et al. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015;14(1):1–10.
Mohammadian M, Zeynali S, Azarbaijani AF, Ansari MHK, Kheradmand F. Cytotoxic effects of the newly-developed chemotherapeutic agents 17-AAG in combination with oxaliplatin and capecitabine in colorectal cancer cell lines. Res Pharm Sci. 2017;12(6):517.
Zeynali-Moghaddam S, Kheradmand F, Aziz SG-G, Abroon S. Combination of capecitabine, irinotecan and 17-AAG on expression of genes in HT-29 colorectal cancer cell line. Ann Med Surg. 2022;78:103850.
Xiong HQ, Ajani JA. Treatment of colorectal cancer metastasis: the role of chemotherapy. Cancer Metastasis Rev. 2004;23(1):145–63.
Ziaee T, Moharreri N, Hosseinzadeh H. Review of pharmacological and toxicological effects of Nigella sativa and its active constituents. J Med Plants. 2012;11(42):16–42.
Tavakkoli A, Mahdian V, Razavi BM, Hosseinzadeh H. Review on clinical trials of black seed (Nigella sativa) and its active constituent, TQ. J Pharmacopuncture. 2017;20(3):179–93.
Yamagishi H, Kuroda H, Imai Y, Hiraishi H. Molecular pathogenesis of sporadic colorectal cancers. Chin J Cancer. 2016;35:1–8.
Al-Joufi FA, Setia A, Salem-Bekhit MM, Sahu RK, Alqahtani FY, Widyowati R, et al. Molecular pathogenesis of colorectal cancer with an emphasis on recent advances in biomarkers, as well as nanotechnology-based diagnostic and therapeutic approaches. Nanomaterials. 2022;12(1):169.
Nguyen HT, Duong HQ. The molecular characteristics of colorectal cancer: Implications for diagnosis and therapy. Oncol Lett. 2018;16(1):9–18.
Hannan MA, Rahman MA, Sohag AAM, Uddin MJ, Dash R, Sikder MH, et al. Black Cumin (Nigella sativa L.): a comprehensive review on phytochemistry, health benefits, molecular pharmacology, and safety. Nutrients. 2021;13(6):1784.
Zheng J, Zhou Y, Li Y, Xu DP, Li S, Li HB. Spices for prevention and treatment of cancers. Nutrients. 2016;8(8):495.
Sutton KM, Greenshields AL, Hoskin DW. TQ, a bioactive component of black caraway seeds, causes G1 phase cell cycle arrest and apoptosis in triple-negative breast cancer cells with mutant p53. Nutr Cancer. 2014;66(3):408–18.
Ibrahim WN, Muizzuddin Bin Mohd Rosli L, Doolaanea AA. Formulation, cellular uptake and cytotoxicity of TQ-loaded PLGA nanoparticles in malignant melanoma cancer cells. Int J Nanomedicine. 2020;15:8059–74.
Kabil N, Bayraktar R, Kahraman N, Mokhlis HA, Calin GA, Lopez-Berestein G, et al. TQ inhibits cell proliferation, migration, and invasion by regulating the elongation factor 2 kinase (eEF-2K) signaling axis in triple-negative breast cancer. Breast Cancer Res Treat. 2018;171:593–605.
Sethi G, Ahn KS, Aggarwal BB. Targeting nuclear factor-κB activation pathway by TQ: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol Cancer Res. 2008;6(6):1059–70.
Manthalkar L, Ajazuddin, Bhattacharya S. Evidence-based capacity of natural cytochrome enzyme inhibitors to increase the effectivity of antineoplastic drugs. Discover Oncol. 2022;13(1):142.
Abdualmjid RJ, Sergi CM. Mitochondrial dysfunction and induction of apoptosis in hepatocellular carcinoma and cholangiocarcinoma cell lines by TQ. Int J Mol Sci. 2022;23(23):14669.
Ansary J, Giampieri F, Forbes-Hernandez TY, Regolo L, Quinzi D, Gracia Villar S, et al. Nutritional value and preventive role of Nigella sativa L. and its main component TQ in cancer: an evidenced-based review of preclinical and clinical studies. Molecules (Basel, Switzerland). 2021;26(8):2108.
Majdalawieh AF, Fayyad MW, Nasrallah GK. Anti-cancer properties and mechanisms of action of TQ, the major active ingredient of Nigella sativa. Crit Rev Food Sci Nutr. 2017;57(18):3911–28.
Homayoonfal M, Asemi Z, Yousefi B. Potential anticancer properties and mechanisms of TQ in osteosarcoma and bone metastasis. Cell Mol Biol Lett. 2022;27(1):21.
Kooti W, Hasanzadeh-Noohi Z, Sharafi-Ahvazi N, Asadi-Samani M, Ashtary-Larky D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). Chin J Nat Med. 2016;14(10):732–45.
Salim EI. Cancer chemopreventive potential of volatile oil from black cumin seeds, Nigella sativa L., in a rat multi-organ carcinogenesis bioassay. Oncol Lett. 2010;1(5):913–24.
Woo CC, Loo SY, Gee V, Yap CW, Sethi G, Kumar AP, et al. Anticancer activity of TQ in breast cancer cells: possible involvement of PPAR-γ pathway. Biochem Pharmacol. 2011;82(5):464–75.
Li F, Rajendran P, Sethi G. TQ inhibits proliferation, induces apoptosis and chemosensitizes human multiple myeloma cells through suppression of signal transducer and activator of transcription 3 activation pathway. Br J Pharmacol. 2010;161(3):541–54.
Kou B, Liu W, Zhao W, Duan P, Yang Y, Yi Q, et al. [Corrigendum] TQ inhibits epithelial-mesenchymal transition in prostate cancer cells by negatively regulating the TGF-β/Smad2/3 signaling pathway. Oncol Rep. 2023;49(3):3592.
Jeong H, Yu SM, Kim SJ. Inhibitory effects on melanogenesis by TQ are mediated through the β-catenin pathway in B16F10 mouse melanoma cells. Int J Oncol. 2020;56(1):379–89.
Saddiq AA, El-Far AH, Mohamed Abdullah SA, Godugu K, Almaghrabi OA, Mousa SA. Curcumin, TQ, and 3, 3’-diindolylmethane combinations attenuate lung and liver cancers progression. Front Pharmacol. 2022;13: 936996.
Adinew GM, Messeha SS, Taka E, Badisa RB, Antonie LM, Soliman KFA. TQ alterations of the apoptotic gene expressions and cell cycle arrest in genetically distinct triple-negative breast cancer cells. Nutrients. 2022;14(10):2120.
El-Far AH, Saddiq AA, Mohamed SA, Almaghrabi OA, Mousa SA. Curcumin and TQ combination attenuates breast cancer cell lines’ progression. Integr Cancer Ther. 2022;21:15347354221099536.
Al-Rawashde FA, Wan Taib WR, Ismail I, Johan MF, Al-Wajeeh AS, Al-Jamal HAN. TQ induces downregulation of BCR-ABL/JAK/STAT pathway and apoptosis in K562 leukemia cells. Asian Pac J Cancer Prev. 2021;22(12):3959–65.
Al-Rawashde FA, Johan MF, Taib WRW, Ismail I, Johari S, Almajali B, et al. TQ inhibits growth of acute myeloid leukemia cells through reversal SHP-1 and SOCS-3 hypermethylation: in vitro and in silico evaluation. Pharmaceuticals (Basel, Switzerland). 2021;14(12):1287.
Tadros SA, Attia YM, Maurice NW, Fahim SA, Abdelwahed FM, Ibrahim S, et al. TQ suppresses angiogenesis in DEN-induced hepatocellular carcinoma by targeting miR-1–3p. Int J Mol Sci. 2022;23(24):15904.
Bhattacharya S, Ahir M, Patra P, Mukherjee S, Ghosh S, Mazumdar M, et al. PEGylated-TQ-nanoparticle mediated retardation of breast cancer cell migration by deregulation of cytoskeletal actin polymerization through miR-34a. Biomaterials. 2015;51:91–107.
Yi T, Cho SG, Yi Z, Pang X, Rodriguez M, Wang Y, et al. TQ inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol Cancer Ther. 2008;7(7):1789–96.
Zhang M, Du H, Wang L, Yue Y, Zhang P, Huang Z, et al. TQ suppresses invasion and metastasis in bladder cancer cells by reversing EMT through the Wnt/β-catenin signaling pathway. Chem Biol Interact. 2020;320: 109022.
Haiaty S, Rashidi MR, Akbarzadeh M, Bazmani A, Mostafazadeh M, Nikanfar S, et al. TQ inhibited vasculogenic capacity and promoted mesenchymal-epithelial transition of human breast cancer stem cells. BMC Complement Med Therap. 2021;21(1):83.
Dragoni S, Laforenza U, Bonetti E, Lodola F, Bottino C, Berra-Romani R, et al. Vascular endothelial growth factor stimulates endothelial colony forming cells proliferation and tubulogenesis by inducing oscillations in intracellular Ca2+ concentration. Stem Cells (Dayton, Ohio). 2011;29(11):1898–907.
Zhou X, Wang F, Wu H, Chen X, Zhang Y, Lin J, et al. TQ Suppresses the proliferation, migration and invasiveness through regulating ROS, autophagic flux and miR-877-5p in human bladder carcinoma cells. Int J Biol Sci. 2021;17(13):3456–75.
Racoma IO, Meisen WH, Wang QE, Kaur B, Wani AA. TQ inhibits autophagy and induces cathepsin-mediated, caspase-independent cell death in glioblastoma cells. PLoS ONE. 2013;8(9): e72882.
Ünal TD, Hamurcu Z, Delibaşı N, Çınar V, Güler A, Gökçe S, et al. TQ inhibits proliferation and migration of MDA-MB-231 triple negative breast cancer cells by suppressing autophagy, beclin-1 and LC3. Anticancer Agents Med Chem. 2021;21(3):355–64.
Rajput S, Kumar BP, Dey KK, Pal I, Parekh A, Mandal M. Molecular targeting of Akt by TQ promotes G1 arrest through translation inhibition of cyclin D1 and induces apoptosis in breast cancer cells. Life Sci. 2013;93(21):783–90.
El-Shehawy AA, Elmetwalli A, El-Far AH, Mosallam SAE, Salama AF, Babalghith AO, et al. TQ, piperine, and sorafenib combinations attenuate liver and breast cancers progression: epigenetic and molecular docking approaches. BMC Complement Med Ther. 2023;23(1):69.
Gali-Muhtasib HU, Abou Kheir WG, Kheir LA, Darwiche N, Crooks PA. Molecular pathway for TQ-induced cell-cycle arrest and apoptosis in neoplastic keratinocytes. Anticancer Drugs. 2004;15(4):389–99.
Roepke M, Diestel A, Bajbouj K, Walluscheck D, Schonfeld P, Roessner A, et al. Lack of p53 augments TQ-induced apoptosis and caspase activation in human osteosarcoma cells. Cancer Biol Ther. 2007;6(2):160–9.
el Arafa SA, Zhu Q, Shah ZI, Wani G, Barakat BM, Racoma I, et al. TQ up-regulates PTEN expression and induces apoptosis in doxorubicin-resistant human breast cancer cells. Mutat Res. 2011;706(1–2):28–35.
Acharya BR, Chatterjee A, Ganguli A, Bhattacharya S, Chakrabarti G. TQ inhibits microtubule polymerization by tubulin binding and causes mitotic arrest following apoptosis in A549 cells. Biochimie. 2014;97:78–91.
Ashour AE, Abd-Allah AR, Korashy HM, Attia SM, Alzahrani AZ, Saquib Q, et al. TQ suppression of the human hepatocellular carcinoma cell growth involves inhibition of IL-8 expression, elevated levels of TRAIL receptors, oxidative stress and apoptosis. Mol Cell Biochem. 2014;389(1–2):85–98.
Xu D, Ma Y, Zhao B, Li S, Zhang Y, Pan S, et al. TQ induces G2/M arrest, inactivates PI3K/Akt and nuclear factor-κB pathways in human cholangiocarcinomas both in vitro and in vivo. Oncol Rep. 2014;31(5):2063–70.
Hasan S, Ahmed WA, Galeb FM, El Taweel MA, Abu BA. In vitro challenge using TQ on hepatocellular carcinoma (HepG2) cell line. Toxicol Lett. 2008. https://doi.org/10.1016/j.toxlet.2009.06.400.
Raghunandhakumar S, Paramasivam A, Senthilraja S, Naveenkumar C, Asokkumar S, Binuclara J, et al. TQ inhibits cell proliferation through regulation of G1/S phase cell cycle transition in N-nitrosodiethylamine-induced experimental rat hepatocellular carcinoma. Toxicol Lett. 2013;223(1):60–72.
Salim LZ, Mohan S, Othman R, Abdelwahab SI, Kamalidehghan B, Sheikh BY, et al. TQ induces mitochondria-mediated apoptosis in acute lymphoblastic leukaemia in vitro. Molecules (Basel, Switzerland). 2013;18(9):11219–40.
Alhosin M, Abusnina A, Achour M, Sharif T, Muller C, Peluso J, et al. Induction of apoptosis by TQ in lymphoblastic leukemia Jurkat cells is mediated by a p73-dependent pathway which targets the epigenetic integrator UHRF1. Biochem Pharmacol. 2010;79(9):1251–60.
Kłos P, Perużyńska M, Baśkiewicz-Hałasa M, Skupin-Mrugalska P, Majcher M, Sawczuk M, et al. Response of skin-derived and metastatic human malignant melanoma cell lines to TQ and TQ-loaded liposomes. Pharmaceutics. 2022;14(11):2309.
Farrash WF, Aslam A, Almaimani R, Minshawi F, Almasmoum H, Alsaegh A, et al. Metformin and TQ co-treatment enhance 5-fluorouracil cytotoxicity by suppressing the PI3K/mTOR/HIF1α pathway and increasing oxidative stress in colon cancer cells. BioFactors. 2023. https://doi.org/10.1002/biof.1947.
Khyavi PA, Valizadeh A, Shanehbandi D, Yousefi B, Soleimanpour J. TQ potentiates methotrexate mediated-apoptosis in Saos-2 osteosarcoma cell line. Drug Res. 2022;72(7):390–5.
Sanapour N, Malakoti F, Shanebandi D, Targhazeh N, Yousefi B, Soleimanpour J, et al. TQ augments methotrexate-induced apoptosis on osteosarcoma cells. Drug Res. 2022;72(4):220–5.
Adinew G, Messeha SS, Badisa R, Taka E, Soliman KFA. TQ Anticancer effects through the upregulation of NRF2 and the downregulation of PD-L1 in MDA-MB-231 triple-negative breast cancer cells. FASEB J. 2022;36(Suppl):1.
Raut PK, Lee HS, Joo SH, Chun KS. TQ induces oxidative stress-mediated apoptosis through downregulation of Jak2/STAT3 signaling pathway in human melanoma cells. Food Chem Toxicol. 2021;157: 112604.
Berehab M, Rouas R, Akl H, Duvillier H, Journe F, Fayyad-Kazan H, et al. Apoptotic and non-apoptotic modalities of TQ-induced lymphoma cell death: highlight of the role of cytosolic calcium and necroptosis. Cancers (Basel). 2021;13(14):3579.
Kaseb AO, Chinnakannu K, Chen D, Sivanandam A, Tejwani S, Menon M, et al. Androgen receptor and E2F–1 targeted TQ therapy for hormone-refractory prostate cancer. Can Res. 2007;67(16):7782–8.
Mu GG, Zhang LL, Li HY, Liao Y, Yu HG. TQ pretreatment overcomes the insensitivity and potentiates the antitumor effect of gemcitabine through abrogation of Notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic cancer. Dig Dis Sci. 2015;60(4):1067–80.
Torres MP, Ponnusamy MP, Chakraborty S, Smith LM, Das S, Arafat HA, et al. Effects of TQ in the expression of mucin 4 in pancreatic cancer cells: implications for the development of novel cancer therapies. Mol Cancer Ther. 2010;9(5):1419–31.
Kolli-Bouhafs K, Boukhari A, Abusnina A, Velot E, Gies JP, Lugnier C, et al. TQ reduces migration and invasion of human glioblastoma cells associated with FAK, MMP-2 and MMP-9 down-regulation. Invest New Drugs. 2012;30(6):2121–31.
Ha JH, Jayaraman M, Radhakrishnan R, Gomathinayagam R, Yan M, Song YS, et al. Differential effects of TQ on lysophosphatidic acid-induced oncogenic pathways in ovarian cancer cells. J Tradit Complement Med. 2020;10(3):207–16.
Al-Rawashde FA, Al-Wajeeh AS, Vishkaei MN, Saad HKM, Johan MF, Taib WRW, et al. TQ inhibits JAK/STAT and PI3K/Akt/ mTOR signaling pathways in MV4–11 and K562 myeloid leukemia cells. Pharmaceuticals (Basel, Switzerland). 2022;15(9):1123.
Almajali B, Johan MF, Al-Wajeeh AS, Wan Taib WR, Ismail I, Alhawamdeh M, et al. Gene expression profiling and protein analysis reveal suppression of the C-Myc oncogene and inhibition JAK/STAT and PI3K/AKT/mTOR signaling by TQ in acute myeloid leukemia cells. Pharmaceuticals (Basel, Switzerland). 2022;15(3):307.
Alhosin M, Ibrahim A, Boukhari A, Sharif T, Gies JP, Auger C, et al. Anti-neoplastic agent TQ induces degradation of α and β tubulin proteins in human cancer cells without affecting their level in normal human fibroblasts. Invest New Drugs. 2012;30(5):1813–9.
Chehl N, Chipitsyna G, Gong Q, Yeo CJ, Arafat HA. Anti-inflammatory effects of the Nigella sativa seed extract, TQ, in pancreatic cancer cells. HPB (Oxford). 2009;11(5):373–81.
Park EJ, Chauhan AK, Min KJ, Park DC, Kwon TK. TQ induces apoptosis through downregulation of c-FLIP and Bcl-2 in renal carcinoma Caki cells. Oncol Rep. 2016;36(4):2261–7.
Lee YM, Kim GH, Park EJ, Oh TI, Lee S, Kan SY, et al. TQ Selectively Kills Hypoxic Renal Cancer Cells by Suppressing HIF-1α-Mediated Glycolysis. Int J Mol Sci. 2019;20(5):1092.
Lee SR, Mun JY, Jeong MS, Lee HH, Roh YG, Kim WT, et al. TQ-induced tristetraprolin inhibits tumor growth and metastasis through destabilization of MUC4 mRNA. Int J Mol Sci. 2019;20(11):2614.
Jafri SH, Glass J, Shi R, Zhang S, Prince M, Kleiner-Hancock H. TQ and cisplatin as a therapeutic combination in lung cancer: in vitro and in vivo. J Exp Clin Cancer Res. 2010;29(1):87.
Shin SB, Woo SU, Yim H. Differential cellular effects of Plk1 inhibitors targeting the ATP-binding domain or polo-box domain. J Cell Physiol. 2015;230(12):3057–67.
Hossen MJ, Yang WS, Kim D, Aravinthan A, Kim JH, Cho JY. TQ: An IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci Rep. 2017;7:42995.
Zenkov RG, Kirsanov KI, Ogloblina AM, Vlasova OA, Naberezhnov DS, Karpechenko NY, et al. Effects of G-quadruplex-binding plant secondary metabolites on c-MYC expression. Int J Mol Sci. 2022;23(16):9209.
Peng L, Liu A, Shen Y, Xu HZ, Yang SZ, Ying XZ, et al. Antitumor and anti-angiogenesis effects of TQ on osteosarcoma through the NF-κB pathway. Oncol Rep. 2013;29(2):571–8.
Siveen KS, Mustafa N, Li F, Kannaiyan R, Ahn KS, Kumar AP, et al. TQ overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-κB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget. 2014;5(3):634–48.
Shanmugam MK, Ahn KS, Hsu A, Woo CC, Yuan Y, Tan KHB, et al. TQ inhibits bone metastasis of breast cancer cells through abrogation of the CXCR4 signaling axis. Front Pharmacol. 2018;9:1294.
Liou YF, Hsieh YS, Hung TW, Chen PN, Chang YZ, Kao SH, et al. TQ inhibits metastasis of renal cell carcinoma cell 786-O-SI3 associating with downregulation of MMP-2 and u-PA and suppression of PI3K/Src signaling. Int J Med Sci. 2019;16(5):686–95.
Alshyarba M, Otifi H, Al Fayi M, Dera A A, Rajagopalan P. TQ inhibits IL-7-induced tumor progression and metastatic invasion in prostate cancer cells by attenuating matrix metalloproteinase activity and Akt/NF-κB signaling. Biotechnol Appl Biochem. 2021;68(6):1403–11.
Kim MJ, Lee HJ, Choi MY, Kang SS, Kim YS, Shin JK, et al. UHRF1 induces methylation of the TXNIP promoter and down-regulates gene expression in cervical cancer. Mol Cells. 2021;44(3):146–59.
Yang J, Kuang XR, Lv PT, Yan XX. TQ inhibits proliferation and invasion of human nonsmall-cell lung cancer cells via ERK pathway. Tumour Biol. 2015;36(1):259–69.
Das S, Dey KK, Dey G, Pal I, Majumder A, MaitiChoudhury S, et al. Antineoplastic and apoptotic potential of traditional medicines TQ and diosgenin in squamous cell carcinoma. PLoS ONE. 2012;7(10): e46641.
Ahmad I, Muneer KM, Tamimi IA, Chang ME, Ata MO, Yusuf N. TQ suppresses metastasis of melanoma cells by inhibition of NLRP3 inflammasome. Toxicol Appl Pharmacol. 2013;270(1):70–6.
Park G, Song NY, Kim DH, Lee SJ, Chun KS. TQ suppresses migration of human renal carcinoma Caki-1 cells through inhibition of the PGE(2)-mediated activation of the EP2 receptor pathway. Biomol Ther. 2021;29(1):64–72.
Almajali B, Al-Jamal HAN, Wan Taib WR, Ismail I, Johan MF, Doolaanea AA, et al. TQ suppresses cell proliferation and enhances apoptosis of HL60 leukemia cells through re-expression of JAK/STAT negative regulators. Asian Pac J Cancer Prev. 2021;22(3):879–85.
Pandita A, Kumar B, Manvati S, Vaishnavi S, Singh SK, Bamezai RN. Synergistic combination of gemcitabine and dietary molecule induces apoptosis in pancreatic cancer cells and down regulates PKM2 expression. PLoS ONE. 2014;9(9): e107154.
El-Mahdy MA, Zhu Q, Wang QE, Wani G, Wani AA. TQ induces apoptosis through activation of caspase-8 and mitochondrial events in p53-null myeloblastic leukemia HL-60 cells. Int J Cancer. 2005;117(3):409–17.
Kou B, Kou Q, Ma B, Zhang J, Sun B, Yang Y, et al. TQ inhibits metastatic phenotype and epithelial-mesenchymal transition in renal cell carcinoma by regulating the LKB1/AMPK signaling pathway. Oncol Rep. 2018;40(3):1443–50.
Subburayan K, Thayyullathil F, Pallichankandy S, Rahman A, Galadari S. Par-4-dependent p53 up-regulation plays a critical role in TQ-induced cellular senescence in human malignant glioma cells. Cancer Lett. 2018;426:80–97.
Rajput S, Kumar BN, Banik P, Parida S, Mandal M. TQ restores radiation-induced TGF-β expression and abrogates EMT in chemoradiotherapy of breast cancer cells. J Cell Physiol. 2015;230(3):620–9.
Lee HJ, Kim MJ, Kim YS, Choi MY, Cho GJ, Choi WS. UHRF1 silences gelsolin to inhibit cell death in early stage cervical cancer. Biochem Biophys Res Commun. 2020;526(4):1061–8.
Shen S, Wei C, Fu J. RNA-sequencing reveals heat shock 70-kDa protein 6 (HSPA6) as a novel TQ-upregulated gene that inhibits growth, migration, and invasion of triple-negative breast cancer cells. Front Oncol. 2021;11: 667995.
Khan MA, Zheng M, Fu J, Tania M, Li J, Fu J. TQ upregulates IL17RD in controlling the growth and metastasis of triple negative breast cancer cells in vitro. BMC Cancer. 2022;22(1):707.
Helmy SA, El-Mesery M, El-Karef A, Eissa LA, El Gayar AM. TQ upregulates TRAIL/TRAILR2 expression and attenuates hepatocellular carcinoma in vivo model. Life Sci. 2019;233: 116673.
Hussain AR, Uddin S, Ahmed M, Al-Dayel F, Bavi PP, Al-Kuraya KS. Phosphorylated IκBα predicts poor prognosis in activated B-cell lymphoma and its inhibition with TQ induces apoptosis via ROS release. PLoS ONE. 2013;8(3): e60540.
Narayanan P, Farghadani R, Nyamathulla S, Rajarajeswaran J, Thirugnanasampandan R, Bhuwaneswari G. Natural quinones induce ROS-mediated apoptosis and inhibit cell migration in PANC-1 human pancreatic cancer cell line. J Biochem Mol Toxicol. 2022;36(5): e23008.
Attoub S, Sperandio O, Raza H, Arafat K, Al-Salam S, Al Sultan MA, et al. TQ as an anticancer agent: evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivo. Fundam Clin Pharmacol. 2013;27(5):557–69.
Upadhyay P, Sarker S, Ghosh A, Gupta P, Das S, Ahir M, et al. Transferrin-decorated TQ-loaded PEG-PLGA nanoparticles exhibit anticarcinogenic effect in non-small cell lung carcinoma via the modulation of miR-34a and miR-16. Biomater Sci. 2019;7(10):4325–44.
Imran M, Rauf A, Khan IA, Shahbaz M, Qaisrani TB, Fatmawati S, et al. TQ: A novel strategy to combat cancer: a review. Biomed Pharmacother. 2018;106:390–402.
Atteia HH, Arafa MH, Mohammad NS, Amin DM, Sakr AT. TQ upregulates miR-125a-5p, attenuates STAT3 activation, and potentiates doxorubicin antitumor activity in murine solid Ehrlich carcinoma. J Biochem Mol Toxicol. 2021;35(12): e22924.
Bashir AO, El-Mesery ME, Anwer R, Eissa LA. TQ potentiates miR-16 and miR-375 expressions in hepatocellular carcinoma. Life Sci. 2020;254: 117794.
Pazhouhi M, Sariri R, Khazaei MR, Moradi MT, Khazaei M. Synergistic effect of temozolomide and TQ on human glioblastoma multiforme cell line (U87MG). J Cancer Res Ther. 2018;14(5):1023–8.
Lei X, Lv X, Liu M, Yang Z, Ji M, Guo X, et al. TQ inhibits growth and augments 5-fluorouracil-induced apoptosis in gastric cancer cells both in vitro and in vivo. Biochem Biophys Res Commun. 2012;417(2):864–8.
Ahmadzadeh H, Ahmadi M, Golchin A, Malakoti F, Maleki M, Alemi F, et al. The effect of TQ and Cis in OS. Drug Res. 2022;72(3):171–6.
Rajput S, Kumar BN, Sarkar S, Das S, Azab B, Santhekadur PK, et al. Targeted apoptotic effects of TQ and tamoxifen on XIAP mediated Akt regulation in breast cancer. PLoS ONE. 2013;8(4): e61342.
Khalife R, El-Hayek S, Tarras O, Hodroj MH, Rizk S. Antiproliferative and proapoptotic effects of topotecan in combination with TQ on acute myelogenous leukemia. Clin Lymphoma Myeloma Leuk. 2014;14(Suppl):S46-55.
Alam S, Mohammad T, Padder RA, Hassan MI, Husain M. TQ and quercetin induce enhanced apoptosis in non-small cell lung cancer in combination through the Bax/Bcl2 cascade. J Cell Biochem. 2022;123(2):259–74.
Bhattacharjee M, Upadhyay P, Sarker S, Basu A, Das S, Ghosh A, et al. Combinatorial therapy of TQ and Emodin synergistically enhances apoptosis, attenuates cell migration and reduces stemness efficiently in breast cancer. Biochim Biophys Acta. 2020;1864(11): 129695.
Hassani S, Maghsoudi H, Fattahi F, Malekinejad F, Hajmalek N, Sheikhnia F, et al. Flavonoids nanostructures promising therapeutic efficiencies in colorectal cancer. Int J Biol Macromol. 2023;241:124508.
Gao J, Kumari A, Zeng XA, Chan S, Farooq MA, Alee M, et al. Coating of chitosan on poly D, L-lactic-co-glycolic acid TQ nanoparticles enhances the anti-tumor activity in triple-negative breast cancer. Front Chem. 2023;11:1044953.
Salmani JM, Asghar S, Lv H, Zhou J. Aqueous solubility and degradation kinetics of the phytochemical anticancer TQ; probing the effects of solvents, pH and light. Molecules (Basel, Switzerland). 2014;19(5):5925–39.
Ong YS, Saiful Yazan L, Ng WK, Abdullah R, Mustapha NM, Sapuan S, et al. TQ loaded in nanostructured lipid carrier showed enhanced anticancer activity in 4T1 tumor-bearing mice. Nanomedicine (Lond). 2018;13(13):1567–82.
Shariare MH, Khan MA, Al-Masum A, Khan JH, Uddin J, Kazi M. Development of stable liposomal drug delivery system of TQ and its in vitro anticancer studies using breast cancer and cervical cancer cell lines. Molecules (Basel, Switzerland). 2022;27(19):6744.
Khan A, Alsahli MA, Aljasir MA, Maswadeh H, Mobark MA, Azam F, et al. Experimental and theoretical insights on chemopreventive effect of the liposomal TQ against Benzo[a]pyrene-induced lung cancer in swiss albino mice. J Inflamm Res. 2022;15:2263–80.
Banupriya SJS, Kavithaa K, Poornima A, Haribalan P, Sri Renukadevi B, Sumathi S. Modulation of gene expression by TQ conjugated zinc oxide nanoparticles arrested cell cycle, DNA damage and increased apoptosis in triple negative breast cancer cell line MDA-MB-231. Drug Dev Ind Pharm. 2021;47(12):1943–51.
Goel S, Mishra P. TQ loaded mesoporous silica nanoparticles retard cell invasion and enhance in vitro cytotoxicity due to ROS mediated apoptosis in HeLa and MCF-7 cell lines. Mater Sci Eng C. 2019;104: 109881.
Imam SS, Gilani SJ, Bin Jumah MN, Rizwanullah M, Zafar A, Ahmed MM, et al. Harnessing lipid polymer hybrid nanoparticles for enhanced oral bioavailability of TQ: in vitro and in vivo assessments. Polymers. 2022;14(18):3705.
El-Najjar N, Chatila M, Moukadem H, Vuorela H, Ocker M, Gandesiri M, et al. Reactive oxygen species mediate TQ-induced apoptosis and activate ERK and JNK signaling. Apoptosis. 2010;15(2):183–95.
Eftekhar SP, Kazemi S, Moghadamnia AA. Effect of TQ on pharmacokinetics of 5-fluorouracil in rats and its effect on human cell line in vitro. Hum Exp Toxicol. 2022;41:9603271221145422.
Thabet NA, El-Khouly D, Sayed-Ahmed MM, Omran MM. TQ chemosensitizes human colorectal cancer cells to imatinib via uptake/efflux genes modulation. Clin Exp Pharmacol Physiol. 2021;48(6):911–20.
Zhang L, Bai Y, Yang Y. TQ chemosensitizes colon cancer cells through inhibition of NF-κB. Oncol Lett. 2016;12(4):2840–5.
Osorio-Pérez SM, Estrada-Meza C, Ruiz-Manriquez LM, Arvizu-Espinosa MG, Srivastava A, Sharma A, et al. TQ potentially modulates the expression of key onco- and tumor suppressor miRNAs in prostate and colon cancer cell lines: insights from PC3 and HCT-15 cells. Genes (Basel). 2023;14(9):1730.
Al Bitar S, Ballout F, Monzer A, Kanso M, Saheb N, Mukherji D, et al. TQ radiosensitizes human colorectal cancer cells in 2D and 3D culture models. Cancers. 2022;14(6):1363.
Jrah-Harzallah H, Ben-Hadj-Khalifa S, Almawi WY, Maaloul A, Houas Z, Mahjoub T. Effect of TQ on 1,2-dimethyl-hydrazine-induced oxidative stress during initiation and promotion of colon carcinogenesis. Eur J Cancer. 2013;49(5):1127–35.
Mohamed AM, Refaat BA, El-Shemi AG, Kensara OA, Ahmad J, Idris S. TQ potentiates chemoprotective effect of Vitamin D3 against colon cancer: a pre-clinical finding. Am J Transl Res. 2017;9(2):774–90.
Ramzy L, Metwally AA, Nasr M, Awad GAS. Novel TQ lipidic core nanocapsules with anisamide-polymethacrylate shell for colon cancer cells overexpressing sigma receptors. Sci Rep. 2020;10(1):10987.
Kortüm B, Campregher C, Lang M, Khare V, Pinter M, Evstatiev R, et al. Mesalazine and TQ attenuate intestinal tumour development in Msh2(loxP/loxP) Villin-Cre mice. Gut. 2015;64(12):1905–12.
El Far AH, Salaheldin TA, Godugu K, Darwish NH, Mousa SA. TQ and its nanoformulation attenuate colorectal and breast cancers and alleviate doxorubicin-induced cardiotoxicity. Nanomedicine (Lond). 2021. https://doi.org/10.2217/nnm-2021-0103.
Selmi M, Salek A, Barboura M, Njim L, Trabelsi A, Lahmar A, et al. TQ-loaded lipid nanocapsules with promising anticancer activity for colorectal cancer. Nanoscale Adv. 2023;5(19):5390–8.
El-Baba C, Mahadevan V, Fahlbusch FB, Mohan SS, Rau TT, Gali-Muhtasib H, et al. TQ-induced conformational changes of PAK1 interrupt prosurvival MEK-ERK signaling in colorectal cancer. Mol Cancer. 2014;13:201.
Karim S, Burzangi AS, Ahmad A, Siddiqui NA, Ibrahim IM, Sharma P, et al. PI3K-AKT pathway modulation by TQ limits tumor growth and glycolytic metabolism in colorectal cancer. Int J Mol Sci. 2022;23(4):2305.
Idris S, Refaat B, Almaimani RA, Ahmed HG, Ahmad J, Alhadrami M, et al. Enhanced in vitro tumoricidal effects of 5-Fluorouracil, TQ, and active vitamin D(3) triple therapy against colon cancer cells by attenuating the PI3K/AKT/mTOR pathway. Life Sci. 2022;296: 120442.
Hsu HH, Chen MC, Day CH, Lin YM, Li SY, Tu CC, et al. TQ suppresses migration of LoVo human colon cancer cells by reducing prostaglandin E2 induced COX-2 activation. World J Gastroenterol. 2017;23(7):1171–9.
Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8(5):387–98.
Lang M, Borgmann M, Oberhuber G, Evstatiev R, Jimenez K, Dammann KW, et al. TQ attenuates tumor growth in ApcMin mice by interference with Wnt-signaling. Mol Cancer. 2013;12(1):41.
Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann NY Acad Sci. 2009;1171:59–76.
Kundu J, Choi BY, Jeong CH, Kundu JK, Chun KS. TQ induces apoptosis in human colon cancer HCT116 cells through inactivation of STAT3 by blocking JAK2- and Src-mediated phosphorylation of EGF receptor tyrosine kinase. Oncol Rep. 2014;32(2):821–8.
Gali-Muhtasib H, Diab-Assaf M, Boltze C, Al-Hmaira J, Hartig R, Roessner A, et al. TQ extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int J Oncol. 2004;25(4):857–66.
Al-Hayali M, Garces A, Stocks M, Collins H, Bradshaw TD. Concurrent reactive oxygen species generation and aneuploidy induction contribute to TQ anticancer activity. Molecules. 2021;26(17):5136.
Rooney S, Ryan M. Effects of alpha-hederin and TQ, constituents of Nigella sativa, on human cancer cell lines. Anticancer Res. 2005;25(3B):2199–204.
Gali-Muhtasib H, Kuester D, Mawrin C, Bajbouj K, Diestel A, Ocker M, et al. TQ triggers inactivation of the stress response pathway sensor CHEK1 and contributes to apoptosis in colorectal cancer cells. Can Res. 2008;68(14):5609–18.
Gali-Muhtasib H, Ocker M, Kuester D, Krueger S, El-Hajj Z, Diestel A, et al. TQ reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J Cell Mol Med. 2008;12(1):330–42.
Thomas JV, Mohan ME, Prabhakaran P, Das SS, Maliakel B, I MK,. A phase I clinical trial to evaluate the safety of TQ-rich black cumin oil (BlaQmax®) on healthy subjects: randomized, double-blinded, placebo-controlled prospective study. Toxicol Rep. 2022;9:999–1007.
Shoaei-Hagh P, Kamelan Kafi F, Najafi S, Zamanzadeh M, Heidari Bakavoli A, Ramezani J, et al. A randomized, double-blind, placebo-controlled, clinical trial to evaluate the benefits of Nigella sativa seeds oil in reducing cardiovascular risks in hypertensive patients. Phytother Res. 2021;35(8):4388–400.
Ali SM, Chen P, Sheikh S, Ahmad A, Ahmad M, Paithankar M, et al. TQ with metformin decreases fasting, post prandial glucose, and HbA1c in type 2 diabetic patients. Drug Res. 2021;71(6):302–6.
Soleymani S, Zargaran A, Farzaei MH, Iranpanah A, Heydarpour F, Najafi F, et al. The effect of a hydrogel made by Nigella sativa L. on acne vulgaris: a randomized double-blind clinical trial. Phytother Res. 2020;34(11):3052–62.
Ammar IMM, Salem MAA. Amelioration of polycystic ovary syndrome-related disorders by supplementation of TQ and metformin. Middle East Fertil Soc J. 2021;26(1):29.
Kwan K, Han AY, Mukdad L, Barragan F, Selim O, Alhiyari Y, et al. Anticancer effects of TQ in head and neck squamous cell carcinoma: a scoping review. Laryngoscope Investig Otolaryngol. 2023;8(4):876–85.
Saraf S, Rajpal V, Pal RR. TQ and Cancer.
El-Far AH, Godugu K, Noreldin AE, Saddiq AA, Almaghrabi OA, Al Jaouni SK, et al. TQ and costunolide induce apoptosis of both proliferative and doxorubicin-induced-senescent colon and breast cancer cells. Integr Cancer Ther. 2021;20:15347354211035450.
Venkataraman B, Almarzooqi S, Raj V, Alhassani AT, Alhassani AS, Ahmed KJ, et al. TQ, a dietary bioactive compound, exerts anti-inflammatory effects in colitis by stimulating expression of the colonic epithelial PPAR-γ transcription factor. Nutrients. 2021;13(4):1343.
Ballout F, Monzer A, Fatfat M, Ouweini HE, Jaffa MA, Abdel-Samad R, et al. TQ induces apoptosis and DNA damage in 5-Fluorouracil-resistant colorectal cancer stem/progenitor cells. Oncotarget. 2020;11(31):2959–72.
El-Far AH, Darwish NHE, Mousa SA. Senescent colon and breast cancer cells induced by doxorubicin exhibit enhanced sensitivity to curcumin, caffeine, and TQ. Integr Cancer Ther. 2020;19:1534735419901160.
Kensara OA, El-Shemi AG, Mohamed AM, Refaat B, Idris S, Ahmad J. TQ subdues tumor growth and potentiates the chemopreventive effect of 5-fluorouracil on the early stages of colorectal carcinogenesis in rats. Drug Des Dev Ther. 2016;10:2239–53.
Khalife R, Hodroj MH, Fakhoury R, Rizk S. TQ from Nigella sativa seeds promotes the antitumor activity of noncytotoxic doses of topotecan in human colorectal cancer cells in vitro. Planta Med. 2016;82(4):312–21.
Chen MC, Lee NH, Hsu HH, Ho TJ, Tu CC, Chen RJ, et al. Inhibition of NF-κB and metastasis in irinotecan (CPT-11)-resistant LoVo colon cancer cells by TQ via JNK and p38. Environ Toxicol. 2017;32(2):669–78.
Chen MC, Lee NH, Hsu HH, Ho TJ, Tu CC, Hsieh DJ, et al. TQ induces caspase-independent, autophagic cell death in CPT-11-resistant lovo colon cancer via mitochondrial dysfunction and activation of JNK and p38. J Agric Food Chem. 2015;63(5):1540–6.
Sheikhnia F, Maghsoudi H, Majidinia M. The critical function of microRNAs in developing resistance against 5-fluorouracil in cancer cells. Mini Rev Med Chem. 2023. https://doi.org/10.2174/1389557523666230825144150.
Acknowledgements
None.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
FS wrote the article and prepared the table and figures; VR and HM prepared the figures; MM designed and revised the article. All the authors studied and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sheikhnia, F., Rashidi, V., Maghsoudi, H. et al. Potential anticancer properties and mechanisms of thymoquinone in colorectal cancer. Cancer Cell Int 23, 320 (2023). https://doi.org/10.1186/s12935-023-03174-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-023-03174-4