Abstract
Colorectal cancer (CRC) is the third cause of cancer death in the world that arises from the glandular and epithelial cells of the large intestine, during a series of genetic or epigenetic alternations. Recently, long non-coding RNAs (lncRNAs) has opened a separate window of research in molecular and translational medicine. Emerging evidence has supported that lncRNAs can regulate cell cycle of CRC cells. LncRNA NEAT1 has been verified to participate in colon cancer development and progression. NEAT1 as a competing endogenous RNA could suppress the expression of miRNAs, and then regulate molecules downstream of these miRNAs. In this review, we summarized emerging roles of NEAT1 in CRC cells.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is one of the most prevalent cancers and the third leading cause of cancer death in both men and women [1, 2]. CRC starts at the inner lining of the colon, rectum, and appendix [3, 4]. In 2020, there will be over 10,000 new cases, with a growing number of young individuals [5]. A variety of etiological factors may have a role in the development of CRC, including environmental, genetic, and epigenetic factors [1, 6]. Based on the genetics and etiology of the disease, CRC is commonly classified into sporadic, hereditary, or familial [7, 8]. Sporadic disease, in which there is no family history, accounts for about 70% of all CRC [9]. The majority of CRCs are patients over 50 years old, dietary, environmental factors, and genetic changes in the adenoma-carcinoma sequences [10,11,12]. There are fewer than 10% of patients who have an inherited predisposition to CRC, and these cases are subdivided based on the presence of polyps (level 0 to 4) [13]. These conditions have high risks of developing CRC, and many of them have underlying genetic mutations [14]. Familial CRC is the third and least well understood pattern [15]. A family history of CRC occurs in up to 25% of affected patients but the pattern is not consistent with those of the inherited syndromes [16]. Individuals from these families are more likely to develop CRC, although the risk is not as high as those with inherited syndromes [17]. Despite substantial advances in current CRC treatments such as adjuvant chemo-radiotherapies and immunological therapy, the prognosis for patients with the advanced-stage disease remains poor and the 5-year survival rate remains unsatisfactory [18, 19]. To increase the survival rate of affected individuals, a better knowledge of the processes of CRC beginning and development is urgently required and more research is needed to identify and develop new biomarkers and targets for its diagnosis and treatment [20,21,22]. Recently, it has been shown that long non-coding RNAs (lncRNAs) are participated in the pathogenesis of CRC [23]. LncRNAs are non-coding RNA molecules with a length of more than 200 nucleotides without protein-coding potential [24]. Although they lack coding ability, the majorities of them are transcribed by RNA polymerase II and share similarities with messenger RNAs (mRNAs) [25]. LncRNAs as an initially transcribed RNA or a mature spliced RNA can regulate the expression of significant genes at multiple levels through epigenetic regulation and by modulating transcription, post-transcriptional processes, translation, and protein modification [26]. Additionally, lncRNAs by targeting microRNAs (miRNAs) play critical roles in physiological processes such as development, tissue differentiation, reproduction, immunity, tumor formation, and development [27, 28]. miRNAs are a small single-stranded non-coding RNAs that play fundamental roles in gene expression and posttranscriptional gene silencing [29, 30]. LncRNAs also play a critical role in peripheral blood components, including serum and plasma [31]. Some of these lncRNAs are upregulated in tumors and act as oncogenes, while others serve as tumor suppressors [32]. There is an association between overexpressed lncRNAs and poor prognoses and metastasis in CRC patients [33]. These findings support the idea that lncRNAs are important therapeutic targets in CRC (Table 1). Furthermore, lncRNA-mediated treatment of patients with CRC might be a promising approach [34]. LncRNAs play a key role in colon carcinogenesis and progression [35, 36], with one study used RNA sequencing data from the TCGA dataset to find approximately 200 differently expressed lncRNAs in CRC [37]. The patient outcome, cell proliferation, apoptosis, metastasis, invasion, cell cycle, epithelial-mesenchymal transition (EMT), cancer stem cells (CSCs), and drug resistance are controlled by lncRNAs [38]. Nuclear enriched abundant transcript 1 (NEAT1) is a novel lncRNA that participated in a variety of cancers, such as breast, gastric, and lung [39,40,41]. Accumulating evidences reported that NEAT1 play critical roles in the tumorigenesis of CRC [42]. In the present manuscript we summarized functional roles of NEAT1 in the pathogenesis and progression of CRC.
Characteristics of NEAT1
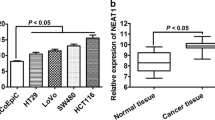
Nuclear paraspeckle assembly transcript 1 (NEAT1) is a lncRNA that is transcribed by RNA polymerase II from the familial tumor syndrome multiple endocrine neoplasia (MEN) type 1 loci on chromosome 11q13.1, encodes two transcriptionally distinct variants, NEAT1-1 (3756 bp) and NEAT1-2 (22,743 bp) [43]. Two isoforms share an identical promoter and 5′-end but they differ at the 3′-end, thus making them different subtypes. This lncRNA assists in the formation and assembly of nuclear paraspeckles, a group of highly dynamic nuclear subdomains [44, 45]. Paraspeckles have a core–shell spheroidal structure and the middle part of NEAT1-2 forms a core, which is encircled by NEAT1-1 and the NEAT1-2, -5′ and 3′-ends [46]. In the formation of paraspeckles, NEAT1-2 has been implicated strongly [47, 48]. Paraspeckle protein (PSP) 1, PSP2 and p54nrb bind to the NEAT1 transcriptional start site to create paraspeckles [47, 49]. These nuclear bodies could act as a “reservoir” for mRNA retention in the nucleus. They’ve also been demonstrated to migrate to the cytoplasm and modulate the function of cytoplasmic proteins and RNA [47]. While NEAT1 is mostly identified in the nucleus, a small amount is also found in the cytoplasm, and this contains miRNA-binding sites that allow it to regulate and communicate with mRNAs by competing for shared miRNAs [39, 41, 50, 51]. miRNAs are a group of small noncoding regulatory RNA molecules that have modulatory roles in a variety of biological processes [52]. Dysregulation of miRNA would have a significant impact on cancer development [53]. Evidence has shown that NEAT1 not only participated in vital physiological processes, including immune responses, organogenesis, and myogenesis, but also plays an important role in pathological processes [54]. The NEAT1 gene exhibits characteristics of cancer drivers since it initiates and progresses tumors, and its frequent dysregulation in cancer is correlated with metastasis, recurrence rate, and survival [55]. There has recently been a great deal of interest in lncRNAs for potential functions in the different stages of CRC formation, invasion, and progression [56]. According to recent studies, NEAT1 is overexpressed and plays an oncogenic role in CRC [57,58,59]. Here, we described how this lncRNA contributes to the biological process of CRC (Fig. 1).
Functional roles of NEAT1 in colorectal cancer
NEAT1/miR-34a/SIRT1/Wnt/β-catenin
It has been reported that NEAT1 expression was dramatically induced in tissues of patients with CRC in comparison to those with normal tissues. High expression of NEAT1 is correlated with clinicopathological significance of CRC including the tumor size, distant metastasis, decreased overall survival, and disease-free survival rate. Moreover, NEAT1 induced the proliferative and invasion activity of CRC cells. So, upregulated NEAT1 may be a predictive risk factor for CRC diagnosis and prognosis [60]. It has been shown that NEAT1 via direct targeting of miR-34a can induce CRC [60, 61]. miR-34a has the tumor suppression effect on silent information regulator 1 (SIRT1) in CRC [62]. SIRT1 as a member of the Sirtuin family not only regulates different cellular biological events but also upregulates in a variety of cancers including CRC [63, 64]. Recent findings indicated that SIRT1 expression has a positive and negative correlation with NEAT1 and miR-34a, respectively. So, NEAT1 increased SIRT1 expression through targeting miR-34a, thereby inducing pathogenesis of CRC. Furthermore, crucial function of the SIRT1/Wnt/β-catenin signaling pathway-dependent manner has been reported in different cell process. Overexpression of NEAT1 increased SIRT1 and Wnt/β-catenin member expressions, whereas miR-34a mimics reversed the NEAT1-induced overexpression of SIRT1 and Wnt/β-catenin. Altogether, NEAT1 promoted pathogenesis of CRC via the miR-34a/SIRT1/Wnt/β-catenin axis, thereby standing as a promising biomarker for the management of CRC [60].
NEAT1/miR-185-5p/IGF2
A study reported that high expression of NEAT1 was associated with lower overall survival of patients with CRC. Such upregulation increased invasion along with migration of colorectal malignant cells through overexpression of vimentin and downregulation of cytokeratin 19 and E-cadherin as EMT-correlated genes. Based on RNA pull-down analysis, there was a special interaction between NEAT1 and miR-185-5p. Insulin-like growth factor 2 (IGF2) was found to function as the main target of NEAT1 and miR-185-5p. NEAT1 by targeting IGF2 stimulated the pathogenesis of CRC [65].
NEAT1/miR-495-3p/CDK6
In contrast to overexpression of NEAT1 in CRC, miR-495-3p as a predicted target of NEAT1 was downregulated. Knockdown of NEAT1 using LV-shNEAT1 repressed CRC cell proliferation along with inhibited expression of cyclinD1 and cyclin E as cell cycle-correlated protein markers. Therefore, NEAT1 suppression promoted apoptosis and inhibited migration and invasion of CRC cells. Such biological function of inhibited NEAT1 is reported to be regulated through promoting miR-495-3p [66]. Cyclin-dependent kinase 6 (CDK6) which is modulated by cyclins was identified as a possible target of miR-495-3p and its expression was regulated in a negative manner by miR-495-3p. As a result, NEAT1 participated in CRC development via sponging miR-495-3p and promoting CDK6 expression [66, 67].
NEAT1/miR-193a-3p
High expression of NEAT1 was demonstrated to be associated with clinicopathological significance of CRC, including poor prognosis, TNM stage, low survival, and high cancer recurrence. Silencing NEAT1 was revealed to induce E-cadherin protein expression and reduce N-cadherin and Vimentin levels, thereby decreasing the invasion of CRC cells. Further analysis showed other biological functions of NEAT1 knockdown such as inhibited proliferation, colony formation potential, and increased apoptosis of CRC. Furthermore, inhibition of miR-193a-3p as a sponge target of NEAT1 has been suggested to induce proliferation and invasion of CRC cells. Hence, the oncogenic function of NEAT1 induced CRC tumorigenesis through targeting miR-193a-3p [68].
NEAT1/miR-205-5p/VEGFA
High expression of both NEAT1 and vascular endothelial growth factor A (VEGFA), and low expression of miR-205-5p have been proved in CRC [69]. VEGFA as key regulator of angiogenesis [70], cancer progression, and metastasis has been found to be a direct target of miR-205-5p. Upregulation of NEAT1 intensified cancer growth by regulating miR-205-5p. Besides, downregulation of NEAT1 and VEGFA not only suppressed the proliferative activity of CRC cells but also decreased matrix metalloproteinase-2 (MMP2) and MMP9 as cell-migration- and invasion-correlated proteins. So, upregulated NEAT1 induced CRC pathogenesis by modulating the miR-205-5p/VEGFA pathway, thereby suggesting being an intriguing marker in CRC therapy and diagnosis [69].
NEAT1/miR-196a-5p/GDNF
An increase of cell proliferation and migration in CRC cells was reported following NEAT1 overexpression. Direct regulation of miR-196a-5p by NEAT1 and their inverse expressions in CRC revealed that miR-196a-5p along with NEAT1 participated in CRC pathogenesis [71]. Further investigation demonstrated that miR-196a-5p represented its function through modulation of glial cell line-derived neurotrophic factor (GDNF) as a neurotrophic factor affecting tumor invasion and metastasis [71, 72]. It can be concluded that NEAT1 exerted its regulatory mechanism in CRC pathogenesis through miR-196a-5p inhibition and GDNF induction.
NEAT1/DDX5/Wnt/β-catenin
Downregulated NEAT1 has been reported to reduce the proliferative activity of CRC cells, increased poly (ADP-ribose) polymerase-1 (PARP-1) and cleaved caspase-3 as hallmark of apoptotic proteins [71, 73, 74]. Furthermore, NEAT1 repression led to downregulate MMP2, MMP9, and N-cadherin along with E-cadherin upregulation. Hence, NEAT1 increased proliferation and metastasis of CRC cells by attenuating apoptosis [74]. Since the discovery of direct binding of DEAD box helicase 5 (DDX5) as a key protein involved in tumorigenesis [75], recent finding revealed NEAT1 modulated DDX5 stability in a direct binding manner, consequently activated Axin2, c-myc, and cyclinD1 as the Wnt/β-catenin pathway targets. It is suggested that NEAT1 elevated CRC pathogenesis through activating the Wnt/β-catenin pathway in a DDX5-regulated manner. Recent pharmacological approaches could focus on the NEAT1/DDX5/Wnt/β-catenin axis as a possible therapeutic axis in CRC [74].
NEAT1/miR-150-5p/CPSF4
The inverse expression trend between NEAT1 and miR-150-5p in CRC could predict their possible correlation. NEAT1 silencing promoted the 5-fluorouracil (5-Fu) sensitivity and apoptosis and repressed the invasion and the expression of resistance-correlated proteins including P-gp and GST-π in CRC cells. It was found that inhibition of miR-150-5p as a target of NEAT1 reversed NEAT1 silencing on the progression of CRC [76]. Moreover, upregulation of cleavage and polyadenylation specific factor 4 (CPSF4) as a target of miR-150-5p reversed the NEAT1 silencing effect on CRC pathogenesis [76, 77]. Further investigation indicated that NEAT1 promoted CPSF4 expression through targeting miR-150-5p to elevate CRC progression. The NEAT1/miR-150-5p/CPSF4 network highlighted new approach for CRC drug resistance [76].
NEAT1/miR-138/SLC38A1
The expression of solute carrier family 38 member 1 (SLC38A1) as a tumor-inducing agent has been found to be upregulated in CRC. NEAT1 silencing or SLC38A1 low expression prevented the proliferative and invasion ability of CRC cells but induced CRC apoptosis and autophagy. In addition, NEAT1 modulated SLC38A1 expression through sponging miR-138. Therefore, knockdown of NEAT1 suppressed CRC cells tumor growth and progression with the miR-138/SLC38A1 axis, exerting an underlying strategy for CRC management [78].
NEAT1/KDM5A/Cul4A/Wnt/β-catenin
NEAT1 silencing repressed the malignant features of CRC cells [79]. Lysine-specific demethylase 5A (KDM5A) which is participated in human cancer has been inhibited through binding of NEAT1 to the E2F transcription factor 1 (E2F1) protein [79, 80]. E2F1 is known to be associated with the pathogenesis, metastasis, and chemoresistance of CRC cells [81]. KDM5A also suppressed cullin 4A (Cul4A) as a ubiquitin ligase promoting tumorigenesis [82]. Cul4A upregulation facilitated the malignant features of the si-NEAT1-transfected CRC cells. Besides, activation of the Wnt pathway through KDM5A/Cul4A is exerted via NEAT1 [79]. Regarding dysregulated the Wnt pathway in CRC development [83], NEAT1 promoted CRC progression through the KDM5A/Cul4A/Wnt axis [79].
NEAT1/miR-486-5p/NR4A1/Wnt/β-catenin
Nuclear orphan receptor 4A1 (NR4A1) as a pro-oncogenic and poor prognosis factor for CRC survival has been overexpressed in CRC [84, 85]. NEAT1 or NR4A1 loss of function suppressed the proliferation along with motility but promoted apoptosis of CRC cells. In contrast, NR4A1 knockdown-mediated impacts on CRC cells. Considering miR-486-5p targeting by NEAT1 and the role of NEAT1 in inducing β-catenin, c-myc, and cyclinD1, this lncRNA facilitated the progression of CRC through sponging miR-486-5p to regulate the NR4A1/Wnt/β-catenin pathway [85].
NEAT1/miR-193a-3p/KRAS
High expression of NEAT1 along with low expression of miR-193a-3p as a target of NEAT1 has been reported in CRC. Downregulated NEAT1 impaired the viability of CRC cells whereas miR-193a-3p inhibition increased tumor cell proliferation and migration, and reduced CRC apoptosis by targeting Kirsten rat sarcoma viral oncogene homology (KRAS) expression [86]. KRAS as a major pathway in tumorigenesis was introduced as a downstream target of miR-193a-3p [86, 87]. NEAT1 silencing or miR-193a-3p induction prevented CRC progression by controlling KRAS expression. The NEAT1/miR-193a-3p/KRAS network could exert intriguing advancement for diagnostic and management of CRC [86].
NEAT1/Akt
A recent study demonstrated that NEAT1 silencing resulted in low expression of Bcl2 and high expression of Bax in CRC cells, which are involved in cell growth and apoptosis [58]. Based on the roles of Akt signaling in cell growth and apoptosis [88], recent finding revealed that NEAT1 knockdown prevented Akt phosphorylation at Ser473, thereby repressing Akt activation [58]. Considering Akt activation in CRC progression [89], NEAT1 affected the proliferation and apoptosis of CRC via modulation of the Akt pathway and the NEAT1/Akt pathway may act as a possible target for cancer therapy [58].
NEAT1/ALKBH5
RNA modification like N6-methyladenosine (m6A) is regarded as the most leading way of gene expression regulation and unusual changes of such modification led to cancer recurrence [90,91,92]. Silencing ALKBH5 as a demethylated enzyme of m6A was reported to reduce the tumor behavior of CRC cells and inhibited the expression of proliferating cell nuclear antigen (PCNA) and the migration induced by NEAT1. Knockdown of ALKBH5 promoted cell apoptosis of CRC cells. Thus, the NEAT1/ALKBH5 axis may regard as possible therapeutic target for CRC management [93].
NEAT1/miR-377-3p
Small interfering RNA (siRNA)-related therapies are a possible approach for targeting different cancers but loss of a desired delivery system still restricts their development [94,95,96]. Chitosan nanoparticles (CNPs) have been developed for targeted delivery of siRNA [97, 98]. In a recent finding, nano-NEAT1 siRNA was shown to reverse upregulated NEAT1 in CRC along with decreased CRC viability. Moreover, nano-NEAT1 siRNA therapy significantly induced CRC apoptosis through upregulating Bax as pro-apoptotic protein and downregulating Bcl-2 as an anti-apoptotic marker [99, 100]. Furthermore, inhibition of miR-377-3p as a target of NEAT1 could neutralize NEAT1 knockdown properties in CRC. Hence, the NEAT1/miR-377-3p axis might be a promising candidate for CNP-based therapy for siRNA-related CRC gene management [99].
Clinical significance of NEAT1
Chemotherapy has been demonstrated to diminish cancer recurrence and life quality of CRC patients but chemotherapy resistance is known as a poor prognosis and recurrence factor in CRC [101]. 5-FU stands as an efficient drug for CRC and knowledge of its resistance in recovering CRC survival is unknown [102, 103]. Silencing autophagy as a regulator of lysosomal degradation phenomenon has been reported to promote the efficacy of chemotherapy [104, 105]. Recent findings revealed that NEAT1 targeted miR-34a to induce autophagy, thereby facilitating chemoresistance of 5-FU in CRC. Knockdown of NEAT1 and consequent inhibited autophagy can be a chemotherapeutic approach to increase CRC sensitivity of 5-FU through targeting miR-34a [106]. Besides, CSCs are introduced as an important cause of chemoresistance [107, 108]. NEAT1 silencing was found to repress the ALDH1 activity and CD133 expression in CRC cells, thereby affecting CRC stemness maintenance. NEAT1 is also involved in chromatin remodeling, histone acetylation, and the expression of ALDH1 and c-Myc as the stemness-related genes. Co-expression of NEAT1/ALDH1/c-Myc is associated with 5-FU resistance, recurrence, and poorer prognosis of CRC patients [101]. Altogether, NEAT1 may be regarded as a promising approach for the therapy of CRC drug resistance. According to previous finding, the miR-124/iASPP/p53 axis showed significant impact on CRC photodynamic therapy (PDT) resistance [109, 110]. NEAT1 knockdown facilitated the sensitivity of CRC cells to PDT and suppressed the effect of PDT on CRC growth through sponging miR-124It has been demonstrated that the p53 deletion or mutation can induce CRC cells resistance to PDT. The c-Myc/miR-124/NEAT1/p53/iASPP axis exerted their regulatory role in CRC response to the chemotherapy [111]. It was revealed that cleavage of gasdermin E (GSDME) as a member of the gasdermin family altered apoptosis to pyroptosis as a lytic cell death which is correlated with chemotherapy along with anticancer immunity in CRC [112,113,114,115].
Recent findings showed that ionizing radiation (IR) of CRC induced caspases and Bax-regulated apoptosis along with GSDME-regulated pyroptosis. The luciferase reporter assay revealed GSDME was a downstream target of miR-448. Hence, high expression of NEAT1 reduced the expression of miR-448 and increased GSDME expression levels. Altogether, NEAT1 induced radioresistance of CRC cells through promoting IR-increased pyroptosis by modulating GSDME expression. The NEAT1/miR-448/GSDME axisparticipated in radioresistance of CRC [117]. Therefore, NEAT1 is implicated in the pathogenesis of CRC through regulating the chemo and radioresistance of CRC cells. Clinical significance of NEAT1 expression displayed its positive association with tumor size, TNM stage, carcinoembryonic antigen (CEA) level, lymphatic metastasis, and presence of distant metastasis of CRC [60]. Among clinicopathologic features of CRC, CEA level along with TNM stage have been reported to be dramatically correlated with overall survival of patients with CRC [118]. Moreover, high expression of NEAT1 was associated with poorer disease-free survival as well as tumor recurrence in tumor tissues of CRC patients, thereby highlighting NEAT1 as a possible predictive marker for the diagnosis and prognosis of CRC patients [101].
Conclusion
NEAT1 plays a role in chemo-resistance, tumor growth, and metastasis, according to several lines of evidence. This lncRNA induced stem cell properties in tumoral tissues, as well. NEAT1 could regulate a variety of miRNAs and their target genes to stimulate tumorigenesis. In contrast to solid tumors, NEAT1 expression is downregulated in acute promyelocytic leukemia and functions as a tumor suppressor by promoting leukocyte differentiation. In solid tumors and haematological malignancies, different gene expression patterns may be responsible for this discrepancy. Such different roles should be considered in designing specific treatments for each type of cancer. This lncRNA is considered as a potential biomarker in several cancer types and developing NEAT1-targeting therapies might be a novel strategy against CRC. CRISPR/Cas-9 genome editing technology may be used to target NEAT1 loci for therapeutic purposes, but there are still challenges, such as the risk of disease occurrence due to unwanted mutations, the immune response to the delivery system, and other toxic side effects. In addition, evaluation of its expression levels in cancer patients' serum in order to replace invasive biopsy with liquid biopsy should be evaluated. Although the chemical stability of NEAT1 in biological samples (e.g., serum) is also unknown. Further studies are required to determine the exact mechanism of NEAT1 involved in carcinogenesis and the underlying mechanism of NEAT1 dysregulation in human cancers.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CNPs:
-
Chitosan nanoparticles
- CSCs:
-
Cancer stem cells
- CRC:
-
Colorectal cancer
- CPSF4:
-
Cleavage and polyadenylation specific factor 4
- Cul4A:
-
Cullin 4A
- DDX5:
-
DEAD box helicase 5
- EMT:
-
Epithelial-mesenchymal transition
- E2F1:
-
E2F transcription factor 1
- GDNF:
-
Glial cell line-derived neurotrophic factor
- GSDME:
-
Cleavage f gasdermin E
- IR:
-
Ionizing radiation
- incRNAs:
-
Intergenic lncRNAs
- IGF2:
-
Insulin-like growth factor 2
- lncRNAs:
-
Long non-coding RNAs
- KDM5A:
-
Lysine-specific demethylase 5A
- KRAS:
-
Kirsten rat sarcoma viral oncogene homology
- mRNAs:
-
Messenger RNAs
- MMP2:
-
Matrix metalloproteinase-2
- m6A:
-
N6-methyladenosine
- NR4A1:
-
Nuclear orphan receptor 4A1
- PARP-1:
-
Poly (ADP-ribose) polymerase-1
- PDT:
-
Photodynamic therapy
- PCNA:
-
Proliferating cell nuclear antigen
- SLC38A1:
-
Solute carrier family 38 member 1
- siRNA:
-
Small interfering RNA
- SIRT1:
-
Silent information regulator 1
- TILs:
-
Tumor infiltrating lymphocytes
- VEGFA:
-
Vascular endothelial growth factor A
- 5-FU:
-
5-Fluorouracil
References
Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89–103.
Giovannucci E. Molecular Biologic and Epidemiologic Insights for Preventability of Colorectal Cancer. J Natl Cancer Inst. 2022. https://doi.org/10.1093/jnci/djab229.
Sameer AS. Colorectal cancer: molecular mutations and polymorphisms. Front Oncol. 2013;3:114–114.
Ottaiano A, Santorsola M, Perri F, Pace U, Marra B, Correra M, Sabbatino F, Cascella M, Petrillo N, Ianniello M. Clinical and molecular characteristics of rare malignant tumors of colon and rectum. Biology. 2022;11:267.
Siegel Rebecca L, Kimberly D, Miller Ann Goding sauer SA, Fedewa Lynn F, Butterly Joseph C, Anderson Andrea Cercek RAS. Jemal Ahmedin. “Colorectal cancer statistics.” Cancer J Clin. 2020;70:145–64.
Rattray NJW, Charkoftaki G, Rattray Z, Hansen JE, Vasiliou V, Johnson CH. Environmental influences in the etiology of colorectal cancer: the premise of metabolomics. Curr Pharmacol Rep. 2017;3:114–25.
Fischer J, Walker LC, Robinson BA, Frizelle FA, Church JM, Eglinton TW. Clinical implications of the genetics of sporadic colorectal cancer. ANZ J Surg. 2019;89:1224–9.
Garcia FA, de Andrade ES, de Campos Reis Galvo H, da Silva Sabato C, Campacci N, de Paula AE, Evangelista AF, Santana IVV, Melendez ME, Reis RM. New insights on familial colorectal cancer type X syndrome. Sci Rep. 2022;12:1–11.
Karp I, Latulippe J, Charlebois P, Emami E. Periodontal disease as a risk factor for sporadic colorectal cancer: results from COLDENT study. Cancer Causes Control. 2022;33:463–72.
Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14: 101174.
Si H, Yang Q, Hu H, Ding C, Wang H, Lin X. Colorectal cancer occurrence and treatment based on changes in intestinal flora. Semin Cancer Biol. 2021. https://doi.org/10.1016/j.semcancer.2020.05.004.
Steck SE, Murphy EA. Dietary patterns and cancer risk. Nat Rev Cancer. 2020;20:125–38.
Valle L, Vilar E, Tavtigian SV, Stoffel EM. Genetic predisposition to colorectal cancer: syndromes, genes, classification of genetic variants and implications for precision medicine. J Pathol. 2019;247:574–88.
Valle L, de Voer RM, Goldberg Y, Sjursen W, Försti A, Ruiz-Ponte C, Caldés T, Garré P, Olsen MF, Nordling M, Castellvi-Bel S, Hemminki K. Update on genetic predisposition to colorectal cancer and polyposis. Mol Aspects Med. 2019;69:10–26.
Boland PM, Yurgelun MB, Boland CR. Recent progress in Lynch syndrome and other familial colorectal cancer syndromes. Cancer J Clin. 2018;68:217–31.
Muller C, Ihionkhan E, Stoffel EM, Kupfer SS. Disparities in early-onset colorectal cancer. Cells. 2021;10:1018.
Souglakos J. Genetic alterations in sporadic and hereditary colorectal cancer: implementations for screening and follow-up. Dig Dis. 2007;25:9–19.
Chen W, Zheng R, Baade P, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in china. CA Cancer J Clin. 2016;66:115–32.
Eefsen R, Vermeulen P, Christensen I, Laerum O, Mogensen M, Rolff H, Van den Eynden G, Høyer-Hansen G, Osterlind K, Vainer B. Growth pattern of colorectal liver metastasis as a marker of recurrence risk. Clin Exp Metas. 2015;32:369–81.
Al-Joufi FA, Setia A, Salem-Bekhit MM, Sahu RK, Alqahtani FY, Widyowati R, Aleanizy FS. Molecular pathogenesis of colorectal cancer with an emphasis on recent advances in biomarkers, as well as nanotechnology-based diagnostic and therapeutic approaches. Nanomaterials (Basel). 2022;12:169.
de Assis JV, Coutinho LA, Oyeyemi IT, Oyeyemi OT, Grenfell RFEQ. Diagnostic and therapeutic biomarkers in colorectal cancer: a review. Am J Cancer Res. 2022;12:661–80.
Xu WW, Jin J, Wu XY, Ren Q, Farzaneh M. MALAT1-related signaling pathways in colorectal cancer. Cancer Cell Int. 2022;22:1–9.
Yang Y, Junjie P, Sanjun C, Ma Y. Long non-coding RNAs in colorectal cancer: progression and future directions. J Cancer. 2017;8:3212–25.
Halasz H, Carpenter S, Challenges and Future Directions for LncRNAs and Inflammation, in: Long Noncoding RNA, Springer, 2022, pp. 179–183.
Marchese FP, Raimondi I, Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18:1–13.
Statello L, Guo C-J, Chen L-L, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:96–118.
Zeni PF, Mraz M. LncRNAs in adaptive immunity: role in physiological and pathological conditions. RNA Biol. 2021;18:619–32.
Taniue K, Akimitsu N. The functions and unique features of LncRNAs in cancer development and tumorigenesis. Int J Mol Sci. 2021;22:632.
Ying H, Ebrahimi M, Keivan M, Khoshnam SE, Salahi S, Farzaneh M. miRNAs; a novel strategy for the treatment of COVID-19. Cell Biol Int. 2021;45:2045–53.
Azizidoost S, Farzaneh M. MicroRNAs as a novel player for differentiation of mesenchymal stem cells into cardiomyocytes. Curr Stem Cell Res Ther. 2022. https://doi.org/10.2174/1574888X17666220422094150.
Xie X, Tang B, Xiao Y-F, Xie R, Li B-S, Dong H, Zhou J-Y, Yang S-M. Long non-coding RNAs in colorectal cancer. Oncotarget. 2016;7:5226.
Smolle M, Uranitsch S, Gerger A, Pichler M, Haybaeck J. Current status of long non-coding RNAs in human cancer with specific focus on colorectal cancer. Int J Mol Sci. 2014;15:13993–4013.
Kam Y, Rubinstein A, Naik S, Djavsarov I, Halle D, Ariel I, Gure AO, Stojadinovic A, Pan H, Tsivin V. Detection of a long non-coding RNA (CCAT1) in living cells and human adenocarcinoma of colon tissues using FIT–PNA molecular beacons. Cancer Lett. 2014;352:90–6.
Chen S, Fang Y, Sun L, He R, He B, Zhang S. Long non-coding RNA: a potential strategy for the diagnosis and treatment of colorectal cancer. Front Oncol. 2021;11:762752.
Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Can Res. 2017;77:3965–81.
Deng H, Wang JM, Li M, Tang R, Tang K, Su Y, Hou Y, Zhang J. Long non-coding RNAs: New biomarkers for prognosis and diagnosis of colon cancer. Tumor Biol. 2017;39:1010428317706332.
Forrest ME, Saiakhova A, Beard L, Buchner DA, Scacheri PC, LaFramboise T, Markowitz S, Khalil AM. Colon cancer-upregulated long non-coding RNA lincDUSP regulates cell cycle genes and potentiates resistance to apoptosis. Sci Rep. 2018;8:1–12.
Chen S, Shen X. Long noncoding RNAs: functions and mechanisms in colon cancer. Mol Cancer. 2020;19:1–13.
Qian K, Liu G, Tang Z, Hu Y, Fang Y, Chen Z, Xu X. The long non-coding RNA NEAT1 interacted with miR-101 modulates breast cancer growth by targeting EZH2. Arch Biochem Biophys. 2017;615:1–9.
Zhang J, Zhao B, Chen X, Wang Z, Xu H, Huang B. Silence of long noncoding RNA NEAT1 inhibits malignant biological behaviors and chemotherapy resistance in gastric cancer. Pathol Oncol Res. 2018;24:109–13.
Sun C, Li S, Zhang F, Xi Y, Wang L, Bi Y, Li D. Long non-coding RNA NEAT1 promotes non-small cell lung cancer progression through regulation of miR-377-3p-E2F3 pathway. Oncotarget. 2016;7:51784.
Wang H, Huang C, Yao X. The functions of long non-coding RNAs in colorectal cancer. Transl Cancer Res. 2019;8:2192–204.
Bond CS, Fox AH. Paraspeckles: nuclear bodies built on long noncoding RNA. J Cell Biol. 2009;186:637–44.
Shi X, Sun M, Liu H, Yao Y, Song Y. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339:159–66.
Clemson CM, Hutchinson JN, Sara SA, Ensminger AW, Fox AH, Chess A, Lawrence JB. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell. 2009;33:717–26.
Souquere S, Beauclair G, Harper F, Fox A, Pierron G. Highly ordered spatial organization of the structural long noncoding NEAT1 RNAs within paraspeckle nuclear bodies. Mol Biol Cell. 2010;21:4020–7.
Nakagawa S, Hirose T. Paraspeckle nuclear bodies—useful uselessness? Cell Mol Life Sci. 2012;69:3027–36.
Sunwoo H, Dinger ME, Wilusz JE, Amaral PP, Mattick JS, Spector DL. MEN ε/β nuclear-retained non-coding RNAs are up-regulated upon muscle differentiation and are essential components of paraspeckles. Genome Res. 2009;19:347–59.
Mao YS, Sunwoo H, Zhang B, Spector DL. Direct visualization of the co-transcriptional assembly of a nuclear body by noncoding RNAs. Nat Cell Biol. 2011;13:95–101.
Wang H, Zhang M, Sun G. Long non-coding RNA NEAT1 regulates the proliferation, migration and invasion of gastric cancer cells via targeting miR-335-5p/ROCK1 axis. Pharmazie. 2018;73:150–5.
Li J-H, Zhang S-Q, Qiu X-G, Zhang S-J, Zheng S-H, Zhang D-H. Long non-coding RNA NEAT1 promotes malignant progression of thyroid carcinoma by regulating miRNA-214. Int J Oncol. 2017;50:708–16.
Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17:118–26.
Volinia S, Calin GA, Liu C-G, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci. 2006;103:2257–61.
Lo P-K, Wolfson B, Zhou Q. Cellular, physiological and pathological aspects of the long non-coding RNA NEAT1. Front Biol. 2016;11:413–26.
Lanzós A, Carlevaro-Fita J, Mularoni L, Reverter F, Palumbo E, Guigó R, Johnson R. Discovery of cancer driver long noncoding RNAs across 1112 tumour genomes: new candidates and distinguishing features. Sci Rep. 2017;7:1–16.
Chen S, Fang Y, Sun L, He R, He B, Zhang S, Long Non-Coding RNA. A potential strategy for the diagnosis and treatment of colorectal cancer. Front Oncol. 2021;11: 762752.
Xiong D-D, Feng Z-B, Cen W-L, Zeng J-J, Liang L, Tang R-X, Gan X-N, Liang H-W, Li Z-Y, Chen G. The clinical value of lncRNA NEAT1 in digestive system malignancies: a comprehensive investigation based on 57 microarray and RNA-seq datasets. Oncotarget. 2017;8:17665.
Peng W, Wang Z, Fan H. LncRNA NEAT1 impacts cell proliferation and apoptosis of colorectal cancer via regulation of Akt signaling. Pathol Oncol Res. 2017;23:651–6.
Wu Y, Yang L, Zhao J, Li C, Nie J, Liu F, Zhuo C, Zheng Y, Li B, Wang Z. Nuclear-enriched abundant transcript 1 as a diagnostic and prognostic biomarker in colorectal cancer. Mol Cancer. 2015;14:1–12.
Luo Y, Chen J-J, Lv Q, Qin J, Huang Y-Z, Yu M-H, Zhong M. Long non-coding RNA NEAT1 promotes colorectal cancer progression by competitively binding miR-34a with SIRT1 and enhancing the Wnt/β-catenin signaling pathway. Cancer Lett. 2019;440:11–22.
Zhang Q, Wang J, Li N, Liu Z, Chen Z, Li Z, Lai Y, Shen L, Gao J. miR-34a increases the sensitivity of colorectal cancer cells to 5-fluorouracil in vitro and in vivo. Am J Cancer Res. 2018;8:280.
Lai M, Du G, Shi R, Yao J, Yang G, Wei Y, Zhang D, Xu Z, Zhang R, Li Y. MiR-34a inhibits migration and invasion by regulating the SIRT1/p53 pathway in human SW480 cells. Mol Med Rep. 2015;11:3301–7.
Casatta N, Porro A, Orlandi I, Brambilla L, Vai M. Lack of Sir2 increases acetate consumption and decreases extracellular pro-aging factors. Biochim Biophys Acta. 2013;1833:593–601.
Yu DF, Jiang SJ, Pan ZP, Cheng WD, Zhang WJ, Yao XK, Li YC, Lun YZ. Expression and clinical significance of Sirt1 in colorectal cancer. Oncol Lett. 2016;11:1167–72.
Zhuang ST, Cai YJ, Liu HP, Qin Y, Wen JF. LncRNA NEAT1/miR-185-5p/IGF2 axis regulates the invasion and migration of colon cancer. Mol Genet Genomic Med. 2020;8:e1125.
He Z, Dang J, Song A, Cui X, Ma Z, Zhang Z. NEAT1 promotes colon cancer progression through sponging miR-495-3p and activating CDK6 in vitro and in vivo. J Cell Physiol. 2019;234:19582–91.
Ferrer J-L, Dupuy J, Borel F, Jacquamet L, Noel JP, Dulic V. Structural basis for the modulation of CDK-dependent/independent activity of cyclin D1. Cell Cycle. 2006;5:2760–8.
Yu HM, Wang C, Yuan Z, Chen GL, Ye T, Yang BW. LncRNA NEAT1 promotes the tumorigenesis of colorectal cancer by sponging miR-193a-3p. Cell Prolif. 2019;52: e12526.
Liu H, Li A, Sun Z, Zhang J, Xu H. Long non-coding RNA NEAT1 promotes colorectal cancer progression by regulating miR-205-5p/VEGFA axis. Hum Cell. 2020;33:386–96.
Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273:114–27.
Zhong F, Zhang W, Cao Y, Wen Q, Cao Y, Lou B, Li J, Shi W, Liu Y, Luo R. LncRNA NEAT1 promotes colorectal cancer cell proliferation and migration via regulating glial cell-derived neurotrophic factor by sponging miR-196a-5p. Acta Biochim Biophys Sin. 2018;50:1190–9.
Huang S-M, Chen T-S, Chiu C-M, Chang L-K, Liao K-F, Tan H-M, Yeh W-L, Chang G, Wang M-Y, Lu D-Y. GDNF increases cell motility in human colon cancer through VEGF-VEGFR1 interaction. Endocr Relat Cancer. 2014;21:73–84.
Los M, Mozoluk M, Ferrari D, Stepczynska A, Stroh C, Renz A, Herceg Z, Wang Z-Q, Schulze-Osthoff K. Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol Biol Cell. 2002;13:978–88.
Zhang M, Weng W, Zhang Q, Wu Y, Ni S, Tan C, Xu M, Sun H, Liu C, Wei P. The lncRNA NEAT1 activates Wnt/β-catenin signaling and promotes colorectal cancer progression via interacting with DDX5. J Hematol Oncol. 2018;11:1–13.
Wu N, Jiang M, Han Y, Liu H, Chu Y, Liu H, Cao J, Hou Q, Zhao Y, Xu B. O-GlcNAcylation promotes colorectal cancer progression by regulating protein stability and potential catcinogenic function of DDX5. J Cell Mol Med. 2019;23:1354–62.
Wang X, Jiang G, Ren W, Wang B, Yang C, Li M. LncRNA NEAT1 regulates 5-Fu sensitivity, apoptosis and invasion in colorectal cancer through the MiR-150-5p/CPSF4 axis. Onco Targets Ther. 2020;13:6373.
Kaufmann I, Martin G, Friedlein A, Langen H, Keller W. Human Fip1 is a subunit of CPSF that binds to U-rich RNA elements and stimulates poly (A) polymerase. EMBO J. 2004;23:616–26.
Wang S, Du H, Sun P. Long noncoding RNA NEAT1 contributes to the tumorigenesis of colorectal cancer through regulating SLC38A1 expression by sponging miR-138. Cancer Biother Radiopharm. 2021;36:793–802.
Shen X, Ye Z, Wu W, Zhao K, Cheng G, Xu L, Gan L, Wu Y, Yang Z. lncRNA NEAT1 facilitates the progression of colorectal cancer via the KDM5A/Cul4A and Wnt signaling pathway. Int J Oncol. 2021;59:1–12.
Yang G-J, Zhu M-H, Lu X-J, Liu Y-J, Lu J-F, Leung C-H, Ma D-L, Chen J. The emerging role of KDM5A in human cancer. J Hematol Oncol. 2021;14:1–18.
Fang Z, Lin M, Li C, Liu H, Gong C. A comprehensive review of the roles of E2F1 in colon cancer. Am J Cancer Res. 2020;10:757.
Hannah J, Zhou P-B. The CUL4A ubiquitin ligase is a potential therapeutic target in skin cancer and other malignancies. Chin J Cancer. 2013;32:478.
Nie X, Liu H, Liu L, Wang Y-D, Chen W-D. Emerging roles of Wnt ligands in human colorectal cancer. Front Oncol. 2020;10:1341.
Mohankumar K, Karki K, Safe S, Abdelrahim M. Nuclear receptor 4A1 (NR4A1) antagonists target PD-L1 in colon cancer. AACR. 2021. https://doi.org/10.1158/1538-7445.AM2021-1149.
Liu Z, Gu Y, Cheng X, Jiang H, Huang Y, Zhang Y, Yu G, Cheng Y, Zhou L. Upregulation lnc-NEAT1 contributes to colorectal cancer progression through sponging miR-486-5p and activating NR4A1/Wnt/β-catenin pathway. Cancer Biomark. 2021;30:309–19.
Zhu Z, Du S, Yin K, Ai S, Yu M, Liu Y, Shen Y, Liu M, Jiao R, Chen X. Knockdown long noncoding RNA nuclear paraspeckle assembly transcript 1 suppresses colorectal cancer through modulating miR-193a-3p/KRAS. Cancer Med. 2019;8:261–75.
Mustachio LM, Chelariu-Raicu A, Szekvolgyi L, Roszik J. Targeting KRAS in cancer: promising therapeutic strategies. Cancers. 2021;13:1204.
Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase–AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501.
Koveitypour Z, Panahi F, Vakilian M, Peymani M, Seyed Forootan F, Nasr Esfahani MH, Ghaedi K. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019;9:1–14.
Lee M, Kim B, Kim VN. Emerging roles of RNA modification: m6A and U-tail. Cell. 2014;158:980–7.
Zhang J, Guo S, Piao HY, Wang Y, Wu Y, Meng XY, Yang D, Zheng ZC, Zhao Y. ALKBH5 promotes invasion and metastasis of gastric cancer by decreasing methylation of the lncRNA NEAT1. J Physiol Biochem. 2019;75:379–89.
He Y, Hu H, Wang Y, Yuan H, Lu Z, Wu P, Liu D, Tian L, Yin J, Jiang K. ALKBH5 inhibits pancreatic cancer motility by decreasing long non-coding RNA KCNK15-AS1 methylation. Cell Physiol Biochem. 2018;48:838–46.
Guo T, Liu D-F, Peng S-H, Xu A-M. ALKBH5 promotes colon cancer progression by decreasing methylation of the lncRNA NEAT1. Am J Transl Res. 2020;12:4542.
Moazed D. Small RNAs in transcriptional gene silencing and genome defence. Nature. 2009;457:413–20.
Xu X, Wu J, Liu S, Saw PE, Tao W, Li Y, Krygsman L, Yegnasubramanian S, De Marzo AM, Shi J. Redox-responsive nanoparticle-mediated systemic RNAi for effective cancer therapy. Small. 2018;14:1802565.
Xin Y, Huang M, Guo WW, Huang Q, Jiang G. Nano-based delivery of RNAi in cancer therapy. Mol Cancer. 2017;16:1–9.
Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12:967–77.
Williford J-M, Wu J, Ren Y, Archang MM, Leong KW, Mao H-Q. Recent advances in nanoparticle-mediated siRNA delivery. Annu Rev Biomed Eng. 2014;16:347–70.
Li T, Deng N, Xu R, Fan Z, He J, Zheng Z, Deng H, Liao R, Lv X, Pang C. NEAT1 siRNA packed with chitosan nanoparticles regulates the development of colon cancer cells via lncRNA NEAT1/miR-377–3p axis. BioMed Res Int. 2021;2021:8.
Naseri MH, Mahdavi M, Davoodi J, Tackallou SH, Goudarzvand M, Neishabouri SH. Up regulation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells. Cancer Cell Int. 2015;15:1–9.
Zhu Y, Hu H, Yuan Z, Zhang Q, Xiong H, Hu Z, Wu H, Huang R, Wang G, Tang Q. LncRNA NEAT1 remodels chromatin to promote the 5-Fu resistance by maintaining colorectal cancer stemness. Cell Death Dis. 2020;11:1–11.
Joag MG, Sise A, Murillo JC, Sayed-Ahmed IO, Wong JR, Mercado C, Galor A, Karp CL. Topical 5-fluorouracil 1% as primary treatment for ocular surface squamous neoplasia. Ophthalmology. 2016;123:1442–8.
Guo Z, Liu Z, Yue H, Wang J. Retracted: Beta-elemene increases chemosensitivity to 5-fluorouracil through down-regulating microRNA-191 expression in colorectal carcinoma cells. J Cell Biochem. 2018;119:7032–9.
Dalby K, Tekedereli I, Lopez-Berestein G, Ozpolat B. Targeting the pro-death and pro-survival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. 2010;6:322–9.
Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Can Res. 2008;68:1485–94.
Liu F, Ai FY, Zhang DC, Tian L, Yang ZY, Liu SJ. LncRNA NEAT1 knockdown attenuates autophagy to elevate 5-FU sensitivity in colorectal cancer via targeting miR-34a. Cancer Med. 2020;9:1079–91.
Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:1–12.
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60.
Ju E, Dong K, Chen Z, Liu Z, Liu C, Huang Y, Wang Z, Pu F, Ren J, Qu X. Copper (II)–graphitic carbon nitride triggered synergy: improved ROS generation and reduced glutathione levels for enhanced photodynamic therapy. Angew Chem Int Ed. 2016;55:11467–71.
Liu K, Chen W, Lei S, Xiong L, Zhao H, Liang D, Lei Z, Zhou N, Yao H, Liang Y. Wild-type and mutant p53 differentially modulate miR-124/iASPP feedback following pohotodynamic therapy in human colon cancer cell line. Cell Death Dis. 2017;8:e3096–e3096.
Liu K, Lei S, Kuang Y, Jin Q, Long D, Liu C, Jiang Y, Zhao H, Yao H. A novel mechanism of the c-Myc/NEAT1 axis mediating colorectal cancer cell response to photodynamic therapy treatment. Front Oncol. 2021. https://doi.org/10.3389/fonc.2021.652831.
Lu F, Lan Z, Xin Z, He C, Guo Z, Xia X, Hu T. Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. J Cell Physiol. 2020;235:3207–21.
Broz P, Pelegrin P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20:143–57.
Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103.
Yu J, Li S, Qi J, Chen Z, Wu Y, Guo J, Wang K, Sun X, Zheng J. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis. 2019;10:1–20.
Skandarajah A, Lynch A, Mackay J, Ngan S, Heriot A. The role of intraoperative radiotherapy in solid tumors. Ann Surg Oncol. 2009;16:735–44.
Su F, Duan J, Zhu J, Fu H, Zheng X, Ge C. Long non-coding RNA nuclear paraspeckle assembly transcript 1 regulates ionizing radiation-induced pyroptosis via microRNA-448/gasdermin E in colorectal cancer cells. Int J Oncol. 2021;59:1–11.
Lakemeyer L, Sander S, Wittau M, Henne-Bruns D, Kornmann M, Lemke J. Diagnostic and prognostic value of CEA and CA19-9 in colorectal cancer. Diseases. 2021;9:21.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SA, FG, OA, RAA, and MC, have made contributions to the writing of the manuscript. MF has made contribution to the revision of the manuscript. All authors have approved the submitted version of the article and have agreed to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Azizidoost, S., Ghaedrahmati, F., Anbiyaee, O. et al. Emerging roles for lncRNA-NEAT1 in colorectal cancer. Cancer Cell Int 22, 209 (2022). https://doi.org/10.1186/s12935-022-02627-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-022-02627-6