Abstract
PTENP1 is a long non-coding RNA which has been regarded as a pseudogene of the PTEN tumor suppressor gene. However, it has been shown to be a biologically active transcript that can function as a competing endogenous RNA and enhance expression of PTEN protein. This lncRNA has two transcripts, namely PTENP1-202 and PTENP1-202 with sizes of 3996 and 1215 bps, respectively. PTENP1 acts as a sponge for some PETN-targeting miRNAs, such as miR-17, miR-20a, miR-19b, miR-106b, miR-200c, miR-193a-3p, miR-499-5p and miR-214. Besides, it can affect miR-20a/PDCD4, miR-27a-3p/EGR1, miR-17‐5p/SOCS6 and miR-19b/TSC1 axes. This long non-coding RNA participates in the pathoetiology of several types of cancers as well as non-malignant conditions such as alcohol-induced osteopenia, insulin resistance, osteoporosis, sepsis-associated cardiac dysfunction and spinal cord injury. In the current review, we elucidate the role of PTENP1 in human disorders, particularly malignant conditions based on evidence acquired from cell line assays, animal studies and investigations on human samples.
Similar content being viewed by others
Introduction
Long non-coding RNAs (lncRNAs) are a group of RNAs with sizes longer than 200 nucleotides, several shared features with mRNAs, the ability to regulate gene expression and lack of significant open reading frames. This novel group of epigenetic regulators mainly resides in the nucleus where they affect histone or DNA modification, chiefly methylation and acetylation [1]. Through influencing alternative splicing, cell differentiation, and cell cycle transition, lncRNAs contribute in the evolution of many diseases [2,3,4]. Moreover, lncRNAs can affect the organization and function of nuclear bodies, modify the stability and expression of cytoplasmic mRNAs and regulate activity of signaling pathways [5]. Functions and contribution of several lncRNAs in human diseases have been reviewed [6,7,8].
Phosphatase and Tensin Homolog Pseudogene 1 (PTENP1) is an example of lncRNAs which has been regarded as a pseudogene of the PTEN tumor suppressor gene. However, it has been shown to be a biologically active transcript that can function as a competing endogenous RNA (ceRNA) and enhance expression of PTEN protein [9]. In fact, PTENP1 exerts a growth-suppressive effect through obstructing the binding of miRNAs to the 3′ UTR of PTEN and protecting it from degradation [9].
The gene coding this lncRNA is located on chromosome 9: 33,673,504−33,677,499 reverse strand. This lncRNA has two transcripts, namely PTENP1-202 and PTENP1-202 with sizes of 3996 and 1215 bps, respectively (https://asia.ensembl.org/Homo_sapiens/Gene/Summary?g=ENSG00000237984;r=9:33673504-33677499). In the current review, we elucidate the function of PTENP1 in human disorders, particularly malignant conditions based on evidence obtained from cell line assays, animal studies and investigations on human samples.
Cell line studies
An in vitro experiment in HL-60 promyeoloblastic cells infected with the pCDH1-PTENP1 vectors has shown up-regulation of both PTENP1 and PTEN mRNA levels. However, protein levels of PTEN have not been affected by this intervention. Authors have suggested that PTENP1 can affect PTEN expression at mRNA level [10].
In addition to hematopoietic cells, PTENP1 can affect malignant properties of cell lines originated from solid tumors. Normal cells can secret PTENP1 in their exosomes. Exosome-mediated transmission of this lncRNA to bladder cancer cells could inhibit the malignant features in these cells through induction of cell apoptosis and reduction of invasion and migration abilities of bladder cancer cells. Functionally, exosomal PTENP1 could increase PTEN expression through sponging miR-17 [11]. The PTENP1/miR-20a/PTEN molecular route has been shown to affect malignant behavior of bladder cancer cells. While up-regulation of miR-20a could promote proliferation and migration of T24 cells, PDCD4 over-expression could exert the opposite effects [12].
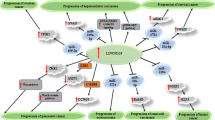
Expression levels of PTENP1 have also been assessed in breast cancer cells. PTENP1 has also been shown to influence proliferation, invasive properties and resistance of breast cancer cells to Adriamycin. These effects are most probably mediated through sponging miR-20a and further regulating expression of PTEN and activity of PI3K/AKT pathway [13]. Moreover, this lncRNA could affect breast cancer pathogenesis through modulation of miR-19b/PTEN axis [14]. PTENP1 could also suppress proliferation and migratory aptitude of breast cancer cells via decreasing expressions of cell cycle regulators cyclin A2 and CDK2 and regulating activity of AKT and MAPK pathways [15]. Finally, the sponging role of PTENP1 on miR-19b has been shown to be implicated in the suppression of proliferation and of breast cancer cells [16] (Fig. 1).
Similarly, PTENP1 could inhibit progression of cervical cancer through different mechanisms including suppression of miR-106b [17], miR-27a-3p [18] and miR-19b [19]. These miRNAs target PTEN, EGR1 and MTUS1, respectively (Fig. 2).
Figure 1. Summary of the role of PTENP1 in progression of cancers. PTENP1 can serve as molecular sponge for miR-19b, miR-20a and miR-17. Down-regulation of these miRNAs by PTENP1 affects proliferation, migration and invasiveness of cancer cells. Detailed information about the impact of this lncRNA on suppression of carcinogenesis is provided in Table 1.
Figure 2. Summary of the role of PTENP1 in progression of cancers. PTENP1 can serve as molecular sponge for miR-21, miR-10a-5p, miR-19b, miR-27a-3p, miR-193a-3p, miR-19b, miR-20a and miR-17. Down-regulation of these miRNAs by PTENP1 induces anti-tumor effects. Detailed information about the impact of this lncRNA on suppression of carcinogenesis is provided in Table 1.
PTENP1 can also affect pathoetiology of non-malignant conditions (Table 2). For instance, it can affect pathogenesis of alcohol-induced osteopenia. Ethanol stimulation has resulted in up-regulation of expression of PTEN and PTENP1 transcripts in a time-dependent mode, leading to up-regulation of PTEN protein levels. Moreover, ethanol could decrease PTEN phosphorylation, representing an upsurge in functional PTEN level. Up-regulation of PTEN could impair downstream Akt/GSK3β/β-catenin signals and osteogenic differentiation of bone mesenchymal stem cells [32]. Moreover, PTENP1 binding to miR-499-5p leads to deficiency in the insulin-signaling pathway, thus participating in insulin resistance [33]. Furthermore, up-regulation of PTENP1 or silencing of miR-214 could inhibit expressions of osteoclast markers and RANKL-induced osteoclast differentiation. These interventions could also inhibit phosphorylation of PI3K and AKT, nuclear transport of p65, destruction of IκBα and NFATc1 expression. On the other hand, PTENP1 silencing has enhanced osteoclast differentiation. Taken together, PTENP1 acts as a sponge for miR-214 to escalate expression of PTEN and suppress osteoclast differentiation. This mode of action attenuates osteoporosis through inhibition of PI3K/AKT/NF-κB signaling [34].
Animal studies
Impact of PTENP1 up-regulation and exosomal PTENP1 on growth of tumors has been investigated in vivo. Authors have injected EJ cells with PTENP1-expressing vectors as well as PTENP1-containing exosomes into nude mice. The results of conducted experiments have indicated that up-regulation of PTENP1 can decrease tumor weight and burden. Moreover, PTENP1-containing exosomes could attenuate tumor size and weight. Besides, over-expression of this lncRNA could reduce Ki67 expression in tumors [11]. Other studies in esophageal carcinoma, head and neck squamous cell carcinoma, hepatocellular cancer and oral squamous cell carcinoma have confirmed the impact of PTENP1 up-regulation on attenuation of tumor growth (Table 3). In animal models of renal cell carcinoma, up-regulation of this lncRNA has enhanced sensitivity to cisplatin and gemcitabine [31].
Animal models have also been used to evaluate the impact of PTENP1 in insulin resistance. An experiment in db/db mice and high fat diet-fed mice has shown up-regulation of PTENP1. Moreover, up-regulation of PTENP1 has led to impairment in activation of Akt/GSK and production of glycogen, while suppression of this lncRNA has enhanced activity of Akt/GSK and increased glycogen content [33]. In an in vivo study, it has shown that the effect of matrine on improvement of cardiac function and attenuation of the inflammatory responses is mediated through down-regulation of PTENP1 expression and up-regulation of miR-106b-5p levels [35].
Clinical studies
Expression of PTEN and PTENP1 mRNAs has been demonstrated to be lower in bone marrow samples of AML patients compared to healthy subjects. Moreover, expressions of these transcripts have been positively correlated. However, when AML patients have been classified based on the prognostic classification of 2011 NCCN, authors have detected no remarkable difference in the expression of PTENP1 among subgroups [10].
Expression of PTENP1 has also been shown to be diminished in bladder cancer tissues as well as exosomes extracted from plasma samples of these patients. In fact, this lncRNA has been found to be principally carried by exosomes. Exosomal levels of PTENP1 have the potential to discriminate bladder cancer patients from healthy subjects with area under receiver characteristic curve of 0.743. Thus, exosomal PTENP1 has been recommended as a putative marker for diagnostic purposes in bladder cancer [11]. In bladder cancer cells, PTENP1 target miR-20a has been shown to be up-regulated, while PDCD4 has been down-regulated [12].
In breast cancer, cervical cancer, head and neck squamous cell carcinoma, hepatocellular carcinoma and oral squamous cell carcinoma, down-regulation of PTENP1 has been linked with poor survival of patients (Table 4). Moreover, down-regulation of this lncRNA has been correlated with advanced histological grade and TNM stage, deep infiltration depth, and lymph node metastasis in cancer patients.
Association between a number of tag single nucleotide polymorphisms within PTENP1, including rs7853346 C > G, rs865005 C > T, and rs10971638 G > A and susceptibility to gastric cancer has been assessed in a Chinese population. Results have shown association between rs7853346 G allele and lower risk of gastric cancer. This association has been stronger in patients aged more than 60 years, non-smokers, non-drinkers, and those without family history of gastric cancer. Notably, expression assays have shown higher levels of PTENP1 in carriers of rs7853346 CG/GG genotype [37].
PTENP1 has also been shown to be down-regulated in osteoporosis patients, parallel with up-regulation of miR-214 [34].
Discussion
PTENP1 is an lncRNA which primarily functions as a ceRNA to enhance expression of PTEN. This lncRNA acts as a sponge for some PETN-targeting miRNAs, such as miR-17, miR-20a, miR-19b, miR-106b, miR-200c, miR-193a-3p, miR-499-5p and miR-214. Besides, it can serve as a molecular sponge for other miRNAs such as miR-20a, miR-27a-3p, miR-17‐5p and miR-19b to influence expressions of PDCD4, EGR1, SOCS6 and TSC1, respectively.
The role of PTENP1 has been mostly evaluated in the pathoetiology of cancer. In this context, the results of in vitro, in vivo and clinical studies have been consistent. This lncRNA is regarded as a tumor suppressor lncRNA in all cancers except for multiple myeloma.
In addition, a number of investigations have shown its influence on development of non-malignant conditions such as alcohol-induced osteopenia, insulin resistance, osteoporosis, sepsis-associated cardiac dysfunction and spinal cord injury.
As an lncRNAs secreted in the exosomes, it has the potential to be used as a biomarker for early detection of cancers. This application has been evaluated in the context of bladder cancer. However, further studies in other cancers are needed to appraise the potential of PTENP1 in diagnostic purposes.
Although forced up-regulation of PTENP1 in cancer cell lines using different vectors could attenuate in vitro cancer cell proliferation and in vivo tumor growth, this field of study is still in its initial phases, needing further evaluations in animal models particularly focusing on bioavailability and biosafety issues. Additionally, a comprehensive evaluation of PTENP1 targets and related signaling pathways is necessary to avoid unwanted side effects.
Since up-regulation of PTENP1 can also enhance the cytotoxic effects of chemotherapeutic agents on cancer cells, therapies aimed at over-expression of this lncRNA are potential ways for combating chemoresistance.
Conclusions
Association between PTENP1 polymorphisms and susceptibility to cancer has been evaluated in Chinese gastric cancer patients. Additional studies in other types of cancers in different populations are needed to find the influence of genetic variants in this lncRNA on cancer risk.
Taken together, PTENP1 is an important modulator of cancer progression which not only increases expression of the important tumor suppressor PTEN, but also affects expression of other cancer-related genes such as those regulating cell cycle progression. Thus, this lncRNA represent a promising target for design of novel anti-cancer therapies.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
References
Zhang X, Wang W, Zhu W, Dong J, Cheng Y, Yin Z, et al. Mechanisms and functions of long non-coding RNAs at multiple regulatory levels. Int J Mol Sci. 2019;20(22):5573.
Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15(1):7–21.
Kitagawa M, Kitagawa K, Kotake Y, Niida H, Ohhata T. Cell cycle regulation by long non-coding RNAs. Cell Mol Life Sci. 2013;70(24):4785–94.
Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochimica et Biophysica Acta (BBA). 2014;1839(11):1097–109.
Statello L, Guo C-J, Chen L-L, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22(2):96–118.
Ghafouri-Fard S, Khoshbakht T, Hussen BM, Taheri M, Mokhtari M. A review on the role of AFAP1-AS1 in the pathoetiology of cancer. Front Oncol. 2021;11:777849.
Ghafouri-Fard S, Khoshbakht T, Taheri M, Jamali E. A concise review on the role of CircPVT1 in tumorigenesis, drug sensitivity, and cancer prognosis. Front Oncol. 2021;11:762960.
Ghafouri-Fard S, Khoshbakht T, Taheri M, Ebrahimzadeh K. A review on the carcinogenic roles of DSCAM-AS1. Front Cell Develop Biol. 2021;9:758513.
Wang Z. Antisense RNA and cancer. Cancer and noncoding RNAs. Amsterdam: Elsevier; 2018. p. 203–27.
Wang C, Huai L, Zhang C, Jia Y, Li Q, Chen Y, et al. Study on expression of PTEN gene and its pseudogene PTENP1 in acute leukemia and correlation between them. Zhonghua xue ye xue za zhi Zhonghua xueyexue zazhi. 2012;33(11):896–901.
Zheng R, Du M, Wang X, Xu W, Liang J, Wang W, et al. Exosome–transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol Cancer. 2018;17(1):1–13.
Zhong X, Wang L, Yan X, Yang X, Xiu H, Zhao M, et al. MiR-20a acted as a ceRNA of lncRNA PTENPL and promoted bladder cancer cell proliferation and migration by regulating PDCD4. Eur Rev Med Pharmacol Sci. 2020;24:2955–64.
Gao X, Qin T, Mao J, Zhang J, Fan S, Lu Y, et al. PTENP1/miR-20a/PTEN axis contributes to breast cancer progression by regulating PTEN via PI3K/AKT pathway. J Exp Clin Cancer Res. 2019;38(1):1–14.
Li R, Guo L, Huang G, Luo W. PTENP1 acts as a ceRNA to regulate PTEN by sponging miR-19b and explores the biological role of PTENP1 in breast cancer. Cancer Gene Ther. 2017;24(7):309–15.
Chen S, Wang Y, Zhang J-H, Xia Q-J, Sun Q, Li Z-K, et al. Long non-coding RNA PTENP1 inhibits proliferation and migration of breast cancer cells via AKT and MAPK signaling pathways. Oncol Lett. 2017;14(4):4659–62.
Shi X, Tang X, Su L. Overexpression of long noncoding RNA PTENP1 inhibits cell proliferation and migration via suppression of miR-19b in breast cancer cells. Oncol Res. 2018;26(6):869.
Fan Y, Sheng W, Meng Y, Cao Y, Li R. LncRNA PTENP1 inhibits cervical cancer progression by suppressing miR-106b. Artif cells Nanomed Biotechnol. 2020;48(1):393–407.
Wu C, Wang F, Tan L. Role and the molecular mechanism of lncRNA PTENP1 in regulating the proliferation and invasion of cervical cancer cells. Gene Ther. 2020. https://doi.org/10.1038/s41434-020-00189-8.
Ou L, Xiang T, Hao X, Wang D, Zeng Q. Reduced long non-coding RNA PTENP1 contributed to proliferation and invasion via miR-19b/MTUS1 axis in patients with cervical cancer. Eur Rev Med Pharmacol Sci. 2020;24:4132–44.
Chen R, Zhang M, Liu W, Chen H, Cai T, Xiong H, et al. Estrogen affects the negative feedback loop of PTENP1-miR200c to inhibit PTEN expression in the development of endometrioid endometrial carcinoma. Cell Death Dis. 2018;10(1):1–13.
Gong T, Zheng S, Huang S, Fu S, Zhang X, Pan S, et al. PTENP1 inhibits the growth of esophageal squamous cell carcinoma by regulating SOCS6 expression and correlates with disease prognosis. Mol Carcinog. 2017;56(12):2610–9.
Zhang R, Guo Y, Ma Z, Ma G, Xue Q, Li F, et al. Long non-coding RNA PTENP1 functions as a ceRNA to modulate PTEN level by decoying miR-106b and miR-93 in gastric cancer. Oncotarget. 2017;8(16):26079.
Hu S, Xu L, Li L, Luo D, Zhao H, Li D, et al. Overexpression of lncRNA PTENP1 suppresses glioma cell proliferation and metastasis in vitro. Onco Targets Ther. 2019;12:147.
Hao S, Ma H, Niu Z, Sun S, Zou Y, Xia H. hUC-MSCs secreted exosomes inhibit the glioma cell progression through PTENP1/miR-10a-5p/PTEN pathway. Eur Rev Med Pharmacol Sci. 2019;23(22):10013–23.
Liu J, Xing Y, Xu L, Chen W, Cao W, Zhang C. Decreased expression of pseudogene PTENP1 promotes malignant behaviours and is associated with the poor survival of patients with HNSCC. Sci Rep. 2017;7(1):1–11.
Cao L-q, Yang X-w, Chen Y-b, Zhang D-w, Jiang X-F, Xue P. Exosomal miR-21 regulates the TETs/PTENp1/PTEN pathway to promote hepatocellular carcinoma growth. Mol Cancer. 2019;18(1):1–14.
Qian Y-Y, Li K, Liu Q-Y, Liu Z-S. Long non-coding RNA PTENP1 interacts with miR-193a-3p to suppress cell migration and invasion through the PTEN pathway in hepatocellular carcinoma. Oncotarget. 2017;8(64):107859.
Chen C-L, Tseng Y-W, Wu J-C, Chen G-Y, Lin K-C, Hwang S-M, et al. Suppression of hepatocellular carcinoma by baculovirus-mediated expression of long non-coding RNA PTENP1 and MicroRNA regulation. Biomaterials. 2015;44:71–81.
Zhang Y, Xu C. G allele of rs7853346 polymorphism in PTENP1 enhances the proliferation of multiple myeloma cancer stem cells by promoting the expression of PTENP1 and its downstream signaling molecules. J Cell Biochem. 2019;120(12):19738–48.
Gao L, Ren W, Zhang L, Li S, Kong X, Zhang H, et al. PTENp1, a natural sponge of miR-21, mediates PTEN expression to inhibit the proliferation of oral squamous cell carcinoma. Mol Carcinog. 2017;56(4):1322–34.
Yu G, Yao W, Gumireddy K, Li A, Wang J, Xiao W, et al. Pseudogene PTENP1 functions as a competing endogenous RNA to suppress clear-cell renal cell carcinoma progression. Mol Cancer Ther. 2014;13(12):3086–97.
Chen YX, Zhu DY, Gao J, Xu ZL, Tao SC, Yin WJ, et al. Diminished membrane recruitment of Akt is instrumental in alcohol-associated osteopenia via the PTEN/Akt/GSK‐3β/β‐catenin axis. FEBS J. 2019;286(6):1101–19.
Wang L, Zhang N, Wang Z, Ai D-m, Cao Z-y, Pan H-p. Pseudogene PTENP1 functions as a competing endogenous RNA (ceRNA) to regulate PTEN expression by sponging miR-499-5p. Biochemistry (Moscow). 2016;81(7):739–47.
Wang C-G, Wang L, Yang T, Su S-L, Hu Y-H, Zhong D. Pseudogene PTENP1 sponges miR-214 to regulate the expression of PTEN to modulate osteoclast differentiation and attenuate osteoporosis. Cytotherapy. 2020;22(8):412–23.
Liu Y, Liu L, Zhang J. Protective role of matrine in sepsis-associated cardiac dysfunction through regulating the lncRNA PTENP1/miR-106b-5p axis. Biomed Pharmacother. 2021;134:111112.
Yuan M, Zhao S, Chen R, Wang G, Bie Y, Wu Q, et al. MicroRNA–138 inhibits tumor growth and enhances chemosensitivity in human cervical cancer by targeting H2AX. Exp Ther Med. 2020;19(1):630–8.
Ge Y, He Y, Jiang M, Luo D, Huan X, Wang W, et al. Polymorphisms in lncRNA PTENP1 and the risk of gastric cancer in a Chinese population. Dis Markers. 2017;2017:6807452.
Yang S, Fu Z-z, Zhang Y-q, Fu B-h, Dong L-x. Rs7853346 Polymorphism in lncRNA-PTENP1 and rs1799864 polymorphism in CCR2 are associated with radiotherapy-induced cognitive Impairment in subjects with glioma via regulating PTENP1/miR-19b/CCR2 signaling pathway. Biochem Genet. 2021. https://doi.org/10.1007/s10528-021-10145-9.
Huang J, Zheng Y, Xiao X, Liu C, Lin J, Zheng S, et al. A circulating long noncoding RNA panel serves as a diagnostic marker for hepatocellular carcinoma. Dis Markers. 2020;2020:5417598.
Xin C, Li J, Zhang Y, Yu Z. Polymorphisms in lncRNA PTENP1 and the risk of oral squamous cell carcinoma in a Chinese population. Eur Rev Med Pharmacol Sci. 2018;22(17):5583–7.
Acknowledgements
This study was financially supported by Grant from Medical School of Shahid Beheshti University of Medical Sciences.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SGF wrote the manuscript and revised it. MT supervised and designed the study. TK, NAD and BMH collected the data and designed the figures and tables. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent forms were obtained from all study participants. The study protocol was approved by the ethical committee of Shahid Beheshti University of Medical Sciences. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ghafouri-Fard, S., Khoshbakht, T., Hussen, B.M. et al. A review on the role of PTENP1 in human disorders with an especial focus on tumor suppressor role of this lncRNA. Cancer Cell Int 22, 207 (2022). https://doi.org/10.1186/s12935-022-02625-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-022-02625-8