Abstract
Circular RNAs (circRNAs) are a new class of long non-coding RNAs, that results from a special type of alternative splicing referred to as back-splicing. They are widely distributed in eukaryotic cells and demonstrate tissue-specific expression patterns in humans. CircRNAs actively participate in various important biological activities like gene transcription, pre-mRNA splicing, translation, sponging miRNA and proteins, etc. With such diverse biological functions, circRNAs not only play a crucial role in normal human physiology, as well as in multiple diseases, including cancer. In this review, we summarized our current understanding of circRNAs and their role in renal cell carcinoma (RCC), the most common cancer of kidneys. Studies have shown that the expression level of several circRNAs are considerably varied in RCC samples and RCC cell lines suggesting the potential role of these circRNAs in RCC progression. Several circRNAs promote RCC development and progression mostly via the miRNA/target gene axis making them ideal candidates for novel anti-cancer therapy. Apart from these, there are a few circRNAs that are significantly downregulated in RCC and overexpression of these circRNAs leads to suppression of RCC growth. Differential expression patterns and novel functions of circRNAs in RCC suggest that circRNAs can be utilized as potential biomarkers and therapeutic targets for RCC therapy. However, our current understanding of the role of circRNA in RCC is still in its infancy and much comprehensive research is needed to achieve clinical translation of circRNAs as biomarkers and therapeutic targets in developing effective treatment options for RCC.
Similar content being viewed by others
Introduction
With trillions of cells multiplying in the body, any alterations in the process that lead to uncontrolled growth of cells will result in cancer. When tubular epithelial cells of nephron go cancerous, it gives rise to renal cell carcinoma (RCC) which accounts for over 90% of the renal malignancies, and over 3% of all adult malignancies [1]. The condition is mostly seen in old age (> 60 years), with nearly two times higher prevalence in males than in females [2]. Further, RCC is ranked as the sixth and eighth most common cancer in males and females respectively. Radical nephrectomy is the mainstay therapy for RCC, however distant metastasis and local invasion limits such an approach. In such cases, chemotherapy is an ideal choice but resistance to current drugs significantly impairs the treatment efficiency [3]. Therefore, novel strategies for early detection and targeted therapies are need of a moment for the successful management of RCC. A deeper understanding of the RCC pathophysiology may reveal relevant molecules for further advancement in the therapeutic management of RCC.
Similar to other cancers, the tumorigenesis of RCC involves dysregulation of genetic and epigenetic pathways [4,5,6]. In most RCC patients, the short arm of chromosome 3 is lost, where the tumor suppressor gene von Hippel-Lindau (VHL) is located, resulting in the dysregulation of the hypoxic pathway due to alteration of HIF-2α expression [7] Other pathways involved in cell proliferation and growth, like PI3K-AKT-mTOR pathways, are also activated in RCC [8]. Epigenetic disruption due to altered epigenetic regulators is identified as fundamental to cancer occurrence. Noncoding RNAs are one such epigenetic regulators that are shown to play a potential role in RCC development and progression [9,10,11]. Circular RNAs (circRNA) are a special category of long noncoding RNAs (lncRNAs) that are being extensively studied for their role in the development and progression of various types of cancer [12, 13]. Many studies have demonstrated that the expression of various circRNAs is dysregulated in various cancers including RCC [14,15,16]. Several circRNAs with enhanced expression in RCC models suggest the oncogenic potential of these overexpressed circRNAs in RCC [14]. On contrary, there are a few circRNAs that are downregulated in RCC demonstrating the tumor suppressor effect of circRNAs in RCC progression [14]. These studies demonstrate a critical role of circRNAs in various stages of RCC making them an important topic of research for developing new strategies to improvise RCC management. Hence, in the present review, we performed an extensive literature search for circRNAs associated with RCC and summarized the role of various circRNAs in RCC, demonstrating their potential to be used as biomarkers and targets for RCC therapy.
The overall structure of the article
Overview and biogenesis of circular RNAs.
Biological functions of CircRNAs.
Functional significance of CircRNAs in RCC.
circRNAs and their involvement in other cancer types.
Conclusion
(Please see Fig. 1 for the flowchart of the research methodology).
Overview and biogenesis of CircRNAs
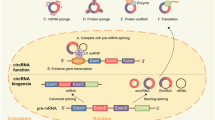
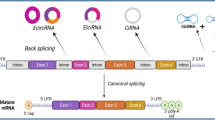
CircRNAs are a subclass of lncRNAs that are generated by the process of back-splicing (Fig. 1), where a 5′ splice site is bonded to the 3′ splice site. Structurally, they are a single chain RNA molecule with a 5′–3′ phosphodiester bond, forming a covalently-looped circular structure [17]. Based on their composition, three types of circRNAs have been identified: exonic circRNAs (EcircRNAs), intronic circRNAs (IcircRNAs) and exon–intron circRNAs (EicircRNA) (Fig. 2) [18]. RNA binding proteins play a major role in the formation of circRNAs. For instance, Quaking supports EcircRNA formation by promoting a 5′–3′ phosphodiester bond formation [19], while muscleblind supports IcircRNA formation by binding to its own pre-mRNA [20]. Due to their unexpected structure, the finding of circRNAs was initially considered an artifact. However, with the use of latest technologies like next-generation sequencing and bioinformatics tools, the existence of circRNAs is not only accepted but over 30,000 types of circRNAs have been predicted to exist in humans [14, 21]. Important bioinformatics tools and databases useful for studying circRNAs are enlisted in Table 1. The expression of circRNAs is tissue specific and they are most abundantly found in neural tissue, where they tend to accumulate with age [22, 23]. The reason for this could be that the neurons exhibit highest rate of alternative splicing, an important process for circRNA biogenesis. Apart from that the circRNAs possess a longer half-life (18–23 h) compared to linear RAs (4–7 h) which could be one of the reasons they might get accumulated in hardly dividing cells like neurons and not much in highly proliferating tissue [24, 25]. The observed longer half-life in circRNA is due to the lack of 5′ and 3′ terminal structure making them relatively resistant to common RNA degradation pathways, and are thus considered stable of all the RNAs. Under in vitro conditions, the enzymatic activity of Rnase H and Rrp44 could cleave circRNAs, although the process is considerably slow [26, 27]. The mechanisms and rate of degradation of circRNAs in vivo are yet to be fully understood.
Biological functions of CircRNAs
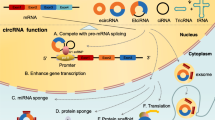
Despite their unusual structure and lack of 5′ and 3′ terminals, circRNAs are biologically active and are a hot topic of research. Various biological and cellular functions of circRNAs have been identified making them an important aspect of current biological research with respect to their role in various diseases such as cancer. Based on various studies, six biological functions of circRNAs have been identified and all the functions have been discussed briefly below. Moreover, all six functions of circRNAS have been represented in Fig. 2.
Functioning as micro RNA (miRNA) sponges
Micro RNA (miRNA) is a type of non-coding RNA with a length of about 18–25 nucleotides, which erroneous expression has been confirmed to be related to cancer, autoimmune diseases, osteoporosis and so on [44,45,46]. CircRNAs can act as competitive endogenous RNAs, where they competitively bind to miRNAs via miRNA response elements and inhibit their functions [47]. For example, Zheng et al., found that the circHIPK3 can sponge 9 miRNAs, especially miR-124, which are known growth-suppressors in different cancer cells [48]. Similarly, circ-ITCH is shown to sponge miR-7 and miR-214 resulting in inhibition of lung cancer via increased expression of the ITCH gene [49]. Sponging miR-9 by circMTO1 increases p21 expression in hepatocellular carcinoma, resulting in the inhibition of its proliferation and invasive abilities [50]. Sponging by circRNA may not always result in inhibition of miRNA, but also serve as its reservoir or transporter. For example, the circRNA sponge for miR-7 (ciRS7) can sponge both miR-7 and miR-671, where the later could trigger the AGO2-mediated cleavage of ciRS7, releasing miR-7 [47, 51].
Regulating transcription and translation
The circRNAs, primarily IcircRNAs and EicircRNAs, are able to influence gene transcription through their retained intronic sequences, by interacting with RNA polymerase II and U1 snRNP. For instance, studies have revealed that circEIF3J, circPAIP2, circANKRD52 and circSIRT7 could enhance the expression of their parental genes [52, 53]. Apart from regulation of gene transcription, the circRNA also influences the protein expression, mostly by acting as modulators of mRNA translation. For example, a study by Chao et al. showed that the circRNAs generated by the mouse formin (Fmn) gene prevented the translation of its mRNA into Fmn protein by harboring itself at its translation site [54].
Competing with linear splicing of pre-mRNA
Both circRNA biogenesis and canonical splicing work on the same splice sites and depend on the same spliceosomal machinery, suggesting that the circRNAs compete with the linear splicing of pre-mRNAs [20, 55]. Studies have shown a negative correlation between circRNAs and their linear isoforms [56]. However, the molecular mechanisms underlying such competition needs to be further elucidated.
Regulating translocation of various RNA binding proteins (RBPs)
CircRNAs can bind to RBPs and transport them to specific subcellular locations, and thus aid in regulating protein functions [57]. Studies have revealed that the RBPs like STAT3, PDK-1, AKT1 and c-myc are translocated into the nucleus by circ-Amotl1 [58,59,60]. While nuclear translocation of MBL protein and translational activator HuR are facilitated by circ-Mbl and circPABPN1, respectively [20, 61].
Acting as a scaffold for protein interaction
By acting as scaffolds, some circRNAs are shown to influence the kinetics of the protein–protein interaction by facilitating the contact between them. For instance, circ-Amotl1 acts as a scaffold for PDK1 mediated AKT1 phosphorylation that aids in its nuclear translocation [62], while circ-Foxo3 can facilitate the interaction between p53 and Mdm2 that results in degradation of p53 [63].
Encoding for peptides and proteins via translation
Owing to its unusual structure, circRNAs were initially thought to be untranslatable. However, recently several studies reported that circRNAs also get encoded [64,65,66]. Despite the absence of 5′ and 3′ terminals, the circRNA demonstrates a cap-independent open reading frame that incorporates internal ribosome entry sites, allowing its translation via membrane-associated ribosomes [64]. Yang et al., showed that in the presence of N6-methyladenosine, some circRNAs within in cancer cell line could encode several peptides [66]. Legnini et al. also demonstrated the presence of heavy polysomes in circ-ZNF609, which can be translated into a protein that may control myoblast proliferation [65]. More studies are needed in the sector to not only reveal the proteins coded by circRNAs but also to elucidate their functional relevance.
Functional significance of CircRNAs in RCC
Owing to such diverse biological activities, circRNAs play a critical role in human physiology and pathology, including cancers. Here we discuss the role of circRNAs in RCC with emphasis on oncogenic and tumor suppressor functions of various circRNAs. The mechanisms of how the circular RNAs impact the tumorigenesis of renal cell carcinoma are shown in Fig. 3. Several genome-wide studies have reported altered transcriptional profiles of circRNA in RCC. Franz et al. identified 13,261 circRNAs in clear cell renal cell carcinoma (ccRCC) samples, of which 78 were upregulated and 91 were downregulated as compared to matched controls [67]. The bioinformatics analysis of the RNA microarray database of ccRCC tissues by Ma et al. revealed that the expression of a total of 542 circRNAs was deviated from normal, among which 218 circRNAs were upregulated while the remaining 324 were downregulated [68]. Mechanism of many of these circRNAs in the regulation of key processes of RCC tumorigenesis, like epithelial–mesenchymal transition (EMT), proliferation, migration, invasion, apoptosis and drug resistance have been identified [14, 69,70,71,72]. Each upregulated or downregulated circRNA can influence multiple key processes via circRNA/miRNA/miRNA-target gene axis, the details of which are discussed briefly in Tables 2 and 3.
CircRNAs facilitating oncogenesis in RCC
Several circRNAs are found upregulated in RCC which is correlated with tumor growth. RNA microarray analysis by Zhou et al. found the upregulation of circPCNXL2 in ccRCC samples, that correlated with poor overall survival of the patients [73]. Knockdown of circPCNXL2 resulted in decreased proliferation and invasion of RCC cells in vitro and significantly reduced the tumor growth in vivo [73]. Further experimental analysis showed that circPCNXL2 functions as a miRNA sponge for miR-153, resulting in increased expression of ZEB2 protein, which is associated with aggressive RCC phenotype and poor prognosis in RCC patients [73, 74]. Similarly, Jin et al. determined an oncogenic role of circ0039569, where it could support the survival and metastasis of RCC by promoting the proliferation, invasion and migration of RCC cells [72]. Circ0039569 was found to achieve this by sponging miR-34a-5p that resulted in upregulation of CCL22 gene, which codes for CCL22 chemokine [72]. CircZNF609 is another oncogenic circRNA which is shown to promote the proliferation and invasion of RCC cells [75]. RNA immunoprecipitation assay and luciferase assay revealed the role of circZNF609/miR-138-5p/FOXP4 axis in RCC tumorigenesis [75]. CircFNDC3B and circNRIP1 are two other circRNAs that are shown to promote the proliferation and migration in RCC cells. CircFNDC3B negatively regulates miR-99a influencing the JAK1/STAT3 and MEK/ERK pathway resulting in increased proliferation and migration of RCC cells [76]. Similarly, circNRIP1 played the oncogenic role in RCC via miR-505/AMPK and miR-505/PI3K/AKT/mTOR pathway [77].
Few circRNAs could impart anti-apoptotic features to RCC cells. Increased SOX12 expression due to sponging of miR-296-5p by circ001895 could not only increase proliferation, invasion and migration of RCC cells but also prevented their apoptosis by increasing Bcl-2 and decreasing Bax and cleaved caspase-3 expression [78]. Moreover, apoptosis of RCC cells was prevented by circEGLN3 via miR-1299/IRF7 [79], and circABCB10 [80], by altering Bax, Bcl2 and caspase-3 protein expression. CircEGLN3, along with circNOX4 and circRHOBTB3 were all found to correlate well, demonstrated by good area under the receiver operating characteristic curve (AUC-ROC), with the clinical features and overall survival of ccRCC patients, indicating their potential as diagnostic biomarkers for the condition [67, 81]. Of these, circEGLN3 demonstrated an excellent AUC-ROC of 0.98, making it a remarkably reliable biomarker [79]. CircABCB10 was found to be associated well with the pathologic grade and TNM staging of RCC and thus may serve as a potential prognostic marker [80].
Epithelial–mesenchymal transition (EMT) is a critical process through which the differentiated epithelial cells acquire the features of stem-like mesenchymal cells, contributing to tumorigenesis, cardiopathy and other diseases [82,83,84]. EMT is a major dysregulated element in RCC, which is reportedly promoted by circ000926 and circPRRC2A via miR-411/CDH2 and miR-514a-5p/miR-6776-5p/TRPM3 axis [69, 85]. Further, circPRRC2A level correlated well with the tumor size, Fuhrman grade and pT stage which makes it an independent prognostic biomarker for overall survival and metastasis-free survival [85]. Yan et al. reported the role of circ0035483 in contributing to gemcitabine resistance in human RCC cells by targeting miR-335/CCNB1 axis [70]. Together, upregulation of these circRNAs contributes to the tumorigenesis and progression of RCC.
CircRNAs suppressing RCC growth and tumorigenesis
Following the same circRNA/miRNA/target gene axis, several circRNAs can exert anti-tumor effects in RCC. Chen T et al. identified that the Hsa-circ0072309 was poorly expressed in RCC specimens [86]. Increasing the expression of Hsa-circ0072309 in RCC cell lines, in which the expression was otherwise suppressed, inhibited their proliferation, migration and invasion abilities, while enhancing their apoptosis. The search for underlying mechanisms revealed the sponging of miR-100 by Hsa-circ0072309 that led to suppression of PI3K/AKT/mTOR pathway in RCC cells [86]. Another circRNA that could induce apoptosis in RCC cells is circ0001451 and knockdown of which resulted in a significant RCC growth under in vitro conditions [81]. Further, an AUC-ROC of 0.704 circ0001451 was found to be correlated well with the clinicopathological features and overall survival of ccRCC patients, making it an attractive diagnostic and prognostic biomarker [81]. By regulating miR-296-3p/E-cadherin axis, circ-AKT3 was shown to inhibit EMT in RCC cells resulting in suppression of metastasis of ccRCC [87]. CircRNAs cRAPGEF5 and circATP2B1 are also shown to suppress the proliferation, migration and invasion of RCC cells by regulating the miR-271-3p/TXNIP and miR-204-3p/FN1 axis, respectively [71, 88]. Further, the expression level of circRAPGEF5 correlated well with tumor stages, overall survival and relapse-free survival of RCC patients and thus may serve as a prognostic biomarker in RCC [71]. circHIAT1 can sponge multiple RNAs that include miR-195-5p, miR-29a-3p and miR-29c-3p to suppress CD-42 expression, which leads to suppression of migration and invasion in ccRCC cells [89]. Another circRNA with an anti-tumor function in RCC is circMTO1, which promotes the expression of tumor suppressor LMX1A by acting as a miR9 sponge and leading to miR9 downregulation. LMX1A is a direct target of miR9 and downregulation of miR9 by circMTO1 leads to higher expression of LMX1A and ultimately leading to suppression of RCC progression demonstrating the role of circMTO1 as a potential therapeutic target for RCC therapy [90]. All these circRNAs with tumor suppressor functions are downregulated in RCC, and the fact that their upregulation can inhibit the RCC progression, metastasis and chemoresistance under in vitro conditions, makes them potential therapeutic targets for treating RCC. Many more circRNAs undoubtedly play a crucial role in RCC tumorigenesis which are yet to be discovered by future research.
CircRNAs and their involvement in other cancer types
Compared with other published articles, we have made a more comprehensive and novel summary of the role of circRNAs in RCC. At the same time, it is not only limited to the role of circRNAs in RCC, we also summarize the reports of circRNAs in other cancers. Different circRNAs and their involvement in various cancer types are summarized in Table 4. According to the information summarized in the table, various studies on different circRNAs suggest that they play an important role not only in RCC but in other types of cancers also including breast cancer, colorectal cancer, gastric cancer, hepatocellular carcinoma, glioma, lung cancer, bladder cancer and hematological malignancies [91,92,93]. In these cancer types, differential expression of various circRNAs has been reported suggesting their crucial role in cancer development and progression. Generally, circRNAs with enhanced expression in different cancer types play an oncogenic role via targeting the expression of important miRNAs and proteins leading to tumorigenesis [91, 92]. On the other hand, circRNAs with diminished expression in different cancer types act as a tumor suppressor and when ectopically expressed leads to suppression of tumor growth [91, 92].
Conclusion and future perspective
Circular RNAs are a group of biologically active long non-coding RNAs that are associated with various biological functions in eukaryotic cells. Although initially neglected as an artifact, advancement in recent research has led to a better understanding of their functions and applicability, particularly in the field of cancer. Here we have demonstrated that there is significant dysregulation in the expression of various circRNAs in cancers including RCC. We further gave an overall idea of how several circRNAs influence RCC growth and progression. We further provided detailed examples and a comprehensive list of circRNAs with oncogenic and tumor suppressive effects in RCC, demonstrating the role of various circRNAs in the complex process of RCC development. However, our knowledge of the role of circRNAs in cancers including RCC is very limited and there is still a need for more research related to circRNAs to determine their internal structure and entire functional spectrum in cancer biology. Our understanding of the role of circRNA in RCC is in its infancy, as only a few circRNAs have been identified. Even within the identified circRNAs, most of our current understanding of their mechanism of action is limited to miRNA sponging activity, while much of their other functions are yet to be understood. The tumor microenvironment (TME) is a complex ecosystem that plays a vital role in the process of RCC development and progression. However, the role of circRNA in shaping the RCC TME and vice versa is still elusive [151]. CircRNAs are abundantly expressed in body fluids [152,153,154], and the fact that they possess a longer half-life and better stability make them attractive biomarkers in liquid biopsy for diagnosis or monitoring of various conditions including RCC. However, not all the current known circRNAs associated with RCC can serve as biomarkers, and those that are considered as potential diagnostic or prognostic biomarkers need more studies to establish their credibility. In fact, the techniques and methods to reliably detect circRNAs need to be further standardized. Within our current understanding, circRNAs seem to be promising agents for targeted therapy, however, we are far from determining the methods to safely and effectively achieve it. One possible way could be to use exosomes to deliver circRNAs without immunologic rejection. Exosomes are shown to contain stable circRNAs and can serve as diagnostic biomarkers for colon cancer detection [155]. A similar role of exosomes in RCC is yet to be revealed. It is evident that differential regulation of various circRNAs and their role in RCC development indicates their importance as potential therapeutic targets and biomarkers for the development of more effective treatment strategies for RCC therapy. However, there is still a need for more extensive research focused on circRNAs and their involvement in RCC.
Abbreviations
- circRNAs:
-
Circular RNAs
- RCC:
-
Renal cell carcinoma
- VHL:
-
Von Hippel-Lindau
- HIF:
-
Hypoxia-inducible factors
- EcircRNAs:
-
Exonic circRNAs
- IcircRNAs:
-
Intronic circRNAs
- EicircRNA:
-
Exon–intron circRNAs
- RNP:
-
RNA binding protein
- ccRCC:
-
Clear cell renal cell carcinoma
- EMT:
-
Epithelial–mesenchymal transition
- TME:
-
Tumor microenvironment
References:
Pandey JSW. Renal Cancer. [Updated 2021 Feb 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing [Internet]. 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK558975/.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin United States. 2020;70:7–30.
Peng J, Mo R, Ma J, Fan J. let-7b and let-7c are determinants of intrinsic chemoresistance in renal cell carcinoma. World J Surg Oncol. 2015;13:175.
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–9.
Li L, Miao W, Huang M, Williams P, Wang Y. Integrated genomic and proteomic analyses reveal novel mechanisms of the methyltransferase SETD2 in renal cell carcinoma development. Mol Cell Proteomics United States. 2019;18:437–47.
Xu Q, Krause M, Samoylenko A, Vainio S. Wnt signaling in renal cell carcinoma. Cancers (Basel). 2016;8:57.
Zhai W, Sun Y, Jiang M, Wang M, Gasiewicz TA, Zheng J, et al. Differential regulation of LncRNA-SARCC suppresses VHL-mutant RCC cell proliferation yet promotes VHL-normal RCC cell proliferation via modulating androgen receptor/HIF-2α/C-MYC axis under hypoxia. Oncogene England. 2016;35:4866–80.
Elfiky AA, Aziz SA, Conrad PJ, Siddiqui S, Hackl W, Maira M, et al. Characterization and targeting of phosphatidylinositol-3 kinase (PI3K) and mammalian target of rapamycin (mTOR) in renal cell cancer. J Transl Med. 2011;9:133.
Ding L, Jiang M, Wang R, Shen D, Wang H, Lu Z, et al. The emerging role of small non-coding RNA in renal cell carcinoma. Transl Oncol. 2021;14:100974.
Liu X, Hao Y, Yu W, Yang X, Luo X, Zhao J, et al. Long non-coding RNA emergence during renal cell carcinoma tumorigenesis. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol Germany. 2018;47:735–46.
Li M, Wang Y, Cheng L, Niu W, Zhao G, Raju JK, et al. Long non-coding RNAs in renal cell carcinoma: a systematic review and clinical implications. Oncotarget. 2017;8:48424–35.
Zhang Z, Xie Q, He D, Ling Y, Li Y, Li J, et al. Circular RNA: new star, new hope in cancer. BMC Cancer. 2018;18:834.
Xu Z, Yan Y, Zeng S, Dai S, Chen X, Wei J, et al. Circular RNAs: clinical relevance in cancer. Oncotarget. 2018;9:1444–60.
Wang Y, Zhang Y, Wang P, Fu X, Lin W. Circular RNAs in renal cell carcinoma: implications for tumorigenesis, diagnosis, and therapy. Mol Cancer. 2020;19:149.
Huang Y, Zhang C, Xiong J, Ren H. Emerging important roles of circRNAs in human cancer and other diseases. Genes Dis. 2021;8:412–23.
Greene J, Baird A-M, Brady L, Lim M, Gray SG, McDermott R, et al. Circular RNAs: biogenesis, function and role in human diseases. Front Mol Biosci. 2017;4:38.
Patop IL, Wüst S, Kadener S. Past, present, and future of circRNAs. EMBO J. 2019;38:e100836.
Huang G, Li S, Yang N, Zou Y, Zheng D, Xiao T. Recent progress in circular RNAs in human cancers. Cancer Lett Ireland. 2017;404:8–18.
Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, et al. The RNA binding protein quaking regulates formation of circRNAs. Cell United States. 2015;160:1125–34.
Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell United States. 2014;56:55–66.
Szabo L, Salzman J. Detecting circular RNAs: bioinformatic and experimental challenges. Nat Rev Genet. 2016;17:679–92.
Rybak-Wolf A, Stottmeister C, Glažar P, Jens M, Pino N, Giusti S, et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell United States. 2015;58:870–85.
You X, Vlatkovic I, Babic A, Will T, Epstein I, Tushev G, et al. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci. 2015;18:603–10.
Enuka Y, Lauriola M, Feldman ME, Sas-Chen A, Ulitsky I, Yarden Y. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 2016;44:1370–83.
Liang D, Tatomer DC, Luo Z, Wu H, Yang L, Chen L-L, et al. The output of protein-coding genes shifts to circular RNAs when the Pre-mRNA processing machinery is limiting. Mol Cell. 2017;68:940-954.e3.
Mackie GA. Ribonuclease E is a 5′-end-dependent endonuclease. Nature England. 1998;395:720–3.
Schaeffer D, Tsanova B, Barbas A, Reis FP, Dastidar EG, Sanchez-Rotunno M, et al. The exosome contains domains with specific endoribonuclease, exoribonuclease and cytoplasmic mRNA decay activities. Nat Struct Mol Biol. 2009;16:56–62.
Ghosal S, Das S, Sen R, Basak P, Chakrabarti J. Circ2Traits: a comprehensive database for circular RNA potentially associated with disease and traits. Front Genet. 2013;4:283.
Wu W, Ji P, Zhao F. CircAtlas: an integrated resource of one million highly accurate circular RNAs from 1070 vertebrate transcriptomes. Genome Biol. 2020;21:101.
Liu M, Wang Q, Shen J, Yang BB, Ding X. Circbank: a comprehensive database for circRNA with standard nomenclature. RNA Biol. 2019;16:899–905.
Glažar P, Papavasileiou P, Rajewsky N. circBase: a database for circular RNAs. RNA. 2014;20:1666–70.
Ma X-K, Wang M-R, Liu C-X, Dong R, Carmichael GG, Chen L-L, et al. CIRCexplorer3: a CLEAR pipeline for direct comparison of circular and linear RNA expression. Genomics Proteomics Bioinformatics. 2019;17:511–21.
Dudekula DB, Panda AC, Grammatikakis I, De S, Abdelmohsen K, Gorospe M. CircInteractome: a web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biol. 2016;13:34–42.
Liu Y-C, Li J-R, Sun C-H, Andrews E, Chao R-F, Lin F-M, et al. CircNet: a database of circular RNAs derived from transcriptome sequencing data. Nucleic Acids Res. 2016;44:D209–15.
Dong R, Ma X-K, Li G-W, Yang L. CIRCpedia v2: an updated database for comprehensive circular RNA annotation and expression comparison. Genomics Proteomics Bioinformatics. 2018;16:226–33.
Meng X, Chen Q, Zhang P, Chen M. CircPro: an integrated tool for the identification of circRNAs with protein-coding potential. Bioinformatics England. 2017;33:3314–6.
Zhao Z, Wang K, Wu F, Wang W, Zhang K, Hu H, et al. circRNA disease: a manually curated database of experimentally supported circRNA-disease associations. Cell Death Dis. 2018;9:475.
Chen X, Han P, Zhou T, Guo X, Song X, Li Y. circRNADb: a comprehensive database for human circular RNAs with protein-coding annotations. Sci Rep. 2016;6:34985.
Jia G-Y, Wang D-L, Xue M-Z, Liu Y-W, Pei Y-C, Yang Y-Q, et al. CircRNAFisher: a systematic computational approach for de novo circular RNA identification. Acta Pharmacol Sin. 2019;40:55–63.
Gao Y, Wang J, Zhao F. CIRI: an efficient and unbiased algorithm for de novo circular RNA identification. Genome Biol. 2015;16:4.
Xia S, Feng J, Chen K, Ma Y, Gong J, Cai F, et al. CSCD: a database for cancer-specific circular RNAs. Nucleic Acids Res. 2018;46:D925–9.
Li S, Li Y, Chen B, Zhao J, Yu S, Tang Y, et al. exoRBase: a database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 2018;46:D106–12.
Li J-H, Liu S, Zhou H, Qu L-H, Yang J-H. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–7.
Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199–227.
Majid AK, Parisa MN, Mohammad MMB, Mohammad H, et al. Increased expression of miR-377-3p in patients with relapsing remitting multiple sclerosis. SciMed J. 2019;1(2):48.
Ge D, Wang W, Chen H, Yang L, Cao X. Functions of MicroRNAs in osteoporosis. Eur Rev Med and Pharmacol Sci. 2017;21:4784–9.
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA circles function as efficient microRNA sponges. Nature England. 2013;495:384–8.
Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:11215.
Wan L, Zhang L, Fan K, Cheng Z-X, Sun Q-C, Wang J-J. Circular RNA-ITCH suppresses lung cancer proliferation via inhibiting the Wnt/β-Catenin pathway. Biomed Res Int. 2016;2016:1579490.
Han D, Li J, Wang H, Su X, Hou J, Gu Y, et al. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology United States. 2017;66:1151–64.
Hansen TB, Wiklund ED, Bramsen JB, Villadsen SB, Statham AL, Clark SJ, et al. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J. 2011;30:4414–22.
Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, et al. Exon–intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol United States. 2015;22:256–64.
Zhang Y, Zhang X-O, Chen T, Xiang J-F, Yin Q-F, Xing Y-H, et al. Circular intronic long noncoding RNAs. Mol Cell United States. 2013;51:792–806.
Chao CW, Chan DC, Kuo A, Leder P. The mouse formin (Fmn) gene: abundant circular RNA transcripts and gene-targeted deletion analysis. Mol Med. 1998;4:614–28.
Chen L-L, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12:381–8.
Liu J, Liu T, Wang X, He A. Circles reshaping the RNA world: from waste to treasure. Mol Cancer. 2017;16:58.
Hentze MW, Preiss T. Circular RNAs: splicing’s enigma variations. EMBO J. 2013;32:923–5.
Yang Z-G, Awan FM, Du WW, Zeng Y, Lyu J, Wu D, et al. The circular RNA interacts with STAT3, increasing its nuclear translocation and wound repair by modulating Dnmt3a and miR-17 function. Mol Ther. 2017;25:2062–74.
Yang Q, Du WW, Wu N, Yang W, Awan FM, Fang L, et al. A circular RNA promotes tumorigenesis by inducing c-myc nuclear translocation. Cell Death Differ. 2017;24:1609–20.
Zheng J, Liu X, Xue Y, Gong W, Ma J, Xi Z, et al. TTBK2 circular RNA promotes glioma malignancy by regulating miR-217/HNF1β/Derlin-1 pathway. J Hematol Oncol. 2017;10:52.
Abdelmohsen K, Panda AC, Munk R, Grammatikakis I, Dudekula DB, De S, et al. Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by CircPABPN1. RNA Biol. 2017;14:361–9.
Zeng Y, Du WW, Wu Y, Yang Z, Awan FM, Li X, et al. A Circular RNA binds to and activates AKT phosphorylation and nuclear localization reducing apoptosis and enhancing cardiac repair. Theranostics. 2017;7:3842–55.
Du WW, Fang L, Yang W, Wu N, Awan FM, Yang Z, et al. Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Differ. 2017;24:357–70.
Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, et al. Translation of CircRNAs. Mol Cell. 2017;66:9-21.e7.
Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66:22-37.e9.
Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017;27:626–41.
Franz A, Ralla B, Weickmann S, Jung M, Rochow H, Stephan C, et al. Circular RNAs in clear cell renal cell carcinoma: their microarray-based identification, analytical validation, and potential use in a clinico-genomic model to improve prognostic accuracy. Cancers (Basel). 2019;11:1473.
Ma C, Qin J, Zhang J, Wang X, Wu D, Li X. Construction and analysis of circular RNA molecular regulatory networks in clear cell renal cell carcinoma. Mol Med Rep. 2020;21:141–50.
Li W, Yang F-Q, Sun C-M, Huang J-H, Zhang H-M, Li X, et al. circPRRC2A promotes angiogenesis and metastasis through epithelial–mesenchymal transition and upregulates TRPM3 in renal cell carcinoma. Theranostics. 2020;10:4395–409.
Yan L, Liu G, Cao H, Zhang H, Shao F. Hsa_circ_0035483 sponges hsa-miR-335 to promote the gemcitabine-resistance of human renal cancer cells by autophagy regulation. Biochem Biophys Res Commun United States. 2019;519:172–8.
Chen Q, Liu T, Bao Y, Zhao T, Wang J, Wang H, et al. CircRNA cRAPGEF5 inhibits the growth and metastasis of renal cell carcinoma via the miR-27a-3p/TXNIP pathway. Cancer Lett Ireland. 2020;469:68–77.
Jin C, Shi L, Li Z, Liu W, Zhao B, Qiu Y, et al. Circ_0039569 promotes renal cell carcinoma growth and metastasis by regulating miR-34a-5p/CCL22. Am J Transl Res. 2019;11:4935–45.
Zhou B, Zheng P, Li Z, Li H, Wang X, Shi Z, et al. CircPCNXL2 sponges miR-153 to promote the proliferation and invasion of renal cancer cells through upregulating ZEB2. Cell Cycle. 2018;17:2644–54.
Fang Y, Wei J, Cao J, Zhao H, Liao B, Qiu S, et al. Protein expression of ZEB2 in renal cell carcinoma and its prognostic significance in patient survival. PLoS ONE. 2013;8:e62558.
Xiong Y, Zhang J, Song C. CircRNA ZNF609 functions as a competitive endogenous RNA to regulate FOXP4 expression by sponging miR-138-5p in renal carcinoma. J Cell Physiol United States. 2019;234:10646–54.
Chen T, Yu Q, Shao S, Guo L. Circular RNA circFNDC3B protects renal carcinoma by miR-99a downregulation. J Cell Physiol United States. 2020;235:4399–406.
Dong Z, Liu Y, Wang Q, Wang H, Ji J, Huang T, et al. The circular RNA-NRIP1 plays oncogenic roles by targeting microRNA-505 in the renal carcinoma cell lines. J Cell Biochem United States. 2020;121:2236–46.
Chen Z, Xiao K, Chen S, Huang Z, Ye Y, Chen T. Circular RNA hsa_circ_001895 serves as a sponge of microRNA-296-5p to promote clear cell renal cell carcinoma progression by regulating SOX12. Cancer Sci. 2020;111:713–26.
Lin L, Cai J. Circular RNA circ-EGLN3 promotes renal cell carcinoma proliferation and aggressiveness via miR-1299-mediated IRF7 activation. J Cell Biochem United States. 2020;121:4377–85.
Huang Y, Zhang Y, Jia L, Liu C, Xu F. Circular RNA ABCB10 promotes tumor progression and correlates with pejorative prognosis in clear cell renal cell carcinoma. Int J Biol Markers United States. 2019;34:176–83.
Wang G, Xue W, Jian W, Liu P, Wang Z, Wang C, et al. The effect of Hsa_circ_0001451 in clear cell renal cell carcinoma cells and its relationship with clinicopathological features. J Cancer. 2018;9:3269–77.
Piva F, Giulietti M, Santoni M, Occhipinti G, Scarpelli M, Lopez-Beltran A, et al. Epithelial to mesenchymal transition in renal cell carcinoma: implications for cancer therapy. Mol Diagn Ther New Zealand. 2016;20:111–7.
Salina N, Beata W-S. Beyond thrombosis: the role of platelets in pulmonary hypertension. SciMed J. 2020;2(4):243.
Carew RM, Wang B, Kantharidis P. The role of EMT in renal fibrosis. Cell Tissue Res. 2012;347(1):103–16.
Zhang D, Yang X-J, Luo Q-D, Fu D-L, Li Z-L, Zhang P, et al. Down-regulation of circular RNA_000926 attenuates renal cell carcinoma progression through miRNA-411-dependent CDH2 inhibition. Am J Pathol United States. 2019;189:2469–86.
Chen T, Shao S, Li W, Liu Y, Cao Y. The circular RNA hsa-circ-0072309 plays anti-tumour roles by sponging miR-100 through the deactivation of PI3K/AKT and mTOR pathways in the renal carcinoma cell lines. Artif cells nanomedicine Biotechnol England. 2019;47:3638–48.
Xue D, Wang H, Chen Y, Shen D, Lu J, Wang M, et al. Circ-AKT3 inhibits clear cell renal cell carcinoma metastasis via altering miR-296-3p/E-cadherin signals. Mol Cancer. 2019;18:151.
Han Z, Zhang Y, Sun Y, Chen J, Chang C, Wang X, et al. ERβ-mediated alteration of circATP2B1 and miR-204-3p signaling promotes invasion of clear cell renal cell carcinoma. Cancer Res United States. 2018;78:2550–63.
Wang K, Sun Y, Tao W, Fei X, Chang C. Androgen receptor (AR) promotes clear cell renal cell carcinoma (ccRCC) migration and invasion via altering the circHIAT1/miR-195-5p/29a-3p/29c-3p/CDC42 signals. Cancer Lett Ireland. 2017;394:1–12.
Li K, Wan C-L, Guo Y. Circular RNA circMTO1 suppresses RCC cancer cell progression via miR9/LMX1A axis. Technol Cancer Res Treat. 2020;19:1533033820914286.
Tang X, Ren H, Guo M, Qian J, Yang Y, Gu C. Review on circular RNAs and new insights into their roles in cancer. Comput Struct Biotechnol J. 2021;19:910–28.
Su M, Xiao Y, Ma J, Tang Y, Tian B, Zhang Y, et al. Circular RNAs in cancer: emerging functions in hallmarks, stemness, resistance and roles as potential biomarkers. Mol Cancer. 2019;18:90.
Cheng D, Wang J, Dong Z, Li X. Cancer-related circular RNA: diverse biological functions. Cancer Cell Int. 2021;21:11.
Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao F, et al. Novel role of FBXW7 Circular RNA in repressing Glioma Tumorigenesis. J Natl Cancer Inst. 2018;110:304–15.
Li F, Ma K, Sun M, Shi S. Identification of the tumor-suppressive function of circular RNA ITCH in glioma cells through sponging miR-214 and promoting linear ITCH expression. Am J Transl Res. 2018;10:1373–86.
Liu J, Du F, Chen C, Li D, Chen Y, Xiao X, et al. CircRNA ITCH increases bortezomib sensitivity through regulating the miR-615-3p/PRKCD axis in multiple myeloma. Life Sci Netherlands. 2020;262:118506.
Yang C, Yuan W, Yang X, Li P, Wang J, Han J, et al. Circular RNA circ-ITCH inhibits bladder cancer progression by sponging miR-17/miR-224 and regulating p21, PTEN expression. Mol Cancer. 2018;17:19.
Li J, Guo R, Liu Q, Sun J, Wang H. Circular RNA Circ-ITCH inhibits the malignant behaviors of cervical cancer by microRNA-93-5p/FOXK2 Axis. Reprod Sci United States. 2020;27:860–8.
Wang ST, Liu LB, Li XM, Wang YF, Xie PJ, Li Q, et al. Circ-ITCH regulates triple-negative breast cancer progression through the Wnt/β-catenin pathway. Neoplasma Slovakia. 2019;66:232–9.
Ren C, Liu J, Zheng B, Yan P, Sun Y, Yue B. The circular RNA circ-ITCH acts as a tumour suppressor in osteosarcoma via regulating miR-22. Artif Cells Nanomed Biotechnol England. 2019;47:3359–67.
Luo L, Gao Y, Sun X. Circ-ITCH correlates with small tumor size, decreased FIGO stage and prolonged overall survival, and it inhibits cells proliferation while promotes cells apoptosis in epithelial ovarian cancer. Cancer Biomark Netherlands. 2018;23:505–13.
Zhao L, Ma N, Liu G, Mao N, Chen F, Li J. Lidocaine inhibits hepatocellular carcinoma development by modulating circ_ITCH/miR-421/CPEB3 axis. Dig Dis Sci United States. 2021.
Barbagallo D, Caponnetto A, Cirnigliaro M, Brex D, Barbagallo C, D’Angeli F, et al. CircSMARCA5 inhibits migration of glioblastoma multiforme cells by regulating a molecular axis involving splicing factors SRSF1/SRSF3/PTB. Int J Mol Sci. 2018;19:480.
Yu J, Xu Q-G, Wang Z-G, Yang Y, Zhang L, Ma J-Z, et al. Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma. J Hepatol Netherlands. 2018;68:1214–27.
Wang Y, Li H, Lu H, Qin Y. Circular RNA SMARCA5 inhibits the proliferation, migration, and invasion of non-small cell lung cancer by miR-19b-3p/HOXA9 axis. Onco Targets Ther. 2019;12:7055–65.
Zhang M, Huang N, Yang X, Luo J, Yan S, Xiao F, et al. A novel protein encoded by the circular form of the SHPRH gene suppresses glioma tumorigenesis. Oncogene England. 2018;37:1805–14.
Yao Z, Luo J, Hu K, Lin J, Huang H, Wang Q, et al. ZKSCAN1 gene and its related circular RNA (circZKSCAN1) both inhibit hepatocellular carcinoma cell growth, migration, and invasion but through different signaling pathways. Mol Oncol. 2017;11:422–37.
Lu Q, Liu T, Feng H, Yang R, Zhao X, Chen W, et al. Circular RNA circSLC8A1 acts as a sponge of miR-130b/miR-494 in suppressing bladder cancer progression via regulating PTEN. Mol Cancer. 2019;18:111.
Liu T, Liu S, Xu Y, Shu R, Wang F, Chen C, et al. Circular RNA-ZFR inhibited cell proliferation and promoted apoptosis in gastric cancer by sponging miR-130a/miR-107 and modulating PTEN. Cancer Res Treat. 2018;50:1396–417.
Wang L, Tong X, Zhou Z, Wang S, Lei Z, Zhang T, et al. Circular RNA hsa_circ_0008305 (circPTK2) inhibits TGF-β-induced epithelial–mesenchymal transition and metastasis by controlling TIF1γ in non-small cell lung cancer. Mol Cancer. 2018;17:140.
Zhang X, Luo P, Jing W, Zhou H, Liang C, Tu J. circSMAD2 inhibits the epithelial–mesenchymal transition by targeting miR-629 in hepatocellular carcinoma. Onco Targets Ther. 2018;11:2853–63.
Wu W, Wu Z, Xia Y, Qin S, Li Y, Wu J, et al. Downregulation of circ_0132266 in chronic lymphocytic leukemia promoted cell viability through miR-337-3p/PML axis. Aging (Albany NY). 2019;11:3561–73.
Feng Y, Zhang L, Wu J, Khadka B, Fang Z, Gu J, et al. CircRNA circ_0000190 inhibits the progression of multiple myeloma through modulating miR-767-5p/MAPK4 pathway. J Exp Clin Cancer Res. 2019;38:54.
Zhang X, Yang D, Wei Y. Overexpressed CDR1as functions as an oncogene to promote the tumor progression via miR-7 in non-small-cell lung cancer. Onco Targets Ther. 2018;11:3979–87.
Tang W, Ji M, He G, Yang L, Niu Z, Jian M, et al. Silencing CDR1as inhibits colorectal cancer progression through regulating microRNA-7. Onco Targets Ther. 2017;10:2045–56.
Su Y, Lv X, Yin W, Zhou L, Hu Y, Zhou A, et al. CircRNA Cdr1as functions as a competitive endogenous RNA to promote hepatocellular carcinoma progression. Aging (Albany NY). 2019;11:8183–203.
Zeng K, Chen X, Xu M, Liu X, Hu X, Xu T, et al. CircHIPK3 promotes colorectal cancer growth and metastasis by sponging miR-7. Cell Death Dis. 2018;9:417.
Kai D, Yannian L, Yitian C, Dinghao G, Xin Z, Wu J. Circular RNA HIPK3 promotes gallbladder cancer cell growth by sponging microRNA-124. Biochem Biophys Res Commun United States. 2018;503:863–9.
Feng XQ, Nie SM, Huang JX, Li TL, Zhou JJ, Wang W, et al. Circular RNA circHIPK3 serves as a prognostic marker to promote chronic myeloid leukemia progression. Neoplasma Slovakia. 2020;67:171–7.
Ding C, Wu Z, You H, Ge H, Zheng S, Lin Y, et al. CircNFIX promotes progression of glioma through regulating miR-378e/RPN2 axis. J Exp Clin Cancer Res. 2019;38:506.
Lu J, Zhu Y, Qin Y, Chen Y. CircNFIX acts as a miR-212-3p sponge to enhance the malignant progression of non-small cell lung cancer by up-regulating ADAM10. Cancer Manag Res. 2020;12:9577–87.
Cheng J, Nie D, Li B, Gui S, Li C, Zhang Y, et al. CircNFIX promotes progression of pituitary adenoma via CCNB1 by sponging miR-34a-5p. Mol Cell Endocrinol Ireland. 2021;525:111140.
Wang R, Zhang S, Chen X, Li N, Li J, Jia R, et al. CircNT5E acts as a sponge of miR-422a to promote glioblastoma tumorigenesis. Cancer Res United States. 2018;78:4812–25.
Dong L, Zheng J, Gao Y, Zhou X, Song W, Huang J. The circular RNA NT5E promotes non-small cell lung cancer cell growth via sponging microRNA-134. Aging (Albany NY). 2020;12:3936–49.
Yang J, Liu X, Dai G, Qu L, Tan B, Zhu B, et al. CircNT5E promotes the proliferation and migration of bladder cancer via sponging miR-502-5p. J Cancer. 2021;12:2430–9.
Liu Y, Li R, Wang X, Yang W. CircTTBK2 contributes to the progression of glioma through regulating miR-145-5p/CPEB4 axis. Cancer Manag Res. 2020;12:8183–95.
Li G, Yang H, Han K, Zhu D, Lun P, Zhao Y. A novel circular RNA, hsa_circ_0046701, promotes carcinogenesis by increasing the expression of miR-142-3p target ITGB8 in glioma. Biochem Biophys Res Commun United States. 2018;498:254–61.
Liu Y-D, Liu L-P. Circ100284 promotes invasion and migration of osteosarcoma cells by down-regulating PTEN and EMP1. Eur Rev Med Pharmacol Sci Italy. 2020;24:6540–50.
Du WW, Yang W, Li X, Awan FM, Yang Z, Fang L, et al. A circular RNA circ-DNMT1 enhances breast cancer progression by activating autophagy. Oncogene England. 2018;37:5829–42.
Zhu M, Xu Y, Chen Y, Yan F. Circular BANP, an upregulated circular RNA that modulates cell proliferation in colorectal cancer. Biomed Pharmacother France. 2017;88:138–44.
Han J, Zhao G, Ma X, Dong Q, Zhang H, Wang Y, et al. CircRNA circ-BANP-mediated miR-503/LARP1 signaling contributes to lung cancer progression. Biochem Biophys Res Commun United States. 2018;503:2429–35.
Shen F, Liu P, Xu Z, Li N, Yi Z, Tie X, et al. CircRNA_001569 promotes cell proliferation through absorbing miR-145 in gastric cancer. J Biochem England. 2019;165:27–36.
Xie H, Ren X, Xin S, Lan X, Lu G, Lin Y, et al. Emerging roles of circRNA_001569 targeting miR-145 in the proliferation and invasion of colorectal cancer. Oncotarget. 2016;7:26680–91.
Xu J-H, Wang Y, Xu D. Hsa_circ_001569 is an unfavorable prognostic factor and promotes cell proliferation and metastasis by modulating PI3K-AKT pathway in breast cancer. Cancer Biomark Netherlands. 2019;25:193–201.
Shen X, Chen Y, Li J, Huang H, Liu C, Zhou N. Identification of Circ_001569 as a potential biomarker in the diagnosis and prognosis of pancreatic cancer. Technol Cancer Res Treat. 2021;20:1533033820983302.
Zhang H, Yan J, Lang X, Zhuang Y. Expression of circ_001569 is upregulated in osteosarcoma and promotes cell proliferation and cisplatin resistance by activating the Wnt/β-catenin signaling pathway. Oncol Lett. 2018;16:5856–62.
Liu H, Xue L, Song C, Liu F, Jiang T, Yang X. Overexpression of circular RNA circ_001569 indicates poor prognosis in hepatocellular carcinoma and promotes cell growth and metastasis by sponging miR-411-5p and miR-432-5p. Biochem Biophys Res Commun United States. 2018;503:2659–65.
Ding L, Yao W, Lu J, Gong J, Zhang X. Upregulation of circ_001569 predicts poor prognosis and promotes cell proliferation in non-small cell lung cancer by regulating the Wnt/β-catenin pathway. Oncol Lett. 2018;16:453–8.
Shang J, Chen W-M, Liu S, Wang Z-H, Wei T-N, Chen Z-Z, et al. CircPAN3 contributes to drug resistance in acute myeloid leukemia through regulation of autophagy. Leuk Res England. 2019;85:106198.
Wang Y, Lin Q, Song C, Ma R, Li X. Circ_0007841 promotes the progression of multiple myeloma through targeting miR-338-3p/BRD4 signaling cascade. Cancer Cell Int. 2020;20:383.
Huang K, Liu D, Su C. Circ_0007841 accelerates ovarian cancer development through facilitating MEX3C expression by restraining miR-151-3p activity. Aging (Albany NY). 2021;13:12058–66.
Zhang P-F, Pei X, Li K-S, Jin L-N, Wang F, Wu J, et al. Circular RNA circFGFR1 promotes progression and anti-PD-1 resistance by sponging miR-381-3p in non-small cell lung cancer cells. Mol Cancer. 2019;18:179.
Cheng Z, Yu C, Cui S, Wang H, Jin H, Wang C, et al. circTP63 functions as a ceRNA to promote lung squamous cell carcinoma progression by upregulating FOXM1. Nat Commun. 2019;10:3200.
Deng Y, Xia J, Xu Y-E. Circular RNA circTP63 enhances estrogen receptor-positive breast cancer progression and malignant behaviors through the miR-873-3p/FOXM1 axis. Anticancer Drugs England. 2021;32:44–52.
Wang J, Che J. CircTP63 promotes hepatocellular carcinoma progression by sponging miR-155-5p and upregulating ZBTB18. Cancer Cell Int. 2021;21:156.
Zhang X, Wang S, Wang H, Cao J, Huang X, Chen Z, et al. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol Cancer. 2019;18:20.
Li X, Ma N, Zhang Y, Wei H, Zhang H, Pang X, et al. Circular RNA circNRIP1 promotes migration and invasion in cervical cancer by sponging miR-629-3p and regulating the PTP4A1/ERK1/2 pathway. Cell Death Dis. 2020;11:399.
Li M, Cai J, Han X, Ren Y. Downregulation of circNRIP1 suppresses the paclitaxel resistance of ovarian cancer via regulating the miR-211-5p/HOXC8 axis. Cancer Manag Res. 2020;12:9159–71.
Lin J, Qin H, Han Y, Li X, Zhao Y, Zhai G. CircNRIP1 modulates the miR-515-5p/IL-25 axis to control 5-Fu and cisplatin resistance in nasopharyngeal carcinoma. Drug Des Devel Ther. 2021;15:323–30.
Meng Y, Hao D, Huang Y, Jia S, Zhang J, He X et al. Circular RNA circNRIP1 plays oncogenic roles in the progression of osteosarcoma. Mamm Genome. United States; 2021.
Ma Z, Shuai Y, Gao X, Wen X, Ji J. Circular RNAs in the tumour microenvironment. Mol Cancer. 2020;19:8.
Vea A, Llorente-Cortes V, de Gonzalo-Calvo D. Circular RNAs in blood. Adv Exp Med Biol United States. 2018;1087:119–30.
Kölling M, Haddad G, Wegmann U, Kistler A, Bosakova A, Seeger H, et al. Circular RNAs in urine of kidney transplant patients with acute T cell-mediated allograft rejection. Clin Chem England. 2019;65:1287–94.
Jafari GF. Circular RNA in saliva. Adv Exp Med Biol United States. 2018;1087:131–9.
Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25:981–4.
Acknowledgements
We appreciated the help from Professor Robin Reed and other lab members in Reed Lab, Cell Biology Department of Harvard Medical School. This work was supported by the Promising Talents Plan Program Funding of Shengjing Hospital, China Medical University. We apologize to those whose study we could not cite due to the limitations of our topic and space.
Funding
Promising Talents Plan Program Funding of Shengjing Hospital, China Medical University. Shuzhen Chen Shengjing Surgical Development Funding.
Author information
Authors and Affiliations
Contributions
ZL: project development, data collection, and manuscript writing and editing. ML: project development, data collection, and manuscript writing and editing. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors approved for publication.
Competing interests
The authors have no conflicts of interest to declare.
Availability of data and materials
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Z., Li, M. Circular RNAs and their role in renal cell carcinoma: a current perspective. Cancer Cell Int 21, 469 (2021). https://doi.org/10.1186/s12935-021-02181-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-021-02181-7