Abstract
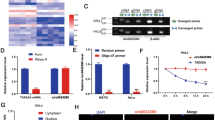
Growing evidence has been demonstrated that circular RNA circ-ITCH plays an important role in the development of several cancers. However, the role of circ-ITCH in cervical cancer has not been evaluated. The aim of the present study was to investigate the biological function of circ-ITCH in cervical cancer both in vitro and in vivo. Our results showed that circ-ITCH was lowly expressed in both human cervical cancer tissues and cell lines. Overexpression of circ-ITCH in HeLa cells significantly suppressed cell proliferation, migration, and invasion. A xenograft tumor model was established to evaluate the role of circ-ITCH in vivo. The results showed that overexpression of circ-ITCH significantly inhibited tumorigenesis of cervical cancer. Mechanism investigations proved that circ-ITCH executed its tumor suppressive activity through sponging microRNA-93-5p (miR-93-5p) and regulating the expression of forkhead box K2 (FOXK2). These findings suggest that circ-ITCH may be a therapeutic target for the management of cervical cancer.





Similar content being viewed by others
References
Fang J, Zhang H, Jin S. Epigenetics and cervical cancer: from pathogenesis to therapy. Tumour Biol. 2014;35:5083–93.
Kumar L, Harish P, Malik PS, Khurana S. Chemotherapy and targeted therapy in the management of cervical cancer. Curr Probl Cancer. 2018;42:120–8.
Seol HJ, Ulak R, Ki KD, Lee JM. Cytotoxic and targeted systemic therapy in advanced and recurrent cervical cancer: experience from clinical trials. Tohoku J Exp Med. 2014;232:269–76.
Szalmas A, Konya J. Epigenetic alterations in cervical carcinogenesis. Semin Cancer Biol. 2009;19:144–52.
Chen I, Chen CY, Chuang TJ. Biogenesis, identification, and function of exonic circular RNAs. Wiley Interdiscip Rev RNA. 2015;6:563–79.
Ebbesen KK, Kjems J, Hansen TB. Circular RNAs: identification, biogenesis and function. Biochim Biophys Acta. 1859;2016:163–8.
Hsiao KY, Sun HS, Tsai SJ. Circular RNA - new member of noncoding RNA with novel functions. Exp Biol Med. 2017;242:1136–41.
Haque S, Harries LW. Circular RNAs (circRNAs) in health and disease. Genes. 2017;8:353–369.
Kristensen LS, Hansen TB, Veno MT, Kjems J. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018;37:555–65.
Zhang Y, Liang W, Zhang P, et al. Circular RNAs: emerging cancer biomarkers and targets. J Exp Clin Cancer Res. 2017;36:152.
Yang C, Yuan W, Yang X, et al. Circular RNA circ-ITCH inhibits bladder cancer progression by sponging miR-17/miR-224 and regulating p21, PTEN expression. Mol Cancer. 2018;17:19.
Guo W, Zhang J, Zhang D, et al. Polymorphisms and expression pattern of circular RNA circ-ITCH contributes to the carcinogenesis of hepatocellular carcinoma. Oncotarget. 2017;8:48169–77.
Luo L, Gao Y, Sun X. Circ-ITCH correlates with small tumor size, decreased FIGO stage and prolonged overall survival, and it inhibits cells proliferation while promotes cells apoptosis in epithelial ovarian cancer. Cancer Biomark. 2018;23:505–13.
Wang ST, Liu LB, Li XM, Wang YF, Xie PJ, Li Q, et al. Circ-ITCH regulates triple-negative breast cancer progression through the Wnt/beta-catenin pathway. Neoplasma. 2019;66:232–9.
Wang M, Chen B, Ru Z, Cong L. CircRNA circ-ITCH suppresses papillary thyroid cancer progression through miR-22-3p/CBL/beta-catenin pathway. Biochem Biophys Res Commun. 2018;504:283–8.
Xu XY, Zhou LL, Yu C, Shen B, Feng JF, Yu SR. Advances of circular RNAs in carcinoma. Biomed Pharmacother. 2018;107:59–71.
Rong D, Sun H, Li Z, Liu S, Dong C, Fu K, et al. An emerging function of circRNA-miRNAs-mRNA axis in human diseases. Oncotarget. 2017;8:73271–81.
Hu B, Mao Z, Du Q, et al. miR-93-5p targets Smad7 to regulate the transforming growth factor-beta1/Smad3 pathway and mediate fibrosis in drug-resistant prolactinoma. Brain Res Bull. 2019;149:21–31.
Choi JY, Shin HJ, Bae IH. miR-93-5p suppresses cellular senescence by directly targeting Bcl-w and p21. Biochem Biophys Res Commun. 2018;505:1134–40.
Xiang Y, Liao XH, Yu CX, et al. MiR-93-5p inhibits the EMT of breast cancer cells via targeting MKL-1 and STAT3. Exp Cell Res. 2017;357:135–44.
Li J, Chu ZP, Han H, et al. Suppression of miR-93-5p inhibits high-risk HPV-positive cervical cancer progression via targeting of BTG3. Hum Cell. 2019;32:160–71.
Wang L, Liu S, Mao Y, et al. CircRNF13 regulates the invasion and metastasis in lung adenocarcinoma by targeting miR-93-5p. Gene. 2018; 10;671:170–177.
Hua Q, Chen Y, Liu Y, et al. Circular RNA 0039411 is involved in neodymium oxide-induced inflammation and anti-proliferation in a human bronchial epithelial cell line via sponging miR-93-5p. Toxicol Sci. 2019;170:69–81.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33.
Liu MX, Liao J, Xie M, et al. miR-93-5p transferred by exosomes promotes the proliferation of esophageal cancer cells via intercellular communication by targeting PTEN. Biomed Eniviron Sci. 2018;31:171–85.
Shyamasundar S, Lim JP, Bay BH. MiR-93 inhibits the invasive potential of triple-negative breast cancer cells in vitro via protein kinase WNK1. Int J Oncol. 2016;49:2629–36.
Ma DH, Li BS, Liu JJ, Xiao YF, Yong X, Wang SM, et al. miR-93-5p/IFNAR1 axis promotes gastric cancer metastasis through activating the STAT3 signaling pathway. Cancer Lett. 2017;408:23–32.
Nestal de Moraes G, Carneiro LDT, Maia RC, Lam EW, Sharrocks AD. FOXK2 transcription factor and its emerging roles in cancer. Cancers (Basel). 2019;11: E393–412.
Shan L, Zhou X, Liu X, Wang Y, Su D, Hou Y, et al. FOXK2 elicits massive transcription repression and suppresses the hypoxic response and breast cancer carcinogenesis. Cancer Cell. 2016;30:708–22.
Liu X, Wei X, Niu W, Wang D, Wang B, Zhuang H. Downregulation of FOXK2 is associated with poor prognosis in patients with gastric cancer. Mol Med Rep. 2018;18:4356–64.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consents were obtained from all patients before the study. The study was approved by the Ethics Committee of the Third Hospital of Jinan.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig. 1
The expression of FOXK2 in HeLa cells. (A) qRT-PCR analysis of FXOK2 expression levels after transfection with si-FOXK2 in miR-93-5p inhibitor transfected HeLa cells. (B) Western blot analysis of FOXK2 protein expression levels after transfection with si-FOXK2 in miR-93-5p inhibitor transfected HeLa cells. *p < 0.05 vs anti-NC group; # p < 0.05 vs miR-93-5p inhibitor group (PNG 246 kb).
Supplementary Fig. 2
The expression of miR-93-5p and FOXK2 in isolated tumor tissues. After four weeks later, the mice were sacrificed and tumor tissues were separated. (A) qRT-PCR analysis of miR-93-5p expression levels in the isolated tumor tissues. (B and C) qRT-PCR and Western blot analysis of FOXK2 mRNA and protein expression levels in the isolated tumor tissues. *p < 0.05 (PNG 196 kb).
Rights and permissions
About this article
Cite this article
Li, J., Guo, R., Liu, Q. et al. Circular RNA Circ-ITCH Inhibits the Malignant Behaviors of Cervical Cancer by microRNA-93-5p/FOXK2 Axis. Reprod. Sci. 27, 860–868 (2020). https://doi.org/10.1007/s43032-020-00140-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00140-7