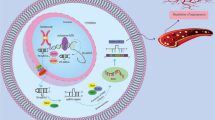
Abstract
MicroRNAs (miRNAs) are a class of small noncoding RNA molecules containing only 20–22 nucleotides. MiRNAs play a role in gene silencing and translation suppression by targeting and binding to mRNA. Proper control of miRNA expression is very important for maintaining a normal physiological environment because miRNAs can affect most cellular pathways, including cell cycle checkpoint, cell proliferation, and apoptosis pathways, and have a wide range of target genes. With these properties, miRNAs can modulate multiple signalling pathways involved in cancer development, such as cell proliferation, apoptosis, and migration pathways. MiRNAs that activate or inhibit the molecular pathway related to tumour angiogenesis are common topics of research. Angiogenesis promotes tumorigenesis and metastasis by providing oxygen and diffusible nutrients and releasing proangiogenic factors and is one of the hallmarks of tumour progression. CRC is one of the most common tumours, and metastasis has always been a difficult issue in its treatment. Although comprehensive treatments, such as surgery, radiotherapy, chemotherapy, and targeted therapy, have prolonged the survival of CRC patients, the overall response is not optimistic. Therefore, there is an urgent need to find new therapeutic targets to improve CRC treatment. In a series of recent reports, miRNAs have been shown to bidirectionally regulate angiogenesis in colorectal cancer. Many miRNAs can directly act on VEGF or inhibit angiogenesis through other pathways (HIF-1a, PI3K/AKT, etc.), while some miRNAs, specifically many exosomal miRNAs, are capable of promoting CRC angiogenesis. Understanding the mechanism of action of miRNAs in angiogenesis is of great significance for finding new targets for the treatment of tumour angiogenesis. Deciphering the exact role of specific miRNAs in angiogenesis is a challenge due to the high complexity of their actions. Here, we describe the latest advances in the understanding of miRNAs and their corresponding targets that play a role in CRC angiogenesis and discuss possible miRNA-based therapeutic strategies.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most common cancer in the world, and is also the second most common cause of cancer-related deaths. Furthermore, in 2018, there were more than 1.8 million new cases of and 881,000 deaths due to CRC [1]. Although most primary colorectal tumours can be surgically removed, the 5-year survival rate of patients with advanced CRC remains low. Metastasis is the leading cause of cancer-related deaths in CRC patients, and it is estimated that more than 50% of patients die of metastasis [2]. Since the concept of angiogenesis was proposed by Maniotis et al. in 1999 [3], a growing number of studies have demonstrated the critical role of abnormal angiogenesis in the invasion and metastasis of CRC. For example, elevated levels of vascular endothelial growth factor-A (VEGF-A) are closely correlated with adverse clinical outcomes in CRC patients [4, 5]. Hence, VEGF-A has been considered a prognostic marker for CRC. In summary, angiogenesis is an important factor that contributes to metastasis in the majority of cancers, including CRC. Undoubtedly, understanding the mechanisms of angiogenesis is necessary to reduce the risk of recurrence and metastasis of CRC.
Angiogenesis mechanism in CRC
The formation and progression of CRC are inseparable from angiogenesis, and angiogenesis plays an important role in CRC proliferation and metastasis [6]. In general, when a tumour is more than 2 mm in diameter, its growth can no longer be maintained by tissue penetration, resulting in a hypoxic microenvironment of the tumour [7]; therefore, the tumour requires the formation of new blood vessels to provide oxygen and nutrients [8]. After vascular overgrowth, tumour growth and metastasis are promoted by the release of proangiogenic factors, the intensity of which depends on the level of the activation pathway and proangiogenic signals [9]. This process is regulated by a variety of angiogenic factors, such as vascular endothelial growth factor (VEGF), thrombospondin-1 (TSP-1), platelet-derived growth factor (PDGF), transforming growth factor (TGF), endothelial growth factor (EGF), and fibroblast growth factor (FGF) [10,11,12,13,14,15]. With the continuous research on antiangiogenic therapy that has occurred over the years, targeting angiogenesis has become an important therapeutic strategy for a variety of tumours, including CRC.
As a member of the VEGF family, VEGF-A has been widely recognized as a major participant in tumour angiogenesis [16]. Multiple pathways in a variety of cancers, including CRC, induce VEGF-A expression and promote tumour angiogenesis [17, 18]. Vascular endothelial growth factor receptor 2 (VEGFR2), as a receptor for VEGF, has been shown to be a target for blocking tumour angiogenesis in a number of studies [19]. Bevacizumab is an antagonist of VEGF that significantly inhibits tumour angiogenesis and tumour progression and has been recognized as a first-line treatment for liver metastases in CRC [20, 21].
However, similar traditional antiangiogenic therapies sometimes cause hypoxia and metastasis during treatment, which in turn accelerate tumour growth [22, 23]. The miR-125 family consists of miR-125a, miR-125b-1 and miR-125b-2 [24] and has been shown to be involved in a variety of cancer processes. Members of the miR-125 family appear to have opposite effects in different cancers. For example, miR-125b has been shown to have tumour-suppressing properties in various cancers, including liver cancer [25], oral squamous cell carcinoma (OSCC) [26], and breast cancer [27]. In contrast, miR-125b also is an oncogene in several cancers, including pancreatic cancer [28] and glioblastoma [29]. The different properties of miR-125 family members expressed in various cancers indicate that these miRNAs have highly diverse regulatory functions in cancer progression and that their underlying mechanisms in different cancer environments may differ. Therefore, the molecular mechanism of angiogenesis in CRC must be clarified in a more precise manner. CRC treatment features antiangiogenic agents. Recent studies have found that microRNAs (miRNAs) also play an important regulatory role in the molecular mechanism of tumour angiogenesis, and the identification of key miRNAs has served an important role in the development of more precise CRC targeted therapy strategies.
Introduction to miRNAs
MiRNAs are small noncoding RNAs of ~ 20–24 nucleotides in length that translationally inhibit or degrade targets by recognizing the 3′ untranslated region (3′-UTR) of mRNAs. As gene expression regulators, miRNAs control ~ 30% of human genes [30,31,32,33].
The first miRNA was discovered in nematodes in 1993, and since then, the development of molecular biology techniques has allowed the function of miRNAs to be increasingly and comprehensively studied; as such, the importance of miRNAs has been widely recognized. In recent years, some studies have indicated that miRNAs play a key role in the tumour growth, metastasis and immune response in CRC and that miRNAs can act as oncogenes or tumour suppressors. MiR-196a-5p is a proto-oncogene that regulates CRC epithelial–mesenchymal transition (EMT) by binding to IκBα and promoting CRC proliferation, metastasis and invasion [34]. MiR-500a-5p was confirmed to be a tumour suppressor that was significantly downregulated in CRC, inhibited CRC tumour growth and metastasis by regulating HDAC2 and was negatively regulated by YY1 [35]. Based on recent advances in miRNA-based therapies [36, 37], miRNAs have become an effective treatment and prognostic indicator for CRC. That is, serum miRNAs have been shown to be predictors of CRC tumour recurrence and treatment outcomes [38].
The biogenesis and function of miRNAs are constantly being researched, the understanding of these factors is constantly being improved, and the effect of miRNA regulation on tumour angiogenesis is becoming increasingly clear. MiRNAs regulate angiogenesis during normal physiological processes, such as wound healing, and the aberrant expression of miRNAs can promote and inhibit angiogenesis during tumour progression [9, 39, 40]. Therefore, identifying key miRNAs involved in vascular angiogenesis will play an important role in the development of better therapeutic strategies. In this review, we summarized the exact targets of miRNA that act on CRC angiogenesis in recent years, and explored possible methods for targeted treatment of CRC angiogenesis.
MiRNAs that inhibit angiogenesis (Fig. 1)
The functions of miRNAs are diverse and affect tumorigenesis, invasion, and metastasis. A series of recent studies have reported the mechanisms by which many miRNAs inhibit angiogenesis in CRC. These reports provide new targets for treatment strategies targeting angiogenesis in CRC (Table 1). VEGFA has been shown to be a good therapeutic target in antiangiogenic strategies [41]. However, the long-term use of VEGF-related drugs often has various side effects. For example, the long-term use of the VEGFA monoclonal antibody bevacizumab induces osteonecrosis [42]. Hence, it is important to search for better targets for angiogenesis therapy and eliminate side effects.
MiRNAs targeting VEGF inhibit CRC angiogenesis
Among miRNAs, miR-622 is an miRNA involved in various cancers, such as ovarian cancer, liver cancer, and gastric cancer [43,44,45], and it has been recently reported to regulate the angiogenesis of CRC by inhibiting CXCR4-VEGFA [46]. VEGF-A is an important receptor for VEGF, is involved in angiogenesis, and triggers the germination of prevascular endothelial cells to induce new vasculature formation. Similarly, miR-590-5p also inhibits the angiogenesis of CRC by affecting VEGF-A [47]. It has been reported that miR-590-5p acts as an oncogene in cervical cancer and a tumour suppressor in renal cancer [48, 49]; miR-590-5p is downregulated in normal tissues compared with CRC tissues, particularly compared with nonmetastatic CRC tissues. MiR-590-5p has been shown to inhibit tumour angiogenesis mainly by inhibiting NF-90/VEGF-A, thereby reducing the enhanced migration ability of CRC cells [47]. MiR-520 was first discovered to act as a tumour suppressor in breast cancer, in which it targets the NF-κB and TBF-β pathways [50], and its family members have also been found to be downregulated in CRC. Furthermore, miR-520a can inhibit the proliferation of oesophageal squamous cell carcinoma by downregulating cysteine-rich C-terminal 1 (CRCT1) [51], and miR-520a-3p can accelerate apoptosis and inhibit cell migration by targeting epidermal growth factor receptor (EGFR) [52]. In addition, miR-520a acts as a direct target of VEGFA, while ATAD2 can inhibit VEGFA secretion by increasing the expression of miR-520a, thereby reducing angiogenesis in CRC [53]. MiR-126 is dysregulated in a variety of cancers, is highly expressed in endothelial cells, and is an angiogenesis inhibitor [54]. Furthermore, miR-126 is usually epigenetically silenced in CRC, and the recovery of miR-126 directly inhibits VEGF expression and reduces angiogenesis, invasion and migration in CRC [55]. MiR-27b inhibits the angiogenesis of CRC by targeting VEGF-C and downregulating DNA hypermethylation, thereby inhibiting the growth of CRC tumours [56]. MiR-150-5p acts as a tumour suppressor in CRC, in which it inactivates Akt/mTOR signalling through direct inhibition of VEGF-A [57]. MiR-125 inhibits VEGF expression by targeting the 3′ untranslated region of VEGF mRNA, thereby promoting apoptosis in RKO CRC cells [58]. The central role of VEGF in the pathogenesis of angiogenesis has also become evident. Instructions of the molecular mechanisms of VEGF and the transformative development of multiple therapeutic pathways targeting VEGF directly or indirectly is a powerful case study of how fundamental research can guide clinical. There are many ways that miRNA targets VEGF to inhibit angiogenesis, which also provides a new theoretical basis for us to search for new targeted drugs.
MiRNA inhibits CRC angiogenesis via PIK3/AKT
The fucosyltransferase (FUT) family is involved in a variety of cancers, including CRC. In recent reports, miR-125a-3p was shown to be negatively correlated with the expression of FUT5 and FUT6. FUT5 and FUT6 can be used as direct targets of miR-125a-3p, and miR-125a-3p/FUT5-FUT6 attenuates angiogenesis in CRC cells and inhibits tumour growth by affecting the PI3K/AKT signalling pathway [59]. PI3K/AKT signalling plays an important role in the development of tumours, which may cause tumour growth and angiogenesis, and these functions are abnormally activated in a variety of cancers [60, 61]. Several strong inhibitors of tumour angiogenesis targeting the PI3K/AKT pathway have been developed [62]. However, the PI3K/AKT pathway is involved in the biological functions of various normal cells, and thus, the cellular process that depend on the PI3K/AKT pathway can be affected by such treatments. Hence, there is a need to reduce the side effects of related drugs. MiR-143 is a tumour suppressor that is downregulated in CRC and inhibits tumour angiogenesis via the PI3K/AKT/HIF-1α/VEGF pathway [63]. MiR-143 inactivates AKT and inhibits HIF-1α, and VEGF inhibits tumour angiogenesis. Similarly, insulin-like growth factor-I receptor (IGF-IR) had been identified as a direct target of miR-143. MiR-143 can reduce the resistance to oxaliplatin by binding to IGF-IR. Treatment of CRC with different agents has provided new information [63]. MiRNA inhibits the angiogenesis of CRC through the PI3K/AKT pathway, and in recent reports, miR-182 and miR-135b were also shown to promote CRC invasion and angiogenesis via the PI3K/AKT pathway [64]. The expression of miR-182 and miR-135b in tumour tissues and cells is higher than that in normal tissues, and the angiogenesis of CRC is promoted by direct targeting of ST6GALNAC2 to activate the PI3K/AKT pathway. Both ST8SIA1 and ST6GALNAC2 belong to the sialyltransferase (ST) family of enzymes, which promote tumour growth and metastasis [65]. In CRC, miR-33a inhibits tumour angiogenesis and metastasis by regulating ST8SIA1, and the overexpression of miR-33a can inhibit CRC cell resistance [66]. The PI3K/AKT pathway modulates the expression of many angiogenic factors such as VEGF nitric oxide and angiopoietins. Numerous inhibitors targeting the PI3K/AKT pathway have been developed, and these drugs reduce VEGF secretion and angiogenesis. However, their effect on the tumor's vascular system can be difficult to predict. The discovery of miRNA targeting PI3K/ Akt may provide new ideas for this purpose. Activation of the PI3K/ Akt pathway in tumor cells also increases VEGF secretion through hypoxia-inducible factor 1α (HIF-1α) dependent and independent mechanisms.
MiRNAs that inhibit angiogenesis via HIF-1α
HIF-1α is one of the central molecules that mediate the development of cancer and is a key regulator of VEGF. HIF-1α is involved in tumour angiogenesis in a variety of pathways [17, 67, 68] and is one of the most promising targets for tumour angiogenesis. Various miRNAs are also involved in the regulation of HIF-1α [69]. HIF-1α can regulate miRNAs, and miRNAs can also act on HIF-1α. This bidirectional regulation plays a very important role in tumour progression. Therefore, the mechanism by which miRNAs affect HIF-1α is modulated and is necessary. MiR-145 is downregulated in the early stage of intestinal cancer and is a tumour suppressor [70]. In CRC, miR-145 can inhibit HIF-1α and VEGF by targeting p70S6K1, thereby reducing the angiogenic ability of CRC [71]. MiR-206 is a tumour suppressor, and the downregulation of miR-206 promotes angiogenesis in breast cancer [72]. In CRC, miR-206 acts on the Met/ERK/Elk-1/HIF-1α/VEGF-A pathway to inhibit tumour angiogenesis, and CCL19 can drive this process by promoting miR-206 expression [73]. MiR-148a downregulates VEGF via the pERK/HIF-1α pathway, inhibits tumour angiogenesis and reduces the risk of early recurrence in CRC patients [74]. MiR-195-5p is a multifunctional miRNA that acts as a tumour suppressor in CRC and has not only multiple targets that inhibit CRC cell migration by regulating EMT but also multiple targets that affect blood vessels, such as HIF-1α and VEGF. To downregulate production factors, miR-195-5p has an inhibitory effect on invasion and angiogenic mediators in invasive CRC cells [75].
MiR-107 is a tumour suppressor expressed in human colon cancer specimens and is regulated by P53, which reduces hypoxia signalling by inhibiting HIF-1β expression and reduces tumour angiogenic capacity [76]. P53 is a tumour suppressor. However, due to its susceptibility to mutation, P53 mutation or loss is considered to be a critical step in tumour progression and often suggests poor prognosis of tumours [77]. In a recent report, P53-induced miR-1249 expression was decreased in CRC tissues and cell lines and inhibited CRC metastasis and angiogenesis by affecting VEGFA and HMGA2 both in vivo and in vitro [78]. HMGA2 is a member of the HMGB family, is expressed in a variety of cancers, and is associated with immunopositivity and tumour aggressiveness. Hence, HMGA2 is used as a tumour marker [79]. HMGA1 is another member of the HMGA family and plays a key role in CRC, and HMGA1 overexpression is associated with a lower overall survival rate in patients with CRC [80]. MiR-216b inhibits the proliferation, invasion and angiogenesis of CRC by directly targeting HMGB1. Furthermore, downregulation of miR-216b promotes the progression of CRC by affecting JAK2/STAT3 signalling [81]. As a very important member of the STAT family, STAT3 has been shown to be involved in cancer cell proliferation, metastasis, and angiogenesis in multiple reports, and STAT3 regulates tumour angiogenesis by regulating VEGF and HIF-1α [82]. MiR-1299 promotes the apoptosis of CRC cells by inhibiting the STAT3 pathway and inhibiting CRC. Furthermore, miR-1299 may be a related regulator of angiogenesis. However, further studies are needed [83].
MiRNAs targeting EGFR to regulate CRC angiogenesis
EGFR is overexpressed in a variety of tumours. Furthermore, it is a carcinogenic factor. EGFR regulates cell proliferation by activating extracellular regulatory protein kinase (ERK) [84]. In addition, EGFR also plays an important role in the vascular growth of CRC, and its targeted inhibitors also play an important role in the treatment of cancer [85]. The expression of EGFR can be regulated by microRNA: miR-7 inhibits the angiogenesis of CRC by downregulating ERK signalling through EGFR. The overexpression of miR-7 downregulates EGFR, ERK1/2 and VEGF and upregulates TSP-1 [86]. EGFR is involved in a variety of cellular responses, such as cell proliferation, differentiation and migration [87, 88]. EGFR binds to CTGF to phosphorylate and activate downstream signalling. MiR-375 inhibits CRC tumour growth, migration and angiogenesis by targeting the CTGF/EGFR-induced downregulation of the PIK3CA-AKT and BRAF-ERK1/2 cascades, thereby acting as a tumour suppressor. In addition, miR-375 and cetuximab also work together to induce an anticancer effect [89]. The results of miR-375 overexpression and cetuximab treatment experiments revealed synergistic enhancement of apoptosis and necrosis of colon cells.
Recent reports have revealed that miR-193a-3P has both cancer-promoting and tumour-suppressing effects, which may be correlated with its environment. However, most studies have reported that miR-193a-3P can inhibit tumour invasion [90,91,92], and only a few reports have indicated that miR-193a-3P can promote the progression of oesophageal squamous cell carcinoma as a cancer-promoting gene [93]. However, miR-193a-3P is expressed at low levels in CRC tissues and is associated with the prognosis of patients with CRC [94]. In a recent report, miR-193a-3P inhibited the proliferation, migration and angiogenesis of CRC by targeting plasminogen activator urokinase (PLAU) [95]. PLAU is a highly expressed urokinase plasminogen activator (uPA), and its high expression often indicates a poor prognosis. PLAU accelerates tumour metastasis by affecting the ECM and basement membrane, accelerating cell migration and angiogenesis [96]. In ovarian cancer, uPA regulates the AKT/mTOR/MMP-2/Laminin5γ2 signalling pathway to promote angiogenesis [97]. However, in CRC, miR-193a-3P can inhibit tumour angiogenesis by downregulating PLAU [94]. Since the exact downstream pathway remains unclear, further research is still needed. Recent reports have indicated that miR-193a-3P can inhibit tumour progression by targeting KRas in lung cancer.
KRAS and miRNAs affect angiogenesis
The KRAS (k-ras, p21) gene plays an important role in regulating tumour growth and angiogenesis. Activation of KRAS mutations induces CRC cell growth, invasion, and metastasis; and thus is considered a critical step in the progression of CRC [98]. KRAS gene detection is the most direct and effective method to understand the status of oncogenes in patients with colorectal cancer. Through detection of KRAS, we can understand the status of oncogenes, so as to screen out targeted drugs against EGFR. Recently, highly expressed miR-19a was shown to downregulate KRAS to reduce angiogenesis, and specifically angiogenesis in CRC, and this effect was restored after the re-expression of KRAS, indicating that miR-19a can directly regulate KRAS and reduce the angiogenesis of CRC [99]. Other studies have shown that miR-19a is negatively correlated with TF expression in patients with early colon cancer, and can inhibit TF expression in vitro and inhibit the migration and invasion of CRC [100]. Interestingly, in other studies, miR-19a showed a different effect. These studies have shown that miR-19a promotes the proliferation and migration of colorectal cancer [101,102,103], and that miR-19a is also associated with lymphatic metastasis and mediates TNF-α-induced epithelial mesenchymal transformation in colorectal cancer [104]. The differences in the above research results, may be related to the different cell lines selected, or may be caused by an undiscovered mechanism, just like miRNAs play the opposite role in different cancers, such as miR-125b in breast cancer both promote cancer [105] and inhibiting cancer [106]. Therefore, it is particularly necessary to clarify the specific mechanism of miRNA's regulation of angiogenesis, which is of great significance for the application of miRNA to target angiogenesis in the treatment of tumors.
FOXM1 and miRNAs in angiogenesis
Forkhead box M1 (FOXM1) is a member of the FOX superfamily. It is overexpressed in a variety of cancers, including CRC [107], in which it promotes EMT, angiogenesis, cell proliferation, stem cell self-renewal, etc. As an activator of tumour metastasis, it exhibits a wide variety of cancer-promoting properties. FOXM1 is a major regulator of CRC and can be used as an indicator of poor prognosis [108, 109]. Recent studies have shown that miR-6868-5p is able to inhibit tumour angiogenesis by inhibiting the FOXM1-IL-8 axis. In turn, FOXM1 also downregulates miR-6868-5p by stimulating EZH2-mediated transcription [110]. In general, the expression of miR-6868-5p is downregulated in CRC, and downregulation of miR-6868-5p inhibits tumour angiogenesis through inhibition of FOXM1; FOXM1 can also inhibit miR-6868-5p expression through promoter histone methylation. The pro-angiogenic factor IL-8 has been identified as a transcriptional target of FOXM1, and it was revealed that miR-6868-5p reduces angiogenesis and IL-8 expression by inhibiting FOXM1 expression. FOXM1 can promote the expression of EZH2 and inhibit the transcription of miR-6868-5p by enhancing the level of H3K27me3 at the miR-6868 promoter [110]. These findings provide a new perspective on the mechanism of CRC angiogenesis.
BMPs affect angiogenesis with miRNAs
Bone morphogenetic proteins (BMPs) are also involved in angiogenesis; they trigger signalling through BMP/Smad to affect angiogenesis, and ID1 is their immediate downstream effector. The DNA-binding protein inhibitor ID-1 (ld1) is overexpressed in tumours and inhibits tumour angiogenesis in mice. As one of the most potent angiogenic factors, ID1 can serve as a direct antiangiogenic target [111, 112]. In a recent study, it was reported that miR-885-3p could inhibit the angiogenesis of CRC by regulating BMPR1A to disrupt the BMP/Smad/Id1 signalling pathway, thereby inhibiting the growth of CRC cells [113].
Other miRNAs that regulate angiogenesis
MiR-15-16 is a tumour suppressor that has been shown to promote apoptosis and inhibit cell proliferation in various independent studies. In addition, recent research reported that c-Myc/Max, HIF-2α, miR-15-16 and FGF2 signalling regulation can modulate CRC angiogenesis under hypoxic conditions. Under hypoxic conditions, HIF-2α-induced c-Myc/Max heterodimer stability is much stronger than HIF-1α-induced c-Myc degradation, resulting in the inhibition of miR-15-16 under hypoxic conditions. The enhanced expression of FGF2 promotes tumour angiogenesis and metastasis [114].
MiR-181a-5p belongs to the miR-181 family, which includes miR-181a, miR-181b, miR-181c and miR-181d. Recent reports have indicated that such miRNAs play an important role in tumour transformation. Furthermore, some studies have shown that miR-181 members promote the formation of tumours [115]. However, miR-181a-5p is specific tumour marker, and it has been reported to promote the progression of cervical cancer [116]. On the other hand, miR-181a-5p has been shown to inhibit tumour growth in liver cancer [117]. In breast cancer and colon cancer, there is evidence that miR-181a-5p can inhibit the invasion and migration of cancer cells and angiogenesis by inhibiting the expression of MMP-14 [118]. These controversial findings indicate the complexity of miRNA functions and that the functions of miRNAs in different types of tumours may significantly differ. However, various studies use tumour tissues, which include a variety of normal cells and tumour cells, and this practice may lead to incorrect conclusions about the expression levels of specific miRNAs. As such, further research is needed.
MiRNAs that promote angiogenesis (Table 2)
MiR-181a also plays a role in a variety of tumours. For example, in chondrosarcoma, miR-181a increases VEGF to promote tumour growth [119]. Unlike miR-181a-5p, which was shown to inhibit angiogenesis in CRC, miR-181a was shown to inhibit angiogenesis in CRC in a recent study. MIR-181a activates SRC by inhibiting SRCIN1, which ultimately leads to increased secretion of VEGF and promotes angiogenesis in CRC. This study demonstrated that the miR-181a-SRCIN1-SRC-VEGF cascade plays an important role in the regulation of tumour angiogenesis and that blocking this pathway can significantly reduce tumour angiogenesis [120]. MiR-194 reduces platelet-reactive protein-1 (TSP-1), reducing its damage to endothelial cells and effects on VEGF and promoting angiogenesis in colon cancer [121].
Exosomes are extracellular small vesicles that contain lipids, proteins and various nucleic acids, including RNA, DNA and miRNA. Most cells secrete exosomes under normal and pathological conditions, while tumour exosomes are derived from outside the tumour. The body includes many species of miRNA [122, 123]. Exosomes are one of the cancer-derived factors that cause distant organ metastasis and promote tumour angiogenesis and metastasis [124, 125]. However, determining how tumour-derived exosomes specifically regulate the tumour microenvironment before metastasis by inducing angiogenesis requires more research. In a recent study, it was shown that exomiR-1229 can promote CRC angiogenesis by directly regulating HIPK2. HIPK2 can inhibit several angiogenic genes, including MMP10 and VEGF, to inhibit angiogenesis, while exomi-1229 promotes CRC tumour angiogenesis by inhibiting HIPK2 and inhibiting P-AKT and VEGFA in CRC [126]. CRC-secreted miR-25-3p can inhibit the activity of the VEGFR2 promoter and disrupt the integrity of the endothelial barrier by regulating KLF2 and KLF4, resulting in increased vascular permeability and angiogenesis and thereby promoting CRC metastasis. In addition, blocking its secretion can reduce the angiogenesis and metastasis of CRC [127]. It has been well demonstrated that miR-25-3p can be used as a therapeutic target to disrupt CRC angiogenesis and metastasis. MiR-1246 secreted by colorectal cancer exosomes directly regulates promyelocytic leukaemia (PML) mRNA, which inactivates Smad2/3 signalling and activates Smad-1/5/8 signalling, leading to endothelial cell growth and tumour angiogenesis [128].
Clinical studies based on miRNAs affecting angiogenesis
In recent years, antiangiogenic strategies have become an important treatment for metastatic colorectal cancer (mCRC), and various antiangiogenic agents have emerged. However, there are still no validated markers for the treatment of angiogenesis. MiRNAs have been shown to regulate angiogenesis in a variety of cancers. Comprehensive miRNA-based therapies may have better efficacy and fewer adverse effects than conventional chemotherapy. Bevacizumab, which inhibits VEGF-A, has been used in a variety of therapeutic regimens [129]. Furthermore, recent studies have shown that miR-20b-5p, miR-29b-3p and miR-155-5p can be used to monitor the response to bevacizumab, and high expression levels of these miRNAs often suggest good prognosis in mCRC patients treated with bevacizumab. Measuring the expression of these miRNAs before treatment helps to identify patients who will likely be resistant to bevacizumab therapy, thereby enabling a better treatment regimen to be administered [130]. Hanen et al. [131, 132] demonstrated that miR-126 has predictive value for the first-line capecitabine and oxaliplatin treatment in mCRC. Highly expressed miR-126 is often associated with anti-VEGFA chemotherapy response in mCRC, in which high expression of miR-126 is related to good prognosis.
Conclusion
Angiogenesis plays an important role from the early stage of colon cancer to the late phase of metastasis. There is ample evidence that angiogenesis is a complex process that involves multiple factors and processes. MiRNAs have a large impact on such factors and processes, and they are potential targets for chemoprevention and chemotherapy. Studies have shown that miRNAs, as a new therapeutic target for tumours, can be successfully regulated through a series of techniques. For example, miRNA antagonists and mimetics may be important new classes of drugs that regulate angiogenesis in colon cancer. A good understanding of the role of miRNAs in the regulation of angiogenesis can help to fully understand the function of miRNAs and their targets and can provide more pathways to target with miRNA-based therapeutic applications. However, there are relatively few clinical studies on the role of miRNAs in CRC angiogenesis. Despite some challenges, numerous discoveries have been made over the past few years, providing a broad perspective for the use of miRNAs in future targeted therapies.
Availability of data and materials
Not applicable.
Abbreviations
- CRC:
-
Colorectal cancer
- VEGF:
-
Vascular endothelial growth factor
- TSP-1:
-
Thrombospondin-1
- PDGF:
-
Platelet-derived growth factor
- TGF:
-
Transforming growth factor
- EGF:
-
Endothelial growth factor
- FGF:
-
Fibroblast growth factor
- OSCC:
-
Oral squamous cell carcinoma
- PI3K:
-
Phosphatidylinositol 3-kinase
- AKT:
-
Protein kinase B
- EMT:
-
Epithelial–mesenchymal transition
- HDAC2:
-
Histone deacetylase
- CRCT1:
-
Cysteine-rich C-terminal 1
- ATAD2:
-
ATPase family AAA domain-containing protein 2
- FUT:
-
Fucosyltransferase
- IGF-IR:
-
Insulin-like growth factor-I receptor
- STs:
-
Sialyltransferases
- HMGA2:
-
High‑mobility group AT‑hook 2
- PLAU:
-
Plasminogen activator urokinase
- uPA:
-
Rrokinase plasminogen activator
- FOXM1:
-
Forkhead box M1
- BMP:
-
Bone morphogenetic protein
- PML:
-
Promyelocytic leukaemia
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Cui YM, Jiao HL, Ye YP, Chen CM, Wang JX, Tang N, Li TT, Lin J, Qi L, Wu P, et al. FOXC2 promotes colorectal cancer metastasis by directly targeting MET. Oncogene. 2015;34(33):4379–90.
Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155(3):739–52.
Burgermeister E, Battaglin F, Eladly F, Wu W, Herweck F, Schulte N, Betge J, Hartel N, Kather JN, Weis CA, et al. Aryl hydrocarbon receptor nuclear translocator-like (ARNTL/BMAL1) is associated with bevacizumab resistance in colorectal cancer via regulation of vascular endothelial growth factor A. EBioMedicine. 2019;45:139–54.
Marisi G, Scarpi E, Passardi A, Nanni O, Ragazzini A, Valgiusti M, Casadei Gardini A, Neri LM, Frassineti GL, Amadori D, et al. Circulating VEGF and eNOS variations as predictors of outcome in metastatic colorectal cancer patients receiving bevacizumab. Sci Rep. 2017;7(1):1293.
Mihalache A, Rogoveanu I. Angiogenesis factors involved in the pathogenesis of colorectal cancer. Curr Health Sci J. 2014;40(1):5–11.
Viallard C, Larrivee B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20(4):409–26.
Alonso-Camino V, Santos-Valle P, Ispizua MC, Sanz L, Alvarez-Vallina L. Engineered human tumor xenografts with functional human vascular networks. Microvasc Res. 2011;81(1):18–25.
Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17(11):1359–70.
Brautigam J, Bischoff I, Schurmann C, Buchmann G, Epah J, Fuchs S, Heiss E, Brandes RP, Furst R. Narciclasine inhibits angiogenic processes by activation of Rho kinase and by downregulation of the VEGF receptor 2. J Mol Cell Cardiol. 2019;135:97–108.
Li Z, Ding X, Wu H, Liu C. Artemisinin inhibits angiogenesis by regulating p38 MAPK/CREB/TSP-1 signaling pathway in osteosarcoma. J Cell Biochem. 2019;120:11462.
Cheng X, Jin Z, Ji X, Shen X, Feng H, Morgenlander W, Ou B, Wu H, Gao H, Ye F, et al. ETS variant 5 promotes colorectal cancer angiogenesis by targeting platelet-derived growth factor BB. Int J Cancer. 2019;145(1):179–91.
Batlle R, Andres E, Gonzalez L, Llonch E, Igea A, Gutierrez-Prat N, Berenguer-Llergo A, Nebreda AR. Regulation of tumor angiogenesis and mesenchymal-endothelial transition by p38alpha through TGF-beta and JNK signaling. Nat Commun. 2019;10(1):3071.
Li L, Fan P, Chou H, Li J, Wang K, Li H. Herbacetin suppressed MMP9 mediated angiogenesis of malignant melanoma through blocking EGFR-ERK/AKT signaling pathway. Biochimie. 2019;162:198–207.
Hegab AE, Ozaki M, Kameyama N, Gao J, Kagawa S, Yasuda H, Soejima K, Yin Y, Guzy RD, Nakamura Y, et al. Effect of FGF/FGFR pathway blocking on lung adenocarcinoma and its cancer-associated fibroblasts. J Pathol. 2019;249:193–205.
Pandey AK, Singhi EK, Arroyo JP, Ikizler TA, Gould ER, Brown J, Beckman JA, Harrison DG, Moslehi J. Mechanisms of VEGF (vascular endothelial growth factor) inhibitor-associated hypertension and vascular disease. Hypertension. 2018;71(2):e1–8.
Sun X, Hu F, Hou Z, Chen Q, Lan J, Luo X, Wang G, Hu J, Cao Z. SIX4 activates Akt and promotes tumor angiogenesis. Exp Cell Res. 2019;383:111495.
Peng T, Deng X, Tian F, Li Z, Jiang P, Zhao X, Chen G, Chen Y, Zheng P, Li D et al. The interaction of LOXL2 with GATA6 induces VEGFA expression and angiogenesis in cholangiocarcinoma. Int J Oncol.2019 Sep;55(3):657-670.
Lien JC, Chung CL, Huang TF, Chang TC, Chen KC, Gao GY, Hsu MJ, Huang SW. A novel 2-aminobenzimidazole-based compound Jzu 17 exhibits anti- angiogenesis effects via targeting VEGFR-2 signaling. Br J Pharmacol. 2019;176:4034–49.
Saltz LB. Bevacizumab in colorectal cancer: it should have worked. Lancet Oncol. 2016;17(11):1469–70.
Smeets D, Miller IS, O’Connor DP, Das S, Moran B, Boeckx B, Gaiser T, Betge J, Barat A, Klinger R, et al. Copy number load predicts outcome of metastatic colorectal cancer patients receiving bevacizumab combination therapy. Nat Commun. 2018;9(1):4112.
Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH, Wu CY, Kuo PL. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36(34):4929–42.
Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17(3):471–94.
Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14(10a):1902–10.
Liu W, Hu J, Zhou K, Chen F, Wang Z, Liao B, Dai Z, Cao Y, Fan J, Zhou J. Serum exosomal miR-125b is a novel prognostic marker for hepatocellular carcinoma. Onco Targets Ther. 2017;10:3843–51.
Chen YF, Wei YY, Yang CC, Liu CJ, Yeh LY, Chou CH, Chang KW, Lin SC. miR-125b suppresses oral oncogenicity by targeting the anti-oxidative gene PRXL2A. Redox Biol. 2019;22:101140.
Li Y, Wang Y, Fan H, Zhang Z, Li N. miR-125b-5p inhibits breast cancer cell proliferation, migration and invasion by targeting KIAA1522. Biochem Biophys Res Commun. 2018;504(1):277–82.
Ottaviani S, Stebbing J, Frampton AE, Zagorac S, Krell J, de Giorgio A, Trabulo SM, Nguyen VTM, Magnani L, Feng H, et al. TGF-beta induces miR-100 and miR-125b but blocks let-7a through LIN28B controlling PDAC progression. Nat Commun. 2018;9(1):1845.
Chen YY, Ho HL, Lin SC, Ho TD, Hsu CY. Upregulation of miR-125b, miR-181d, and miR-221 predicts poor prognosis in MGMT promoter-unmethylated glioblastoma patients. Am J Clin Pathol. 2018;149(5):412–7.
He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–31.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97.
Lai EC. Micro RNAs are complementary to 3’ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet. 2002;30(4):363–4.
Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34(Database issue):D140-144.
Xin H, Wang C, Liu Z. miR-196a-5p promotes metastasis of colorectal cancer via targeting IkappaBalpha. BMC Cancer. 2019;19(1):30.
Tang W, Zhou W, Xiang L, Wu X, Zhang P, Wang J, Liu G, Zhang W, Peng Y, Huang X, et al. The p300/YY1/miR-500a-5p/HDAC2 signalling axis regulates cell proliferation in human colorectal cancer. Nat Commun. 2019;10(1):663.
To KK, Tong CW, Wu M, Cho WC. MicroRNAs in the prognosis and therapy of colorectal cancer: from bench to bedside. World J Gastroenterol. 2018;24(27):2949–73.
Deng S, Wang H, Fan H, Zhang L, Hu J, Tang Q, Shou Z, Liu X, Zuo D, Yang J, et al. Over-expressed miRNA-200b ameliorates ulcerative colitis-related colorectal cancer in mice through orchestrating epithelial–mesenchymal transition and inflammatory responses by channel of AKT2. Int Immunopharmacol. 2018;61:346–54.
Ji D, Qiao M, Yao Y, Li M, Chen H, Dong Q, Jia J, Cui X, Li Z, Xia J, et al. Serum-based microRNA signature predicts relapse and therapeutic outcome of adjuvant chemotherapy in colorectal cancer patients. EBioMedicine. 2018;35:189–97.
Kuehbacher A, Urbich C, Dimmeler S. Targeting microRNA expression to regulate angiogenesis. Trends Pharmacol Sci. 2008;29(1):12–5.
Hong L, Han Y, Zhou Y, Nita A. Angiogenesis-related microRNAs in colon cancer. Expert Opin Biol Ther. 2013;13(1):77–84.
Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6 Suppl 16):15–8.
Tabouret T, Gregory T, Dhooge M, Brezault C, Mir O, Dreanic J, Chaussade S, Coriat R. Long term exposure to antiangiogenic therapy, bevacizumab, induces osteonecrosis. Invest New Drugs. 2015;33(5):1144–7.
Choi YE, Meghani K, Brault ME, Leclerc L, He YJ, Day TA, Elias KM, Drapkin R, Weinstock DM, Dao F, et al. Platinum and PARP inhibitor resistance due to overexpression of microrna-622 in BRCA1-mutant ovarian cancer. Cell Rep. 2016;14(3):429–39.
Liu H, Liu Y, Liu W, Zhang W, Xu J. EZH2-mediated loss of miR-622 determines CXCR4 activation in hepatocellular carcinoma. Nat Commun. 2015;6:8494.
Xu L, Hou Y, Tu G, Chen Y, Du YE, Zhang H, Wen S, Tang X, Yin J, Lang L, et al. Nuclear Drosha enhances cell invasion via an EGFR-ERK1/2-MMP7 signaling pathway induced by dysregulated miRNA-622/197 and their targets LAMC2 and CD82 in gastric cancer. Cell Death Dis. 2017;8(3):e2642.
Fang Y, Sun B, Wang J, Wang Y. miR-622 inhibits angiogenesis by suppressing the CXCR4-VEGFA axis in colorectal cancer. Gene. 2019;699:37–42.
Zhou Q, Zhu Y, Wei X, Zhou J, Chang L, Sui H, Han Y, Piao D, Sha R, Bai Y. MiR-590-5p inhibits colorectal cancer angiogenesis and metastasis by regulating nuclear factor 90/vascular endothelial growth factor A axis. Cell Death Dis. 2016;7(10):e2413.
Xiao X, Tang C, Xiao S, Fu C, Yu P. Enhancement of proliferation and invasion by MicroRNA-590-5p via targeting PBRM1 in clear cell renal carcinoma cells. Oncol Res. 2013;20(11):537–44.
Chu Y, Ouyang Y, Wang F, Zheng A, Bai L, Han L, Chen Y, Wang H. MicroRNA-590 promotes cervical cancer cell growth and invasion by targeting CHL1. J Cell Biochem. 2014;115(5):847–53.
Keklikoglou I, Koerner C, Schmidt C, Zhang JD, Heckmann D, Shavinskaya A, Allgayer H, Guckel B, Fehm T, Schneeweiss A, et al. MicroRNA-520/373 family functions as a tumor suppressor in estrogen receptor negative breast cancer by targeting NF-kappaB and TGF-beta signaling pathways. Oncogene. 2012;31(37):4150–63.
Wu N, Song Y, Pang L, Chen Z. CRCT1 regulated by microRNA-520 g inhibits proliferation and induces apoptosis in esophageal squamous cell cancer. Tumour Biol. 2016;37(6):8271–9.
Zhang R, Liu R, Liu C, Niu Y, Zhang J, Guo B, Zhang CY, Li J, Yang J, Chen X. A novel role for MiR-520a-3p in regulating EGFR expression in colorectal cancer. Cell Physiol Biochem. 2017;42(4):1559–74.
Hong S, Chen S, Wang X, Sun D, Yan Z, Tai J, Bi M. ATAD2 silencing decreases VEGFA secretion through targeting has-miR-520a to inhibit angiogenesis in colorectal cancer. Biochem Cell Biol. 2018;96(6):761–8.
Wang S, Olson EN. AngiomiRs-key regulators of angiogenesis. Curr Opin Genet Dev. 2009;19(3):205–11.
Zhang Y, Wang X, Xu B, Wang B, Wang Z, Liang Y, Zhou J, Hu J, Jiang B. Epigenetic silencing of miR-126 contributes to tumor invasion and angiogenesis in colorectal cancer. Oncol Rep. 2013;30(4):1976–84.
Ye J, Wu X, Wu D, Wu P, Ni C, Zhang Z, Chen Z, Qiu F, Xu J, Huang J. miRNA-27b targets vascular endothelial growth factor C to inhibit tumor progression and angiogenesis in colorectal cancer. PLoS ONE. 2013;8(4):e60687.
Chen X, Xu X, Pan B, Zeng K, Xu M, Liu X, He B, Pan Y, Sun H, Wang S. miR-150-5p suppresses tumor progression by targeting VEGFA in colorectal cancer. Aging (Albany NY). 2018;10(11):3421–37.
Wu QB, Chen J, Zhu JW, Yin X, You HY, Lin YR, Zhu HQ. MicroRNA125 inhibits RKO colorectal cancer cell growth by targeting VEGF. Int J Mol Med. 2018;42(1):665–73.
Liang L, Gao C, Li Y, Sun M, Xu J, Li H, Jia L, Zhao Y. miR-125a-3p/FUT5-FUT6 axis mediates colorectal cancer cell proliferation, migration, invasion and pathological angiogenesis via PI3K-Akt pathway. Cell Death Dis. 2017;8(8):e2968.
Sun D, Zhang F, Qian J, Shen W, Fan H, Tan J, Li L, Xu C, Yang Y, Cheng H. 4′-hydroxywogonin inhibits colorectal cancer angiogenesis by disrupting PI3K/AKT signaling. Chem Biol Interact. 2018;296:26–33.
Noorolyai S, Shajari N, Baghbani E, Sadreddini S, Baradaran B. The relation between PI3K/AKT signalling pathway and cancer. Gene. 2019;698:120–8.
Avan A, Narayan R, Giovannetti E, Peters GJ. Role of Akt signaling in resistance to DNA-targeted therapy. World J Clin Oncol. 2016;7(5):352–69.
Qian X, Yu J, Yin Y, He J, Wang L, Li Q, Zhang LQ, Li CY, Shi ZM, Xu Q, et al. MicroRNA-143 inhibits tumor growth and angiogenesis and sensitizes chemosensitivity to oxaliplatin in colorectal cancers. Cell Cycle. 2013;12(9):1385–94.
Jia L, Luo S, Ren X, Li Y, Hu J, Liu B, Zhao L, Shan Y, Zhou H. miR-182 and miR-135b mediate the tumorigenesis and invasiveness of colorectal cancer cells via targeting ST6GALNAC2 and PI3K/AKT pathway. Dig Dis Sci. 2017;62(12):3447–59.
Nguyen K, Yan Y, Yuan B, Dasgupta A, Sun J, Mu H, Do KA, Ueno NT, Andreeff M, Battula VL. ST8SIA1 regulates tumor growth and metastasis in TNBC by activating the FAK-AKT-mTOR signaling pathway. Mol Cancer Ther. 2018;17(12):2689–701.
Shan Y, Liu Y, Zhao L, Liu B, Li Y, Jia L. MicroRNA-33a and let-7e inhibit human colorectal cancer progression by targeting ST8SIA1. Int J Biochem Cell Biol. 2017;90:48–58.
Calvo N, Carriere P, Martin MJ, Gigola G, Gentili C. PTHrP treatment of colon cancer cells promotes tumor associated-angiogenesis by the effect of VEGF. Mol Cell Endocrinol. 2019;483:50–63.
Cai S, Cheng X, Liu Y, Lin Z, Zeng W, Yang C, Liu L, Chukwuebuka OA, Li W. EYA1 promotes tumor angiogenesis by activating the PI3K pathway in colorectal cancer. Exp Cell Res. 2018;367(1):37–46.
Yang W, Ma J, Zhou W, Cao B, Zhou X, Zhang H, Zhao Q, Hong L, Fan D. Reciprocal regulations between miRNAs and HIF-1alpha in human cancers. Cell Mol Life Sci. 2019;76(3):453–71.
Sadeghiyeh N, Sehati N, Mansoori B, Mohammadi A, Shanehbandi D, Khaze V, Baradaran B. MicroRNA-145 replacement effect on growth and migration inhibition in lung cancer cell line. Biomed Pharmacother. 2019;111:460–7.
Xu Q, Liu L-Z, Qian X, Chen Q, Jiang Y, Li D, Lai L, Jiang B-H. MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res. 2012;40(2):761–74.
Liang Z, Bian X, Shim H. Downregulation of microRNA-206 promotes invasion and angiogenesis of triple negative breast cancer. Biochem Biophys Res Commun. 2016;477(3):461–6.
Xu Z, Zhu C, Chen C, Zong Y, Feng H, Liu D, Feng W, Zhao J, Lu A. CCL19 suppresses angiogenesis through promoting miR-206 and inhibiting Met/ERK/Elk-1/HIF-1alpha/VEGF-A pathway in colorectal cancer. Cell Death Dis. 2018;9(10):974.
Tsai HL, Miao ZF, Chen YT, Huang CW, Yeh YS, Yang IP, Wang JY. miR-148a inhibits early relapsed colorectal cancers and the secretion of VEGF by indirectly targeting HIF-1alpha under non-hypoxia/hypoxia conditions. J Cell Mol Med. 2019;23(5):3572–82.
Forouzan Jahromi Z, Javeri A, Fakhr Taha M. Tumor suppressive effects of the pleiotropically acting miR-195 in colorectal cancer cells. Excli j. 2019;18:243–52.
Yamakuchi M, Lotterman CD, Bao C, Hruban RH, Karim B, Mendell JT, Huso D, Lowenstein CJ. P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc Natl Acad Sci USA. 2010;107(14):6334–9.
Muller PA, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell. 2014;25(3):304–17.
Chen X, Zeng K, Xu M, Liu X, Hu X, Xu T, He B, Pan Y, Sun H, Wang S. P53-induced miR-1249 inhibits tumor growth, metastasis, and angiogenesis by targeting VEGFA and HMGA2. Cell Death Dis. 2019;10(2):131.
Liu Y, Liang H, Jiang X. MiR-1297 promotes apoptosis and inhibits the proliferation and invasion of hepatocellular carcinoma cells by targeting HMGA2. Int J Mol Med. 2015;36(5):1345–52.
Yao X, Zhao G, Yang H, Hong X, Bie L, Liu G. Overexpression of high-mobility group box 1 correlates with tumor progression and poor prognosis in human colorectal carcinoma. J Cancer Res Clin Oncol. 2010;136(5):677–84.
Chen X, Liu X, He B, Pan Y, Sun H, Xu T, Hu X, Wang S. MiR-216b functions as a tumor suppressor by targeting HMGB1-mediated JAK2/STAT3 signaling way in colorectal cancer. Am J Cancer Res. 2017;7(10):2051–69.
Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, Gritsko T, Turkson J, Kay H, Semenza GL, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24(36):5552–60.
Wang Y, Lu Z, Wang N, Zhang M, Zeng X, Zhao W. MicroRNA-1299 is a negative regulator of STAT3 in colon cancer. Oncol Rep. 2017;37(6):3227–34.
Clauditz TS, Gontarewicz A, Lebok P, Tsourlakis MC, Grob TJ, Munscher A, Sauter G, Bokemeyer C, Knecht R, Wilczak W. Epidermal growth factor receptor (EGFR) in salivary gland carcinomas: potentials as therapeutic target. Oral Oncol. 2012;48(10):991–6.
Matos I, Noguerido A, Ros J, Mulet N, Argiles G, Elez E, Tabernero J. Triple-drug chemotherapy regimens in combination with an anti-EGFR agent in metastatic colorectal cancer—prospects from phase II clinical trials. Expert Opin Investig Drugs. 2019;28(5):463–71.
Fan X, Liu M, Tang H, Leng D, Hu S, Lu R, Wan W, Yuan S. MicroRNA-7 exerts antiangiogenic effect on colorectal cancer via ERK signaling. J Surg Res. 2019;240:48–59.
Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96(8):4240–5.
Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, Ding M, Bamford S, Cole C, Ward S, et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43(Database issue):D805-811.
Alam KJ, Mo JS, Han SH, Park WC, Kim HS, Yun KJ, Chae SC. MicroRNA 375 regulates proliferation and migration of colon cancer cells by suppressing the CTGF-EGFR signaling pathway. Int J Cancer. 2017;141(8):1614–29.
Liu Y, Xu X, Xu X, Li S, Liang Z, Hu Z, Wu J, Zhu Y, Jin X, Wang X, et al. MicroRNA-193a-3p inhibits cell proliferation in prostate cancer by targeting cyclin D1. Oncol Lett. 2017;14(5):5121–8.
Pekow J, Meckel K, Dougherty U, Huang Y, Chen X, Almoghrabi A, Mustafi R, Ayaloglu-Butun F, Deng Z, Haider HI, et al. miR-193a-3p is a key tumor suppressor in ulcerative colitis-associated colon cancer and promotes carcinogenesis through upregulation of IL17RD. Clin Cancer Res. 2017;23(17):5281–91.
Takahashi H, Takahashi M, Ohnuma S, Unno M, Yoshino Y, Ouchi K, Takahashi S, Yamada Y, Shimodaira H, Ishioka C. microRNA-193a-3p is specifically down-regulated and acts as a tumor suppressor in BRAF-mutated colorectal cancer. BMC Cancer. 2017;17(1):723.
Yi Y, Chen J, Jiao C, Zhong J, Song Z, Yu X, Lu X, Lin B. Upregulated miR-193a-3p as an oncogene in esophageal squamous cell carcinoma regulating cellular proliferation, migration and apoptosis. Oncol Lett. 2016;12(6):4779–84.
Lin M, Duan B, Hu J, Yu H, Sheng H, Gao H, Huang J. Decreased expression of miR-193a-3p is associated with poor prognosis in colorectal cancer. Oncol Lett. 2017;14(1):1061–7.
Lin M, Zhang Z, Gao M, Yu H, Sheng H, Huang J. MicroRNA-193a-3p suppresses the colorectal cancer cell proliferation and progression through downregulating the PLAU expression. Cancer Manag Res. 2019;11:5353–63.
Mekkawy AH, Pourgholami MH, Morris DL. Involvement of urokinase-type plasminogen activator system in cancer: an overview. Med Res Rev. 2014;34(5):918–56.
Tang J, Wang J, Fan L, Li X, Liu N, Luo W, Wang J, Wang Y, Wang Y. cRGD inhibits vasculogenic mimicry formation by down-regulating uPA expression and reducing EMT in ovarian cancer. Oncotarget. 2016;7(17):24050–62.
Boutin AT, Liao WT, Wang M, Hwang SS, Karpinets TV, Cheung H, Chu GC, Jiang S, Hu J, Chang K, et al. Oncogenic Kras drives invasion and maintains metastases in colorectal cancer. Genes Dev. 2017;31(4):370–82.
Chen M, Lin M, Wang X. Overexpression of miR-19a inhibits colorectal cancer angiogenesis by suppressing KRAS expression. Oncol Rep. 2018;39(2):619–26.
Yu G, Li H, Wang X, Wu T, Zhu J, Huang S, Wan Y, Tang J. MicroRNA-19a targets tissue factor to inhibit colon cancer cells migration and invasion. Mol Cell Biochem. 2013;380(1–2):239–47.
Zhang J, Xiao Z, Lai D, Sun J, He C, Chu Z, Ye H, Chen S, Wang J. miR-21, miR-17 and miR-19a induced by phosphatase of regenerating liver-3 promote the proliferation and metastasis of colon cancer. Br J Cancer. 2012;107(2):352–9.
Cellura D, Pickard K, Quaratino S, Parker H, Strefford JC, Thomas GJ, Mitter R, Mirnezami AH, Peake NJ. miR-19-mediated inhibition of transglutaminase-2 leads to enhanced invasion and metastasis in colorectal cancer. Mol Cancer Res. 2015;13(7):1095–105.
Liu Y, Liu R, Yang F, Cheng R, Chen X, Cui S, Gu Y, Sun W, You C, Liu Z, et al. miR-19a promotes colorectal cancer proliferation and migration by targeting TIA1. Mol Cancer. 2017;16(1):53.
Huang L, Wang X, Wen C, Yang X, Song M, Chen J, Wang C, Zhang B, Wang L, Iwamoto A, et al. Hsa-miR-19a is associated with lymph metastasis and mediates the TNF-α induced epithelial-to-mesenchymal transition in colorectal cancer. Sci Rep. 2015;5:13350.
Vu LT, Peng B, Zhang DX, Ma V, Mathey-Andrews CA, Lam CK, Kiomourtzis T, Jin J, McReynolds L, Huang L, et al. Tumor-secreted extracellular vesicles promote the activation of cancer-associated fibroblasts via the transfer of microRNA-125b. J Extracell Vesicles. 2019;8(1):1599680.
Hong L, Pan F, Jiang H, Zhang L, Liu Y, Cai C, Hua C, Luo X, Sun J, Chen Z. miR-125b inhibited epithelial–mesenchymal transition of triple-negative breast cancer by targeting MAP2K7. Onco Targets Ther. 2016;9:2639–48.
Myatt SS, Lam EW. Targeting FOXM1. Nat Rev Cancer. 2008;8(3):242.
Li M, Yang J, Zhou W, Ren Y, Wang X, Chen H, Zhang J, Chen J, Sun Y, Cui L, et al. Activation of an AKT/FOXM1/STMN1 pathway drives resistance to tyrosine kinase inhibitors in lung cancer. Br J Cancer. 2017;117(7):974–83.
Wu XR, Chen YH, Liu DM, Sha JJ, Xuan HQ, Bo JJ, Huang YR. Increased expression of forkhead box M1 protein is associated with poor prognosis in clear cell renal cell carcinoma. Med Oncol. 2013;30(1):346.
Wang Y, Wu M, Lei Z, Huang M, Li Z, Wang L, Cao Q, Han D, Chang Y, Chen Y, et al. Dysregulation of miR-6868-5p/FOXM1 circuit contributes to colorectal cancer angiogenesis. J Exp Clin Cancer Res. 2018;37(1):292.
Qiu H, Yang B, Pei ZC, Zhang Z, Ding K. WSS25 inhibits growth of xenografted hepatocellular cancer cells in nude mice by disrupting angiogenesis via blocking bone morphogenetic protein (BMP)/Smad/Id1 signaling. J Biol Chem. 2010;285(42):32638–46.
Perk J, Gil-Bazo I, Chin Y, de Candia P, Chen JJ, Zhao Y, Chao S, Cheong W, Ke Y, Al-Ahmadie H, et al. Reassessment of id1 protein expression in human mammary, prostate, and bladder cancers using a monospecific rabbit monoclonal anti-id1 antibody. Cancer Res. 2006;66(22):10870–7.
Xiao F, Qiu H, Cui H, Ni X, Li J, Liao W, Lu L, Ding K. MicroRNA-885-3p inhibits the growth of HT-29 colon cancer cell xenografts by disrupting angiogenesis via targeting BMPR1A and blocking BMP/Smad/Id1 signaling. Oncogene. 2015;34(15):1968–78.
Xue G, Yan HL, Zhang Y, Hao LQ, Zhu XT, Mei Q, Sun SH. c-Myc-mediated repression of miR-15-16 in hypoxia is induced by increased HIF-2alpha and promotes tumor angiogenesis and metastasis by upregulating FGF2. Oncogene. 2015;34(11):1393–406.
Liu X, Peng H, Liao W, Luo A, Cai M, He J, Zhang X, Luo Z, Jiang H, Xu L. MiR-181a/b induce the growth, invasion, and metastasis of neuroblastoma cells through targeting ABI1. Mol Carcinog. 2018;57(9):1237–50.
Yang M, Zhai X, Ge T, Yang C, Lou G. miR-181a-5p promotes proliferation and invasion and inhibits apoptosis of cervical cancer cells via regulating inositol polyphosphate-5-phosphatase A (INPP5A). Oncol Res. 2018;26(5):703–12.
Bi JG, Zheng JF, Li Q, Bao SY, Yu XF, Xu P, Liao CX. MicroRNA-181a-5p suppresses cell proliferation by targeting Egr1 and inhibiting Egr1/TGF-beta/Smad pathway in hepatocellular carcinoma. Int J Biochem Cell Biol. 2019;106:107–16.
Li Y, Kuscu C, Banach A, Zhang Q, Pulkoski-Gross A, Kim D, Liu J, Roth E, Li E, Shroyer KR, et al. miR-181a-5p inhibits cancer cell migration and angiogenesis via downregulation of matrix metalloproteinase-14. Cancer Res. 2015;75(13):2674–85.
Sun X, Charbonneau C, Wei L, Chen Q, Terek RM. miR-181a targets RGS16 to promote chondrosarcoma growth, angiogenesis, and metastasis. Mol Cancer Res. 2015;13(9):1347–57.
Sun W, Wang X, Li J, You C, Lu P, Feng H, Kong Y, Zhang H, Liu Y, Jiao R, et al. MicroRNA-181a promotes angiogenesis in colorectal cancer by targeting SRCIN1 to promote the SRC/VEGF signaling pathway. Cell Death Dis. 2018;9(4):438.
Sundaram P, Hultine S, Smith LM, Dews M, Fox JL, Biyashev D, Schelter JM, Huang Q, Cleary MA, Volpert OV, et al. p53-responsive miR-194 inhibits thrombospondin-1 and promotes angiogenesis in colon cancers. Cancer Res. 2011;71(24):7490–501.
Liu Y, Gu Y, Cao X. The exosomes in tumor immunity. Oncoimmunology. 2015;4(9):e1027472.
Wu K, Xing F, Wu SY, Watabe K. Extracellular vesicles as emerging targets in cancer: recent development from bench to bedside. Biochim Biophys Acta Rev Cancer. 2017;1868(2):538–63.
Alipoor SD, Mortaz E, Varahram M, Movassaghi M, Kraneveld AD, Garssen J, Adcock IM. The potential biomarkers and immunological effects of tumor-derived exosomes in lung cancer. Front Immunol. 2018;9:819.
Chen S, Lv M, Fang S, Ye W, Gao Y, Xu Y. Poly(I:C) enhanced anti-cervical cancer immunities induced by dendritic cells-derived exosomes. Int J Biol Macromol. 2018;113:1182–7.
Hu HY, Yu CH, Zhang HH, Zhang SZ, Yu WY, Yang Y, Chen Q. Exosomal miR-1229 derived from colorectal cancer cells promotes angiogenesis by targeting HIPK2. Int J Biol Macromol. 2019;132:470–7.
Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, Zhou K, Liu X, Ren X, Wang F, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9(1):5395.
Yamada N, Tsujimura N, Kumazaki M, Shinohara H, Taniguchi K, Nakagawa Y, Naoe T, Akao Y. Colorectal cancer cell-derived microvesicles containing microRNA-1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down-regulation in endothelial cells. Biochim Biophys Acta. 2014;1839(11):1256–72.
Marques RP, Heudtlass P, Pais HL, Quintela A, Martins AP. Patient-reported outcomes and health-related quality of life for cetuximab versus bevacizumab in metastatic colorectal cancer: a prospective cohort study. J Cancer Res Clin Oncol. 2019;145(7):1719–28.
Ulivi P, Canale M, Passardi A, Marisi G, Valgiusti M, Frassineti GL, Calistri D, Amadori D, Scarpi E. Circulating plasma levels of miR-20b, miR-29b and miR-155 as predictors of bevacizumab efficacy in patients with metastatic colorectal cancer. Int J Mol Sci. 2018;19(1):307.
Hansen TF, Sorensen FB, Lindebjerg J, Jakobsen A. The predictive value of microRNA-126 in relation to first line treatment with capecitabine and oxaliplatin in patients with metastatic colorectal cancer. BMC Cancer. 2012;12:83.
Hansen TF, Christensen R, Andersen RF, Sorensen FB, Johnsson A, Jakobsen A. MicroRNA-126 and epidermal growth factor-like domain 7-an angiogenic couple of importance in metastatic colorectal cancer. Results from the Nordic ACT trial. Br J Cancer. 2013;109(5):1243–51.
Acknowledgements
Not applicable.
Funding
This work was supported by the Scientific Research Foundation of Traditional Chinese Medicine of the Shanghai Health Bureau, ZY (2018-2020)-FWTX-6008 and the Scientific Research Plan Project of the Shanghai Science and Technology Committee (16401970503).
Author information
Authors and Affiliations
Contributions
YT and FH conceived the study and designed the experiments. YT, HZ, SZ, and SH contributed to the drafting of the article and the final approval of the submitted version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was not primary research involving human samples from public databases.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tang, Y., Zong, S., Zeng, H. et al. MicroRNAs and angiogenesis: a new era for the management of colorectal cancer. Cancer Cell Int 21, 221 (2021). https://doi.org/10.1186/s12935-021-01920-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-021-01920-0