Abstract
Background
Streptomyces is renowned for its robust biosynthetic capacity in producing medically relevant natural products. However, the majority of natural products biosynthetic gene clusters (BGCs) either yield low amounts of natural products or remain cryptic under standard laboratory conditions. Various heterologous production hosts have been engineered to address these challenges, and yet the successful activation of BGCs has still been limited. In our search for a valuable addition to the heterologous host panel, we identified the strain Streptomyces sp. A4420, which exhibited rapid initial growth and a high metabolic capacity, prompting further exploration of its potential.
Results
We engineered a polyketide-focused chassis strain based on Streptomyces sp. A4420 (CH strain) by deleting 9 native polyketide BGCs. The resulting metabolically simplified organism exhibited consistent sporulation and growth, surpassing the performance of most existing Streptomyces based chassis strains in standard liquid growth media. Four distinct polyketide BGCs were chosen and expressed in various heterologous hosts, including the Streptomyces sp. A4420 wild-type and CH strains, alongside Streptomyces coelicolor M1152, Streptomyces lividans TK24, Streptomyces albus J1074, and Streptomyces venezuelae NRRL B-65442. Remarkably, only the Streptomyces sp. A4420 CH strain demonstrated the capability to produce all metabolites under every condition outperforming its parental strain and other tested organisms. To enhance visualization and comparison of the tested strains, we developed a matrix-like analysis involving 15 parameters. This comprehensive analysis unequivocally illustrated the significant potential of the new strain to become a popular heterologous host.
Conclusion
Our engineered Streptomyces sp. A4420 CH strain exhibits promising attributes for the heterologous expression of natural products with a focus on polyketides, offering an alternative choice in the arsenal of heterologous production strains. As genomics and cloning strategies progress, establishment of a diverse panel of heterologous production hosts will be crucial for expediting the discovery and production of medically relevant natural products derived from Streptomyces.
Similar content being viewed by others
Background
The Actinobacteria phylum comprises high GC content microbes, featuring distinct fungal-like morphological development, sporulation, and unique biosynthetic capabilities [1]. Predominantly, natural product production, especially antibiotics, is centered in the Streptomyces genus [2, 3]. Their wide habitat range and interactions with diverse organisms led to the evolution of intricate natural product biosynthesis, captivating the drug discovery field [4]. Commercially, Actinobacteria have contributed an impressive array of natural product based drugs, including immunosuppressants like ascomycin and rapamycin [5, 6], antibiotics such as erythromycin and tetracycline [7, 8], and other compounds with applications spanning antifungal, antiviral, immunostimulant, anti-cancer, and agriculturally valuable properties [9, 10].
Native strains have complex regulatory systems, lack genetic manipulation tools and exhibit poor growth patterns, which result in ∼90% of biosynthetic gene clusters (BGCs) being cryptic under standard laboratory conditions or expressed at very low levels [11, 12]. However, recent breakthroughs in molecular biology and the development of innovative tools have redirected research interest toward the heterologous expression of these elusive BGCs in genetically well-understood and engineered chassis hosts [13,14,15]. Numerous Streptomyces strains have emerged as prime candidates for this purpose, with Streptomyces coelicolor leading the way as one of the most characterized and extensively studied species. Leveraging well-established molecular techniques and a deeply understood metabolism, S. coelicolor has proven invaluable in heterologous BGC expression [16]. In addition, closely related species like Streptomyces lividans, Streptomyces avermitilis, and Streptomyces albus have also played crucial roles in activating and enhancing the production of industrially relevant compounds [17,18,19]. These chosen heterologous hosts share common attributes, including rapid growth, abundant biosynthetic precursor availability, genetic manipulability, conjugation compatibility, and ideally, a low background of native metabolites [20]. However, the intricate genetic circuits and the complex nature of natural product biosynthesis necessitate the use of a diverse panel of heterologous hosts for expressing unknown BGCs [21, 22]. Consequently, there is an escalating demand for an array of Streptomyces heterologous hosts, as no single host alone can fulfill the ever-expanding requirements of this burgeoning field [23].
In the pursuit of enhancing the activation, discovery, and production of natural products, rigorous engineering efforts have been invested in various heterologous hosts [14]. Among these, the S. coelicolor M145 strain has undergone substantial modifications, initially involving the elimination of competing pathways responsible for actinorhodin, prodiginine, coelimycin, and calcium-dependent antibiotic production. This transformation yielded the "cleaner" background M1146 strain, facilitating the identification of heterologously expressed natural products. Subsequent iterations introduced previously identified advantageous rpoB (rifampicin resistance) or the double rpoB and rpsL mutations (streptomycin resistance) [24, 25], resulting in strains M1152 and M1154, respectively [26]. While these introduced mutations impacted growth, they led to remarkable increases in natural product yields, ranging from 20 to 40-fold.
Another closely related and extensively studied strain, S. lividans 66, garnered attention for its unique ability to accept methylated DNA and its low protease activity. Through the removal of self-replicating plasmids SLP2 and SLP3, the engineered strain TK24 emerged as one of the most widely adopted heterologous hosts [27]. Various modified S. lividans strains have displayed promising results in the production of the antibiotic daptomycin and the anti-cancer compound mithramycin A [28, 29]. Recently, a comprehensive re-evaluation of the S. lividans TK24 engineering concept was undertaken, involving the knockout of a total of nine metabolically active BGCs and the introduction of two additional attB integration sites to facilitate higher copy numbers of integrated heterologous BGCs [30]. The resultant strain, ΔYA11, exhibited superior production levels for three metabolites compared to its progenitor TK24 while maintaining robust growth performance, which outperformed even S. coelicolor M1152 strains.
Another chassis strain developed for natural products production was the minimized version of S. albus J1074 named Del14, where 15 native secondary metabolite biosynthetic pathways were deleted from the parental chromosome [31]. Interestingly, the introduction of additional attB integration sites led to only marginal improvements in production, while simultaneously compromising conjugation rates, casting doubts on the high BGC copy number strategy [32]. Both S. albus Del14 and S. lividans ΔYA11 strains displayed a proclivity for the expression of various BGCs sourced from the bacterial artificial chromosome (BAC) library of Streptomyces albus subsp. chlorinus NRRL B-24108. Overall, the highlighted studies demonstrated that the native competing pathways affected production of heterologous BGCs dramatically and selective knock-out strains improved final yields significantly. Furthermore, the reduction of background interference has not only streamlined the process but also improved the detection of heterologously expressed exogenous natural products.
In this study, we evaluate the prospectives of unique strains from an in-house collection as novel Streptomyces host for heterologous BGC production. The strain Streptomyces sp. A4420 was identified as part of the screening process for bacterial alkaloid producers, as the host for streptazolin natural product. Due to its innate affinity towards production of high levels of polyketides, which is one of the largest groups of natural products produced by bacteria, we decided to initially focus on evaluation of PKS-related gene clusters as even among polyketide class of natural products, there is a great diversity of scaffolds and post-PKS modifications to be investigated. To evaluate its capacity as a heterologous expression host, this strain was sequenced initially, followed by identification and deletion of 9 endogenous BGCs. The resulting engineered strain showed a similar growth pattern to that of the parental strain and outperformed commonly used Streptomyces heterologous expression hosts. Finally, by utilizing four heterologous Type I and II polyketide BGCs of varying chemical diversity as benchmarks including benzoisochromanequinone, glycosylated macrolide, glycosylated polyene macrolactam and heterodimeric aromatic polyketide products, a series of activation and production yield assessments were performed and juxtaposed against model organisms. These experiments demonstrate the feasibility and potential of our newly engineered strain to complement and significantly bolster the processes of natural product discovery and production. Collectively, our findings reveal that the newly engineered strain exhibits a range of promising features, positioning it as a valuable chassis strain to complement existing well-characterized heterologous hosts for natural product discovery and production.
Results
Strain identification and genetic analysis
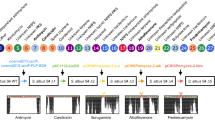
Streptomyces sp. A4420 was identified from the private Natural Organism Library (NOL) collection housed within the Agency for Science and Technology (A*STAR) in Singapore [33]. Initial fermentation studies using solid SFM and ISP2 media showed high production of the piperidine alkaloid streptazolin reaching up to 10 mg L−1 yield, prompting us to investigate its potential as a chassis strain for natural products synthesis, in particular polyketides. At the same time, this strain also displayed a growth rate comparable to the commonly used Streptomyces heterologous host strains, and a uniquely high sporulation rate. To characterize its phylogenetic relationship to other Streptomyces strains, its 16S rDNA sequence was amplified using the established actinomycetes primer pair 243F and A3R [34] (Additional File 1: Fig. S1), and a phylogenetic tree comprising a total of 30 species was constructed using the MEGA 11 software with the neighbor-joining method [35, 36] (Fig. 1). This analysis revealed that Streptomyces sp. A4420 is distantly related to the Streptomyces strains commonly employed as heterologous hosts, including S. albus J1074, and the closely related S. lividans TK24 and S. coelicolor M1152. Among the strains previously used as heterologous hosts, Streptomyces sp. A4420 is most closely related to Streptomyces avermitilis MA-4680 [18], while among the sequenced strains, it is most closely related to Streptomyces neopeptinius strain F18.
Neighbor-joining phylogenetic tree based on 16S rRNA gene sequences, showing the position of Streptomyces sp. A4420 relative to other well established heterologous hosts S. lividans TK24, S. coelicolor M1152, S. albus J1074 and S. venezuelae NRRL B-65442. Neighbor-joining tree shows distribution based on 243F and A3R amplified 16S rDNA samples from Streptomyces sp. A4420 strain. The root position was determined using Micrococcus lylae, Corynebacterium glutamicum and Mycrobacterium smegmatis as an outgroup. Bootstrap values are given as a percentage of 1000 replicates (only bootstrap values above 50% are shown). GenBank accession numbers are given in parentheses
Construction of Streptomyces sp. A4420 CH strain for heterologous production of natural products
To obtain the genome sequence of the Streptomyces sp. A4420 strain, its genomic DNA (gDNA) was extracted, sequenced, and assembled following a recently described method for hybrid long-short read assembly of Illumina and Oxford Nanopore sequencing data [37]. This approach provides a cost-effective alternative to traditional whole genome sequencing techniques, enabling the investigation of a broader range of organisms as potential chassis strains. The assembled genome was analyzed using AntiSMASH [38] (Table 1), resulting in the identification of 9 Type I, II and NRPS hybrid polyketide BGCs, including the streptazolin BGC (Additional File 1: Fig. S2), which could potentially compete with exogenous BGCs for production. To improve precursor flux and eliminate production of undesired natural products in Streptomyces sp. A4420, these 9 polyketide BGCs were knocked out according to their identified boundaries. Due to concerns regarding Cas9-induced toxicity, potential off-target effects, and genome instability resulting from double stranded breaks [39,40,41], as well as lack of a comprehensive CRISPR-based genetic toolkit in this strain, we chose to employ traditional recombination-based techniques for genetic engineering.
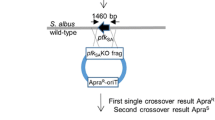
Two homology arms flanking the target sequence were PCR-amplified from the purified gDNA of the wild-type Streptomyces sp. A4420 strain (WT) and cloned into a pIJ101 plasmid pYH7 [42, 43] that had been linearized with NdeI and HindIII by using HiFi assembly. The strain demonstrated high efficacy for intergeneric conjugation with E. coli ET12567 harboring plasmid pUZ8002, and this system was efficiently applied for the introduction of pYH7 plasmid. To identify mutants with double cross-over events, individual colonies were isolated for crude gDNA extractions, and successful recombination events were validated by PCR (Additional File 1: Table S2). This strategy was successfully employed to sequentially knock out all 9 target BGCs (Table 1), generating a chassis strain Streptomyces sp. A4420 CH (hereon annotated as CH strain). The engineered strain retained sensitivity to apramycin, indicating elimination of pYH7 suicide plasmid in the last round of mutations, allowing apramycin selection to be used for subsequent expression of exogenous BGCs.
Biomass accumulation studies
Growth of the WT, CH, and three model chassis Streptomyces strains, S. lividans TK24, S. coelicolor M1152 and S. albus J1074, were compared in standard TSB media. Both WT and CH strains displayed a strong propensity for coagulation, therefore glass beads were added for agitation in all pre-culture and growth experiments. To accurately evaluate growth rates and minimize variations in initial density, inoculation was normalized based on colony forming unit (CFU), and dry biomass was quantified by freeze drying the collected samples. Under said conditions, both WT and CH displayed germination and growth rates similar to S. lividans TK24 strain and outperformed the other strains (Fig. 2a). In addition, WT and CH had the highest accumulated dry biomass, which peaked at 24 h before reaching a plateau at 39 h. The highest final biomass was observed for S. albus J1074, although it had the slowest germination and growth rate. No discernable differences in sporulation and growth were observed between WT and CH strains on SFM agar (Fig. 2b).
Biomass accumulation and morphological comparison studies. Growth curves for Streptomyces sp. A4420 (WT), Streptomyces sp. A4420 CH, S. lividans TK24, S. coelicolor M1152 and S. albus J1074 strains in TSB media supplemented with glass beads were established by plotting freeze dried cell pellet weight (a). Inoculation was normalized based on measured CFU of each spore stock. The S. venezuelae NRRL B-65442 strain was not used for cell growth experiments due to low observed sporulation. Solid phase growth of sporulation phenotype for Streptomyces sp. A4420 (WT) and Streptomyces sp. A4420 CH at was observed at 20, 34 and 59 h on SFM media (b)
Heterologous expression of exogenous BGCs
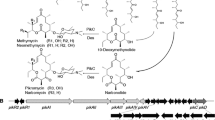
To evaluate the biosynthetic capacity of the CH strain, we selected four heterologous polyketide BGCs producing antibiotic and anti-fungal polyketides, including the well-studied actinorhodin [44] and erythromycin [45], together with the recently discovered auroramycin [46, 47] and bipentaromycin [48] (Fig. 3). The gene clusters were introduced into WT, CH, S. lividans TK24 [49], S. coelicolor M1152 [26], S. albus J1074 [50], and S. venezuelae NRRL B-65442 [51] via intergeneric conjugation using E. coli ET12567 containing a pUZ8002 plasmid donor. The auroramycin BGC, which has not previously been heterologously expressed, was cloned from S. roseosporus NRRL 15998 using the CAPTURE method [22] for this study. All BGCs were successfully conjugated with different efficiencies, as confirmed by PCR using internal primers (data not shown). Isolated exconjugants were inoculated into TSB media for pre-culture, followed by transfer into SFM and R5 fermentation media for a growth period of 7 days. Actinorhodin production rates were directly evaluated using a colorimetric assay [52], while other fermentation samples were freeze dried, extracted with methanol, and analysed by LC–MS. In general, production of the compounds by different strains varied between the two media. The CH strain produced all four compounds in both media (Fig. 4) (Additional File 1: Fig. S3–S15), and significantly outperformed the parental strain under all conditions, except for erythromycin in SFM media where both strains showed similar production levels.
Heterologous production level of actinorhodin, erythromycin, and auroramycin (a), as well as bipentaromycin in R5 and SFM (b, and c respectively) in Streptomyces sp. A4420 (WT), Streptomyces sp. A4420 CH, S. lividans TK24, S. coelicolor M1152, S. albus J1074, S. venezuelae NRRL B-65442 in R5 and SFM media. Bipentaromycin congeners A–H as well as different strains for actinorhodin, erythromycin and auroramycin are highlighted in different colors. To facilitate comparison, the production scale is normalized to the highest producer depicted as 100% yield for each condition (highlighted with star on top). All production levels are represented with a linear scale, using A640 absorption (actinorhodin), peak area in BPC + mode (erythromycin and auroramycin) and peak area using A280 absorption (bipentaromycin). Expanded version is available at Additional File 1: Fig. S18
For actinorhodin, S. coelicolor M1152 showed the highest production in SFM media, with M1152 and the CH strain being matched for the highest production in R5 media (Fig. 4a). The WT strain showed only 50% of the production levels of the CH strain in both media. Erythromycin production was detected for CH in both media, and a similar production level was detected for WT in SFM, but not in R5 media. Interestingly, no erythromycin production was detected in either of S. lividans TK24, S. coelicolor M1152, S. albus J1074 in both media, except for low levels in S. venezuelae NRRL B-65442 (Fig. 4a). Auroramycin production was observed for the CH strain, S. coelicolor M1152 and S. albus J1074 at different levels in both media (Fig. 4a), while only trace levels were observed for WT, S. lividans TK24 and S. venezuelae NRRL B-65442 in R5 media. Bipentaromycins are heterodimeric aromatic molecules comprising two distinctive pentacyclic ring systems, with two specific modification sites R1 and R2, allowing us to investigate the ability of the various strains to produce a range of congeners (A-H in Figs. 3, 4b, c). In R5 media, the highest production level was observed for S. albus J1074, followed by the CH and WT strains, with much lower or undetectable levels for the other strains. In SFM media, production of all seven congeners was observed for the CH and WT strains, with much lower or undetectable levels for the other strains.
Consequently, we proceeded to assess the performance and suitability of our engineered CH strain as a candidate for heterologous production based on 15 parameters, which encompass data from this study and previously published sources (Additional File 1: Table S4, Fig. S18 and S19). We introduced a scoring system to rank the performance of each strain across these individual parameters. To mitigate bias towards any single criterion, we summed the performance scores to create a heterologous fitness score. This allowed for a direct comparison of all the strains tested under the specific conditions in this study (Fig. 5). S. venezuelae NRRL B-65442, which is not commonly used as a heterologous host, exhibited a notably lower heterologous fitness score compared to the other strains. Surprisingly, S. lividans TK24, one of the commonly explored heterologous producers, scored lower than other strains. This outcome may be attributed to the specific nature of the BGCs that were tested.
Multi parameter evaluation and comparison of heterologous fitness score of Streptomyces sp. A4420 (WT), Streptomyces sp. A4420 CH, S. lividans TK24, S. coelicolor M1152, S. albus J1074, S. venezuelae NRRL B-65442 based on determined parameters (Additional File 1: Table S4)
Discussion
Actinobacteria offer a rich source of natural products for medical applications, and research into their secondary metabolism has contributed immensely to drug discovery. Their genomes host a multitude of BGCs, most of which remain dormant under standard lab conditions, suggesting that their full biosynthetic capabilities remain underexplored [3, 53]. Advances in DNA sequencing have expanded our knowledge of their native regulatory circuits and BGC organization, setting the stage for the exploration of new BGCs through heterologous expression. Streptomyces strains like S. coelicolor, S. lividans, S. albus, and S. avermitilis have been instrumental in discovering natural products, and in advancing our knowledge of their genetics, biochemistry, and physiology [19]. The recently developed CAPTURE direct cloning strategy enables large-scale cloning of BGCs from various Streptomyces strains, and has revealed the limited capacity of widely used heterologous hosts to functionally express exogenous gene clusters [22], with only approximately 16% of the studied natural product pathways being successfully expressed.
To broaden the selection of Streptomyces strains available as heterologous hosts, we investigated Streptomyces sp. A4420, which is a prolific producer of streptazolin, suggesting a strong potential for production of other polyketides as well. A 16S rRNA analysis revealed substantial divergence from well-characterized Streptomyces strains, prompting a comprehensive assessment of its genomics and metabolomics. To obtain the genomic sequence of the strain for engineering, we explored the cost-effective combination of Illumina and Oxford Nanopore sequencing [37]. AntiSMASH analysis [38] resulted in identification of the streptazolin cluster and other potentially competing polyketide BGCs, facilitating the construction of a Streptomyces sp. A4420-based CH strain by sequential knockouts of 9 annotated and potentially competing polyketide BGCs. Alternatively, comprehensive understanding native secondary metabolite biosynthetic pathways as well as of genetic elements responsible for growth cycle, genetic stability, intracellular energy dynamics and redox potential can be effectively used to pre-select and rationally design large deletions as demonstrated for Streptomyces chattanoogensis L10 [54]. However, determining core conserved regions as well as dispensable sub-telomeric areas is time consuming and was predominantly explored in model strains only [55]. The metabolically streamlined chassis strain exhibited no changes in sporulation or growth, and yielded comparable biomass in liquid culture.
To assess the potential of our engineered CH strain as a novel heterologous producer, we selected four diverse polyketides, including actinorhodin (benzoisochromanequinone, produced by a type II PKS), erythromycin (glycosylated, macrolide, produced by a type I PKS), auroramycin (glycosylated, polyene macrolactam, produced by a type I PKS) and bipentaromycin (heterodimeric aromatic polyketide, produced by a type II PKS). The engineered CH strain and parental strain were compared against well-established heterologous hosts such as S. coelicolor M1152, S. lividans TK24, S. albus J1074, and the model strain S. venezuelae NRRL B-65442 commonly used in morphological studies (Fig. 4). Variable conjugation rates were observed depending on the tested BGC, with bipentaromycin displaying the lowest efficiency, resulting in fewer than 10 exconjugants for each strain. The deletion of endogenous competing BGCs led to enhanced natural product production in the CH strain compared to the parental strain in all cases, except for a single condition involving the erythromycin BGC. This suggests that complete genome minimization may not be imperative for the development of a heterologous producer, at least in the context of improving the yields of the investigated compounds. Simultaneously, glycosylated polyketides like erythromycin and auroramycin were not detected in the parental Streptomyces sp. A4420 (except for erythromycin in SFM medium) but were successfully reconstituted in the metabolically simplified strain when cultured in R5 and SFM media, respectively.
Interestingly, erythromycin was undetectable in any of the model heterologous secondary metabolite expression strains, including S. lividans TK24, S. coelicolor M1152, and S. albus J1074, which led us to suspect that part of the BGC might have been lost due to recombination events. However, PCR analysis using five pairs of primers, annealing at different regions of the BGC, indicated that the cluster remained intact (Additional File 1: Figs. S16, S17). Indeed, heterologous expression of glycosylated erythromycin could not be reconstituted in S. coelicolor and S. lividans production hosts, only yielding intermediate metabolites [56]. Simultaneously, S. venezuelae NRRL B-65442 showed detectable levels of erythromycin, emphasizing that the issue likely stems from intrinsic regulatory circuits. It is worth noting that both S. lividans TK24 and S. coelicolor M1152 share many metabolic pathways and potentially regulatory circuits due to their close genetic relatedness [27]. This conclusion is supported by our 16S rRNA analysis, which revealed their similar phylogenetic niche. It further underscores the necessity for distinctively diverse heterologous hosts with a supply of unusual precursors and diverse regulatory systems.
S. coelicolor M1152 is a metabolically streamlined derivative of the strain M145. The parental strain is known for being a prolific producer of the pigmented antibiotic actinorhodin, and the derivative strain is equally prolific when the actinorhodin gene cluster in re-introduced [26, 57], while the closely related S. lividans strain demonstrates only moderate yields. This observation suggests that these strains possess well-established precursor flux, regulatory, and secretion systems for antibiotic resistance [52]. Thus, we anticipated that our engineered CH strain might encounter challenges with this particular gene cluster. Indeed, in SFM media, S. coelicolor M1152 exhibited significantly higher production levels. However, to our surprise, the production rates in R5 media were comparable between the CH and the latter S. coelicolor M1152 strain. To push the boundaries further, we selected the cryptic auroramycin BGC, spanning approximately 106 kb, from S. roseosporus NRRL 15 998 [46, 58]. Using our recently described CAPTURE method [22] and from our in-house strain library, we cloned and expressed this BGC heterologously. This approach enabled the decoupling of native regulatory elements, and the glycosylated polyketide was detectable in three of the tested strains, though at varying production levels. In this instance, the removal of competing BGCs from the parental Streptomyces sp. A4420 was indispensable for detecting and producing auroramycin, albeit at relatively low levels. The most structurally diverse BGC investigated in this study, bipentaromycin, could be effectively expressed only in S. avermitilis SUKA 17, as reported previously [48]. Both the parental and metabolically simplified CH strains demonstrated the capability to produce all main derivatives A to H at detectable levels compared to the other tested strains. Interestingly, the limitations in this case did not stem from a lack of available precursors but rather from the heterologous expression of modifying enzymes, such as Bpa9 and Bpa15, which give rise to hydroxylated and methylated derivatives. BGC arrangements are intricate, encompassing not only core biosynthetic genes but also regulatory circuits, transporters, and post-tailoring enzymes [59].
These findings affirm that, under specified conditions, each of the strains can serve as a highly efficient host for heterologous production of natural products. Our analysis suggests that simplifying the secondary metabolite profile of Streptomyces sp. A4420 significantly improved the heterologous fitness score (Fig. 5), surpassing even the performance of S. coelicolor M1152 and S. albus J1074 strains. While this analysis can be subject to the definition and criteria of the scoring system, it represents our initial attempt to develop a more comprehensive approach to visualize the impact of extensive strain selection and engineering. In the future, we aim to expand the range of tested conditions and refine our comparison strategy. This expansion should encompass recently engineered and validated strains such as S. lividans TK24 ΔYA11 [30], S. albus J1074 Del14 [31], and S. avermitilis SUKA strains [18]. Additionally, a broader array of BGCs and different culture media could be included to provide a more comprehensive performance evaluation.
Conclusion
In summary, we have discovered and engineered an alternative CH chassis strain derived from Streptomyces sp. A4420 for natural product biosynthesis. Both the engineered and parental strains exhibited faster growth rates and higher accumulated biomass than other commonly used Streptomyces strains. Compared to the parental strain, the engineered strain exhibited improved heterologous production of polyketide natural products, with yields on par with or surpassing those of commonly used strains under specific conditions. To facilitate a comprehensive comparison, we introduce a heterologous fitness score, which demonstrates that the engineered CH strain outperforms the parental strain under all evaluated criteria, and also compares favorably to commonly used strains, including S. albus J1074, S. coelicolor M1152, and S. lividans TK24. The favorable growth and production characteristics of this metabolically streamlined Streptomyces strain make it an attractive chassis strain for the discovery and production of polyketides, while further assessments are underway for other classes of natural products. In-depth genomic, transcriptional and translational analysis of the CH strain and the associated BGCs will aid in understanding the basis of its biosynthetic capacity for polyketides. This will also guide the continued genomic optimization of the CH strain, and development of its genome engineering tools and strategies, to enable its integration into the repertoire of commonly employed heterologous hosts.
Methods
Strains, plasmids, and culturing conditions
All the E. coli and Streptomyces strains, and plasmids used in this study are provided in Additional File 1: Table S1. Streptomyces sp. A4420 was obtained from private Natural Organism Library (NOL) collection housed within the Agency for Science and Technology (A*STAR, Singapore). S. coelicolor M1152, S. albus J1074 and S. venezuelae NRRL B-65442 were kindly provided by Jason Micklefield (University of Manchester, UK). NEB10β cell line was used for propagation of plasmids encoding heterologous BGC and E. coli ET12567 harboring pUZ8002 plasmid, for intergeneric conjugation with Streptomyces strains. E. coli strains were cultivated in LB (lysogeny broth) and 2xYT (Sigma-Aldrich, USA) for general cloning and conjugation respectively. Streptomyces strains were grown on soy flour mannitol (SFM) solid medium for sporulation (20 g soya flour, 20 g D-mannitol, 20 g agar, 1 L ddH2O) or tryptic soy broth (TSB) for pre-culture (Sigma-Aldrich, USA). Soy flour mannitol (MS) (20 g soya flour, 20 g D-mannitol, 1 L ddH2O) and R5 liquid medium [27] were used for heterologous BGC expression and natural product production. E. coli and Streptomyces strains were cultured at 37 °C and 30 °C respectively with 250 rpm agitation for liquid cultures. Antibiotics were used as following: apramycin (50 µg/mL), thiostrepton (25 µg/mL) and chloramphenicol (25 µg/mL).
Genomic DNA isolation, manipulation, and sequencing
Genomic DNA from Streptomyces was isolated using lysozyme-based lysis with phenol/chloroform extraction method. Full genome sequence was obtained according to recently described Illumina and Oxford Nanopore hybrid sequencing strategy [37]. Intergeneric conjugation between E. coli ET12567/pUZ8002 and Streptomyces strains was performed according to previously established protocols [27]. PrimeSTAR Max (Takara Bio, Japan) and GoTaq® Green (Promega, USA) polymerases were used for cloning and PCR verification, respectively. The primers used in the study are listed in Additional File 1: Table S3. PCR-amplified fragments were analyzed and purified from agarose gels using the QIAquick Gel Extraction Kit (Qiagen, Germany). All restriction enzymes and NEBuilder HiFi DNA assembly mastermix were used according to manufacturer’s recommendations (New England Biolabs, USA).
Biomass accumulation studies
Biomass accumulation was measured for all strains (except S. venezuelae NRRL B-65442) at 30 °C and 250 rpm agitation in 500 mL baffled flasks. 66 × 106 CFU of each spore stock was used to inoculate 100 mL TSB growth medium supplemented with 55 g, 5 mm glass beads (Sigma-Aldrich, USA). 1 mL of each culture was collected at respective time points in pre-weighted Eppendorf tubes and centrifuged at 5000×g for 10 min at 4 °C. Supernatant was discarded and cell pellets were washed twice with equal amount of ddH2O. Wet cell pellets were freeze-dried overnight, and dry biomass weight was measured using Ohaus PA 214C Pioneer Series Analytical Balance.
Construction of Streptomyces sp. A4420 CH strain for heterologous production of natural products
To generate a strain with desired genotype, sequential knock out steps were performed iteratively using pYH7 suicide plasmid and homologous recombination [42, 43] removing entire BGCs based on AntiSMASH prediction of the cluster boundaries. Streptomyces—E. coli shuttle vector pYH7 was digested with NdeI and HindIII followed by HiFi cloning with the two flanking homology arms amplified by PCR to generate pYH7_XW1 (Additional File 1: Table S1). The verified construct was transformed to E. coli ET12567/pUZ8002 strain followed by conjugation with Streptomyces sp. A4420 strain. Thiostrepton and apramycin resistant exconjugants were initially selected on SFM media supplemented with 10 mM MgCl2. After sporulation double cross-over mutants were selected based on apramycin and thiostrepton sensitivity, yielding XW1 strain (Additional File 1: Table S2). The genotype of mutants was confirmed using PCR analysis of amplified bands. Knock out process was repeated with the latter strain and subsequently generated strains until the last mutant was acquired with the genotype containing all selected mutations.
Heterologous production and extraction of natural products
Spores harboring heterologous BGCs were used to inoculate 30 mL TSB supplemented with 12.5 mL 5 mm glass beads. The pre-culture was grown for 24–36 h at 30 °C, 250 rpm until cloudy culture observed, and 50 mg of wet biomass used to inoculate 25 mL fermentation media in triplicates. Strains were incubated for 7 days at 30 °C and 250 rpm agitation. The metabolites were extracted as following: actinorhodin fermentation samples were mixed with 25 mL 1 M KOH (1:1 ratio) according to previously established protocol [52]. For erythromycin, bipentaromycin and auroramycin the whole culture was freeze-dried until all moisture was removed. 35 mL of MeOH was added to dry cultures and extracted using sonication bath at room temperature for 30 min. Samples then vortexed for 30 s and soluble supernatant directly injected into LCMS.
Analysis and evaluation of natural products
Actinorhodin samples were measured directly at 640 nm after addition of 1 M KOH. 10 µL of erythromycin, bipentaromycin and auroramycin extracts were separated by using Thermo Scientific Ultimate3000 RS system equipped with a Phenomenex Kinetex 2.6 µm XB-C18 100 Å (150 mm × 4.6 mm) and running the following program: acetonitrile + 0.1% formic acid against water + 0.1% formic acid from 5 to 50% (0–15 min), 50 to 100% (15–20 min), 100% (isocratic elution, 20–24 min), 100 to 5% (24–25 min), 5% (isocratic elution, 25–30 min) at 0.6 mL/min flow rate. Eluents were analyzed using Bruker amaZon SL mass spectrophotometer system. Mass spectra were acquired in centroid mode ranging from 100 to 2000 m/z at a 2 Hz scan rate. Production of target natural products and yields were evaluated as following: erythromycin peak was identified based on the acquired standard and relative yields were compared using BPC + mode; bipentaromycin peaks were identified based on previously determined m/z data [48] and yields were compared using A280 absorption; auroramycin peak was identified using a combination of native activated producer and m/z data published [46], and relative yields were evaluated using BPC + mode.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
van Bergeijk DA, Terlouw BR, Medema MH, van Wezel GP. Ecology and genomics of Actinobacteria: new concepts for natural product discovery. Nat Rev Microbiol. 2020;18(10):546–58.
Lo Grasso L, Chillura-Martino D, Alduina R. Production of antibacterial compounds from actinomycetes. In Actinobacteria-basics and biotechnological applications. London: InTech; 2016.
Watve MG, Tickoo R, Jog MM, Bhole BD. How many antibiotics are produced by the genus Streptomyces? Arch Microbiol. 2001;176(5):386–90.
van der Meij A, Worsley SF, Hutchings MI, van Wezel GP. Chemical ecology of antibiotic production by actinomycetes. FEMS Microbiol Rev. 2017;41(3):392–416.
Sierra-Paredes G, Sierra-Marcuno G. Ascomycin and FK506: pharmacology and therapeutic potential as anticonvulsants and neuroprotectants. CNS Neurosci Ther. 2008;14(1):36–46.
Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19(3):373–9.
Platon VM, Dragoi B, Marin L. Erythromycin formulations—a journey to advanced drug delivery. Pharmaceutics. 2022;14(10):2180.
Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65(2):232–60.
Alam K, Mazumder A, Sikdar S, Zhao YM, Hao J, Song C, Wang Y, Sarkar R, Islam S, Zhang Y, Li A. Streptomyces: the biofactory of secondary metabolites. Front Microbiol. 2022;13: 968053.
Zhang MM, Qiao Y, Ang EL, Zhao H. Using natural products for drug discovery: the impact of the genomics era. Expert Opin Drug Discov. 2017;12(5):475–87.
Walsh CT, Fischbach MA. Natural products version 2.0: connecting genes to molecules. J Am Chem Soc. 2010;132(8):2469–93.
Ren H, Wang B, Zhao H. Breaking the silence: new strategies for discovering novel natural products. Curr Opin Biotechnol. 2017;48:21–7.
Xu W, Klumbys E, Ang EL, Zhao H. Emerging molecular biology tools and strategies for engineering natural product biosynthesis. Metab Eng Commun. 2020;10: e00108.
Luo Y, Li BZ, Liu D, Zhang L, Chen Y, Jia B, Zeng BX, Zhao H, Yuan YJ. Engineered biosynthesis of natural products in heterologous hosts. Chem Soc Rev. 2015;44(15):5265–90.
Zhang MM, Wang Y, Ang EL, Zhao H. Engineering microbial hosts for production of bacterial natural products. Nat Prod Rep. 2016;33(8):963–87.
Borodina IKP, Nielsen J. Genome-scale analysis of Streptomyces coelicolor A3(2) metabolism. Genome Res. 2005;15(6):820–9.
Ke J, Yoshikuni Y. Multi-chassis engineering for heterologous production of microbial natural products. Curr Opin Biotechnol. 2020;62:88–97.
Komatsu M, Komatsu K, Koiwai H, Yamada Y, Kozone I, Izumikawa M, Hashimoto J, Takagi M, Omura S, Shin-ya K, et al. Engineered Streptomyces avermitilis host for heterologous expression of biosynthetic gene cluster for secondary metabolites. ACS Synth Biol. 2013;2(7):384–96.
Nepal KK, Wang G. Streptomycetes: surrogate hosts for the genetic manipulation of biosynthetic gene clusters and production of natural products. Biotechnol Adv. 2019;37(1):1–20.
Kang HS, Kim ES. Recent advances in heterologous expression of natural product biosynthetic gene clusters in Streptomyces hosts. Curr Opin Biotechnol. 2021;69:118–27.
Malcolmson SJ, Young TS, Ruby JG, Skewes-Cox P, Walsh CT. The posttranslational modification cascade to the thiopeptide berninamycin generates linear forms and altered macrocyclic scaffolds. Proc Natl Acad Sci U S A. 2013;110(21):8483–8.
Enghiad B, Huang C, Guo F, Jiang G, Wang B, Tabatabaei SK, Martin TA, Zhao H. Cas12a-assisted precise targeted cloning using in vivo Cre-lox recombination. Nat Commun. 2021;12(1):1171.
Huo L, Hug JJ, Fu C, Bian X, Zhang Y, Muller R. Heterologous expression of bacterial natural product biosynthetic pathways. Nat Prod Rep. 2019;36(10):1412–36.
Shima JHA, Okamoto S, Kawamoto S, Ochi K. Induction of Actinorhodin production by rpsL (encoding ribosomal protein S12) mutations that confer streptomycin resistance in Streptomyces lividans and Streptomyces coelicolor A3(2). J Bacteriol. 1996;178(24):7276–84.
Hu H, Zhang Q, Ochi K. Activation of antibiotic biosynthesis by specified mutations in the rpoB gene (encoding the RNA polymerase beta subunit) of Streptomyces lividans. J Bacteriol. 2002;184(14):3984–91.
Gomez-Escribano JP, Bibb MJ. Engineering Streptomyces coelicolor for heterologous expression of secondary metabolite gene clusters. Microb Biotechnol. 2011;4(2):207–15.
Kieser TBMJ BM, Charter KF, Hopwood D. Practical Streptomyces genetics. Norwich: John Innes Foundation. 2000.
Penn J, Li X, Whiting A, Latif M, Gibson T, Silva CJ, Brian P, Davies J, Miao V, Wrigley SK, Baltz RH. Heterologous production of daptomycin in Streptomyces lividans. J Ind Microbiol Biotechnol. 2006;33(2):121–8.
Novakova R, Nunez LE, Homerova D, Knirschova R, Feckova L, Rezuchova B, Sevcikova B, Menendez N, Moris F, Cortes J, Kormanec J. Increased heterologous production of the antitumoral polyketide mithramycin A by engineered Streptomyces lividans TK24 strains. Appl Microbiol Biotechnol. 2018;102(2):857–69.
Ahmed Y, Rebets Y, Estevez MR, Zapp J, Myronovskyi M, Luzhetskyy A. Engineering of Streptomyces lividans for heterologous expression of secondary metabolite gene clusters. Microb Cell Fact. 2020;19(1):5.
Myronovskyi M, Rosenkranzer B, Nadmid S, Pujic P, Normand P, Luzhetskyy A. Generation of a cluster-free Streptomyces albus chassis strains for improved heterologous expression of secondary metabolite clusters. Metab Eng. 2018;49:316–24.
Bilyk B, Luzhetskyy A. Unusual site-specific DNA integration into the highly active pseudo-attB of the Streptomyces albus J1074 genome. Appl Microbiol Biotechnol. 2014;98(11):5095–104.
Ng SB, Kanagasundaram Y, Fan H, Arumugam P, Eisenhaber B, Eisenhaber F. The 160K Natural Organism Library, a unique resource for natural products research. Nat Biotechnol. 2018;36(7):570–3.
Monciardini P, Sosio M, Cavaletti L, Chiocchini C, Donadio S. New PCR primers for the selective amplification of 16S rDNA from different groups of actinomycetes. FEMS Microbiol Ecol. 2002;42(3):419–29.
Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38(7):3022–7.
Saitou NNM. The neighbor-joining method a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–25.
Heng E, Tan LL, Tay DWP, Lim YH, Yang LK, Seow DCS, Leong CY, Ng V, Ng SB, Kanagasundaram Y, et al. Cost-effective hybrid long-short read assembly delineates alternative GC-rich Streptomyces hosts for natural product discovery. Synth Syst Biotechnol. 2023;8(2):253–61.
Medema MH, Blin K, Cimermancic P, de Jager V, Zakrzewski P, Fischbach MA, Weber T, Takano E, Breitling R. antiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011;39(Web Server issue):W339–46.
Tong Y, Weber T, Lee SY. CRISPR/Cas-based genome engineering in natural product discovery. Nat Prod Rep. 2019;36(9):1262–80.
Tong YWC, Robertsen HL, Blin K, Jørgensen TS, Klitgaard AK, Gren T, Jiang X, Weber T, Lee SY. Highly efficient DSB-free base editing for streptomycetes with CRISPR-BEST. Proc Natl Acad Sci U S A. 2019;116(41):20366–75.
Alberti F, Corre C. Editing streptomycete genomes in the CRISPR/Cas9 age. Nat Prod Rep. 2019;36(9):1237–48.
He Q, Li L, Yang T, Li R, Li A. Functional characterization of a Ketoreductase-encoding gene med-ORF12 Involved in the formation of a Stereospecific Pyran Ring during the biosynthesis of an antitumor antibiotic medermycin. PLoS ONE. 2015;10(7): e0132431.
Sun Y, He X, Liang J, Zhou X, Deng Z. Analysis of functions in plasmid pHZ1358 influencing its genetic and structural stability in Streptomyces lividans 1326. Appl Microbiol Biotechnol. 2009;82(2):303–10.
Rudd BAHD. Genetics of actinorhodin biosynthesis by Streptomyces coelicolor A3(2). J Gen Microbiol. 1979;114(1):35–43.
Weber JMWC, Hutchinson CR. Genetic analysis of erythromycin production in Streptomyces erythreus. J Bacteriol. 1985;164(1):425–33.
Lim YH, Wong FT, Yeo WL, Ching KC, Lim YW, Heng E, Chen S, Tsai DJ, Lauderdale TL, Shia KS, et al. Auroramycin: a potent antibiotic from Streptomyces roseosporus by CRISPR-Cas9 activation. ChemBioChem. 2018. https://doi.org/10.1002/cbic.201800266.
Yeo WL, Heng E, Tan LL, Lim YW, Ching KC, Tsai DJ, Jhang YW, Lauderdale TL, Shia KS, Zhao H, et al. Biosynthetic engineering of the antifungal, anti-MRSA auroramycin. Microb Cell Fact. 2020;19(1):3.
Huang C, Cui H, Ren H, Zhao H. Investigation of the biosynthetic mechanism of bipentaromycin featuring an unprecedented cyclic head-to-tail dimeric scaffold. JACS Au. 2023;3(1):195–203.
Ruckert C, Albersmeier A, Busche T, Jaenicke S, Winkler A, Friethjonsson OH, Hreggviethsson GO, Lambert C, Badcock D, Bernaerts K, et al. Complete genome sequence of Streptomyces lividans TK24. J Biotechnol. 2015;199:21–2.
Zaburannyi NRM, Ostash B, Fedorenko V, Luzhetskyy A. Insights into naturally minimised Streptomyces albus J1074 genome. BMC Genomics. 2014;15:97.
Som NF, Heine D, Holmes NA, Munnoch JT, Chandra G, Seipke RF, Hoskisson PA, Wilkinson B, Hutchings MI. The conserved actinobacterial two-component system MtrAB coordinates chloramphenicol production with sporulation in Streptomyces venezuelae NRRL B-65442. Front Microbiol. 2017;8:1145.
Xu Y, Willems A, Au-Yeung C, Tahlan K, Nodwell JR. A two-step mechanism for the activation of actinorhodin export and resistance in Streptomyces coelicolor. MBio. 2012;3(5):e00191-e212.
Hoskisson PA, Seipke RF. Cryptic or silent? The known unknowns, unknown knowns, and unknown unknowns of secondary metabolism. MBio. 2020;11(5):e02642-e2720.
Bu QT, Yu P, Wang J, Li ZY, Chen XA, Mao XM, Li YQ. Rational construction of genome-reduced and high-efficient industrial Streptomyces chassis based on multiple comparative genomic approaches. Microb Cell Fact. 2019;18(1):16.
Bu QT, Li YP, Xie H, Wang J, Li ZY, Chen XA, Mao XM, Li YQ. Comprehensive dissection of dispensable genomic regions in Streptomyces based on comparative analysis approach. Microb Cell Fact. 2020;19(1):99.
Fayed B, Ashford DA, Hashem AM, Amin MA, El Gazayerly ON, Gregory MA, Smith MC. Multiplexed integrating plasmids for engineering of the erythromycin gene cluster for expression in Streptomyces spp. and combinatorial biosynthesis. Appl Environ Microbiol. 2015;81(24):8402–13.
Bystrykh LV, Fernández-Moreno M, Herrema JK, Malpartida F, Hopwood DA, Dijkhuizen L. Production of actinorhodin-related blue pigments by Streptomyces coelicolor A3(2). J Bacteriol. 1996;178(8):2238–44.
Wong JH, Alfatah M, Kong KW, Hoon S, Yeo WL, Ching KC, Jie Hui Goh C, Zhang MM, Lim YH, Wong FT, Arumugam P. Chemogenomic profiling in yeast reveals antifungal mode-of-action of polyene macrolactam auroramycin. PLoS ONE. 2019;14(6): e0218189.
Risdian C, Mozef T, Wink J. Biosynthesis of polyketides in Streptomyces. Microorganisms. 2019;7(5):124.
Acknowledgements
We would like to thank Prof. Jason Micklefield and Prof. Mervyn Bibb for providing the S. coelicolor M1152, S. albus J1074 and S. venezuelae NRRL B-65442 strains and Dr. Siew Bee Ng for providing Streptomyces sp. A4420.
Funding
We gratefully acknowledge financial support from the National Research Foundation, Singapore (NRF-CRP19-2017-05-00, EK, WX, LK, EH, FTW, and ELA), and Agency for Science, Technology and Research (A*STAR), Singapore (C211917003, FTW and LK), and the U.S. National Institutes of Health (AI144967 to HZ) for this work.
Author information
Authors and Affiliations
Contributions
X.W., E.L.A and H.Z. conceived the project and designed the experiments, E.K designed the experiments, E.K, X.W., E.H., and F.T.W. conducted the wet lab experiments, L.K. and Y.W. conducted the bioinformatics studies. E.K. wrote the paper with input from all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict or competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary tables and figures.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Klumbys, E., Xu, W., Koduru, L. et al. Discovery, characterization, and engineering of an advantageous Streptomyces host for heterologous expression of natural product biosynthetic gene clusters. Microb Cell Fact 23, 149 (2024). https://doi.org/10.1186/s12934-024-02416-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-024-02416-y