Abstract
Background
In this study, the effect of static magnetic fields (SMFs) on improving the performance of activated sludge process to enhance the higher rate of microbial growth biomass and improve sludge settling characteristics in real operation conditions of wastewater treatment plants has been investigated. The effect of SMFs (15 mT), hydraulic retention time, SRT, aeration time on mixed liquor suspended solids (MLSS) concentrations, mixed liquor volatile suspended solids (MLVSS) concentrations, α-factor, and pH in the complete-mix activated sludge (CMAS) process during 30 days of the operation, were evaluated.
Results
There were not any differences between the concentration of MLSS in the case (2148.8 ± 235.6 mg/L) and control (2260.1 ± 296.0 mg/L) samples, however, the mean concentration of MLVSS in the case (1463.4 ± 419.2 mg/L) was more than the control samples (1244.1 ± 295.5 mg/L). Changes of the concentration of MLVSS over time, follow the first and second-order reaction with and without exposure of SMFs respectively. Moreover, the slope of the line and, the mean of α-factor in the case samples were 6.255 and, − 0.001 higher than the control samples, respectively. Changes in pH in both groups of the reactors were not observed. The size of the sluge flocs (1.28 µm) and, the spectra of amid I' (1440 cm−1) and II' (1650 cm−1) areas related to hydrogenase bond in the case samples were higher than the control samples.
Conclusions
SMFs have a potential to being considered as an alternative method to stimulate the microbial growth rate in the aeration reactors and produce bioflocs with the higher density in the second clarifiers.
Similar content being viewed by others
Introduction
Activated sludge (AS) process is the most well-known and worldwide technic for the wastewater treatment. This process has been commonly applied to remove pollutants by a microbial community of the active biomass [14, 50]. Microbial communities has a basic role on the operation of the wastewater treatment plants (WWTPs). The stability of present this kind of organisms is related to design the process, wastewater characteristics, the operational factors and, conditions of the environmental [11].
There are various modifications of AS processes for wastewater treatment, such as the complete-mix activated sludge (CMAS) process. In CMAS, the suspended growth of the microorganisms has the most critical component in the aeration reactors and, the operation of a system [7].
The aerobic methods which are used for any type of the wastewater treatment have disadvantages. Bulking of the sludge in the clarifier, due to the overgrowth of filamentous bacteria is well-known problem [10]. Rising costs of sludge disposal and, the risk of the presence of toxic materials in unsanitary disposal of solids in the environment, are the other disadvantages of this process [41].
Nowadays, for improving bio-chemical reactions, application of new combination methods such as nonosheets for active enzyems of cells [18], biochar to bioremediation of pollutants [52] and, removal contamination of the sewage sludge by use of biochar [23] have been considered by many researchers. The improvement in the AS process in terms of the higher sludge settling by a combination of the new technics is the target of many studies, too [21]. An important point in the operation of AS processes is the higher gravity settling of the bioflocs in the secondary clarifiers. Higher separation of the flocs from the liquid phase in the clarifier basins has a basic effect on the effectivness of the sludge removal [8].
The application of magnetic fields (MFs) and the growth rate of the microorganisms is an interesting discussion. Recently, studies focused on the positive effect of MFs on stimulating the growth rate of microorganisms in term of higher biodegradation of the organic components in the wastewater treatment processes [56]. A classification of MFs is according to the frequency of the electric current. If the frequency of the electric current is 0 Hz (no change is observed in the intensity overtime) the generated MFs are called static magnetic fields (SMFs) and, when the electric current has a frequency more than 0 Hz, the generated MFs are called dynamic MFs [54].
MFs are an alternative method for the treatment of wastewater [56]. Some of the fundamental properties of the liquids are viscosity, density and, tension of the surface. These components are affected when exposed to MFs that are present in the surrounding [44]. Łebkowska et al. summarized the effect of SMFs on living things in 9 categories. Changes in the properties of water, pH, higher rate of coagulation and, settling of solids are certain of them [30].
Improvement of the biomass and settling of sludge has been studied by many researchers when the application of MFs in AS is considered [39, 51]. In the process of the wastewater treatment, more growth of the microorganisms is a critical point to degradation of the organic compounds. In other words, the higher growth rate of microorganisms is equal to the higher consumption of the organic materials [56].
Many researchers are interested to use non-chemical techniques for the removal pollutants according to the side effects of the chemical materials on the environment. Non-thermal plasma catalysis [32], adsorption of the colloidal suspended particles for removal pollutants [4] and exposure of SMFs to treatment of wastewater [35] are the new aspects.
Based on available articles, there are few scientific reports on the discussion in course of the biological and the chemical changes in the components of the real CMAS process with the application of SMFs. In this case–control study, the impact of SMFs on temperature, pH, α-factor (dissolved oxygen transfer rate into the biomass), the concentration of mixed liquor suspended solids (MLSS) and, mixed liquor volatile suspended solids (MLVSS) in the aeration reactors of CMAS during the 30 days operation Lab-scale in order to estimated the role of SMFs on the optimization of oxygen transfer in the aeration reactor have been considered. Moreover, stimulate the microbial biomass growth rate and, generate the sludge flocs with higher density and, increasing the rate of the efficiency of process for removing the sludge flocs in the clarifier reactor, were the other main topics of this research.
Materials and methods
Source of wastewater sampling
All samples of this study were taken from the effluent of the primary clarifier of CMAS process in Sanandaj (a city located in the west of Iran) wastewater treatment plants (WWTPs) during the summer of 2022 and sent to a small feeding container (40 cm × 100 cm × 100 cm = 40 L) for distribution among the two series reactors.
Experimental setup
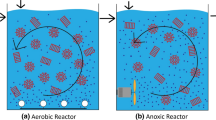
The setup of this study consisted of the two series reactors (feeding and distribution containers, aeration and settling devices, air and peristaltic pumps, and sludge return systems). These devices and other accessories such as DC power, valves, and a solenoid are illustrated in Fig. 1.
It must be mentioned that, the design parameters of Lab-scale reactors (just like CMAS) were according to the real wastewater treatment plants and effluent guideline criteria [7]. Therefore, the rate of flow (mL/min) into the aeration reactors when microbial retention time was 5 days can be calculate using the following equation:
S0 is primary BOD5 (mg/L), S is the effluent BOD5 (mg/L), Kd is a kinetic coefficient which is equal to 0.06 d−1 and, \(\Theta\)c is the microbial retention time (d), X is MLVSS in the aeration tank (mg/L), V is the volume of the reactors (L), Y is a degradable portion of the organic materials (kg VSS/kg COD), Q is the flow rate of wastewater (mL/min) [7].
Hydraulic retention time (HRT) was estimated using the following equation:
The amount of 40 L of the wastewater from the primary sedimentation outlet of the WWTPs was transferred to the feeding reactor and distributed into the two series reactors (case and control), daily. By application of a digital pH meter (827 pH Lab Meter), a digital DO meter (HQ30D–Multi/1Channel) and, a digital infrared thermometer (Testo 831) the desired parameters were measured. The concentration of MLSS, MLVSS measured based on the standard methods, daily.
Type of aeration system
Aeration and agitation of MLSS in the aeration reactors are carried out by an electric ambient air pump. Diffuser aeration has two main benefits in this study. Provided oxygen between 2 and 3 mg/L that was regulated by airflow meter (Yokogawa RAGL 41 Laboratory Rotameter) during the operation periods (30 days) and, mix the contents of the aeration reactors with uniform distribution of air by stone air diffusers (0.6 ± 0.1 L/min) to obtain complete-mix conditions. DO concentration in the aeration reactors was measured by DO meter (Hach HQ30D) daily.
The rate of DO concentration in the aeration basin of AS must be 1.5–4 mg/L [7].
Static magnetic fields generation
DC power (DAZHENG PS-305D) was used for SMFS generation in solenoid at the lab-scale. The number of turns of the coil was 750 rounds (0.5 mm thickness) in three rows and, they were wrapped around a sheet of the galvanized iron in order to the ever-increasing intensity of MFs. Based on the measurement by Tesla meter (GM-511 Polytronic), the intensity of generated MFs was 15 mT.
It must be mentioned that the field intensity of 15 mT was chosen because it is a intensity that can be easily produced in the laboratory using a DC power device, and it has been determined in past studies that this field intensity can stimulate the growth rate of the microorganisms.
Seeding the aeration reactors
At the beginning of the processes, seeding of the aeration reactors as initial inoculum was done by MLSS (1750 ± 100 mg/L) from the effluent of the primery clarifier of WWTPs. For this reason, a half volume of the aeration reactors (1250 mL) was filled with MLSS. We have to do seeding the reactors because, even after 30 days of the starting the reactors without seeding, the mean concentration of MLSS in the case and control samples were only, 176.8 ± 118.9 and 120.3 ± 73.7 (mg/L), respectively.
The concentrations of MLSS that is recommended for the CMAS process are from 2500 to 6000 (mg/L) [7].
Design of experiment
A basic parameter that has the main effect on the CMAS process is the number of the microorganisms in the bioreactor or the aeration reactor. Therefore, in terms of evaluating the efficiency of the process, the concentration of MLVSS in the aeration reactors is the key factor. The time required to reach this concentration (at least 2500 mg/L) is called start-up time and, this takes 7–28 days [7]. The flow rate of return sludge from a secondary clarifier reactor has the main effect on MLVSS concentration and performance the aeration reactors and, is calculated as the following equation:
100–150% of average flow rate is suggested to be returned to the aeration reactors in AS process [38].
In Table 1, Design parameters of CMAS reactors (\(\Theta\)c, HRT, Q, the aeration flow rate, and 100 Qr/Q) are illustrated.
In this study, 100% of the sludge was returned to the aeration reactors by the peristaltic pumps. In Table 2. The mean of the fundamental parameters in the feeding container and, the case and control samples with and without seeding are illustrated.
Results and discussion
The main goal of this study was answer to this question that by exposure of 15 mT on the aeration reactor of CMAS what happened to the mean of temperature, DO concentration, oxygen mass transfer rate, pH, MLSS and MLVSS concentration in the aeration reactor and, flocs density and bonds structures of the settled sludge in the clarifiers in the case and control samples.
Change of the temperature in the reactors
The community of the micrroorganisms in the AS is related to the optimum temperature of the environmrnt and liquied phase and has the main effect on the microbial growth rate [49]. Nevertheless, some of the researchers reported that the effect of the temperature (in the low range of changes, especially) on the category of kind and community of bacteria, is not noticeable. The community of microorganisms can be affected by other main factors, such as pH, total phosphorus concentration, and, BOD loading rate than the temperature [34, 53].
The differences between the temperature (°C) of the wastewater in the feeding container, case samples (after exposure of SMFs for one hour) and, control samples (without exposure of SMFs) during the time of the operation is demonstrated in Fig. 2.
There was a statistical difference (p < 0.05) between the mean temperature of MLSS in the feeding container (22.86 ± 0.80 °C) and, the case (29..42 ± 2.34 °C) samples, and, between the case and control (23.70 ± 0.74 °C) samples. However, there weren’t any differences between the mean of feeding container temperature with control samples.
In the operation of the biological wastewater treatments processes, the maintenance of temperature in the recommended ranges is the basic challenge [40]. Furthermore, as the pattern of the flow rate in our study was continuous, the mean temperature in the control samples was only 0.88 °C more than feeding container.
Due to the passage of direct current (DC) through the solenoid or wiers and, the generation of the internal resistance, heat generation is inevitable. This mechanism is known as Joule heating [31]. Since the only difference between three groups of samples was the exposure of the SMFs, it can be concluded that the application of SMFs causes the higher temperature in the case samples 6.56 °C and, 5.72 °C more than the feeding container and the control samples, respectively. However, increasing the reactor temperature can affect the dissolution of dissolved oxygen in bioreactors, too.
Finally, it seems that the interaction between the SMFs in the increasing the liquid temperature of the aeration reactors in the short period of time and, according to the continuous flow pattern rate and, keeping the concentration of dissolved oxygen between 2 and 3 mg/L during the operation of the systems in the aeration reactors, has no significant effect on the mean concentration rate of MLVSS in the case samples.
DO concentration in the aeration reactors
The concentration of oxygen in water is called dissolved oxygen (DO) and, in the wastewater treatment processes, DO has basic role on the community and physiology of microorganisms [22]. It has been proven that DO concentration is a factor that can limit the community and distribution of the microorganisms [47]. Nevertheless, providing oxygen in the aeration reactor is one of the nain challenge and energy consumption step in WWTPs operation. Each process that could improve oxygenation efficiency, has a positive effect on the reduce the total cost of wastewater treatment [33].
The mean DO concentration in the case samples was 2.6 ± 0.32 (mg/L) and, in the control, samples was 2.6 ± 0.31 (mg/L), during the operation of reactors, intentionally. The mean of this parameter in the feeding container was 0.38 ± 0.15 (mg/L). Based on statistical analysis, there were differences between the DO concentration in the feeding container compared to the case and control samples (p < 0.05), Nevertheless, this difference between the case and control samples was not observed (p = 1.000). The concentration of DO (mg/L) in the feeding container and the aeration reactors is demonstrated in Fig. 3.
One of the main aim of this study was to increase the transfer of DO from the wastewater into the biomass by applying SMFs to make the optimal use of oxygen in the aeration reactor at the proposed concentration of DO (2–4 mg/L), which is discussed in the following section.
O2 concentration in the aeration reactors with application of the SMFs increases, as by exposure of SMFs surface tension of the solution decresed and transmission of O2 from solution into the air decrease [20].
Oxygen mass transfer (OMT)
Some parameters can affect the rate of oxygen mass transfer (OMT) into the biomass in the aeration processes, such as biomass concentration, type and rate of the aeration, hydrodynamic qualification, solids retention time (SRT) and, biofilms conditions [15, 46]. OMT and volumetric OMT coefficient (KLa) are the two most important parameters to evaluate biofilms quality in the wastewater treatment processes [17, 33]. In this way, KLa and α-factor (0.25–0.65) are used to estimate the OMT quality in the wastewater [42].
In AS process, MLSS (mg/L) is the main propellant agent for controlling KLa and α-factor. MLSS must be between 10 and 15 g/L to have a basic efficiency in the transfer of oxygen into the biomass [33]. The range of α-factor on air diffusers is 0.3–0.85 [6].
α-factor can be estimated from the following equation:
KLa is a volumetric oxygen transfer coefficient (1/h).
α-factor can be estimated according to SRT as following equation:
As consumption of substrate by the microorganisms happened during the operation of the systems, and increasing SRT, so the rate of α-factor increases with increasing SRT [5]. Following equation is suggested to measure this relationship [19]:
There is a linear relationship between the α-factor, and SRT. The relationship between three main parameters (MLVSS, α-factor and SRT) in the case samples of the aeration reactor is shown in Fig. 4.
The correlation between MLVSS (g/L) and α-factor is positive non-linear equation (y = 1147.4e0.0235x). However, this correlation with SRT (d) is positive and linear equation (y = 0.019x + 0.533).
In Fig. 5 the relationship between MLSS, α-factor, and SRT in the control samples is demonstrated.
The patern of the relationship between the MLVSS, α-factor, and operation time of the system in the control samples was look like the case samples, too. By exposure of MFs in the biological processes, the rate of oxygen transfer into the cell of the microorganisms increases. This impact was on the increasing amount of α-factor. At the beginning of the processes, the amount of α-factor in both reactors was 0.45 and over time increased. However, the increase of α-factor at the end of one-month operation of the systems in the case reactor was 0.96 and, in the control samples was 0.98 (slop of line in both groups was 0.019). The mean of α-factor in the case samples only 0.01 unit was less than the mean of that in the control samples.
The aeration method in WWTPs is a costly process (about 15–49% of total energy consumed by a plant) and, saving energy, especially in the discussion of the increasing the efficiency of oxygen to generate higher biomass, must be considered [12]. Nowadays, the operation of the aeration reactors with a low concentration of DO for saving energy suggested [13].
Baquero-Rodríguez et al. argued that the concentration of TSS (total suspended solids) has fundamental effect on the α-factor. when it was less than 6 g/L there is a positive relationship between them and, higher than 6 g/L of TSS has negative effect on the α-factor [6]. Inhibitory of substances in term of the transfer oxygen into the biosorption, suggested for this limitation [1].
Changes of pH in the aeration reactors
Water characteristics such as electrical conductivity and pH could be affected by exposure of MFs [36].
As MFs stimulated the rate of the bacterial growth rate in the wastewater, a fall in pH in the solution or the external of bacteria (such as E. coli) by decomposition of glucose, happened [45]. So it seems that the changes in wastewater pH are unimpressive when the intensity of SMFs is 15 mT and the flow pattern is continuous.
Based on statistical analysis, no significant changes were observed in the pH of all samples in the aeration reactors (p > 0.05). Changes in pH value during 30 days of the operation in the feeding container, the case, and, control samples in the aeration reactors are illustrated in Fig. 6.
It must be considered that the intensity of 15 mT of SMFs for one hour in the continuous flow pattern of CMAS, was not in such a way to cause major changes in the pH of the solution between the case and, control samples. According to this, there is no need to adjust the pH of MLSS in the aeration reactors, as the SMFs did not change the pH of aeration contents more than the recommended range.
Moreover, the typical pH for the most biological processes is 6–9 [7].
Mixed liquor suspended solids (MLSS)
According to the result of the analysis of the data in terms of evaluation of MLSS in the aeration reactors, the concentration of MLSS (mg/L) in the case and, control samples was not statistically different (p = 0.107), as shown in Fig. 7. Nevertheless, In both reactors, the concentration of MLSS increased, daily.
Improve of MLSS concentration in the aeration reactors is based upon first-order kinetic, however, changes of this concentration in the reactor with the application of SMFs were similar to second-order kinetic.
At the beginning of the experiments (first 10 days), the changes in MLSS concentration were similar in both reactors. Moreover, a trend changes of MLSS concentration in the second and third 10 days was growing. This correlation is illustrated in Fig. 8.
The relationship between MLSS, pH, and temperature in both reactors is illustrated in Fig. 9. The red dots of the places indicate the concentration of MLSS. As it is clear, at a temperature of about 30 (°C) and pH around 7.8, the concentration of MLSS was more than the other area in the case samples (Fig. 9 on the left). Nevertheless, all the red dots (concentration of MLSS) for the control samples, accumulated at temperatures under 25.34 (°C) and pH around 7.75 (Fig. 9 on the right).
Zieliński et al. in their research reported that SMFs could improve MLSS in the aeration reactors of AS by about 470 ± 20 mg/L in the case samples (3420 ± 710 mg/L) higher than the control samples (2950 ± 670 mg/L) [56]. The mean concentration of MLSS in the case samples of SBR (sequencing batch reactor) was 1.4 (g/L) more than the control samples. In our study the mean concentration of MLSS in the case and control samples was 1689 ± 351.0 and 1535 ± 235.5 (mg/L). In other words, the differences between the mean concentration of MLSS in the case and control samples was 154 (mg/L) that the most of them was happened after 10th day of the operation of systems.
Mixed liquor volatile suspended solids (MLVSS)
Most of the portion of MLVSS is related to bacteria and the organiv materials. In AS process, the density of the microorganisms in the aeration reactor can be estimated by measurement of the concentration of MLVSS (mg/L), approximately [25, 26].
In Fig. 10. effect of SMFs (15 mT) on MLVSS in the case samples compared to the control samples, was illustrated. Although the changes in MLVSS at the beginning of the process were imperceptible, simultaneously, the difference between the mean of MLVSS in two groups of samples increased and becomes statistically significant, over time (p < 0.05).
The correlation between the concentration of MLSS in both groups (the case and control) was not statistically significant. Moreover, in terms of MLVSS, this difference is significant (p = 0.45). The SMFs, have the properties to affect on the growth rate of living components of MLSS, not the non-living components. The relationship between the improvement of MLVSS over time in both groups (case and control) is shown in Fig. 10.
There was a linear correlation relationship between MLVSS and the time of the operation of the systems in the control samples. Nevertheless, the paterrn of the growth rate of MLVSS in the case samples was look like a second-order reaction. Therefore, it can be said that the rate of growth in the mean concentration of the microorganisms or MLVSS in the case samples (1.44E3 ± 397.7 mg/L) was higher than in the control samples (1.26E3 ± 257.9 mg/L).
The correlation between MFs and the growth rate of microorganisms is not linear [57].
It must be mentioned that the changes of MLVSS concentration in first 10 days of the operation in the case and control was similar with each other as shown in Fig. 11.
In the second 10 days of the operation of the system, increasing the concentration of the MLVSS (mg/L) was observed. To determine the changes in trend and a correlation between temperature, pH value, and concentration of MLVSS, contour line diagrams have been used. In the case samples, the red dots were gathered around a pH of 7.89 and, temperature was gathered between 28.62 and 32.5 (°C) as shown in Fig. 12 on the left. These trends and correlations of pH and temperature are illustrated for the control samples, too (Fig. 12 on the right). As specified, the accumulation of data was between 22.8 and 25.32 (°C) and pH 7.75.
It seems that, despite the positive and incremental trend changes of MLVSS in the case samples, when compared to the control samples, no changes occurred in the pH of both groups. One main reason for this is related to the pattern of flow. As a continuous flow pattern was used in our study, changes in pH in two groups of the samples were not significant.
Shuhao Huo et al. reported that the use of MFs could improve the highest generation of biomass of microalga (4.44 g/L) at 30 °C [24]. In our study the more growth of MLVSS in the soludtion in the case samples was around 30 °C.
Enzymes stimulation and, improve of the microbial community by exposure of 11.4 mT SMFs could increase 85.5% of microbial counts in the case samples [55].
Flocs density and bonds structures
In Fig. 13 the flocs structure (1000 × magnification) at the outlets of the aeration reactors in the case and the control samples on the 30th day of the operation of systems by light microscope, is illustrated.
Microscopic image is one method to determine the generation of the microbial flocs in AS processes [27]. Quality and segments of the flocs can be observed by this method in the wastewater samples [28]. As it is clear, the volume and density of the flocs on the case sample was higher than the control sample.
In Fig. 14 image of Atomic Force Microscopy (AFM) in the case and, control samples on the 15th day of the operation of the system is illustrated.
AFM scan is a new three dimentional image to observed surface roughness and smoothness of the particles and, size of them [29].
SMFs could improve the density of the flocs in the aeration reactors of CMAS when the intensity of SMFs was 15 mT [2]. MFs can improve sedimentation of the sludge due to an inhibitory effect on the growth rate of filamentous bacteria [57].
Generated the low volume of the sludge by sludge dewatering methods in WWTPs is a key factor that has foundamental effects on the cose of the operation [52].
In addition to the generation of the higher density of flocs by SMFs, the size of flocs increased, too. By application of 15 mT SMFs for 1 h daily on the aeration reactor, density of flocs could be increased. An interesting point in this section of the study was that the surface of the flocs when exposed to SMFs was rough. Nevertheless, the surface of the flocs without the application of SMFs was not so uneven (Fig. 14).
In order to determine chemical changes in the sludge properties, FTIR (Fourier-transform infrared spectroscopy) spectra was used for the flocs in output of the settled sludge in the secondary clarifier reactors in two groups of samples that were demonstrated in Fig. 15.
FTIR analysis is a method to determine the functional variation of the chemical groups in the flocs of MLSS. Based on analyses of wavenumbers, the percent of transmittance in the sharp peaks of the case (17 sharp peaks) was higher than the control (10 sharp peaks) sample in all bonds of the chemical substances. These sharp peaks indicated that the density of materials in the case samples was higher. It can be concluded that by application of SMFs (15 mT) the ultimate target of the wastewater treatment in the aeration reactors is obtained by generating a higher density of the flocs for higher settling in the clarifier.
Ren et al. reported that intensity of 15–25 mT of MFs improves the metabolism of bacteria by the effect of more generation of dehydrogenase and, more consumption of substrate [43]. In this study, The spectra of amid I' (1440 cm−1) and II' (1650 cm−1) areas related to hydrogenase bonds in the case samples were higher than the control samples and, this indicates that the microbial growth rate in the case samples in which the SMFs applied was higher due to higher production of hydrogenase enzyme.
Deamici et al. in 2019 reported that the use of MFs method have many adventages for instant, no secondary pollution, low cost and, nontoxic material. They also, mentioned to stimulate of cell metabolism and more carbohydrates synthesis [9].
Energy consumption
One of the basic dissadventages of AS processes is more use of the electrical energy for the apply oxygen into the aeration reactors [16]. In WWTPs 40–75% of the total of the electrical energy consumer is used to the aeration of wastewater [37]. Moreover, application of the MFs for treatment of wastewater have adventages such as improve an efficient, easy-to-use method and, low-cost [48].
In our study we use 150 W of alternative current (220 V) of the electrical energy daily, for supply SMFs (Additional file 1).
For reduce energy consumption in this course of the wastewater treatment scale use of the solar panel during the day, is recommended [3].
Conclusion and outlook
The higher growth rate of the microorganisms in the aeration reactors to the transformation of the soluble organic matter into the flocs and removal of BOD5 is a basic point of the operation of the CMAS process. The supply of oxygen required for the metabolism of the organic matter in the aeration reactors has always been one of the most challenges in the operating of the aeration system due to the need for higher energy consumption. Furthermore, as oxygen solubility in water is low, the use of new methods (such as the application of SMFs) that can increase the efficiency of the aeration process (an alternative method for the process of the wastewater treatment in the aerobic conditions). Application of 15 mT intensity of SMFs on the aeration reactor could effect of the α-factor as the basic parameter in term of the transition of oxygen into the MLVSS and, generation the higher concenteratuon of MLVSS in the case samples. Furthermore, Higher settling of the flocs in the secondary clarifier under the exposure of SMFs is related to the higher density of them, is the main key to improving the effectiveness of the seconed clarifier. By the application of 15 mT intensity of SMFs in the wastewater treatment processes, a new view of the use of the physical methods can be achieved without consuming the chemical materials.
Effect of SMFs on components of the rectors were limited to development of MLVSS. Although MLVSS is one vital part of MLSS, the impact of SMFs on MLSS was not significant. The reason for this issue may be due to the presence of the microbial mass in MLVSS as a portion of MLSS.
The effect of SMFs on α-factor or rate of oxygen transfer is stimulated. Even when the concentration of DO was adjusted by a uniform flow rate during the processes. In other words, the SMFs cause a more efficient use of oxygen in the aeration reactors.
By the applicaton of SMFs in the aeration reactor of the case sample, the size of the generated flocs decreased based on the AFM image and, the sharp peaks of amids related to dehydrogenase bond as the indicator of the microbial growth rate, was higher than the control sample.
Data availability
On request from the corresponding author.
Abbreviations
- AC:
-
Alternative current
- AS:
-
Activated sludge
- AFM:
-
Atomic Force Microscopy
- BOD:
-
Biological oxygen demand
- CMAS:
-
Complete-mix activated sludge
- COD:
-
Chemical oxygen demand
- DC:
-
Direct current
- DO:
-
Dissolved oxygen
- FTIR:
-
Fourier-transform infrared spectroscopy
- HRT:
-
Hydraulic retention time
- kd :
-
Endogenous decay coefficient
- KLa:
-
The volumetric oxygen transfer coefficient
- MFs:
-
Magnetic fields
- MLSS:
-
Mixed liquor suspended solids
- MLVSS:
-
Mixed liquor volatile suspended solids
- OMT:
-
Oxygen mass transfer
- Qr/Q:
-
The recycle ratio
- S0 :
-
Primary substrate concentration
- S:
-
Effluent substrate concentration
- SMFs:
-
Static magnetic fields
- SRT:
-
Solids retention time
- SVI:
-
Sludge volume index
- WWTPs:
-
Wastewater treatment plants
- X:
-
Biomass concentration
- Y:
-
Maximum biomass yield coefficient
- Θc :
-
Microbial retention time
- ρw :
-
Density of water
References
Amaral A, Schraa O, Rieger L, Gillot S, Fayolle Y, Bellandi G, Amerlinck Y, Mortier ST, Gori R, Neves R. Towards advanced aeration modelling: from blower to bubbles to bulk. Water Sci Technol. 2017;75:507–17.
Asgari G, Khoshniyat R, Nazifi M, Shabrandi H. Investigation of the effect of magnetic fields on the volume index of diluted municipal wastewater sludge. J Water Wastewater. 2021;32:113–24.
Asgari G, Seid-Mohammadi A, Shokoohi R, Samarghandi MR, Diger GT, Malekolkalami B, Khoshniyat R. The best location for the application of static magnetic fields based on biokinetic coefficients in complete-mix activated sludge process. Sci Rep. 2023;13:5091.
Bai B, Rao D, Chang T, Guo Z. A nonlinear attachment-detachment model with adsorption hysteresis for suspension-colloidal transport in porous media. J Hydrol. 2019;578:124080.
Baquero-Rodríguez GA, Lara-Borrero JA, Nolasco D, Rosso D. A critical review of the factors affecting modeling oxygen transfer by fine-pore diffusers in activated sludge. Water Environ Res. 2018;90:431–41.
Baquero-Rodríguez GA, Lara-Borrero JA, Nolasco D, Rosso D. Review of the factors affecting modeling oxygen transfer by fine-pore diffusers in activated sludge. Water Environ Res. 2019. https://doi.org/10.1002/wer.1082.
Burton FL, Tchobanoglous G. Wastewater engineering: treatment, disposal, and reuse. New York: McGraw-Hill; 2018.
Daigger GT, Redmond E, Downing L. Enhanced settling in activated sludge: design and operation considerations. Water Sci Technol. 2018;78:247–58.
Deamici KM, Costa JAV, Santos LO. Magnetic fields as triggers of microalga growth: evaluation of its effect on Spirulina sp. Biores Technol. 2016;220:62–7.
Deepnarain N, Nasr M, Kumari S, Stenström TA, Reddy P, Pillay K, Bux F. Artificial intelligence and multivariate statistics for comprehensive assessment of filamentous bacteria in wastewater treatment plants experiencing sludge bulking. Environ Technol Innov. 2020;19:100853.
Dottorini G, Michaelsen TY, Kucheryavskiy S, Andersen KS, Kristensen JM, Peces M, Wagner DS, Nierychlo M, Nielsen PH. Mass-immigration determines the assembly of activated sludge microbial communities. Proc Natl Acad Sci. 2021;118:e2021589118.
Drewnowski J, Remiszewska-Skwarek A, Duda S, Łagód G. Aeration process in bioreactors as the main energy consumer in a wastewater treatment plant. Review of solutions and methods of process optimization. Processes. 2019;7:311.
Fan H, Qi L, Liu G, Zhang Y, Fan Q, Wang H. Aeration optimization through operation at low dissolved oxygen concentrations: evaluation of oxygen mass transfer dynamics in different activated sludge systems. J Environ Sci. 2017;55:224–35.
Fang H, Zhang H, Han L, Mei J, Ge Q, Long Z, Yu Y. Exploring bacterial communities and biodegradation genes in activated sludge from pesticide wastewater treatment plants via metagenomic analysis. Environ Pollut. 2018;243:1206–16.
Garrido-Baserba M, Sobhani R, Asvapathanagul P, Mccarthy GW, Olson BH, Odize V, Al-Omari A, Murthy S, Nifong A, Godwin J. Modelling the link amongst fine-pore diffuser fouling, oxygen transfer efficiency, and aeration energy intensity. Water Res. 2017;111:127–39.
Gu Y, Li Y, Li X, Luo P, Wang H, Wang X, Wu J, Li F. Energy self-sufficient wastewater treatment plants: feasibilities and challenges. Energy Procedia. 2017;105:3741–51.
Guimera X, Dorado AD, Bonsfills A, Gabriel G, Gabriel D, Gamisans X. Dynamic characterization of external and internal mass transport in heterotrophic biofilms from microsensors measurements. Water Res. 2016;102:551–60.
He X, Xie S, Xu J, Yin X-B, Zhang M. Reactive template-engaged synthesis of NiSx/MoS2 nanosheets decorated on hollow and porous carbon microtubes with optimal electronic modulation toward high-performance enzyme-like performance. Inorgan Chem. 2023;62:8033–42.
Henkel J, Cornel P, Wagner M. Oxygen transfer in activated sludge—new insights and potentials for cost saving. Water Sci Technol. 2011;63:3034–8.
Hosseini H, Hosseini H, Jalili M, Apourvari SN, Schaffie M, Ranjbar M. Static adsorption and interfacial tension of sodium dodecyl sulfate via magnetic field application. J Petrol Sci Eng. 2019;178:205–15.
Hreiz R, Latifi M, Roche N. Optimal design and operation of activated sludge processes: state-of-the-art. Chem Eng J. 2015;281:900–20.
Hu B, Quan J, Huang K, Zhao J, Xing G, Wu P, Chen Y, Ding X, Hu Y. Effects of C/N ratio and dissolved oxygen on aerobic denitrification process: a mathematical modeling study. Chemosphere. 2021;272:129521.
Hu J, Zhao L, Luo J, Gong H, Zhu N. A sustainable reuse strategy of converting waste activated sludge into biochar for contaminants removal from water: modifications, applications and perspectives. J Hazard Mater. 2022;438:129437.
Huo S, Chen X, Zhu F, Zhang W, Chen D, Jin N, Cobb K, Cheng Y, Wang L, Ruan R. Magnetic field intervention on growth of the filamentous microalgae Tribonema sp. in starch wastewater for algal biomass production and nutrients removal: influence of ambient temperature and operational strategy. Bioresour Technol. 2020;303:122884.
Jagaba AH, Kutty SRM, Baloo L, Birniwa AH, Lawal IM, Aliyu MK, Yaro NSA, Usman AK. Combined treatment of domestic and pulp and paper industry wastewater in a rice straw embedded activated sludge bioreactor to achieve sustainable development goals. Case Stud Chem Environ Eng. 2022;6:100261.
Jagaba AH, Kutty SRM, Hayder G, Baloo L, Noor A, Yaro NSA, Saeed AAH, Lawal IM, Birniwa AH, Usman AK. A systematic literature review on waste-to-resource potential of palm oil clinker for sustainable engineering and environmental applications. Materials. 2021;14:4456.
Khan MB, Lee XY, Nisar H, Ng CA, Yeap KH, Malik AS. Digital image processing and analysis for activated sludge wastewater treatment. In: Signal and image analysis for biomedical and life sciences; 2015. p. 227–48.
Khan MB, Nisar H, Ng CA, Lo PK, Yap VV. Local adaptive approach toward segmentation of microscopic images of activated sludge flocs. J Electron Imaging. 2015;24:061102–061102.
Khosravizadeh N, Lu D, Liao Y, Liao B, Fatehi P. Simulation and experimental analysis of microalgae and membrane surface interaction. Colloids Interfaces. 2023;7:24.
Łebkowska M, Rutkowska-Narożniak A, Pajor E, Tabernacka A, Załęska-Radziwiłł M. Impact of a static magnetic field on biodegradation of wastewater compounds and bacteria recombination. Environ Sci Pollut Res. 2018;25:22571–83.
Li D, Chen S, Zuo Y, Yun J, Cui B, Wu K, Guo X, Yang D, Wang J, Xi L. Roles of Joule heating and spin-orbit torques in the direct current induced magnetization reversal. Sci Rep. 2018;8:12959.
Liang Y, Li J, Xue Y, Tan T, Jiang Z, He Y, Shangguan W, Yang J, Pan Y. Benzene decomposition by non-thermal plasma: a detailed mechanism study by synchrotron radiation photoionization mass spectrometry and theoretical calculations. J Hazard Mater. 2021;420:126584.
Liu G, Wang J, Campbell K. Formation of filamentous microorganisms impedes oxygen transfer and decreases aeration efficiency for wastewater treatment. J Clean Prod. 2018;189:502–9.
Liu P, Klose M, Conrad R. Temperature effects on structure and function of the methanogenic microbial communities in two paddy soils and one desert soil. Soil Biol Biochem. 2018;124:236–44.
Liu S, Li H, Wang Y. Research on microbial community structure and treatment of dye wastewater with the enhancement of activated sludge by magnetic field at low temperature. RSC Adv. 2023;13:16471–9.
Liu Y, Pan L-M, Liu H, Chen T, Yin S, Liu M. Effects of magnetic field on water electrolysis using foam electrodes. Int J Hydrogen Energy. 2019;44:1352–8.
Mamais D, Noutsopoulos C, Dimopoulou A, Stasinakis A, Lekkas T. Wastewater treatment process impact on energy savings and greenhouse gas emissions. Water Sci Technol. 2015;71:303–8.
Metcalf E, Abu-Orf M, Bowden G, Burton FL, Pfrang W, Stensel HD, Tchobanoglous G, Tsuchihashi R, Aecom,. Wastewater engineering: treatment and resource recovery. New York: McGraw Hill Education; 2014.
Nur Syamimi Z, Muda K, Sohaili J, Sillanpää M. Optimization of activated sludge physical properties by magnetic field via response surface modeling. Appl Mech Mater. 2014;567:98–103.
Obaid H, Shahid S, Basim K, Chelliapan S. Modeling of wastewater quality in an urban area during festival and rainy days. Water Sci Technol. 2015;72:1029–42.
Pang H, Li L, He J, Yan Z, Ma Y, Nan J, Liu Y. New insight into enhanced production of short-chain fatty acids from waste activated sludge by cation exchange resin-induced hydrolysis. Chem Eng J. 2020;388:124235.
Pino-Herrera DO, Fayolle Y, Pageot S, Huguenot D, Esposito G, Van Hullebusch ED, Pechaud Y. Gas-liquid oxygen transfer in aerated and agitated slurry systems with high solid volume fractions. Chem Eng J. 2018;350:1073–83.
Ren Z, Leng X, Zhang Z, Feng H. Effect of low-strength magnetic fields on the oil removal performance of oil-degrading microorganisms. Desalin Water Treat. 2018;120:133–40.
Rusanowska P, Zieliński M, Dębowski M. Impact of static magnetic field on efficiency of fine-bubble aeration of liquid. Ecol Eng Environ Technol. 2017;18:130–5.
Sánchez-Clemente R, Igeño MI, Población AG, Guijo MI, Merchán F, Blasco R. Study of pH changes in media during bacterial growth of several environmental strains. Multidiscip Digit Publ Inst Proc. 2018;2:1297.
Tang B, Qiu B, Huang S, Yang K, Bin L, Fu F, Yang H. Distribution and mass transfer of dissolved oxygen in a multi-habitat membrane bioreactor. Biores Technol. 2015;182:323–8.
Tang B, Song H, Bin L, Huang S, Zhang W, Fu F, Zhao Y, Chen Q. Determination of the profile of DO and its mass transferring coefficient in a biofilm reactor packed with semi-suspended bio-carriers. Biores Technol. 2017;241:54–62.
Wang Y, Gu X, Quan J, Xing G, Yang L, Zhao C, Wu P, Zhao F, Hu B, Hu Y. Application of magnetic fields to wastewater treatment and its mechanisms: a review. Sci Total Environ. 2021;773:145476.
Wu L, Ning D, Zhang B, Li Y, Zhang P, Shan X, Zhang Q, Brown MR, Li Z, Van Nostrand JD. Global diversity and biogeography of bacterial communities in wastewater treatment plants. Nat Microbiol. 2019;4:1183–95.
Xia Y, Wen X, Zhang B, Yang Y. Diversity and assembly patterns of activated sludge microbial communities: a review. Biotechnol Adv. 2018;36:1038–47.
Zaidi NS, Muda K, Sohaili J, Toemen S, Yusof NZ. Optimization of operating parameters for aggregation under magnetic field by response surface methodology. ARPN J Eng Appl Sci. 2016;11:2419–25.
Zhang G, Zhao Z, Yin X-A, Zhu Y. Impacts of biochars on bacterial community shifts and biodegradation of antibiotics in an agricultural soil during short-term incubation. Sci Total Environ. 2021;771:144751.
Zhang L, Shen Z, Fang W, Gao G. Composition of bacterial communities in municipal wastewater treatment plant. Sci Total Environ. 2019;689:1181–91.
Zhang X, Yarema K, Xu A. Impact of static magnetic fields (SMFs) on cells. Biological effects of static magnetic fields. Berlin: Springer; 2017.
Zhao B, Sha H, Li J, Cao S, Wang G, Yang Y. Static magnetic field enhanced methane production via stimulating the growth and composition of microbial community. J Clean Prod. 2020;271:122664.
Zieliński M, Cydzik-Kwiatkowska A, Zielińska M, Dębowski M, Rusanowska P, Kopańska J. Nitrification in activated sludge exposed to static magnetic field. Water Air Soil Pollut. 2017;228:1–8.
Zieliński M, Rusanowska P, Dębowski M, Hajduk A. Influence of static magnetic field on sludge properties. Sci Total Environ. 2018;625:738–42.
Acknowledgements
The authors are grateful for the financial support from Hamadan University of Medical Sciences, Water and Wastewater Company of Kurdistan, and, Iran National Science Foundation.
Funding
This work was a part of a Ph.D. thesis with the financial support from Hamadan University of Medical Sciences (Ethics Committee Code IR.UMSHA.REC.1399.1077 and, No. 140004012661), Water and Wastewater Company of Kurdistan (No. 16.35.2697-1400.04.02) and, based upon research funded by Iran National Science Foundation (INSF) under Project No. 4003096 has been done.
Author information
Authors and Affiliations
Contributions
All authors of this paper have read and, agreed to the published the final version of the manuscript in Microbial Cell Factories Journal.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Aeration and clarifiers reactor (Case sample); Aeration and clarifiers reactor (Control sample); Case and control samples reactors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Asgari, G., Seid-Mohammadi, A., Shokoohi, R. et al. Exposure of the static magnetic fields on the microbial growth rate and the sludge properties in the complete-mix activated sludge process (a Lab-scale study). Microb Cell Fact 22, 195 (2023). https://doi.org/10.1186/s12934-023-02207-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-023-02207-x