Abstract
Background
Nature has provided unique molecular scaffolds for applications including therapeutics, agriculture, and food. Due to differences in ecological environments and laboratory conditions, engineering is often necessary to uncover and utilize the chemical diversity. Although we can efficiently activate and mine these often complex 3D molecules, sufficient production of target molecules for further engineering and application remain a considerable bottleneck. An example of these bioactive scaffolds is armeniaspirols, which are potent polyketide antibiotics against gram-positive pathogens and multi-resistance gram-negative Helicobacter pylori. Here, we examine the upregulation of armeniaspirols in an alternative Streptomyces producer, Streptomyces sp. A793.
Results
Through an incidental observation of enhanced yields with the removal of a competing polyketide cluster, we observed seven-fold improvement in armeniaspirol production. To further investigate the improvement of armeniaspirol production, we examine upregulation of armeniaspirols through engineering of biosynthetic pathways and primary metabolism; including perturbation of genes in biosynthetic gene clusters and regulation of triacylglycerols pool.
Conclusion
With either overexpression of extender unit pathway or late-stage N-methylation, or the deletion of a competing polyketide cluster, we can achieve seven-fold to forty nine-fold upregulation of armeniaspirol production. The most significant upregulation was achieved by expression of heterologous fatty acyl-CoA synthase, where we observed not only a ninety seven-fold increase in production yields compared to wild type, but also an increase in the diversity of observed armeniaspirol intermediates and analogs.
Similar content being viewed by others
Background
Natural products (NPs) are highly bioactive molecules with widespread utility in therapeutic applications; for example, > 50% of current small molecule drugs are NPs or NP-derived [3, 11]. Recent genomics and bioinformatics analyses of Nature’s materials highlighted significant untapped potential in NP chemical diversity as the 400 K NPs characterized to-date represent only about 20% of Nature’s possible repertoire [15]. NP biosynthesis is highly regulated through nutritional, environmental signals, combined with pleiotropic and pathway-specific regulatory mechanisms. Thus, discovery and downstream applications of NPs are oftentimes hindered by their low production under laboratory conditions. To activate and regulate production of these rare NPs, our labs and others have developed various native strain and heterologous engineering strategies aided by synthetic biology techniques (i.e. CRISPR-Cas mediated editing, DNA capture and refactoring [8, 10, 20].
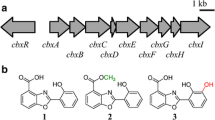
Armenispirols are a family of bioactive polyketide antibiotics with an unique chlorinated spiro[4.4]non-8-ene scaffold. Recent elucidation of the biosynthetic pathways for armeniaspirols in Streptomyces armeniacus have led to discovery and characterization of new biosynthetic enzymes and analogs [5, 12] for this family of NPs. Biosynthesis of armeniaspirols in Streptomyces armeniacus is initiated with the formation of a dichloropyrrolyl starter unit and a 2-alkylmalonyl CoA extender unit. Its biosynthetic gene cluster (BGC) includes three polyketide synthase modules, as well as a variety of tailoring enzymes responsible for chlorination, cyclization, and methylation, which together generate to the final chlorinated spiro[4.4]non-8-ene product (Fig. 1) [5, 12]. Novel analogs have also been generated through manipulation of the tailoring enzymes [12] and feeding of new acyl-CoA derivatives [21]. Besides anti-MRSA (Methicillin-resistant Staphylococcus aureus) and anti-VRE (vancomycin-resistant Enterococcus) bioactivity [2], its proposed mechanism of bacterial cell membrane disruption has also conferred armeniaspirol A potent bioactivity against multi-resistance gram-negative Helicobacter pylori [9].
Biosynthetic pathway for armeniaspirols. Left inset: Fatty acid pathway [19]. Right inset: Putative 2-alkylmalonyl-CoA biosynthesis pathway
Here, we examine strategies to enhance production of armeniaspirols in an alternative producer Streptomyces sp. A793 [7]. These include investigation of competing polyketide synthase, increasing precursors via primary metabolism or extender unit pathway and tailoring enzymes. Through engineering of the BGC and pleiotropic regulator overexpression, we demonstrate up to 97-fold enhancement of armeniaspirol production.
Results
Armeniaspirols in Streptomyces sp. A793
In a prior work examining notonesomycin production in Streptomyces sp. A793, we fermented a mutant strain (A793-Δnbc20, 21, Additional file 1: Fig. S1), where the loading polyketide domains of notonesomycin BGC were deleted via CRISPR-Cas mediated editing [7]. In this mutant strain, the production of notonesomycin was disrupted but we also observed a sevenfold upregulation of armeniaspirols (1–3) production (Fig. 2A, Additional file 1: Fig. S1). In parallel, antiSMASH analysis of its genome also identified a putative gene cluster containing enzymes for biosynthesis of pyrrolomycin, 2-alkylmalonyl-CoA extender unit and polyketide synthases (Fig. 1, Additional file 1: Table S1) that corresponded to armeniaspirols producing BGCs (MiBiG IDs: BGC0001934, BGC0002022) as identified by two other separate groups [5, 12]. The native BGC was also verified by deletion of the polyketide module, armE, which removed armeniaspirol production (Fig. 2A, Additional file 1: Fig. S2, A793-1).
Armeniaspirols in Streptomyces sp. A793. A LCMS spectra comparison of notonesomycin BGC disrupted strain (A793-Δnbc20, 21), polyketide module armE deleted strain (A793-1) and Streptomyces sp. A793 wild type strain (A793 WT). B Structures of notonesomycin and armeniaspirols with their respective substrates from the primary metabolism pathways
The observation of enhanced armeniaspirol production when notonesomycin BGC was disrupted led us to hypothesize that polyketide synthases within the gene clusters of notonesmycin and armeniaspirols might be competing for similar primary substrates, such as acyl-CoAs (Fig. 2B). With only seven-fold yield improvement compared to Streptomyces sp. A793 wild type strain, we reasoned that limits for armeniaspirol production have not been reached and it could be further enhanced.
Strain engineering strategies
Armed with our initial hypothesis that precursors might be limiting, we investigated multiple approaches to improve precursor levels and examined their impacts on armeniaspirol production level (Fig. 1). A recent observation of intracellular triacylglycerols degradation for channelling carbon flux towards polyketide via key enzyme fatty acyl-CoA synthase (FAS, SCO6196, [19] led us to include FAS overexpression as a strategy to improve precursor levels. In this previous study by Wang et al. on Streptomyces coelicolor M145, the expression of SCO6196 resulted in an estimated two-fold to three-fold increase in carbon fluxes directed towards malonyl CoA and methylmalonyl CoA, as well as increased levels of reducing co-factors equivalents (NADH). Besides increasing precursors from primary metabolism, we also wondered if production of the unique extender, 2-alkylmalonyl CoA, could be limiting. Within the BGC, armJKL is highly similar for revRST which encodes for a FabH homologue, a medium chain fatty acyl-CoA ligase, and a crotonyl-CoA reductase/carboxylase (CCRC), and is predicted to generate the unique 2-alkylmalonyl CoA extender unit for armeniaspirol production [13, 21]. ArmN has also been annotated as an acyl-CoA carboxylase ε-subunit domain, which is known to enhance carboxylase activity and in this biosynthesis, it is proposed to enhance ArmL activity [1, 6]. Consequently, we overexpressed ArmJKLN as a strategy to enhance the 2-alkylmalonyl CoA production (Fig. 1). As a comparison to these earlier two approaches targeting substrate precursors levels, we also examined upregulation of N-methyltransferase, ArmO. ArmO is part of the final steps in the biosynthetic pathway [5, 12].
Due to limitations of strain editing in Streptomyces sp. A793, both CRISPR-Cas mediated, and integrative editing techniques were used to realise the above-mentioned engineering strategies (Table 1, Fig. 3, Additional file 1: Fig. S3–6). Mutants were achieved by phiC31 integration of overexpression cassettes for overexpression of ArmJKLN (A793-3) and FAS (A793-4), respectively while CRISPR-Cas mediated insertion of a strong constitutive promoter, kasO*p, in front of the native armO was used to upregulate ArmO expression (A793-2).
Enhancement in armeniaspirol production
With these three strategies, we observed a significant 12-fold to 97-fold increase in the production of armeniaspirols (1–3) and in the case of ArmJKLN and FAS overexpressing strains (A793-3 and A793-4, respectively), a suppression in notonesmycin production was also observed (Fig. 4A). FAS overexpressing mutant (A793-4) demonstrated nearly two orders of magnitude increase in armeniaspirol production (97-fold) followed by ArmJKLN (A793-3, 49-fold) and ArmO (A793-2, 12-fold). This suggested that precursors from primary metabolism were indeed limiting armeniaspirol production in this strain under lab conditions (Fig. 4A, B). Not surprisingly, overexpression of FAS, which might be involved in both acyl-CoA and fatty acid biosynthesis pathways, resulted in the highest fold change. Fatty acid products from fatty acid degradation are expected to feed into the extender unit biosynthetic pathway (ArmJKLN) whilst impact on acyl-CoAs is expected to enhance malonyl CoA levels.
Enhancement of armeniaspirol production. A LCMS spectra comparison of notonesomycin A and armeniaspirol production between Streptomyces sp. A793 wild type strain (A793 WT) and edited strains A793-2, A793-3 and A793-4. B Comparison of the production of armeniaspirols (1–3) between A793 WT and edited strains A793-2, A793-3 and A793-4. C Comparison of the production of armeniaspirols intermediates 4–6, 7–8, 10–12 and 16–18 between A793 WT and edited strains A793-2, A793-3 and A793-4. 9, 13–15 were not observed in our analyses. Mean values in three independent experiments are presented and error bars refer to standard deviation. Significance of differences to A793 WT was calculated with Student’s t-test (* P-values < 0.05)
Along with the significant increase in armeniaspirols (1–3) yields, we also observed a corresponding enhancement in intermediates and analogs production in the mutant strains (Fig. 4C). Compared to the WT strain, A793-2 and A793-3 exhibit the presence of previously unobserved mono-chlorinated armeniaspirol analogs (4–6) and respective precursors 10–12, while 16–18 were observed in all three strains. Interestingly, the corresponding tri-chlorinated precursors 13–15 or dechlorinated precursors 7–9 for armeniaspirols 1–3 were not observed in these strains. Based on these observations, we hypothesized that ArmM activity is likely a limiting factor for conversion of 16–18 to 10–12, resulting in 16–18 accumulation. Moreover, ArmO was known to be specific to doubly chlorinated precursors, 7–9 [12]. Consequently, although some analogs 4–6 are observed in A793-2 and A792-3, there were small accumulation of the mono-chloro-demethylated armeniaspirols 10–12 in A793-2 and A793-3. With overexpression of FAS (A793-4), the ratios of intermediates were altered from earlier observations of A793-2 and A793-3, possibly due to a concurrent increase in reducing equivalents [19]. The increased presence of reducing equivalents may have facilitated ArmM activity and alleviated its bottleneck in converting intermediates 16–18 to 10–12. Notwithstanding, the significant increase in flux may have made ArmO the limiting factor leading to appearance of demethylated analogs, 7 and 8.
Discussion
Traditional biosynthetic engineering within native strains typically involved significant engineering within the relevant BGCs, assuming that silent or low yielding NPs can be mainly contributed to limited genetic expression towards secondary metabolite production [15, 22]. But in recent years, Wang et al. have shown that balancing this interface of primary and secondary metabolisms is important in ensuring optimal production of secondary metabolites and thus NPs. They also demonstrated that this can be tuned by expression of fatty acyl-CoA synthase (FAS). In this work and prior observations [17], we also observed FAS to be significantly efficient in improving production of silent or low-yielding products. Additionally, similar to observations made previously with notonesomycin production in Streptomyces sp. A793 and other prior work [7, 14, 17], we expect that further improvement of yields can be made in regulation of nutrient sources or pleiotropic regulators.
Although the individual engineering strategies had been successful, we were unable to explore them fully due to the genetic inaccessibility of Streptomyces sp. A793. However, we did manage to obtain a mutant strain that was integrated with ArmJKLN overexpression cassette in notonesomycin A disrupted strain (A793-Δnbc20, 21–5). This mutant surprisingly gave rise to lower armeniaspirol production (Additional file 1: Figs. S7 and S8). This observation suggested that there are further considerations for metabolic engineering, including the possibility of regulatory effects of notonesomycin or its regulatory elements on armeniaspirols and vice versa [23]. Further in-depth investigation using metabolomics and transcriptomics would be required to elucidate these relationships [24] which might become important factors to consider in heterologous expression of BGCs—local regulatory and feedback regulation.
Conclusion
To summarize, we demonstrated upregulation of armeniaspirols by up to 97-fold and increased analog diversity through engineering of its native producer. Overexpression of a rare extender unit pathway (ArmJKLN) or even N-methyltransferase (ArmO) within the BGC was also sufficient to effect Twelve-fold to Forty nine-fold improvement in armeniaspirol production. However, pleiotropic regulation (via FAS) towards increasing substrate pools of fatty acids and acyl-CoAs led to the most significant fold improvement. Along with upregulation, we also observed a proportional increase in diversity of analogs and intermediates, which will be important in expanding chemical space and optimization of bioactive candidates.
Materials and methods
Strains and growth conditions
Strains and plasmids used in this study are listed in Table 1 and Additional file 1: Table S2. Unless otherwise strains are propagated in A793 seed media [4] at 30 °C. Spore preparations and conjugation protocols were similar to those described by [22].
Construction of editing plasmids
All DNA manipulations were carried out in Escherichia coli OmniMAX™ (Thermo Fisher). The protospacers were initially incorporated into pCRISPRomyces-2 plasmids [18] using BbsI-mediated Golden Gate assembly, followed by the addition of the corresponding homology flanks via Gibson assembly. The methodology for CRISPR plasmid construction was previously detailed [16]. The insertion of kasO*p into CRISPR plasmids were constructed as described in previous study [22]. The integration plasmids were derived by cloning overexpression cassette, under strong constitutive kasO*p promoter, into pSET152 (Additional file 1: Table S2).
Genome editing
DNA methylation-proficient WM6026 E.coli strains were used to perform conjugation experiments with R2 agar without sucrose. CRISPR-Cas mediated editing protocols were similar as described before [22]. For integration with pSET152 derived plasmids, apramycin selection was used to select for mutants. PCR was performed using genomic DNA extracted from exconjugants to screen for edited strains and positive samples were sent for Sanger sequencing.
Cultivation conditions
Seed culture was grown in seed media (0.4% glucose, 0.4% yeast extract, 1% malt extract, 0.2% CaCO3, pH 7.0) incubated for 3 days at 28 °C, 200 rpm. 5% seed culture was used to inoculate shake flasks fermentation. Strains were fermented in ISP2 (0.4% glucose, 0.4% yeast extract, 1% malt extract, pH 7.0) for 9 days at 28 °C, 200 rpm.
Sample preparation and LCMS analysis
The supernatant was lyophilized, extracted with methanol (1.6 mL), disrupted by sonication and centrifuged to remove the cell debris. The methanol supernatant (40 µL) was analysed by HPLC–MS (Agilent, single quadrupole G6120B and QTOF 6545B) equipped with Acquity Premier UPLC BEH C18 column (50 × 2.1 mm, 1.7 μm, 130 Å). HPLC parameters were as follows: solvent A, 0.1% formic acid in water; solvent B, 0.1% formic acid in acetonitrile; gradient at a constant flow rate of 0.6 mL/min, 5 to 95% B in 37 min, 95% B for 3 min, 95 to 5% B in 1 min, 5% B for 4 min. Identity of armeniaspirols (1–3) was determined by comparison with authentic samples (Additional file 1: Fig. S9). HRMS was also utilized for identification of intermediates and compounds (Additional file 1: Figs. S10–17). The estimated yield (based on MS peak area of authentic sample) for Armeniaspirol A, B and C is 2.7 mg/L, 1.9 mg/L and 2.3 mg/L respectively in A793-4.
Availability of data and materials
The data supporting the conclusions of this article are included within the article and its supplementary data.
Abbreviations
- NP:
-
Natural product
- BGC:
-
Biosynthetic gene cluster
- FAS:
-
Fatty acyl-CoA synthase
- CCRC:
-
Crotonyl-CoA reductase/carboxylase
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- E.coli :
-
Escherichia coli
- HRMS:
-
High-resolution mass spectrometry
References
Arabolaza A, Shillito ME, Lin TW, et al. Crystal structures and mutational analyses of Acyl-CoA carboxylase α subunit of Streptomyces coelicolor. Biochemistry. 2010. https://doi.org/10.1021/bi1005305.
Arisetti N, Fuchs HLS, Coetzee J, et al. Total synthesis and mechanism of action of the antibiotic armeniaspirol A. Chem Sci. 2021;12:16023–34. https://doi.org/10.1039/d1sc04290d.
Challis GL. Genome mining for novel natural product discovery. J Med Chem. 2008;51:2618.
Dufour C, Wink J, Kurz M, et al. Isolation and structural elucidation of Armeniaspirols A-C: potent antibiotics against Gram-positive pathogens. Chem Eur J. 2012;18:16123–8. https://doi.org/10.1002/chem.201201635.
Fu C, Xie F, Hoffmann J, et al. Armeniaspirol antibiotic biosynthesis: chlorination and oxidative dechlorination steps affording Spiro[4.4]non-8-ene. ChemBioChem. 2019. https://doi.org/10.1002/cbic.201800791.
Gago G, Kurth D, Diacovich L, et al. Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. J Bacteriol. 2006. https://doi.org/10.1128/JB.188.2.477-486.2006.
Goh F, Zhang MM, Lim TR, et al. Identification and engineering of 32 membered antifungal macrolactone notonesomycins. Microb Cell Fact. 2020;19:71. https://doi.org/10.1186/s12934-020-01328-x.
Heng E, Tan LL, Zhang MM, Wong FT. CRISPR-Cas strategies for natural product discovery and engineering in actinomycetes. Proc Biochem. 2021;102:261.
Jia J, Zhang C, Liu Y, et al. Armeniaspirol A: a novel anti- Helicobacter pylori agent. Microb Biotechnol. 2022;15:442–54. https://doi.org/10.1111/1751-7915.13807.
Mitousis L, Thoma Y, Musiol-Kroll EM. An update on molecular tools for genetic engineering of actinomycetes—the source of important antibiotics and other valuable compounds. Antibiotics. 2020. https://doi.org/10.3390/antibiotics9080494.
Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83:770.
Qiao Y, Yan J, Jia J, et al. Characterization of the biosynthetic gene cluster for the antibiotic Armeniaspirols in Streptomyces armeniacus. J Nat Prod. 2019. https://doi.org/10.1021/acs.jnatprod.8b00753.
Ray L, Valentic TR, Miyazawa T, et al. A crotonyl-CoA reductase-carboxylase independent pathway for assembly of unusual alkylmalonyl-CoA polyketide synthase extender units. Nat Commun. 2016. https://doi.org/10.1038/ncomms13609.
Romano S, Jackson SA, Patry S, Dobson ADW. Extending the “one strain many compounds” (OSMAC) principle to marine microorganisms. Mar Drugs. 2018;16:244.
Scherlach K, Hertweck C. Mining and unearthing hidden biosynthetic potential. Nat Commun. 2021;12:3864.
Tan LL, Heng E, Zulkarnain N, et al. CRISPR/Cas-mediated genome editing of Streptomyces. New York: Springer; 2022. p. 207–25.
Tay D, Tan LL, Heng E. Training old dogs to do new tricks: A general multi-pronged activation approach for natural product discovery in Actinomycetes. Durham: Reseach Square; 2022.
Tong Y, Weber T, Lee SY. CRISPR/Cas-based genome engineering in natural product discovery. Nat Prod Rep. 2019;36:1262–80. https://doi.org/10.1039/C8NP00089A.
Wang W, Li S, Li Z, et al. Harnessing the intracellular triacylglycerols for titer improvement of polyketides in Streptomyces. Nat Biotechnol. 2020;38:76–83. https://doi.org/10.1038/s41587-019-0335-4.
Xu Z, Ji L, Tang W, et al. Metabolic engineering of Streptomyces to enhance the synthesis of valuable natural products. Eng Microbiol. 2022;2: 100022. https://doi.org/10.1016/j.engmic.2022.100022.
Zhang J, Zheng M, Yan J, et al. A permissive medium chain acyl-coA carboxylase enables the efficient biosynthesis of extender units for engineering polyketide carbon scaffolds. ACS Catal. 2021;11:12179–85. https://doi.org/10.1021/acscatal.1c03818.
Zhang MM, Wong FT, Wang Y, et al. CRISPR–Cas9 strategy for activation of silent Streptomyces biosynthetic gene clusters. Nat Chem Biol. 2017;13:607–9. https://doi.org/10.1038/nchembio.2341.
Zhou Q, Ning S, Luo Y. Coordinated regulation for nature products discovery and overproduction in Streptomyces. Synth Syst Biotechnol. 2020;5:49–58. https://doi.org/10.1016/j.synbio.2020.04.002.
Zhou Z, Gu J, Du Y-L, et al. The -omics era- toward a systems-level understanding of Streptomyces. Curr Genomics. 2011;12:404–16. https://doi.org/10.2174/138920211797248556.
Acknowledgements
We would like to thank Dr Yoganathan Kanagasundaram (Singapore Institute of Food and Biotechnology Innovation, A*STAR, Singapore) for sharing with us authentic samples of armeniaspirol A, B and C.
Funding
We gratefully acknowledge financial support from the National Research Foundation, Singapore (NRF-CRP19-2017-05-00) and Agency for Science, Technology and Research (A*STAR), Singapore (C211917006) for this work.
Author information
Authors and Affiliations
Contributions
FTW and YHL conceptualized, designed, and coordinated the study. The following authors conducted the experiments and acquired the data: EH performed the molecular biology (integration and screening mutants) work; YWL acquired and analysed LCMS data and characterized the compounds. CYL and VWPN performed all the fermentation. EH performed the bioinformatics analysis. FTW supervised the molecular biology design and experiments. SBN supervised fermentation. FTW and YHL supervised the overall data analysis, interpretation, and presentation. EH, YWL, FTW and YHL wrote the manuscript with inputs from all the authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Comparison of the production armeniaspirols (1-3) between A793 WT and notonesomycin BGC disrupted strain (A793-∆nbc20, 21). Figure S2. Streptomyces sp. A793 with armE deletion. Figure S3. Schematic of deletion of nbc20, 21 for disruption of notonesomycin production. Figure S4. Streptomyces sp. A793 with kasO*p insertion before armO. Figure S5. PCR verification of integrated kasO*p-armJKLN cassette plasmid in Streptomyces sp. A793 genome. Figure S6. PCR verification of integrated kasO*p-sco6196 cassette plasmid in Streptomyces sp. A793 genome. Figure S7. LCMS analysis of A793 WT, A793-Δnbc20, 21 and A793-Δnbc20, 21-5. Figure S8. PCR verification of integrated kasO*p-armJKLN cassette plasmid in Streptomyces sp. A793, ∆nbc20, 21 genome. Figure S9. LCMS analysis of authentic samples of 1, 2 and 3. Figure S10. HPLC-MS/MS fragmentation analysis of 1-6. Figure S11. HPLC-MS/MS fragmentation analysis of 7-12. Figure S12. HPLC-MS/MS fragmentation analysis of 16-18. Figure S13. The comparisons of observed isotope pattern and calculated isotope pattern of Notonesomycin A, 1, 2 and 3. Figure S14. The comparisons of observed isotope pattern and calculated isotope pattern of 4, 5 and 6. Figure S15. The comparisons of observed isotope pattern and calculated isotope pattern of 7 and 8. Figure S16. The comparisons of observed isotope pattern and calculated isotope pattern of 10, 11 and 12. Figure S17. The comparisons of observed isotope pattern and calculated isotope pattern of 16, 17 and 18. Table S1. Armeniaspirol biosynthetic gene cluster in Streptomyces sp. A793 Table S2. List of plasmids used in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Heng, E., Lim, Y.W., Leong, C.Y. et al. Enhancing armeniaspirols production through multi-level engineering of a native Streptomyces producer. Microb Cell Fact 22, 84 (2023). https://doi.org/10.1186/s12934-023-02092-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-023-02092-4