Abstract
Background
Inflammatory bowel disease (IBD) is a gastrointestinal disease characterized by diarrhea, rectal bleeding, abdominal pain, and weight loss. Recombinant probiotics producing specific proteins with IBD therapeutic potential are currently considered novel drug substitutes. In this study, a Bifidobacterium bifidum BGN4-SK strain was designed to produce the antioxidant enzymes streptococcal superoxide dismutase (SOD) and lactobacillus catalase (CAT), and a B. bifidum BGN4-pBESIL10 strain was proposed to generate an anti-inflammatory cytokine, human interleukin (IL)-10. In vitro and in vivo efficacy of these genetically modified Bifidobacterium strains were evaluated for colitis amelioration.
Results
In a lipopolysaccharide (LPS)-stimulated HT-29 cell model, tumor necrosis factor (TNF)-α and IL-8 production was significantly suppressed in the B. bifidum BGN4-SK treatment, followed by B. bifidum BGN4-pBESIL10 treatment, when compared to the LPS-treated control. Synergistic effects on TNF-α suppression were also observed. In a dextran sodium sulphate (DSS)-induced colitis mouse model, B. bifidum BGN4-SK treatment significantly enhanced levels of antioxidant enzymes SOD, glutathione peroxidase (GSH-Px) and CAT, compared to the DSS-only group. B. bifidum BGN4-SK significantly ameliorated the symptoms of DSS-induced colitis, increased the expression of tight junction genes (claudin and ZO-1), and decreased pro-inflammatory cytokines IL-6, IL-1β and TNF-α.
Conclusions
These findings suggest that B. bifidum BGN4-SK ameliorated DSS-induced colitis by generating antioxidant enzymes, maintaining the epithelial barrier, and decreasing the production of pro-inflammatory cytokines. Although B. bifidum BGN4-pBESIL10 exerted anti-inflammatory effects in vitro, the enhancement of IL-10 production and alleviation of colitis were very limited.
Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory disease with symptoms that include diarrhea, rectal bleeding, abdominal pain over an extended period of time. Though IBD is not a fatal disease, it can noticeably decrease quality of life and enhance the risk of colorectal cancer [1]. Probiotics are live microorganisms that, when administered in adequate amounts, confer health benefit(s) to the host [2]. Though many clinical trials of probiotic therapies against IBD have been conducted, the results have been inconsistent [3,4,5,6]. To further augment the beneficial effects of probiotics, genetic modification can be used to construct recombinant strains that secrete specific anti-inflammatory proteins and offer additional beneficial effects [7]. The most frequently utilized engineered probiotics include Lactococcus, Lactobacillus sensu strictu, Bifidobacterium, and Escherichia coli Nissle 1917. Their colonization capabilities make them potential candidates as carrier organisms for synthesized therapeutic molecules in situ, and their use can reduce side effects compared to the injection of medication or refined drugs [8]. For instance, IBD requires lifelong medication. It is highly desirable to develop inexpensive, easily administered therapeutics with minimal side effects [9]. Thus, the use of genetically modified probiotics with anti-inflammatory potential can be an effective strategy for IBD treatment [7].
IBD is noted for reactive oxygen species (ROS)-induced oxidative stress generated by neutrophils and macrophages in the inflamed epithelial tissues, with reduced antioxidant capacity in the plasma [10]. Previous studies have indicated that more oxygen free radicals are generated in the colons of IBD patients than those of healthy subjects, and the imbalance between prooxidant and antioxidant compounds leads to the development of IBD [11, 12]. Tissues respond to mild oxidative stress by producing more antioxidants. When oxidative stress becomes severe and persistent, antioxidant reserves in the tissues are exhausted and their capability for antioxidant generation is diminished, resulting in lower antioxidant levels and tissue injury [13]. Usually, superoxide dismutase (SOD) and catalase (CAT) are recruited as antioxidative strategies for reducing IBD inflammatory damage. Specifically, SOD converts the highly reactive superoxide anion O2− to the less reactive species H2O2, then CAT catabolizes the hydrogen peroxide into O2 and H2O [14]. Several recombinant SOD/CAT-expressing lactic acid bacteria have been developed with the capability to reduce inflammation, as demonstrated in different murine models of chemically induced colitis with diminished ROS levels in the gut [15,16,17,18].
Interleukin (IL)-10 is a critical anti-inflammatory cytokine involved in the maintenance of intestinal immune responses, protecting hosts from an excessive response to inflammation [19]. Oral administration of human IL-10 protein is not feasible because it is unstable and destroyed transiting the human gastrointestinal tract [20]. Genetically modified probiotic bacteria have been developed to surge intestinal IL-10 levels via local protein delivery or DNA delivery systems that trigger DNA expression by intestinal cells to produce IL-10 directly at the site of inflammation [7, 21]. The first study related to genetically modified probiotics expressing IL-10 was conducted in 2000. Recombinant Lactococcus lactis expressing murine IL-10 protected IL-10−/− mice from colitis and significantly reduced inflammation in mouse colitis induced by dextran sodium sulphate (DSS) [22].
Research related to genetically modified bacteria has increasingly focused on the direct oral administration of these recombinant bacteria to humans mainly for in situ delivery of proteins of therapeutic interest (i.e., antioxidants, cytokines, and protease inhibitors) [23, 24]. In this study, Bifidobacterium bifidum BGN4 was genetically modified as B. bifidum BGN4-SK to produce streptococcal SOD and lactobacillus CAT, and B. bifidum BGN4-pBESIL10 to produce human IL-10. Although Bifidobacterium sp. offer host health benefits, they are limited as genetically modified bacteria by their strict anaerobic metabolism, multilayered and complex cell walls, and restriction–modification systems. Thus, compared to genetically modified lactic acid bacteria, recombinant Bifidobacteria spp. have only recently been developed [25]. Based on our previous studies, recombinant B. bifidum BGN4-SK was constructed by introducing the SOD (StSodA) and catalase (LpKatL) genes into B. bifidum BGN4 [26]. Bifidobacterium bifidum BGN4-pBESIL10 was constructed by cloning the human IL-10 gene into the E. coli-Bifidobacterium shuttle vector pBES2 [27]. The aim of this study was to evaluate the immunomodulatory effects of B. bifidum BGN4-SK, B. bifidum BGN4-pBESIL10, and their combination in both lipopolysaccharide (LPS)-stimulated HT-29 cell and DSS-induced mouse colitis models, and to compare their effects to that of a control bacterial strain.
Materials and methods
Preparation of bacterial strains
Bifidobacterium bifidum BGN4, B. bifidum BGN4-SK and B. bifidum BGN4-pBESIL10 were cultured in MRS medium containing 0.05% l-cysteine·HCl, 30 µM hematin and 500 µM MnSO4 at 37 ºC for 24 h under anaerobic conditions [27].
Anti-inflammatory effects of B. bifidum BGN4-SK and B. bifidum BGN4-pBESIL10 in a cell line model
Cell line preparation
The HT-29 (KCLB 30,038) cell line was purchased from the Korea Cell Line Bank (Seoul, Korea). The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, NY, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Sigma Aldrich, USA) at 37 ºC in an atmosphere of 5% CO2. Thereafter, HT-29 cells were seeded into 24-well plates at a density of 1 × 106 cells per well and cultured for 24 h at 37 ºC.
Determination of cytokines
Bifidobacterium bifidum BGN4-SK and B. bifidum BGN4-pBESIL10 were adjusted to 108 CFU/mL and the HT-29 cells were treated with 100 ng/mL LPS from Escherichia coli 055: B5 (Sigma Aldrich, USA), and 100 µL neutralized cell-free supernatants (CFSs) of B. bifidum BGN4-SK, B. bifidum BGN4-pBESIL10 or a mixture of 50% (v/v) B. bifidum BGN4-SK and 50% (v/v) B. bifidum BGN4-pBESIL10, followed by incubation for 10 h at 37 ºC. Then, cell culture supernatants were collected to determine the levels of tumor necrosis factor (TNF)-α and IL-8 using enzyme-linked immunosorbent assay kits (BD Biosciences, CA, USA), according to the manufacturer’s instructions.
Anti-inflammatory effects of B. bifidum BGN4-SK and B. bifidum BGN4-pBESIL10 in a DSS-induced colitis model
Animals and treatments
Facilities and protocols employed in this study were approved by the Institutional Animal Care and Use Committee of Seoul National University (Approval Number SNU-200529-1). Forty-eight 8-week-old female C57BL/6 mice were obtained from Central Lab Animal, Inc. (Sungnam, Korea). All mice had free access to American Institute of Nutrition-93G diet (Doo Yeol Biotech Co., Lid., Korea). After acclimatization to the environment for 1 week, the mice were randomly assigned to six groups (n = 8 per group): untreated control (Control), DSS-treated group (DSS), 1010 CFU lyophilized B. bifidum BGN4 treatment (BGN4), 1010 CFU lyophilized B. bifidum BGN4-SK treatment (SK), 1010 CFU lyophilized B. bifidum BGN4-pBESIL10 treatment (IL-10) and a mixture of 5 × 109 CFU B. bifidum BGN4-SK and 5 × 109 CFU B. bifidum BGN4-pBESIL10 (SK + IL-10). The probiotic cultures were lyophilized for 48 h in a Virtis Freezemobile 12EL (SP Scientific, NY). Each lyophilized bifidobacterial stock was tested for bacterial viability and enumeration before oral administration, and the bifidobacterial powder was resuspended daily in the sterilized water for administration to each mouse.
The DSS-induced colitis groups received 2% (w/v) DSS (36–50 kDa) (MP Biomedical, CA, USA) in drinking water ad libitum for 6 days and then drank sterilized water for two days before sacrifice. Treatment groups were orally administered lyophilized B. bifidum strains every day, by gavage, starting one day prior to DSS induction, while the control and the DSS groups received PBS solution at the same volume, as shown in Fig. 1. All mice were euthanized by CO2 asphyxiation.
Antioxidant enzyme activities
Blood samples were collected into 1.5 mL heparinized tubes by cardiac puncture. The blood was centrifuged at 1000×g for 10 min at 4 ºC to harvest serum samples. To determine serum antioxidant enzyme activities, SOD assay, catalase assay and GSH-Px assay kits were utilized according to the manufacturer’s instructions.
Histological analysis
Colon tissues were collected immediately after animal sacrifice. The colonic tissues of three mice per group were prepared as “Swiss rolls” for histological analysis and fixed in 10% buffered formalin, dehydrated in ethanol, and then embedded in paraffin. Then, 5 mm sections were prepared and stained with hematoxylin and eosin and the samples were rated on a scale of 0–4 for the extent of crypt damage, epithelial injury, and inflammatory infiltration (0 = none; 1 = mild; 2 = moderate; 3 = severe; and 4 = very severe).
Tissue myeloperoxidase (MPO) activity
Proximal colonic tissues were homogenized and evaluated for myeloperoxidase (MPO) activity by Myeloperoxidase assay kit (Hycult Biotech, Wayne, PA, USA) according to the manufacturer’s instruction.
Real-time polymerase chain reaction (PCR) of the colonic tissues
The collected colonic tissues (30 mg) were homogenized in 600 µL of RNAlater with TissueLyser II (Qiagen, CA, USA) for 2 min at 50 Hz. Total RNA was isolated from the homogenized tissues using RNeasy Mini kits (Qiagen, CA, USA) following the protocol’s guidance. This extraction system includes the DNase treatment to eliminate genomic DNA. The purity and quantity of obtained RNA were verified using a NanoDrop™ ND-1000 Spectrophotometer (Thermo Fisher Scientific Inc., MA, USA). Extracted RNA was reverse transcribed into cDNA using a GoScript™ Reverse Transcription kit (Promega, WI, USA). Quantitative real-time PCR was conducted with a StepOne™ Real-time PCR System (Applied Biosystems, CA, USA) using SYBR Green PCR Master Mix (Applied Biosystems). qRT-PCR was performed as following: 2 min at 95 ºC for initiation, 15 s at 95 ºC for denaturation and 60 s at 60 ºC for annealing up to 38 cycles. All qRT-PCR reactions were completed in triplicate. Before analyzing the data, melt curves were visually inspected for the presence of a single peak at melting temperature to ensure amplification specificity. Quantitative gene expression of each sample was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression and quantified using the 2−ΔΔCt method. Primer sequences used in the analysis are shown in Additional file 1: Table S1.
Statistical analysis
Data are presented as the mean ± SD. The normality of data was checked using the Shapiro-Wilk normality test. Differential abundance analyses were conducted by one-way ANOVA with Tukey’s multiple comparisons test. All statistical analyses were conducted via Graph-Pad Prism 8 with statistical significance set at P < 0.05.
Results
Inhibitory effects of recombinant B. bifidum BGN4 strains on pro-inflammatory cytokines in vitro
Compared with the LPS-treated control and the control strain B. bifidum BGN4 group, TNF-α production was significantly suppressed when cells were treated with a mixture of B. bifidum BGN4-SK and B. bifidum BGN4-pBESIL10 CFSs, followed by the B. bifidum BGN4-SK-only and B. bifidum BGN4-pBESIL10-only treatments (Fig. 2A). The combination of two recombinant B. bifidum strains resulted in synergistic inhibitory effects on the TNF-α production.
Production of TNF-α (A) and IL-8 (B) in lipopolysaccharide (LPS)-stimulated HT-29 cells when treated with cell-free supernatants (CFSs) of B. bifidum BGN4-SK, B. bifidum BGN4-pBESIL10 or their combination. Treatments with different letters are significantly different at P < 0.05. BGN4, B. bifidum BGN4; SK, B. bifidum BGN4-SK; IL-10, B. bifidum BGN4-pBESIL10
The generation of another pro-inflammatory cytokine IL-8 was significantly inhibited by B. bifidum BGN4-SK treatment, followed by the bacterial combination treatment. In terms of IL-8 suppression, the B. bifidum BGN4 control group also exerted anti-inflammatory effects but B. bifidum BGN4-pBESIL10 did not strengthen the efficacy and subsequently no synergistic effect between the recombinant bacteria was observed, as shown in Fig. 2B.
Anti-inflammatory activities of recombinant B. bifidum BGN4 strains in a DSS-induced mouse colitis model
Clinical symptoms
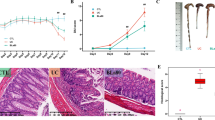
In this study, colitis inflammation was induced by 6-day oral administration of 2% DSS in drinking water. As shown in Fig. 3A, body weight slightly increased during the first four days in the DSS-received treatment group, and then significant body weight loss was observed in the four treatment groups (B. bifidum BGN4-SK group excepted), compared with control. After 2-day recovery period, the body weight in the B. bifidum BGN4-SK group was augmented and significantly larger than the DSS group. Colon length, as a critical indicator of inflammation severity, was significantly shortened in the DSS treatment compared to the control (Fig. 3B). To further assess ulceration and crypt loss in colon tissues, histological analysis was conducted and cumulative damage scores in the proximal, middle, and distal colon sections were recorded as histological scores, displayed in Fig. 3C. The histological scoring of colon pathology indicated that DSS-induced inflammation was significantly ameliorated in B. bifidum BGN4-SK-fed mice. According to the representative histological images of the colons presented in Fig. 3D, the control group showed intact crypt architecture and epithelial layers in the mucosa and submucosa, whereas loss of the entire crypt and epithelium disruption were observed with DSS treatment. Compared with the DSS group, the groups treated with the recombinant B. bifidum BGN4 strains showed less inflammation, indicating substantial protection of the colonic mucosa structure from DSS-induced damages. Of special note was the B. bifidum BGN4-SK-added treatment, which maintained crypt and goblet cell architectures and left intact mucosal and epithelial structures in the colonic tissues.
Effects of B. bifidum BGN4-SK, B. bifidum BGN4-pBESIL10 or their combination on clinical symptoms in DSS-induced colitis model. A Body weight change (%), B colon length (n = 8), C histopathologic score and D histological images of colonic tissues stained with H&E (n = 3). Data are expressed as mean ± SD. *Significant versus control. #Significant versus DSS. *P < 0.05. The scale bars represent 500 μm (whole colon) and 50 μm (colonic segments). BGN4, Bifidobacterium bifidum BGN4; SK, B. bifidum BGN4-SK; IL-10, B. bifidum BGN4-pBESIL10
Antioxidant activities
SOD, GSH-Px and CAT are critical antioxidant enzymes against oxidative stress. As shown in Fig. 4, the DSS-only and B. bifidum BGN4/ BGN4-pBESIL10 administration treatments displayed significantly lower GSH-Px and CAT activities when compared to the control group, while SOD, GSH-Px and CAT activities in the B. bifidum BGN4-SK treatment were significantly higher than the DSS-only treatment. Additionally, the B. bifidum combination treatment displayed significantly higher CAT activity than the DSS group.
Superoxide dismutase (SOD) activity (A), glutathione peroxidase (GSH-Px) activity (B), and catalase (CAT) activity (C) in the blood serum of mice at the final day. Data are expressed as mean ± SD. *Significant versus control. #Significant versus DSS. (n = 8). P < 0.05. BGN4, Bifidobacterium bifidum BGN4; SK, B. bifidum BGN4-SK; IL-10, B. bifidum BGN4-pBESIL10
Intestinal barrier integrity
Relative mRNA expression of tight junction genes involving claudin and ZO-1 were examined to evaluate the integrity of the intestinal barrier in colon (Fig. 5). The expressions of claudin were significantly decreased in the four DSS-induced groups compared with the control group. Only the B. bifidum BGN4-SK treatment presented significantly higher claudin expression when compared to the DSS-only group. Similarly, B. bifidum BGN4-SK treatment showed significantly higher ZO-1 expression than the DSS treatment, while the expressions of ZO-1 significantly dropped in the DSS, B. bifidum BGN4 and B. bifidum BGN4-pBESIL10 groups, compared with the control.
MPO activity and cytokines in the colon
Colonic accumulation of MPO was measured as a neutrophil influx marker in the tissue. The activities of MPO in the DSS, B. bifidum BGN4 and recombinant bacteria combination groups were significantly higher than in the control group, and MPO in the B. bifidum BGN4-SK group was significantly decreased compared to the DSS group, as shown in Fig. 6A. The transcriptional levels of pro-inflammatory cytokines (IL-6, IL-1β, TNF-α and IL-8) and anti-inflammatory cytokine (IL-10) were investigated. The expression of IL-6 and TNF-α were inhibited in the four treatment groups compared with the DSS-only group, while the IL-1β expression was suppressed only in the B. bifidum BGN4-SK group when compared to the DSS-only treatment (Fig. 6B–D). Even though the inhibitory effect of the recombinant bacteria on IL-8 production was detected in vitro, it was not confirmed in this mouse model (Fig. 6E). In addition, the capability of anti-inflammatory IL-10 production in B. bifidum BGN4-pBESIL10 was not observed in vivo (Fig. 6 F).
Myeloperoxidase (MPO) activities (A) and relative mRNA expression of pro-inflammatory cytokines (B–F). Data are expressed as mean ± SD. *Significant versus control. #Significant versus DSS. (n = 5). P < 0.05. BGN4, Bifidobacterium bifidum BGN4; SK, B. bifidum BGN4-SK; IL-10, B. bifidum BGN4-pBESIL10
Discussion
In this study, the effects of two recombinant B. bifidum BGN4 strains and their combination were assessed on the development of inflammation and oxidative intestinal damage in vitro and in vivo. Both recombinant strain treatments led to anti-inflammatory effects in the cell line, and their combination appeared to have synergic effects. However, only B. bifidum BGN4-SK treatment significantly ameliorated colitis inflammation, boosted antioxidant enzymes, and protected the epithelial barrier in the DSS-treated mice.
Oral administration of DSS is toxic to colonic epithelial cells with increased apoptosis and decreased proliferation, leading to leakage of the epithelial barrier and mucosal invasion of intraluminal microorganisms [28]. Histological characteristics of colitis include epithelial distortions (i.e., crypt branching and shortening, reduction in crypt density and goblet cell numbers) and severe infiltration of inflammatory cells into intestinal walls [29]. Previous studies have indicated that probiotics are only slightly effective as IBD treatments (usually in less severe cases), because they generally act by reinforcing regulatory T cell responses in the mucosal immune system, which can be overwhelmed by an ongoing and robust inflammatory response [30]. Consistent with these previous studies, the group treated with the original B. bifidum BGN4 strain slightly alleviated DSS-induced inflammation, based on histological results.
Oxidative stress is seen as the main cause of tissue destruction in IBD patients, which emerges as a manifest imbalance between the production of ROS and their removal by antioxidants [13]. Previous studies have suggested the significance of antioxidant enzymes as a treatment approach for IBD [31,32,33]. Carroll et al. [16] reported that Lactobacillus gasseri secreting SOD alleviated inflammation in IL-10 knockout mice. Carmen et al. [34] revealed that recombinant Streptococcus thermophilus strains producing SOD/CAT enhanced anti-inflammatory activities both in vitro and in a mouse model of colitis. The benefits of these recombinant strains were mainly through their antioxidant mechanisms rather than an immunomodulating mechanism [35]. In addition, MPO is an enzyme in neutrophils, and its activity reflects the degree of neutrophil infiltration in intestinal inflammation [36]. More importantly, MPO catalyzes the formation of potent cytotoxic oxidants (i.e., hypochlorous acid), affecting the severity of inflammation [37]. In this study, we confirmed significant in vivo generation of antioxidant enzymes (SOD, GSH-Px and CAT) in the B. bifidum BGN4-SK treatment compared to the DSS group, which facilitated the attenuation of oxidative stress. Combined with reduced MPO activity and enhanced expression of tight junction genes, the B. bifidum BGN4-SK treatment maintained the functional and structural integrity of the colon tissues. Thus, the genetic modification of B. bifidum BGN4-SK to provide antioxidant production capability makes it superior to the wild-type strain in terms of colon tissue protection.
Cytokines are the critical signaling molecules of immune system [38]. TNF-α directly affects intestinal epithelial tissues and its secretion can lead to the disruption of the epithelial barrier and induction of apoptosis in epithelial cells [39]. IL-1ß and IL-6 are essential mediators of IBD progression [39]. IL-6 stimulates neutrophil chemotaxis, which is linked to necrosis in the colon [37]. Previous research reported that an antimurine IL-1ß antibody decreased IL-6 mRNA expression and alleviated pathological symptoms of DSS-induced colitis, suggesting that IL-1β targets itself and IL-6 for progressing colonic inflammation [40]. In this study, mRNA expressions of IL-6 and TNF-α were markedly reduced in the four bacterial treatments when compared to the DSS group, and only B. bifidum BGN4-SK treatment significantly decreased IL-1ß expression.
IL-10 is an essential anti-inflammatory cytokine, and IL-10-deficient mice have been reported to develop colitis spontaneously [41]. Steidler et al. demonstrated a 50% decrease in DSS-induced murine colitis using recombinant Lactococcus lactis secreting murine IL-10 [22], and then developed the first biocontainment system for L. lactis IL-10 strain. However, a phase IIA clinical trial did not reveal a statistically significant difference in mucosal healing with L. lactis IL-10 versus placebo [42]. Other previous studies implied that recombinant L. lactis and B. bifidum expressing IL-10 exerted mildly anti-inflammatory effects on DSS-induced colitis, but only for some clinical parameters [24, 42], which are consistent with our in vivo study. The low therapeutic efficacy of recombinant IL-10 indicates that a sustained and more mucosa-focused delivery is necessary for IL-10 to be effective against colitis [43]. Furthermore, using probiotics as vectors for IL-10 delivery to the mucosal surface ensures the release of IL-10 within the lumen without deep penetration into the tissues, which probably limits its anti-inflammatory effects [42]. IL-10 has broad immunoregulatory activities, including the inhibition of TH1 lymphocyte differentiation, promotion of regulatory T cell activities, and reduction of IL-12 release, which acts on immune cells in the lamina propria rather than at the mucosal surface [44, 45]. Hence, despite the very limited efficacy of B. bifidum BGN4-pBESIL10 observed in this study, it can potentially provide better results in immunologically driven chronic colitis models.
The biosafety of GMOs is a concern of the general public, and the biological issue is primarily the plasmid. The transfer of plasmids coding for antibiotic resistance has been reported both during food fermentation and in vivo, which indicates the potential for transmission of resistance genes to pathogenic species [46]. Lactobacilli and lactococci are increasingly recognized as reservoirs of antibiotic resistance genes [47], whereas Bifidobacterium spp. have low intrinsic and acquired resistance to antibiotics, suggesting they are safe live vectors for delivery of proteins in humans [48]. The genomic sequence of B. bifidum BGN4 published in GenBank (Accession No. CP001361.1) reveals this strain lacks plasmids capable of transferring antibiotic-resistance genes, the organism has been used as a food ingredient since 2000 [49, 50], and it is certified as Generally Recognized as Safe (GRAS) by U.S. Food and Drug Administration (FDA) (GRN No. 814).
In conclusion, this study demonstrated that both B. bifidum BGN4-SK and B. bifidum BGN4-pBESIL10 diminished the inflammatory levels in HT-29 cells. However, treatment by a recombinant strain expressing IL-10 presented very limited protective effects in the in vivo colitis model, while the B. bifidum BGN4-SK treatment effectively enhanced antioxidant capability, protected colonic epithelial integrity and inhibited colonic inflammation. In addition, there were no synergic effects between B. bifidum BGN4-SK and B. bifidum BGN4-pBESIL10 and the lower efficacy of the combination treatment was primarily due to the insufficient dose of the B. bifidum BGN4-SK strain. Hence, B. bifidum BGN4-SK is a potential candidate for IBD treatment. Clinical trials are a necessary next step to further confirm the potential for B. bifidum BGN4-SK treatment.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29:2727–37.
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S. Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014. https://doi.org/10.1038/nrgastro.2014.66.
Matthes H, Krummenerl T, Giensch M, Wolff C, Schulze J. Clinical trial: probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN). BMC Complement Altern Med. 2010;10:1–8.
Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, Forti G, Morini S, Hassan C, Pistoia MA. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL# 3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010;105:2218.
D’Incà R, Barollo M, Scarpa M, Grillo AR, Brun P, Vettorato MG, Castagliuolo I, Sturniolo GC. Rectal administration of Lactobacillus casei DG modifies flora composition and toll-like receptor expression in colonic mucosa of patients with mild ulcerative colitis. Dig Dis Sci. 2011;56:1178–87.
Wildt S, Nordgaard I, Hansen U, Brockmann E, Rumessen JJ. A randomised double-blind placebo-controlled trial with Lactobacillus acidophilus La-5 and Bifidobacterium animalis subsp. lactis BB-12 for maintenance of remission in ulcerative colitis. J Crohn’s Colitis. 2011;5:115–21.
de Moreno de LeBlanc A, Del Carmen S, Chatel J-M, Miyoshi A, Azevedo V, Langella P, Bermúdez-Humarán LG, LeBlanc JG. Current review of genetically modified lactic acid bacteria for the prevention and treatment of colitis using murine models. Gastroenterol Res Pract. 2015. https://doi.org/10.1155/2015/146972.
Gardlik R, Palffy R, Celec P. Recombinant probiotic therapy in experimental colitis in mice. Folia Biol. 2012;58:238.
Steidler L, Neirynck S, Huyghebaert N, Snoeck V, Vermeire A, Goddeeris B, Cox E, Remon JP, Remaut E. Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nat Biotechnol. 2003;21:785–9.
Larrosa M, González-Sarrías A, Yáñez-Gascón MJ, Selma MV, Azorín-Ortuño M, Toti S, Tomás-Barberán F, Dolara P, Espín JC. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem. 2010;21:717–25.
Shiratora Y, Aoki S, Takada H, Kiriyama H, Ohto K, Hai K, Teraoka H, Matano S, Matsumoto K, Kamii K. Oxygen-derived free radical generating capacity of polymorphonuclear cells in patients with ulcerative colitis. Digestion. 1989;44:163–71.
Hur SJ, Kang SH, Jung HS, Kim SC, Jeon HS, Kim IH, Lee JD. Review of natural products actions on cytokines in inflammatory bowel disease. Nutr Res. 2012;32:801–16.
Lakhan SE, Kirchgessner A. Neuroinflammation in inflammatory bowel disease. J Neuroinflammation. 2010;7:1–12.
Rochat T, Bermúdez-Humarán L, Gratadoux J-J, Fourage C, Hoebler C, Corthier G, Langella P. Anti-inflammatory effects of Lactobacillus casei BL23 producing or not a manganese-dependant catalase on DSS-induced colitis in mice. Microb Cell Fact. 2007;6:1–10.
Bruno-Bárcena JM, Andrus JM, Libby SL, Klaenhammer TR, Hassan HM. Expression of a heterologous manganese superoxide dismutase gene in intestinal lactobacilli provides protection against hydrogen peroxide toxicity. Appl Environ Microbiol. 2004;70:4702–10.
Carroll IM, Andrus JM, Bruno-Bárcena JM, Klaenhammer TR, Hassan HM, Threadgill DS. Anti-inflammatory properties of Lactobacillus gasseri expressing manganese superoxide dismutase using the interleukin 10-deficient mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G729–38.
Han W, Mercenier A, Ait-Belgnaoui A, Pavan S, Lamine F, van Swam II, Kleerebezem M, Salvador-Cartier C, Hisbergues M, Bueno L. Improvement of an experimental colitis in rats by lactic acid bacteria producing superoxide dismutase. Inflamm Bowel Dis. 2006;12:1044–52.
Watterlot L, Rochat T, Sokol H, Cherbuy C, Bouloufa I, Lefèvre F, Gratadoux J-J, Honvo-Hueto E, Chilmonczyk S, Blugeon S. Intragastric administration of a superoxide dismutase-producing recombinant Lactobacillus casei BL23 strain attenuates DSS colitis in mice. Int J Food Microbiol. 2010;144:35–41.
Saraiva M, Vieira P, O’garra A. Biology and therapeutic potential of interleukin-10. J Exp Med. 2020. https://doi.org/10.1084/jem.20190418.
Del Carmen S, de LeBlanc AdM, Levit R, Azevedo V, Langella P, Bermúdez-Humarán LG, LeBlanc JG. Anti-cancer effect of lactic acid bacteria expressing antioxidant enzymes or IL-10 in a colorectal cancer mouse model. Int Immunopharmacol. 2017;42:122–9.
Guimarães V, Innocentin S, Chatel J-M, Lefèvre F, Langella P, Azevedo V, Miyoshi A. A new plasmid vector for DNA delivery using lactococci. Genet Vaccines Ther. 2009;7:1–7.
Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–5.
Kumar M, Yadav AK, Verma V, Singh B, Mal G, Nagpal R, Hemalatha R. Bioengineered probiotics as a new hope for health and diseases: an overview of potential and prospects. Future Microbiol. 2016;11:585–600.
Mauras A, Chain F, Faucheux A, Ruffié P, Gontier S, Ryffel B, Butel M-J, Langella P, Bermúdez-Humarán LG, Waligora-Dupriet A-J. A new Bifidobacteria expression system (BEST) to produce and deliver interleukin-10 in Bifidobacterium bifidum. Front Microbiol. 2018;9:3075.
Zuo F, Chen S, Marcotte H. Engineer probiotic bifidobacteria for food and biomedical applications—current status and future prospective. Biotechnol Adv. 2020. https://doi.org/10.1016/j.biotechadv.2020.107654.
Lin Z, Ku S, Lim T, Park SY, Park MS, Ji GE, O’Brien K, Hwang KT. Antioxidant and anti-inflammatory properties of recombinant Bifidobacterium bifidum BGN4 expressing antioxidant enzymes. Microorganisms. 2021;9:595.
Hong N, Ku S, Yuk K, Johnston TV, Ji GE, Park MS. Production of biologically active human interleukin-10 by Bifidobacterium bifidum BGN4. Microb Cell Fact. 2021;20:1–14.
Araki Y, Mukaisyo K-I, Sugihara H, Fujiyama Y, Hattori T. Increased apoptosis and decreased proliferation of colonic epithelium in dextran sulfate sodium-induced colitis in mice. Oncol Rep. 2010;24:869–74.
Xu X, Lin S, Yang Y, Gong X, Tong J, Li K, Li Y. Histological and ultrastructural changes of the colon in dextran sodium sulfate-induced mouse colitis. Exp Ther Med. 2020;20:1987–94.
Boirivant M, Strober W. The mechanism of action of probiotics. Curr Opin Gastroenterol. 2007;23:679–92.
Kruidenier La, Verspaget H. oxidative stress as a pathogenic factor in inflammatory bowel disease—radicals or ridiculous? Aliment Pharmacol Ther. 2002;16:1997–2015.
Kruidenier L, van Meeteren ME, Kuiper I, Jaarsma D, Lamers CB, Zijlstra FJ, Verspaget HW. Attenuated mild colonic inflammation and improved survival from severe DSS-colitis of transgenic Cu/Zn-SOD mice. Free Radic Biol Med. 2003;34:753–65.
Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, Weissman GS, Katz S, Floyd RA, McKinley MJ. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–86.
Del Carmen S, de Moreno de LeBlanc A, Martin R, Chain F, Langella P, Bermúdez-Humarán LG, LeBlanc JG. Genetically engineered immunomodulatory Streptococcus thermophilus strains producing antioxidant enzymes exhibit enhanced anti-inflammatory activities. Appl Environ Microbiol. 2014;80:869–77.
LeBlanc JG, del Carmen S, Miyoshi A, Azevedo V, Sesma F, Langella P, Bermúdez-Humarán LG, Watterlot L, Perdigon G, de LeBlanc AdM. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn’s disease in mice. J Biotechnol. 2011;151:287–93.
Islam M, Murata T, Fujisawa M, Nagasaka R, Ushio H, Bari A, Hori M, Ozaki H. Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br J Pharmacol. 2008;154:812–24.
Liu X, Wang J. Anti-inflammatory effects of iridoid glycosides fraction of Folium syringae leaves on TNBS-induced colitis in rats. J Ethnopharmacol. 2011;133:780–7.
Sanchez-Muñoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280.
Cho E-j, Shin J-S, Noh Y-S, Cho Y-W, Hong S-J, Park J-H, Lee JY, Lee J-Y, Lee K-T. Anti-inflammatory effects of methanol extract of Patrinia scabiosaefolia in mice with ulcerative colitis. J Ethnopharmacol. 2011;136:428–35.
Kwon KH, Murakami A, Hayashi R, Ohigashi H. Interleukin-1β targets interleukin-6 in progressing dextran sulfate sodium-induced experimental colitis. Biochem Biophys Res Commun. 2005;337:647–54.
Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74.
Bermúdez-Humarán LG, Motta J-P, Aubry C, Kharrat P, Rous-Martin L, Sallenave J-M, Deraison C, Vergnolle N, Langella P. Serine protease inhibitors protect better than IL-10 and TGF-β anti-inflammatory cytokines against mouse colitis when delivered by recombinant lactococci. Microb Cell Fact. 2015;14:1–11.
Fedorak RN, Gangl A, Elson CO, Rutgeerts P, Schreiber S, Wild G, Hanauer SB, Kilian A, Cohard M, LeBeaut A. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn’s disease. Gastroenterology. 2000;119:1473–82.
Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765.
Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, De Vries JE, Roncarolo MG. A CD4+T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42.
Cocconcelli PS, Cattivelli D, Gazzola S. Gene transfer of vancomycin and tetracycline resistances among Enterococcus faecalis during cheese and sausage fermentations. Int J Food Microbiol. 2003;88:315–23.
Devirgiliis C, Zinno P, Perozzi G. Update on antibiotic resistance in foodborne Lactobacillus and Lactococcus species. Front Microbiol. 2013;4:301.
Moubareck C, Gavini F, Vaugien L, Butel M, Doucet-Populaire F. Antimicrobial susceptibility of bifidobacteria. J Antimicrob Chemother. 2005;55:38–44.
Ku S, Park MS, Ji GE, You HJ. Review on Bifidobacterium bifidum BGN4: functionality and nutraceutical applications as a probiotic microorganism. Int J Mol Sci. 2016;17:1544.
Ku S-M, You H-J, Ji G-E. Enhancement of anti-tumorigenic polysaccharide production, adhesion, and branch formation of Bifidobacterium bifidum BGN4 by phytic acid. Food Sci Biotechnol. 2009;18:749–54.
Acknowledgements
Not applicable.
Funding
This work was carried out with the support of the Ministry of Small and Medium-sized Enterprises (SMEs) and Startups (MSS), Korea, under the “Regional Specialized Industry Development Program (R&D, Project number S2848321)” supervised by the Korea Institute for Advancement of Technology (KIAT). This work was also supported by a Faculty Research and Creative Activity Committee (FRCAC) grant (No. 221745) funded by Middle Tennessee State University (U.S.), Collaborative Grant-in-Aid of the HBUT National “111” Center for Cellular Regulation and Molecular Pharmaceutics (XBTK-2021004) and Doctoral start-up fund of Hubei University of Technology (XJ2021000101). Seockmo Ku and Tony V. Johnston participated this work based on a non-disclosure research agreement between Middle Tennessee State University and BIFIDO Co., Ltd.
Author information
Authors and Affiliations
Contributions
Experiment design, SK, ZL, SK and MSP; experiments and data analysis, SK, ZL, YX, MP; writing—original draft preparation, SK and ZL; writing—review and editing, GEJ, MSP, TVJ, and SK; supervising, SK and MSP; funding acquisition, SK, SK, GEJ and MSP. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Myeong Soo Park is directly employed by BIFIDO Co., Ltd. and he also hold BIFIDO Co., Ltd stocks as a CTO. Other authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Real-timepolymerase chain reaction (PCR) primer sequences.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kang, S., Lin, Z., Xu, Y. et al. A recombinant Bifidobacterium bifidum BGN4 strain expressing the streptococcal superoxide dismutase gene ameliorates inflammatory bowel disease. Microb Cell Fact 21, 113 (2022). https://doi.org/10.1186/s12934-022-01840-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-022-01840-2