Abstract
Background
Diabetes mellitus (DM) and Lp(a) are well-established predictors of coronary artery disease (CAD) outcomes. However, their combined association remains poorly understood.
Objective
To investigate the relationship between elevated Lp(a) and DM with CAD outcomes.
Methods
Retrospective analysis of the MGB Lp(a) Registry involving patients ≥ 18 years who underwent Lp(a) measurements between 2000 and 2019. Exclusion criteria were severe kidney dysfunction, malignant neoplasms, and prior atherosclerotic cardiovascular disease (ASCVD). The primary outcome was a combination of cardiovascular death or myocardial infarction (MI). Elevated Lp(a) was defined as > 90th percentile (≥ 216 nmol/L).
Results
Among 6,238 patients who met the eligibility criteria, the median age was 54, 45% were women, and 12% had DM. Patients with DM were older, more frequently male, and had a higher prevalence of additional cardiovascular risk factors. Over a median follow-up of 12.9 years, patients with either DM or elevated Lp(a) experienced higher rates of the primary outcome. Notably, those with elevated Lp(a) had a higher incidence of the primary outcome regardless of their DM status. The annual event rates were as follows: No-DM and Lp(a) < 90th% − 0.6%; No-DM and Lp(a) > 90th% − 1.3%; DM and Lp(a) < 90th% − 1.9%; DM and Lp(a) > 90th% − 4.7% (p < 0.001). After adjusting for confounders, elevated Lp(a) remained independently associated with the primary outcome among both patients with DM (HR = 2.66 [95%CI: 1.55–4.58], p < 0.001) and those without DM (HR = 2.01 [95%CI: 1.48–2.74], p < 0.001).
Conclusions
Elevated Lp(a) constitutes an independent and incremental risk factor for CAD outcomes in patients with and without DM.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a major cardiovascular risk factor and its global prevalence and associated burden continues to increase [1, 2]. Coronary artery disease (CAD) remains the leading cause of morbidity and mortality among patients with DM [3,4,5]. Lipoprotein(a) [Lp(a)] is a lipid-carrying particle that consists of a low-density lipoprotein (LDL)-like structure, incorporating apolipoprotein B-100 connected by a disulfide bond to apolipoprotein(a) [6]. Lp(a) was discovered over 60 years ago and has captured considerable interest in the cardiovascular field due to its established connection with atherosclerotic cardiovascular disease (ASCVD) in general and CAD in particular [7,8,9]. The recent introduction of potential therapies focused on lowering Lp(a) has brought new attention to this biomarker [6, 10].
Reports indicate that DM can impact lipid metabolism and contribute to the accelerated development of coronary atherosclerosis, a condition referred to as “diabetic dyslipidemia” [11, 12]. There are known variations in the distribution of Lp(a) levels among patients with DM, with some showing higher and others lower Lp(a) levels among individuals with DM [13,14,15]. Furthermore, studies suggest that although Lp(a) is associated with an increased risk of adverse cardiovascular events in patients with a history of atherosclerotic cardiovascular disease (ASCVD) (i.e. secondary prevention), the magnitude of this increased risk may vary between those with and without DM [16,17,18,19]. There is a deficiency in high-quality contemporary data evaluating the risk associated with elevated Lp(a) levels among individuals with and without DM, especially among those without a prior history of atherosclerotic cardiovascular disease (ASCVD). Therefore, the purpose of this study was to investigate the association between Lp(a) and CAD outcomes among patients with and without DM.
Methods
Study design and population
The patient population was derived from the Mass General Brigham Lp(a) Registry, as previously described [9, 20]. In brief, this retrospective cohort study included all individuals who underwent Lp(a) testing as part of their routine healthcare from January 2000 to July 2019. The research was carried out at two academic medical centers in Boston, Massachusetts: Brigham and Women’s Hospital and Massachusetts General Hospital.
Ethical considerations
The Mass General Brigham Lp(a) Registry, encompassing the present study, received approval from the Institutional Review Board at Mass General Brigham. This study was conducted in accordance with the principles outlined in the Declaration of Helsinki. Informed consent waived due to retrospective analysis of anonymized data.
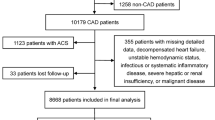
All individuals aged 18 years or older with at least one Lp(a) result were screened for inclusion in the cohort. Exclusion criteria consisted of two factors: (1) severe kidney dysfunction, defined as stage 5 chronic kidney disease (estimated glomerular filtration rate < 15 mL/min/m2), prior renal transplant, or those undergoing renal replacement therapy; and (2) the presence of a diagnostic International Classification of Diseases (ICD) code for malignant neoplasm during the covariate assessment window, except for non-melanoma skin cancer. Moreover, for this study, individuals with a history of atherosclerotic cardiovascular disease (ASCVD), such as a previous myocardial infarction, a history of coronary revascularization, or a history of ischemic stroke, were also excluded.
Data sources and definitions
The following sources were used to collect study data, including: (1) The Research Patient Data Registry (RPDR) [21] at Mass General Brigham, which provides demographic, laboratory, imaging, diagnostic, procedural, medication, vital status (based on the Social Security Administration Death Master File), and clinical documentation for individuals who meet specific search criteria. (2) ICD-coded death information from the National Death Index (NDI) and the Massachusetts Office of Vital Statistics was utilized to establish the causes of death for each patient who passed away during the study period.
To determine the presence of cardiovascular (CV) risk factors, validated natural language processing (NLP) modules [22], laboratory data, and diagnostic and procedural ICD-9, ICD-10, and Current Procedural Terminology (CPT) codes were utilized, as previously described [20]. The baseline covariate assessment period was established as the 12-month period before and 30 days after the Lp(a) measurement. For individuals with more than one Lp(a) test, their covariate assessment period was assessed relative to the first Lp(a) test.
Diabetes mellitus was defined by having at least two diagnostic ICD codes or two references via NLP during the covariate window. Alternatively, a single HbA1c > 6.5% test (for males of age or females > 50 years old) or two elevated HbA1c tests at least 400 days apart for females under the age of 50 to account for the possibility of gestational diabetes.
Non-Lp(a) dyslipidemia was determined through NLP, treatment with a cholesterol lowering medication, or laboratory values (median) that exceeded any one of the following thresholds during the covariate window: (1) total cholesterol ≥ 240 mg/dL, (2) LDL cholesterol ≥ 160 mg/dL, (3) HDL cholesterol < 40 mg/dL (men), (4) HDL cholesterol < 50 mg/dL (women), (5) total triglycerides ≥ 175 mg/dL.
Lipoprotein(a) assays
Lp(a) testing occurred as part of routine medical care using either the Lp(a)-particle assay (measured in nmol/L) or the Lp(a)-mass assay (measured in mg/dL). All Lp(a) lab testing was conducted at commercial laboratories during the study period, utilizing industry-standard assays. To mitigate potential biases stemming from differences in Lp(a) testing techniques over the study period, percentile distributions were established separately for each assay, as previously described [9]. This approach was employed in prior large Lp(a) studies [23,24,25,26]. Considering the well-established distribution of Lp(a), predefined percentile groups were established and applied in this study: 1st-50th, 51st-70th, 71st-90th, and 91st-100th. After merging separate assays across percentiles, we then converted all Lp(a) values to nmol/L using the following conversion formula to best represent the data in a clinically relevant manner: Lp(a) nmol = (2.18 x Lp(a)-M) − 3.83 [25, 27]. Consequently, elevated Lp(a) was defined as a value surpassing the 90th percentile (≥ 216 nmol/L.)
Primary outcome
The primary outcome was a combination of cardiovascular death or myocardial infarction (MI). As previously described [20], MI was defined by the presence of a diagnostic ICD code in the primary hospital discharge position. This methodology has been thoroughly validated and is associated with high specificity, high positive predictive value, and reasonable sensitivity [28,29,30,31]. Cardiovascular mortality was established using the ICD-coded underlying causes of death [32,33,34], as determined by the National Death Index (NDI) or the Massachusetts Office of Vital Statistics. The assessment of the cause of death was conducted blind to the Lp(a) levels or any other clinical factors.
Statistical analysis
Baseline Characteristics were reported as median (interquartile range) for numerical characteristics and frequencies (percentages) for categorical variables. Baseline characteristics were analyzed as Mann-Whitney U tests for numerical characteristics and chi-square tests of association or Fisher exact tests for categorical variables. Patients were censored at either date of death or date of querying vital statistics. Patients without a cardiovascular death defined ICD code were censored at date of death and labeled as not experiencing cardiovascular death.
Log-rank tests were used to compare Kaplan-Meier Curves. Incident rate ratios and their 95% confidence intervals were reported for the primary outcome of cardiovascular death or acute myocardial infarction. Univariable and multivariable Cox proportional hazards regressions were analyzed separately for patients with and without DM to assess the association of Lp(a) percentile group with cardiovascular death or acute myocardial infarction. Separate individual outcomes were assessed as secondary outcomes. Cox proportional hazard regressions were reported as hazard ratios and 95% confidence intervals. Multivariable Cox regression models were assessed for multicollinearity using Spearman rank correlation and proportional hazard assumptions with Schoenfeld residuals, as well as being adjusted for the following covariates: age, sex, self-reported race and ethnicity, hypertension, non-Lp(a) dyslipidemia, and current smoking status. Wald test of coefficient was used to evaluate interaction term between Lp(a) and diabetes mellitus. Spline models were developed to assess a potential nonlinear relationship between continuous Lp(a) levels and the primary composite outcome in groups with and without DM. These models were constructed using 5 knots for each group.
All analyses were performed using Stata MP Version 18 (StataCorp, College Station, TX) and RStudio (version 2022.12.0) ggplot2 package (version 3.4.1). Two-tailed test with an alpha value of 0.05 were considered statistically significant.
Results
A total of 6238 patients met the eligibility criteria, and among them the median age was 54 years, 45% were women, and 12% (733) had DM. The baseline characteristics of the study cohort and comparison between patients with and without DM are presented in Table 1. Patients with DM were older, more frequently male, and had a higher prevalence of other cardiovascular risk factors. There were no significant differences in the distribution of the Lp(a) values between patients with versus without DM (see Supplemental Fig. 1 for distribution). Furthermore, the median Lp(a) levels were similar between patients with and without DM, measuring 29 (IQR 12–99) versus 31 (IQR 11–107) nmol/L, respectively, p = 0.85.
Over a median follow-up period of 12.9 (IQR 7.7–15.3) years, individuals with either DM or elevated Lp(a) experienced higher rates of the primary outcome, as illustrated in Fig. 1. Notably, regardless of Lp(a) levels, patients with DM were found to have the highest annual event rates. For individuals with both DM and elevated Lp(a), the annual event rate of cardiovascular mortality or myocardial infarction (MI) was 4.7%, whereas the annual event rate was only 0.6% for those with neither condition. Findings were similar when annual event rates were adjusted for potential confounders. Notably, those with elevated Lp(a) had a higher incidence of the primary composite outcome regardless of DM status (Figs. 1 and 2).
When examining the association between the four pre-specified percentile subgroups of increasing Lp(a) with the primary outcome, patients with DM had a higher event rate among the group of 71-90th Lp(a) percentile (borderline statistical significance) when compared with the reference group (1-50th percentile). Among patients without DM, a higher event rate was only observed once when Lp(a) was greater than the 90th percentile. The restricted cubic spline analysis (Supplemental Fig. 2) demonstrated that higher Lp(a) levels were consistently associated with an increasing risk of the primary composite outcome for patients with and without DM. Notably among patients with DM the hazard ratio seemed to increase at a lower Lp(a) level.
Following adjustment for confounders (Table 2), elevated Lp(a) remained independently associated with the primary outcome in those with DM (HR = 2.66 [95%CI: 1.55–4.58], p < 0.001) and those without DM (HR = 2.01 [95%CI: 1.48–2.74], p < 0.001). There was no statistically significant interaction term between Lp(a) and DM (interaction p = 0.51).
Similar findings were also found when examining the cumulative incidence of the individual outcomes of cardiovascular death or myocardial infarction stratified by Lp(a) levels and DM status (Supplemental Fig. 3). Specifically, regardless of DM status, patients with elevated Lp(a), had a markedly higher cumulative occurrence of each individual outcome. Following adjustment for confounders (Supplemental Table 1), elevated Lp(a) remained independently associated with each individual outcome in those with DM (cardiovascular Death: HR = 2.59 [95%CI: 1.38–4.84], p = 0.003, MI: HR = 3.79 [95%CI: 1.62–8.85], p = 0.002) and those without DM (CV Death: HR = 1.73 [95%CI: 1.19–2.51], p = 0.004, MI: HR = 3.21 [95%CI: 2.00–5.18], p < 0.001).
Discussion
The present study, utilizing a comprehensive U.S. Lp(a) registry with long term follow-up, assessed the association of DM and Lp(a) levels with adverse cardiovascular events among individuals without a prior history of ASCVD. The main findings were as follows: (1) The incidence of the primary outcome of cardiovascular death or MI was elevated when either DM or elevated Lp(a) was present. However, the highest event rates were observed when both conditions were present. (2) Regardless of DM status, elevated Lp(a) was independently associated with the composite outcome of cardiovascular mortality or myocardial infarction (MI), as well as with each of the individual components. (3) Regardless of Lp(a) levels, patients with DM had significant higher event rates than those without DM.
These results are consistent with earlier studies, predominantly centered on patients with DM in secondary prevention studies, that indicated an association between elevated Lp(a) levels and an increased incidence of adverse cardiovascular outcomes [17,18,19].
The distribution of Lp(a) observed in the current study, particularly the 90th percentile threshold, is comparable to those reported in other observational registries (where the 90th percentile ranges between 43.5 and 75 mg/dL) [35,36,37]. However, this comparison may be limited by the variability in assays, laboratories, and conversion methods.
Our findings are also consistent with a large European study by Waldeyer et al. [36] revealing a higher coronary and cardiovascular risk among individuals with DM and elevated Lp(a). Notably, there are some key differences between our study and the European study. The prevalence of diabetes mellitus was significantly higher in our population (12% vs. 5.4%), likely reflecting recent trends of obesity and diabetes in the US. Additionally, the rate of lipid-lowering treatments, particularly statins, was significantly higher in our cohort, possibly indicating a more contemporary practice. We also evaluated risk associated with different Lp(a) thresholds among patients with and without diabetes separately, and conducted univariate and multivariable analyses of outcome by various Lp(a) values in these populations.
Nonetheless, a recent investigation conducted by Li et al. [16], which involved patients experiencing ST-elevation myocardial infarction (STEMI) and undergoing emergency revascularization, unveiled a distinct association between Lp(a) levels and the risk of adverse cardiovascular outcomes among individuals with and without DM. Specifically, elevated Lp(a) levels were linked to an increased risk in patients with DM, but not in those without DM. The variation in their findings compared to our study might be attributed to the distinction between a secondary prevention population in their cohort versus a primary prevention cohort in our study. Other factors that may contribute to these differences involve variations in Lp(a) thresholds within the Chinese population compared to American populations, a majority of male patients versus a more evenly distributed population in our study, and potential shifts in Lp(a) levels following myocardial infarction [16, 38, 39]. A recent study by Yu et al. [13] explored the threshold value of Lp(a) for the development of coronary artery disease (CAD) in individuals with suspected CAD, both with and without DM, and examined the impact of Lp(a) on CAD at optimal LDL-C levels. Consistent with our findings the authors reported that elevated Lp(a) was an independent risk factor for CAD in patients with or without DM. In addition, they found that Lp(a) had a different threshold value for the occurrence of CAD in patients with and without DM. As also supported by our findings, they reported that the threshold value of Lp(a) for the risk of CAD in DM patients was lower, than for those without DM. Our study aligned with their results concerning the heightened risk of adverse cardiovascular outcomes in patients with DM compared to those without. Additionally, we noted a higher incidence of the primary outcome among patients with DM in the 71-90th Lp(a) percentile range, which was not evident among patients without DM.
Several mechanisms could play a role in differential impact of Lp(a) between patients with versus without DM as following: first, individuals with DM are more likely to coronary atherosclerosis, even in the absence of prior ASCVD events, Thus, it is plausible that elevated Lp(a) may demonstrate a higher propensity for atherothrombotic events once coronary artery disease (CAD) is established. For example, findings from the MESA study indicate that when coronary artery calcium (CAC) is zero, there is no surplus risk associated with elevated Lp(a). However, in the presence of significant coronary atherosclerosis (CAC > 100), the risk associated with elevated Lp(a) becomes more robust [40]. Second, there could be a potential association between Lp(a) levels and the incidence of DM, although this was not consistently shown in the literature [13, 14, 41, 42] and not observed in our study. Third, a higher event rate associated with higher Lp(a) in diabetes could be due to additional confounding effects such as the presence of diabetic dyslipidemia [11, 12] which refers to negative effects of DM over lipid metabolism which contribute to the accelerated development of coronary atherosclerosis. Fourth, elevated levels of Lp(a) could potentially indicate both insulin resistance and pro-inflammation [43, 44]. Fifth, there could be a potentially synergistic effect between Lp(a) and high glucose levels, leading to damage to the vascular endothelium, increased susceptibility to vascular complications, and ultimately a heightened vulnerability to adverse cardiovascular events. Although patients with diabetes mellitus (DM) had higher hazard ratios (HR) compared with those without DM, we did not find a significant interaction between DM and lipoprotein(a) [Lp(a)], which aligns with the findings of by Waldeyer et al. [36]. However, our methodology may have been insufficient to detect such interactions due to limitations in sample size, and the fact that we evaluated a primary prevention population over a long-term follow-up.
Our findings advocate for the routine inclusion of Lp(a) testing for risk stratification and consideration of preventive pharmacotherapies particularly among individuals with DM. Furthermore, future trials exploring treatments aimed at lowering Lp(a) should consider focusing on patients with DM, particularly given the ongoing efforts to identify high-risk “primary” prevention cohorts.
Limitations
The current study has certain limitations that should be acknowledged. First, this study is based on a retrospective registry from a single geographic region of the US, which could limit the generalizability of the findings particularly as our patient population was predominantly white. Second, the evaluation of DM and the other risk factors was conducted retrospectively around the time of Lp(a) measurement, which was a priori defined as the baseline covariate window. However, this could result in some underestimation as patients may develop additional risk factors over additional follow-up time over time. Third, Lp(a) testing was conducted as a routine part of clinical care, with the specific indications for testing unknown, thus potentially introducing selection bias. Nonetheless, our focus was on patients without a history of ASCVD who were referred for testing. Therefore, these findings remain generalizable to the population of patients in the U.S. for whom Lp(a) is presently tested, as routine testing for Lp(a) in patients without ASCVD is not commonly performed. Fourth, Lp(a) was measured using various techniques within our cohort, leading to potential variations, as there are limitations when converting between different units/measurements. Nevertheless, we standardized and assessed Lp(a) levels across assays using percentile distributions, consistent with the approach adopted by numerous previous studies. Fifth since we lacked reliable laboratory data on proteinuria range, this variable was not used for excluding patients, which may have led to biased Lp(a) values in a small subset of patients. However, some patients with proteinuria may be accounted for under the CKD exclusion criteria. Therefore, the likelihood of this biasing our results is low. Other potential confounders such as family history of ASCVD and C-reactive protein were not accounted for. Sixth, due to limited power, we were not able to delineate differences across various thresholds and cardiovascular events between subgroups of patients.
Conclusions
Among individuals without a history ASCVD, there was a higher incidence of cardiovascular death or myocardial infarction when either DM or elevated Lp(a) was present, reaching a particularly high level when both conditions coexisted. While patients with DM had a considerably higher event rate than those without DM, Lp(a) was a robust risk marker among patients both with and without DM. Notably, the threshold of Lp(a) associated with higher risk was lower among individuals with DM. Our findings support the use of Lp(a) testing stratification among individuals with DM.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AMI:
-
Acute myocardial infarction
- ASCVD:
-
Atherosclerotic cardiovascular disease
- CAD:
-
Coronary artery disease
- DM:
-
Diabetes Mellitus
- ICD:
-
International Classification of Diseases (ICD)
- Lp(a):
-
Lipoprotein (a)
- LDL:
-
Low density lipoprotein
- MI:
-
myocardial infarction
- NLP:
-
Natural language processing
References
Stevens GA, Singh GM, Lu Y, et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metrics Nov. 2012;20(1):22.
Plakht Y, Elkis Hirsch Y, Shiyovich A, Abu Tailakh M, Liberty IF, Gilutz H. Heterogenicity of diabetes as a risk factor for all-cause mortality after acute myocardial infarction: age and sex impact. Diabetes Res Clin Pract Dec. 2021;182:109117.
Olsson M, Schnecke V, Cabrera C, Skrtic S, Lind M. Contemporary risk estimates of three HbA1c variables for myocardial infarction in 101,799 patients following diagnosis of type 2 diabetes. Diabetes care Aug. 2015;38(8):1481–6.
Shiyovich A, Gilutz H, Plakht Y. Serum electrolyte/metabolite abnormalities among patients with acute myocardial infarction: comparison between patients with and without diabetes mellitus. Postgrad Med Dec. 2020;24:1–9.
Plakht Y, Gilutz H, Shiyovich A. Changes over Time in Hemoglobin A1C (HbA(1C)) Levels Predict Long-Term Survival Following Acute Myocardial Infarction among Patients with Diabetes Mellitus. J Clin Med Jul. 2021. https://doi.org/10.3390/jcm10153232.
Bhatia HS, Wilkinson MJ. Lipoprotein(a): Evidence for Role as a Causal Risk Factor in Cardiovascular Disease and Emerging Therapies. J Clin Med Oct. 2022. https://doi.org/10.3390/jcm11206040.
Shiyovich A, Berman AN, Besser SA, et al. Cardiovascular outcomes in patients with coronary artery disease and elevated lipoprotein(a): implications for the OCEAN(a)-outcomes trial population. Eur Heart J open Jul. 2023;3(4):oead077.
Shiyovich A, Berman AN, Besser SA, et al. Association of Lipoprotein (a) and Standard Modifiable Cardiovascular Risk factors with Incident Myocardial Infarction: the Mass General Brigham Lp(a) Registry. J Am Heart Association May. 2024;21(10):e034493.
Berman AN, Biery DW, Besser SA, et al. Lipoprotein(a) and major adverse Cardiovascular events in patients with or without Baseline Atherosclerotic Cardiovascular Disease. J Am Coll Cardiol. 2024;83(9):873–86.
Kronenberg F, Mora S, Stroes ESG, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European atherosclerosis society consensus statement. Eur Heart J Oct. 2022;14(39):3925–46.
Lai SCT. Diabetic dyslipidaemia. Practical Lab Med Aug. 2021;26:e00248.
Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes care Jun. 2004;27(6):1496–504.
Yu B, Hu X, Liu J, et al. Lipoprotein(a) as a higher residual risk for coronary artery disease in patients with type 2 diabetes Mellitus than without. Int J Gen Med. 2023;16:3383–91.
Fu Q, Hu L, Xu Y, Yi Y, Jiang L. High lipoprotein(a) concentrations are associated with lower type 2 diabetes risk in the Chinese Han population: a large retrospective cohort study. Lipids Health Disease Jul. 2021;27(1):76.
Wang C, Niu DM, Hu J, et al. Elevated serum β2-glycoprotein-I-lipoprotein(a) complexes levels are associated with the presence and complications in type 2 diabetes mellitus. Diabetes Res Clin Pract May. 2013;100(2):250–6.
Li N, Zhou J, Chen R, et al. Prognostic impacts of diabetes status and lipoprotein(a) levels in patients with ST-segment elevation myocardial infarction: a prospective cohort study. Cardiovasc Diabetol Jun. 2023;26(1):151.
Konishi H, Miyauchi K, Shitara J, et al. Impact of lipoprotein(a) on long-term outcomes in patients with diabetes Mellitus who underwent percutaneous coronary intervention. Am J Cardiol Dec. 2016;15(12):1781–5.
Zhang Y, Jin JL, Cao YX, et al. Lipoprotein (a) predicts recurrent worse outcomes in type 2 diabetes mellitus patients with prior cardiovascular events: a prospective, observational cohort study. Cardiovasc Diabetol Jul. 2020;9(1):111.
Jin JL, Cao YX, Zhang HW, et al. Lipoprotein(a) and Cardiovascular outcomes in patients with coronary artery Disease and Prediabetes or Diabetes. Diabetes care Jul. 2019;42(7):1312–8.
Berman AN, Biery DW, Ginder C, et al. Study of lipoprotein(a) and its impact on atherosclerotic cardiovascular disease: design and rationale of the Mass General Brigham Lp(a) Registry. Clin Cardiol Nov. 2020;43(11):1209–15.
Rudziński PN, Kruk M, Demkow M, et al. Efficacy and safety of coronary computed tomography angiography in patients with a high clinical likelihood of obstructive coronary artery disease. Kardiologia Polska. 2022;80(1):56–63.
Berman AN, Biery DW, Ginder C, et al. Natural language processing for the assessment of cardiovascular disease comorbidities: the cardio-Canary comorbidity project. Clin Cardiol Sep. 2021;44(9):1296–304.
Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol Feb. 2014;11(5):470–7.
Langsted A, Nordestgaard BG, Kamstrup PR. Elevated lipoprotein(a) and risk of ischemic stroke. J Am Coll Cardiol Jul. 2019;9(1):54–66.
Langsted A, Kamstrup PR, Nordestgaard BG. High lipoprotein(a) and high risk of mortality. Eur Heart J Sep. 2019;1(33):2760–70.
Szarek M, Reijnders E, Jukema JW, et al. Relating Lipoprotein(a) Concentrations to Cardiovascular Event Risk After Acute Coronary Syndrome: A Comparison of Three Tests. Circulation. 2024. https://doi.org/10.1161/CIRCULATIONAHA.123.066398.
Madsen CM, Kamstrup PR, Langsted A, Varbo A, Nordestgaard BG. Lipoprotein(a)-Lowering by 50 mg/dL (105 nmol/L) may be needed to reduce Cardiovascular Disease 20% in secondary Prevention: a Population-based study. Arterioscler Thromb Vasc Biol Jan. 2020;40(1):255–66.
Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med Jun. 2010;10(23):2155–65.
Andrade SE, Harrold LR, Tjia J, et al. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf Jan. 2012;21(Suppl 1):100–28.
Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care May. 2005;43(5):480–5.
Tamariz L, Harkins T, Nair V. A systematic review of validated methods for identifying venous thromboembolism using administrative and claims data. Pharmacoepidemiol Drug Saf Jan. 2012;21(Suppl 1):154–62.
Coady SA, Sorlie PD, Cooper LS, Folsom AR, Rosamond WD, Conwill DE. Validation of death certificate diagnosis for coronary heart disease: the atherosclerosis risk in communities (ARIC) Study. J Clin Epidemiol Jan. 2001;54(1):40–50.
Ives DG, Samuel P, Psaty BM, Kuller LH. Agreement between nosologist and cardiovascular health study review of deaths: implications of coding differences. J Am Geriatr Soc Jan. 2009;57(1):133–9.
Chen Y, Freedman ND, Albert PS, et al. Association of Cardiovascular Disease with premature mortality in the United States. JAMA Cardiol Dec. 2019;1(12):1230–8.
Wong ND, Fan W, Hu X, et al. Lipoprotein(a) and Long-Term Cardiovascular Risk in a multi-ethnic pooled prospective cohort. J Am Coll Cardiol Apr. 2024;23(16):1511–25.
Waldeyer C, Makarova N, Zeller T, et al. Lipoprotein(a) and the risk of cardiovascular disease in the European population: results from the BiomarCaRE consortium. Eur Heart J Aug. 2017;21(32):2490–8.
Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J Am Coll Cardiol Mar. 2013;19(11):1146–56.
Li JJ, Ma CS, Zhao D, Yan XW. Lipoprotein(a) and Cardiovascular Disease in Chinese Population: a Beijing Heart Society Expert Scientific Statement. JACC Asia Nov. 2022;2(6):653–65.
Tsimikas S, Witztum JL, Miller ER, et al. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation Sep. 2004;14(11):1406–12.
Mehta A, Vasquez N, Ayers CR, et al. Independent Association of Lipoprotein(a) and coronary artery calcification with atherosclerotic Cardiovascular risk. J Am Coll Cardiol Mar. 2022;1(8):757–68.
Kamstrup PR, Nordestgaard BG. Lipoprotein(a) concentrations, isoform size, and risk of type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol Nov. 2013;1(3):220–7.
Gudbjartsson DF, Thorgeirsson G, Sulem P, et al. Lipoprotein(a) concentration and risks of Cardiovascular Disease and Diabetes. J Am Coll Cardiol Dec. 2019;17(24):2982–94.
Rainwater DL, Haffner SM. Insulin and 2-hour glucose levels are inversely related to lp(a) concentrations controlled for LPA genotype. Arterioscler Thromb Vascular Biology Aug. 1998;18(8):1335–41.
Onat A, Can G. Enhanced proinflammatory state and autoimmune activation: a breakthrough to understanding chronic diseases. Curr Pharm Design. 2014;20(4):575–84.
Acknowledgements
AS, ANB, SB, DWB, RC, SD, AvS, DH, KN, JP: none. BW: Scientific advisory board: Kiniksa, Horizon Therapuetics, and Novo Nordisk. CC: Dr. Cannon reports in calendar years 2021 to 2023: Research Grants from: Amgen, Better Therapeutics, Boehringer-Ingelheim (BI), Daiichi Sankyo, Merck, Novo Nordisk, Pfizer. Consulting fees from Amryt/Chiesi, Alnylam, Amarin, Amgen, Applied Therapeutics, Ascendia, Biogen, BI, BMS, CSL Behring, Eli Lilly, Janssen, Lexicon, Merck, Milestone, Pfizer, Rhoshan, SanofiI serve on Data and Safety Monitoring Boards for the Applied Therapeutics, Areteia and Novo Nordisk. JJ: Dr. Januzzi is a Trustee of the American College of Cardiology, is a Board Member of Imbria Pharmaceuticals, is a director at Jana Care, has received research support from Abbott, Applied Therapeutics, HeartFlow Inc, Innolife and Roche Diagnostics, consulting income from Abbott, AstraZeneca, Bayer, Beckman, Boehringer-Ingelheim, Janssen, Novartis, Merck, and Roche Diagnostics, and participates in clinical endpoint committees/data safety monitoring boards for Abbott, AbbVie, Bayer, CVRx, Intercept, Pfizer and Takeda. MDC: Grant support from Amgen. DLB: Dr. Bhatt discloses the following relationships - Advisory Board: Angiowave, Bayer, Boehringer Ingelheim, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Stasys; Board of Directors: American Heart Association New York City, Angiowave (stock options), Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock); Consultant: Broadview Ventures, GlaxoSmithKline, Hims, SFJ, Youngene; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic, Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical; for ALLAY-HF, funded by Alleviant Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), CSL Behring (AHA lecture), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), WebMD (CME steering committees), Wiley (steering committee); Other: Clinical Cardiology (Deputy Editor); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women’s Hospital who assigned to Lexicon; neither I nor Brigham and Women’s Hospital receive any income from this patent); Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Alnylam, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, Cleerly, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Otsuka, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, 89Bio; Royalties: Elsevier (Editor, Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo. RB: Dr. Blankstein has received research support from Amgen Inc, Novartis Inc, and Beren Therapeutics, and has served as a consultant / advisory board for Caristo Inc, Elucid Inc, Hearflow Inc, Nanox AI, and Beren Therapeutics.
Funding
None.
Author information
Authors and Affiliations
Contributions
Study concept and design: Ron Blankstein, Arthur Shiyovich Acquisition, analysis and interpretation: Ron Blankstein, Arthur Shiyovich, Adam N. Berman, Stephanie A. Besser, David W. Biery. Statistical analysis: Stephanie A. Besser, Ron Blankstein, Arthur Shiyovich. Critical revision of the manuscript for important intellectual content: All authors. Drafting of the manuscript: Arthur Shiyovich, Ron Blankstein. Supervision: Ron Blankstein.
Corresponding author
Ethics declarations
Competing interests
AS, ANB, SB, DWB, RC, SD, AvS, DH, KN, JP: noneBW: Scientific advisory board: Kiniksa, Horizon Therapuetics, and Novo NordiskCC: Dr. Cannon reports in calendar years 2021 to 2023: Research Grants from: Amgen, Better Therapeutics, Boehringer-Ingelheim (BI), Daiichi Sankyo, Merck, Novo Nordisk, Pfizer. Consulting fees from Amryt/Chiesi, Alnylam, Amarin, Amgen, Applied Therapeutics, Ascendia, Biogen, BI, BMS, CSL Behring, Eli Lilly, Janssen, Lexicon, Merck, Milestone, Pfizer, Rhoshan, SanofiI serve on Data and Safety Monitoring Boards for the Applied Therapeutics, Areteia and Novo Nordisk. JJ: Dr. Januzzi is a Trustee of the American College of Cardiology, is a Board Member of Imbria Pharmaceuticals, is a director at Jana Care, has received research support from Abbott, Applied Therapeutics, HeartFlow Inc, Innolife and Roche Diagnostics, consulting income from Abbott, AstraZeneca, Bayer, Beckman, Boehringer-Ingelheim, Janssen, Novartis, Merck, and Roche Diagnostics, and participates in clinical endpoint committees/data safety monitoring boards for Abbott, AbbVie, Bayer, CVRx, Intercept, Pfizer and Takeda; MDC: Grant support from AmgenDLB: Dr. Bhatt discloses the following relationships - Advisory Board: Angiowave, Bayer, Boehringer Ingelheim, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Stasys; Board of Directors: American Heart Association New York City, Angiowave (stock options), Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock); Consultant: Broadview Ventures, GlaxoSmithKline, Hims, SFJ, Youngene; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic, Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical; for ALLAY-HF, funded by Alleviant Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), CSL Behring (AHA lecture), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), WebMD (CME steering committees), Wiley (steering committee); Other: Clinical Cardiology (Deputy Editor); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women’s Hospital who assigned to Lexicon; neither I nor Brigham and Women’s Hospital receive any income from this patent); Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Alnylam, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, Cleerly, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Otsuka, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, 89Bio; Royalties: Elsevier (Editor, Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo.RB: Dr. Blankstein has received research support from Amgen Inc, Novartis Inc, and Beren Therapeutics, and has served as a consultant/advisory board for Caristo Inc, Elucid Inc, Hearflow Inc, Nanox AI, and Beren Therapeutics.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shiyovich, A., Berman, A.N., Besser, S.A. et al. Lipoprotein(a) as a cardiovascular risk factor among patients with and without diabetes Mellitus: the Mass General Brigham Lp(a) Registry. Cardiovasc Diabetol 23, 257 (2024). https://doi.org/10.1186/s12933-024-02348-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02348-2