Abstract
Background
Triglyceride and glucose (TyG) index, a surrogate marker of insulin resistance, has been validated as a predictor of cardiovascular disease. However, effects of TyG-related indices combined with obesity markers on cardiovascular diseases remained unknown. We aimed to investigate the associations between TyG index and modified TyG indices with new-onset cardiovascular disease and the time-dependent predictive capacity using a national representative cohort.
Methods
This study is a retrospective observational cohort study using data from China Health and Retirement Longitudinal Study (CHARLS) of 7 115 participants. The TyG index was calculated as Ln [fasting triglyceride (mg/dL) × fasting glucose (mg/dL)/2]. The modified TyG indices were developed combining TyG with body mass index (BMI), waist circumference (WC) and waist-to‐height ratio (WHtR). We used adjusted Cox proportional hazards regression to analyze the association and predictive capacity based on hazard ratio (HR) and Harrell’s C‐index.
Results
Over a 7-year follow‐up period, 2136 participants developed cardiovascular disease, including 1633 cases of coronary heart disease and 719 cases of stroke. Compared with the lowest tertile group, the adjusted HR (95% CI) for new-onset cardiovascular disease in the highest tertile for TyG, TyG-BMI, TyG-WC, and TyG-WHtR were 1.215 (1.088–1.356), 1.073 (0.967–1.191), 1.078 (0.970–1.198), and 1.112 (1.002–1.235), respectively. The C‐indices of TyG index for cardiovascular disease onset were higher than other modified TyG indices. Similar results were observed for coronary heart disease and stroke.
Conclusion
TyG and TyG-WhtR were significantly associated with new-onset cardiovascular diseases, and TyG outperformed the modified TyG indices to identify individuals at risk of incident cardiovascular event.
Similar content being viewed by others
Background
Insulin resistance plays a key role in the development of diabetes and atherosclerotic cardiovascular diseases [1, 2]. Using insulin resistance assessment to evaluate cardiovascular risk is of particular importance in the general population [3]. In a clinical setting, insulin resistance is commonly evaluated using hyperinsulinemic-euglycemic clamp test and fasting insulin (e.g., HOMA-IR) [4]. In epidemiological studies, triglyceride-glucose (TyG) index, developed using fasting triglyceride and blood glucose, has been proposed as a simple and reliable surrogate marker of insulin resistance [5]. Previous studies have recognized that TyG index is independently associated with atherosclerosis and cardiovascular disease, such as hypertension [6], carotid plaque [7], coronary heart disease and stroke [8].
Recently, modified TyG indices combining TyG and body composition indices, such as body mass index (BMI), waist circumference (WC) and waist-to-height ratio, have been developed to track the risk of metabolic diseases. TyG-WC is a novel and effective predictor of diabetes in first-degree relatives of type 2 diabetes patients [9]. TyG index combined with BMI, WC, and WHtR can further stratify and predict the risk of diabetes [10]. TyG and modified TyG indices are validated to identify individuals at risk of hypertension [11]. However, there is scare evidence examining the relationship between TyG and modified TyG indices with cardiovascular disease.
Using a national representative cohort, we aimed to investigate the association and the predictive capacity of TyG index and modified TyG indices with new-onset cardiovascular diseases in the middle aged and older Chinese population.
Methods
Study population
This current study was a secondary analysis using data from China Health and Retirement Longitudinal Study (CHARLS). CHARLS (http://charls.pku.edu.cn/) is a national project aiming to investigate the policy and health related data among adults aged 45 years or older and solve the population aging issue. There are four surveys between 2011 and 2018, with participants recruited from both rural and urban residence using multistage stratified probability proportional-to-size sampling strategy. Details of the study design and cohort profile have been previously described [12]. CHARLS data have been widely used in the epidemiolocal research [13,14,15]. The CHARLS study was approved by the Institutional Review Board of Peking University (IRB00001052-11015).
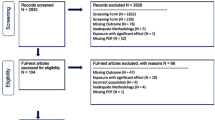
Following the study design of our previous study [8], the first survey (2011–2012) was set as baseline and participants were then followed at three subsequent visits (2013–2014, 2015–2016, 2017–2018). Participants with no history of cardiovascular diseases were primarily included in the current analysis. People lacking sociodemographic characteristics (age and sex), necessary blood tests and anthropometric measurements, or data of cardiovascular diseases or cancer history at baseline were further excluded. Then, a total of 7 115 participants were included in the final analysis.
TyG and modified TyG indices
Plasma glucose (FPG) and triglyceride levels were analyzed using Hitachi 7180 chemistry analyzer (Hitachi, Tokyo, Japan). The coefficient of variation (CV) of blood marker measurement was < 5%. Height, weight, and waist circumference (WC) were physically measured three times, and the average values were adopted as the final measurements. BMI was calculated as weight (in kilograms)/height^2 (in meters squared). Waist-to-height ratio (WHtR) was defined as WC/height. TyG index was calculated as Ln [triglycerides (mg/dL) × glucose (mg/dL)/2]. TyG was modified by multiplying with BMI, WC, and WHtR to produce TyG-BMI, TyG-WC, and TyG-WHtR, respectively. TyG index and modified TyG indices were grouped into tertiles (T) as follows:
-
TyG, T1: <8.36, T2: 8.36–8.87, and T3: >8.87.
-
TyG-BMI, T1: <177.9, T2: 177.9-223.1, and T3: >223.1.
-
TyG-WC, T1: <684.4, T2: 684.4-775.7, and T3: >775.7.
-
TyG-WHtR, T1: <4.30, T2: 4.30–4.93, and T3: >4.93.
New-onset cardiovascular events
The primary study outcome was the incidence of cardiovascular disease, a composite event of coronary heart disease and stroke. In accordance with previous studies [13, 16], new-onset cardiovascular events were assessed by the following standardized questions: “Have you been told by a doctor that you have been diagnosed with a heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems?” or “Have you been told by a doctor that you have been diagnosed with a stroke?” Participants who reported heart disease or stroke during the follow-up period were defined as having new-onset cardiovascular disease. The outcomes were assessed by rigorously trained interviewers through standardized questionnaires that are harmonized to international leading aging surveys.
Covariates
Information on socio-demographic status and health-related factors using a structured questionnaire was collected at baseline. Sociodemographic variables included age, sex, education (elementary school and below, secondary school, and college and above), marital status (married and others), and residence (rural, urban). Health-related factors included smoking and drinking status (yes or no), hypertension, diabetes, and use of medications for hypertension and diabetes. Smoking status was defined as “never smoking”, “current smoker” and “former smoker”. Subjects were diagnosed of hypertension when the systolic blood pressure was ≥ 140 mmHg or the diastolic pressure was ≥ 90 mmHg or self-reported diagnosis history of hypertension or antihypertensive medications currently used. Diabetes was defined as fasting glucose ≥ 7.0 mmol/L or self-reported diagnosis history of diabetes or use of any hypoglycemic medication currently used.
Statistical analysis
Data are presented as means ± standard deviation (SD) for continuous variables and number (percentages) for categorical variables. Baseline characteristics were summarized based on TyG index tertile groups. The correlations of TyG, TyG-BMI, TyG-WC and TyG-WHtR are shown using Spearman’s coefficients.
The cumulative incidence rate of new-onset events based on tertile of TyG and modified TyG indices are presented as Kaplan-Meier curves. Multivariable-adjusted Cox regression models are used to investigate the association between TyG and modified TyG indices with cardiovascular disease onset. Hazard ratio (HR) with 95% confidence interval (CI) are calculated. The proportional hazards assumption is tested using Schoenfeld residuals, and no potential violation was observed. To account for potential confounders, we conducted the analysis in two models: age (continuous) and sex are adjusted in model 1, and in model 2, residence (rural, urban), education level (primary, secondary, third), marital status (married, others), smoking status (current, former, never), current drinking (yes, no), hypertension (yes, no) and diabetes (yes, no) are further adjusted. To illustrate the dose-response relationship of TyG and modified TyG indices, we performed the restricted cubic spline function analysis using 3 knots at the 10th, 50th, and 90th percentiles. The reference point is set as the median value of variables among the corresponding populations. In addition, the effects of TyG and modified TyG indices on coronary heart disease and stroke are analyzed.
We calculated the time-dependent Harrell’s C-index (95% CI) to evaluate the predictive capacity of TyG, TyG-BMI, TyG-WC, and TyG-WHtR for new-onset cardiovascular disease, coronary heart disease and stroke. Given potential sex difference, we performed the subgroup analysis in terms of sex. All statistical analyses were performed using R version 4.1.0 (R Foundation for Statistical Computing), and a two-sided P value < 0.05 was considered to indicate statistical significance.
Results
Characteristics of the study population
A total of 7 115 participants (3894 men and 3221 women) were included in this study. The mean (SD) age was 58.8 (9.0) years. Baseline characteristics according the TyG tertile groups are shown in Table 1. People in the highest tertile of TyG index were more likely to be male (< 0.001) and having diabetes (P < 0.001). During a mean follow-up of 7.0 years, 2136 participants developed cardiovascular disease, including 1633 cases of coronary heart disease and 719 cases of stroke. There is no significant difference of BMI level (P = 0.898). Figure 1 presents the Spearman’s coefficients of paired correlation among TyG, TyG-BMI, TyG-WC, and TyG-WHtR. TyG was not strongly correlated with the modified TyG indices, and the Spearman’s coefficients for TyG-BMI, TyG-WC, and TyG-WHtR were 0.27, 0.49, and 0.44, respectively.
Relationships of TyG and modified TyG indices with cardiovascular disease
Figure 2 presents the positive dose-responsive relationships of TyG and modified TyG indices with the risk of cardiovascular disease, coronary heart disease and stroke. The Kaplan–Meier curves of the cumulative incidence of cardiovascular disease, coronary heart diseases and stroke were shown in Figure S1-Figure S3. In the fully adjusted model (Table 2), when compared with people in the lowest tertile, those in the highest tertile of TyG and TyG-WHtR were significantly associated with a higher risk of new-onset cardiovascular disease, and the adjusted HR (95% CI) were 1.215 (1.088–1.356) and 1.112 (1.002–1.235), respectively. TyG-BMI and TyG-WC were not significantly associated with the incidence of cardiovascular disease, and the adjusted HR (95% CI) were 1.073 (0.967–1.191) and 1.078 (0.970–1.198). Table 3 shows the associations between TyG and modified TyG indices in terms of new-onset coronary heart disease and stroke. TyG index, but not TyG-BMI, TyG-WC and TyG-WHtR, was associated with a higher risk of coronary heart disease (T3 vs. T1: HR, 1.200; 95 CI, 1.057–1.362). TyG, TyG-BMI and TyG-WHtR, but not TyG-WC, were significantly associated with the incidence of stroke, and the adjusted HR (95% CI) were 1.249 (1.039–1.501), 1.200 (1.001–1.438), and 1.236 (1.031–1.483), respectively.
Predictive capacity comparison
We calculated the time-dependent Harrell’s C-indices of TyG, TyG-BMI, TyG-WC, and TyG-WHtR that were significantly related to new-onset cardiovascular disease, coronary heart diseases and stroke (Fig. 3). The overall C-index values were 0.544 (95% CI: 0.528–0.560) for TyG, followed by TyG-WC of 0.525 (95% CI: 0.508–0.541), TyG-WHtR of 0.523 (95% CI: 0.506–0.539), and TyG-BMI of 0.516 (95% CI: 0.503–0.532) in terms of new-onset cardiovascular disease. Similarly, the C-index values were 0.543 (95% CI: 0.525–0.560) for TyG, followed by TyG-WC of 0.525 (95% CI: 0.507–0.542), TyG-WHtR of 0.522 (95% CI: 0.504–0.540), and TyG-BMI of 0.517 (95% CI: 0.502–0.534) in terms of new-onset coronary heart disease, and 0.545 (95% CI: 0.517–0.572) for TyG, followed by TyG-WHtR of 0.538 (95% CI: 0.510–0.565), TyG-WC of 0.536 (95% CI: 0.508–0.563), and TyG-BMI of 0.528 (95% CI: 0.501–0.555) in terms of new-onset stroke. In addition, TyG index shows the highest C-index of cardiovascular diseases both in men and women (Figure S4).
Discussion
In this national longitudinal cohort study based on the Chinese health examination population, we comprehensively investigated the associations between TyG index and modified TyG indices with new-onset cardiovascular disease and compared the time-dependent predictive capacity. Overall, TyG index is moderately correlated with other modified TyG indices. TyG index and TyG-WHtR, but not TyG-BMI nor TyG-WC, are independently predictors of future cardiovascular disease onset. Of note, TyG index has the highest predictive capacity for cardiovascular risk.
Insulin resistance plays a key role in atherosclerosis and cardiovascular disease, recognized by mechanism research, epidemiology study and genetic data. One mechanism by which insulin resistance leads to atherosclerosis and cardiovascular disease is via vascular stiffness, and defective insulin signaling in atherosclerotic lesion cells also links insulin resistance and atherosclerotic vascular disease [17, 18]. Genetically instrumented insulin resistance is causally associated with cardiovascular risk, such as heart failure [19] and ischemic stroke [20]. In epidemiological studies, the association between insulin resistance estimated by HOMA-IR has been confirmed [21].
Given the data of insulin levels are not widely measured, there are several surrogate markers of insulin resistance, of which TyG index is a reliable indicator. Previous studies have recognized the relationship between TyG index and cardiometabolic outcomes, including hypertension [11], diabetes [22], and cardiovascular diseases [23]. In addition to the effects of metabolic biomarkers like triglyceride and glucose, the content and distribution of body fat are closed correlated with insulin resistance. Hormones and cytokines from adipocytes can enhance or inhibit both glycemic response and insulin signaling [24]. Taking account for the crosstalk between insulin resistance and obesity, an increasing number of studies have been investigating whether combing TyG index and obesity-related parameters, such as BMI, WC and WHtR could enhance the risk stratification of cardiometabolic outcomes. In our analysis, we found that TyG index is moderately correlated with the modified TyG indices, of which the correlation coefficients range from 0.27 to 0.49 (Fig. 1). But the correlation coefficients among TyG-BMI, TyG-WC and TyG-WHtR range from 0.67 to 0.94, which means TyG index capture the distinct aspect compared to the modified TyG indices.
The results in terms of modified TyG indices including TyG-BMI, TyG-WC and TyG-WHtR are highly controversial given different disease outcomes and study design. In a cross-sectional survey of an elderly population, Ke et al. found that combining the TyG index with BMI, WC, and WHtR could not further improve the performance of TyG index in recognizing the risk of diabetes [25]. Zhang et al. found that TyG-WC was more strongly related with the prevalence of prediabetes and diabetes, which had the highest prediction accuracy [9]. Kuang et al. found that TyG index combined with BMI, WC, and WHtR can improve the prediction of the risk of diabetes using a cohort design [10]. Li et al. indicated that TyG-WHtR and TyG-BMI outperformed the TyG index alone to predict the new-onset of diabetes [26]. Lee et al. reported the sex-specific relationship between modified TyG indices and the incidence of hypertension [11]. Another cohort study found that TyG-related markers combining obesity parameters are superior to identify metabolic syndrome in both genders [27].
Given that evidence is inconsistent about the predictive capacity of the TyG index and modified TyG indices on cardiovascular outcomes, our study evaluated the association between TyG index and the modified TyG indices with the new-onset cardiovascular diseases using a Chinese national cohort. We found that TyG index along is most strongly associated with the incidence of coronary heart disease, stroke, and the composite outcome. TyG index also outperformed other modified TyG indices in predicting the future cardiovascular risk (Fig. 3), and the C-index was largely consistent from previous study among other population [28]. WC is a useful assessment parameter for abdominal obesity, which is closely associated insulin resistance [29]. WHtR is another a useful tool for assessing central obesity after adjusting for statures [30, 31]. However, the findings did not support the superior role of modified TyG indices for predicting the onset of cardiovascular events. In addition, a previous study indicated that TyG index had a higher predictive power for cardiovascular disease than HOMA-IR [32]. Our findings indicated that there is a J-shaped relationship between modified TyG indices and new-onset coronary heart disease, while Dang et al. reported a potential linear relationship [33]. The distinct study designs of cohort and cross-sectional frameworks could partially explain the difference. In addition, participants aged 45 years and older were included in our study, and the distinct age distribution could be another reason. On the other hand, Park et al. reported that TyG-WC and TyG-WHtR had better predictive performances for new-onset cardiovascular disease than TyG and TyG-BMI only in participants without diabetes [34]. Given the current analysis, findings supported the superiority of TyG index over the modified TyG indices using obesity related parameters for the risk stratification and prediction of future cardiovascular disease in clinical practice among middle-aged and older Chinese adults. Further studies are needed to reveal the distinct effects of TyG index and modified TyG indices on cardiovascular health among different population and subgroups, such as diabetes status [23].
Indeed, the specific reasons for the differential relationship between the TyG index and modified TyG indices with cardiovascular disease are not clear. There are several possible explanations. TyG index, derived from glucose and triglyceride, captures the status of glucose metabolism and hyperlipidemia, which determine the risk of atherosclerosis diseases than body weight and visceral fat [35]. TyG index itself is a comprehensive indicator of adipose volume, density, and distribution [36]. In addition, CHARLS cohort recruited people aged above 45 years. Hyperglycemia and hyperlipidemia could play a more important role in further incidence of cardiovascular disease among the middle-aged and elder population than obesity parameters. Besides, weight loss could be associated with an increase in all-cause and cause-specific mortality in the healthy older adults [37]. Moreover, the effects of TyG index and and modified TyG indices are possibly distinct between low- or middle-income countries and high-income countries, which is suggested by the PURE study [23].
Limitation
Several limitations of the current study should be acknowledged. First, owing to the observational study design, we could not confer the causal association between TyG index and modified TyG indices with cardiovascular disease. Further genetic studies and clinical trials are needed to validate the evidence strengthen. Second, the data of cardiovascular disease onset is self-reported; however, it has been shown the high consistency between self-reported disease diagnosis and medical records in terms of cardiovascular event [38]. Third, there is a possibility of residual or unmeasured confounding bias. Further studies are needed to validate the findings in other populations, not limited to the middled-aged and older.
Conclusions
TyG index and TyG-WHtR were significantly associated with new-onset cardiovascular disease among the middle-aged and older Chinese adults. The findings supported the superiority of TyG index over the modified TyG indices using obesity related parameters for the risk stratification and prediction of future cardiovascular disease.
Data availability
The data sets used and/or analyzed during the current study are publicly available or from the corresponding author upon reasonable request.
References
Louie JZ, Shiffman D, McPhaul MJ, Melander O. Insulin resistance probability score and incident cardiovascular disease. J Intern Med. 2023;294(4):531–5.
Brahimaj A, Rivadeneira F, Muka T, Sijbrands EJG, Franco OH, Dehghan A, Kavousi M. Novel metabolic indices and incident type 2 diabetes among women and men: the Rotterdam Study. Diabetologia. 2019;62(9):1581–90.
Wu Z, Zhou D, Liu Y, Li Z, Wang J, Han Z, Miao X, Liu X, Li X, Wang W, et al. Association of TyG index and TG/HDL-C ratio with arterial stiffness progression in a non-normotensive population. Cardiovasc Diabetol. 2021;20(1):134.
Tahapary DL, Pratisthita LB, Fitri NA, Marcella C, Wafa S, Kurniawan F, Rizka A, Tarigan TJE, Harbuwono DS, Purnamasari D, et al. Challenges in the diagnosis of insulin resistance: focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr. 2022;16(8):102581.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Che B, Zhong C, Zhang R, Pu L, Zhao T, Zhang Y, Han L. Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio as potential cardiovascular disease risk factors: an analysis of UK biobank data. Cardiovasc Diabetol. 2023;22(1):34.
Wu Z, Wang J, Li Z, Han Z, Miao X, Liu X, Li X, Wang W, Guo X, Tao L. Triglyceride glucose index and carotid atherosclerosis incidence in the Chinese population: a prospective cohort study. Nutr Metab Cardiovasc Dis. 2021;31(7):2042–50.
Cui C, Liu L, Zhang T, Fang L, Mo Z, Qi Y, Zheng J, Wang Z, Xu H, Yan H, et al. Triglyceride-glucose index, renal function and cardiovascular disease: a national cohort study. Cardiovasc Diabetol. 2023;22(1):325.
Zheng S, Shi S, Ren X, Han T, Li Y, Chen Y, Liu W, Hou PC, Hu Y. Triglyceride glucose-waist circumference, a novel and effective predictor of diabetes in first-degree relatives of type 2 diabetes patients: cross-sectional and prospective cohort study. J Transl Med. 2016;14(1):260.
Kuang M, Yang R, Huang X, Wang C, Sheng G, Xie G, Zou Y. Assessing temporal differences in the predictive power of baseline TyG-related parameters for future diabetes: an analysis using time-dependent receiver operating characteristics. J Transl Med. 2023;21(1):299.
Lee JH, Heo SJ, Kwon YJ. Sex-specific comparison between triglyceride glucose index and modified triglyceride glucose indices to Predict New-Onset Hypertension in Middle-aged and older adults. J Am Heart Assoc. 2023;12(18):e030022.
Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61–8.
Gao K, Cao LF, Ma WZ, Gao YJ, Luo MS, Zhu J, Li T, Zhou D. Association between Sarcopenia and cardiovascular disease among middle-aged and older adults: findings from the China health and retirement longitudinal study. EClinicalMedicine. 2022;44:101264.
Wu Z, Zhang H, Miao X, Li H, Pan H, Zhou D, Liu Y, Li Z, Wang J, Liu X, et al. High-intensity physical activity is not associated with better cognition in the elder: evidence from the China Health and Retirement Longitudinal Study. Alzheimers Res Ther. 2021;13(1):182.
Cui C, Liu L, Qi Y, Han N, Xu H, Wang Z, Shang X, Han T, Zha Y, Wei X, et al. Joint association of TyG index and high sensitivity C-reactive protein with cardiovascular disease: a national cohort study. Cardiovasc Diabetol. 2024;23(1):156.
Li H, Zheng D, Li Z, Wu Z, Feng W, Cao X, Wang J, Gao Q, Li X, Wang W, et al. Association of depressive symptoms with Incident Cardiovascular diseases in Middle-aged and older Chinese adults. JAMA Netw Open. 2019;2(12):e1916591.
Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, Sowers JR. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119:154766.
Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14(5):575–85.
Mordi IR, Lumbers RT, Palmer CNA, Pearson ER, Sattar N, Holmes MV, Lang CC. Type 2 diabetes, metabolic traits, and risk of Heart failure: a mendelian randomization study. Diabetes Care. 2021;44(7):1699–705.
Georgakis MK, Harshfield EL, Malik R, Franceschini N, Langenberg C, Wareham NJ, Markus HS, Dichgans M. Diabetes Mellitus, glycemic traits, and Cerebrovascular Disease: a mendelian randomization study. Neurology. 2021;96(13):e1732–42.
Wang T, Li M, Zeng T, Hu R, Xu Y, Xu M, Zhao Z, Chen Y, Wang S, Lin H, et al. Association between Insulin Resistance and Cardiovascular Disease Risk varies according to glucose tolerance status: a nationwide prospective cohort study. Diabetes Care. 2022;45(8):1863–72.
Deng H, Hu P, Li H, Zhou H, Wu X, Yuan M, Duan X, Lao M, Wu C, Zheng M, et al. Novel lipid indicators and the risk of type 2 diabetes mellitus among Chinese hypertensive patients: findings from the Guangzhou Heart Study. Cardiovasc Diabetol. 2022;21(1):212.
Lopez-Jaramillo P, Gomez-Arbelaez D, Martinez-Bello D, Abat MEM, Alhabib KF, Avezum Á, Barbarash O, Chifamba J, Diaz ML, Gulec S, et al. Association of the triglyceride glucose index as a measure of insulin resistance with mortality and cardiovascular disease in populations from five continents (PURE study): a prospective cohort study. Lancet Healthy Longev. 2023;4(1):e23–33.
Martyn JA, Kaneki M, Yasuhara S. Obesity-induced insulin resistance and hyperglycemia: etiologic factors and molecular mechanisms. Anesthesiology. 2008;109(1):137–48.
Ke P, Wu X, Xu M, Feng J, Xu H, Gan Y, Wang C, Deng Z, Liu X, Fu W, et al. Comparison of obesity indices and triglyceride glucose-related parameters to predict type 2 diabetes mellitus among normal-weight elderly in China. Eat Weight Disord. 2022;27(3):1181–91.
Li X, Sun M, Yang Y, Yao N, Yan S, Wang L, Hu W, Guo R, Wang Y, Li B. Predictive effect of triglyceride glucose-related parameters, obesity indices, and lipid ratios for diabetes in a Chinese Population: a prospective cohort study. Front Endocrinol (Lausanne). 2022;13:862919.
Zhang X, Zhang T, He S, Jia S, Zhang Z, Ye R, Yang X, Chen X. Association of metabolic syndrome with TyG index and TyG-related parameters in an urban Chinese population: a 15-year prospective study. Diabetol Metab Syndr. 2022;14(1):84.
Rafiee H, Mohammadifard N, Nouri F, Alavi Tabatabaei G, Najafian J, Sadeghi M, Boshtam M, Roohafza H, Haghighatdoost F, Hassannejad R, et al. Association of triglyceride glucose index with cardiovascular events: insights from the Isfahan Cohort Study (ICS). Eur J Med Res. 2024;29(1):135.
Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106(4):473–81.
Ashwell M, Hsieh SD. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr. 2005;56(5):303–7.
Xuan W, Liu D, Zhong J, Luo H, Zhang X. Impacts of triglyceride glucose-Waist to height ratio on diabetes incidence: a secondary analysis of a Population-based Longitudinal Data. Front Endocrinol (Lausanne). 2022;13:949831.
Moon JH, Kim Y, Oh TJ, Moon JH, Kwak SH, Park KS, Jang HC, Choi SH, Cho NH. Triglyceride-glucose index predicts Future Atherosclerotic Cardiovascular diseases: a 16-Year follow-up in a prospective, Community-Dwelling Cohort Study. Endocrinol Metab (Seoul). 2023;38(4):406–17.
Dang K, Wang X, Hu J, Zhang Y, Cheng L, Qi X, Liu L, Ming Z, Tao X, Li Y. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003–2018. Cardiovasc Diabetol. 2024;23(1):8.
Park HM, Han T, Heo SJ, Kwon YJ. Effectiveness of the triglyceride-glucose index and triglyceride-glucose-related indices in predicting cardiovascular disease in middle-aged and older adults: a prospective cohort study. J Clin Lipidol. 2024;18(1):e70–9.
Alizargar J, Bai CH, Hsieh NC, Wu SV. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol. 2020;19(1):8.
Yang Q, Xu H, Zhang H, Li Y, Chen S, He D, Yang G, Ban B, Zhang M, Liu F. Serum triglyceride glucose index is a valuable predictor for visceral obesity in patients with type 2 diabetes: a cross-sectional study. Cardiovasc Diabetol. 2023;22(1):98.
Hussain SM, Newman AB, Beilin LJ, Tonkin AM, Woods RL, Neumann JT, Nelson M, Carr PR, Reid CM, Owen A, et al. Associations of Change in body size with all-cause and cause-specific mortality among healthy older adults. JAMA Netw Open. 2023;6(4):e237482.
Valtorta NK, Kanaan M, Gilbody S, Hanratty B. Loneliness, social isolation and risk of cardiovascular disease in the English Longitudinal Study of Ageing. Eur J Prev Cardiol. 2018;25(13):1387–96.
Acknowledgements
This study used data from China Health and Retirement Longitudinal Study (CHARLS). We would like to thank the CHARLS research team for the time and effort into the CHARLS project.
Funding
None.
Author information
Authors and Affiliations
Contributions
Literature search: CC. Cui, YT. Qi, JQ. Song; Study conception and design: XY. Shang, CC. Cui; Data collection: N. Hang, TJ. Han; Data analysis and interpretation: CC. Cui, SQ. Yue, YN. Zha; Manuscript writing and reviewing: CC. Cui, ZH. Xu; Study supervision: L. Liu, JN. Li. Lin Liu, Jiannan Li and Zhonghang Xu are the co-corresponding authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Funding
None.
Additional contributions
None.
Originality of content
All authors verify that all information and materials in the manuscript are original.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12933_2024_2278_MOESM1_ESM.pdf
Supplementary Material 1: Additional File: Fig. S1-S4. Fig. S1 [K‒M plot of cardiovascular diseases by the tertile groups of TyG and modified indices]. Fig. S2 [K‒M plot of coronary heart diseases by the tertile groups of TyG and modified indices]. Fig. S3 [K‒M plot of stroke by the tertile groups of TyG and modified indices]. Fig. S4 [Sex-specific time-dependent predictive capacity of TyG and modified indices for cardiovascular diseases, coronary heart disease and stroke]
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cui, C., Qi, Y., Song, J. et al. Comparison of triglyceride glucose index and modified triglyceride glucose indices in prediction of cardiovascular diseases in middle aged and older Chinese adults. Cardiovasc Diabetol 23, 185 (2024). https://doi.org/10.1186/s12933-024-02278-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02278-z