Abstract
Background
Atherogenic index of plasma (AIP) represents a novel marker in the current era of cardiovascular diseases. In this meta-analysis, we aimed to evaluate the association of AIP with cardiovascular prognosis in patients with coronary artery disease (CAD).
Methods
PubMed, Scopus, and Web of Science databases were searched from inception through 2024. The primary outcome was major cardiovascular events (MACE). The secondary outcomes included all-causes death, cardiovascular death, myocardial infarction (MI), stroke, revascularization, and no-reflow phenomenon. AIP was determined by taking the logarithm of the ratio of triglyceride (TG) to high-density lipoprotein cholesterol (HDL-C). The data analysis was represented using the risk ratio (RR) along with a 95% confidence interval (CI).
Results
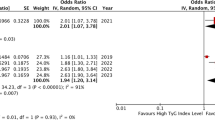
Sixteen studies with a total number of 20,833 patients met the eligible criteria. The pooled-analysis showed a significant increased risk of MACE in the highest AIP group compared with the lowest AIP group (RR = 1.63; 95% CI, 1.44–1.85; P < 0.001). A similar result was observed when AIP was regarded as a continuous variable (RR = 1.54; 95% CI, 1.30–1.83; P < 0.001). Besides, elevated AIP was associated with increased risk of cardiovascular death (RR = 1.79; 95% CI, 1.09–2.78; P = 0.02), MI (RR = 2.21; 95% CI, 1.55–3.13; P < 0.001), revascularization (RR = 1.62; 95% CI, 1.34–1.97; P < 0.001), no-reflow phenomenon (RR = 3.12 95% CI, 1.09–8.96; P = 0.034), and stent thrombosis (RR = 13.46; 95%CI, 1.39-129.02; P = 0.025). However, AIP was not significantly associated with the risk of all-causes death and stroke among patients with CAD.
Conclusions
The results of this study demonstrated that increased AIP is an independent prognostic factors in patients with CAD. Further research is warranted to elucidate the potential development of targeted interventions to modify AIP levels and improve patient outcomes.
Similar content being viewed by others
Introduction
Coronary artery diseases (CAD) are accountable for a high morbidity and mortality rate worldwide, with 17.8 million deaths annually [1]. Many studies have been conducted on the role of risk factors in predicting the risk of CAD; however, fewer studies have addressed the role of various factors in the short and long-term prognosis of patients with CAD. The short-term prognosis is mainly related to percutaneous coronary intervention (PCI) and in-hospital events such as the no-reflow phenomenon and in-hospital death, while the long-term prognosis mainly includes major adverse cardiovascular events (MACE) [2].
The prognosis of patients with CAD is dependent upon multiple factors. Traditional and modifiable risk factors for CAD include hypertension, diabetes mellitus, smoking, obesity, and dyslipidemia, which have been identified to play a role in the prognosis of CAD and, therefore, MACE [3]. However, clinicians frequently come across patients with novel CADs that have been misclassified due to these traditional cardiovascular risk factors in a way that necessitates establishing accurate predictors for CAD [4].
Atherogenic index of plasma (AIP) is calculated by Logarithm [triglyceride (TG) / high-denisity lipoprotein cholesterol (HDL-C)] and can be an independent cardiovascular risk factor by correlating with lipoprotein particle size [5]. A recent meta-analysis concluded that higher values of AIP can significantly increase the risk of CAD after adjusting for other risk factors [6]. Moreover, other studies have revealed the prognostic role of AIP in arterial stiffness, atherosclerotic disease, the risk of AMI, ischemic stroke, and MACE [7,8,9]. Fu et al. demonstrated that diabetic patients with MACE had higher values of AIP, introducing a novel MACE predictor for high-risk patients [10]. Similar results were observed in another study, including non-diabetic older adults with hypertension [11]. Nevertheless, no meta-analysis has been performed to reveal AIP’s prognostic effect in patients with CAD; therefore, we sought to determine the association between the levels of AIP and prognosis in patients with CAD.
Materials and methods
Data sources and searches
This systematic review and meta-analysis is performed according to the guideline of the Preferred Reporting Items for Systematic Review and Meta-analyses statement (PRISMA) [12]. A systematic search of the electronic databases including PubMed, Scopus, and Web of Science was undertaken to identify relevant papers published before January 2024. Search strategy used the terms for AIP (“Atherogenic index of plasma”, “atherogenic index”, AIP) and CAD (“coronary disease”, “coronary diseases”, “disease coronary”, “coronary heart disease”, “coronary heart diseases”, “heart disease coronary”, “heart diseases coronary”, “left main”, “left main coronary disease”, “percutaneous coronary intervention”, “coronary artery disease”, “coronary artery diseases”, “coronary artery bypass”, “coronary artery bypass graft”, “coronary syndrome”, “acute coronary syndrome”, “chronic coronary syndrome”). We also conducted a manual search of reference lists and potential related articles. Two independent reviewers completed the electronic search in databases.
Eligible criteria
Two reviewers independently screened the eligible studies based on the following inclusion criteria: (1) Adult patients who were diagnosed with CAD including myocardial infarction (MI), and acute or chronic CAD; (2) Measured AIP and reported the odds ratios (ORs) or hazard ratios (HRs) were with 95% confidence interval (CI) for association of AIP with the outcomes; and (3) The full text was available and written in the English language. Abstracts, reviews, case reports and case series, nonhuman studies, and letters to editors were excluded. Any disagreement was resolved by consensus.
Data extraction and quality assessment
Major adverse cardiovascular events (MACE) was the primary outcome of interest. The secondary outcomes included all-causes mortality, cardiovascular mortality, MI, stroke, revascularization, and no-reflow phenomenon. The following information was abstracted by two independent investigators: the first author’s last name, publication date, sample size, country, study design, mean age, percent of female participants, type of CAD, length of follow-up, adjusted RRs with their 95% CI for the outcomes. Disagreements were resolved by a third reviewer.
Two reviewers independently conducted a quality assessment of each included study using the Newcastle–Ottawa Quality Assessment Scale (NOS), with scores of ≥ 7 considered as high-quality studies [13]. Any discrepancies were resolved through discussion.
Statistical analyses
Risk ratios (RRs) and 95% CIs from the fully adjusted models were pooled to obtain the association of AIP with the outcomes. In studies where the AIP was examined as categorized variable, the RR of the outcomes for patients with the highest AIP level compared to those with the lowest level were collected. In studies where the AIP was analyzed as a continuous variable, the RR of the outcomes per 1-unit increase in the AIP were extracted.
The I2 statistic and Cochran’s Q test were utilized to assess heterogeneity. In cases where significant heterogeneity was observed (I2 > 50%, p < 0.1) among the studies, a random-effects model was applied. A fixed-effects model was used in case of no significant heterogeneity. Visual inspection of Funnel plot and Egger test were used to evaluate possible publication bias. We performed subgroup analysis to identify the potential sources of heterogeneities. All data were analyzed with STATA (Version14). P value < 0.05 was considered as significant.
Results
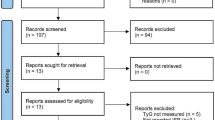
Following the abovementioned systematic search, we identified 1067 papers through databases. After duplicates removing and title/abstract screening, 118 studies eligible for full-text evaluation. After full-text screening, 16 studies [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29] were included (Fig. 1).
Characteristics of included studies
The basic characteristics of included studies are summarized in Table 1. Sixteen studies with a total of 20,883 participants were published from 2020 to 2024. Twelve studies were retrospective cohort, three studies were prospective cohort, and one study was cross-sectional. The mean age and female proportion ranged from 55 to 63 years and 14.7–41.1%, respectively. The duration of follow-up ranged from three day to four years. All included studies evaluated the AIP under fasting condition. According to NOS score, all included studies had high quality (score ≥ 7).
Primary outcome
A total of eight studies investigated the association of AIP as a continues variable and MACE in patients with CAD. Overall, AIP level was found to increase the risk of MACE (RR = 1.54; 95% CI, 1.30–1.83; P < 0.001) with a significant heterogeneity (I2 = 61.9%, P = 0.010) (Fig. 2). Six studies compared the highest vs. lowest category of AIP, and the pooled analysis showed an increased risk of MACE in those with higher AIP (RR = 1.63; 95% CI, 1.44–1.85; P < 0.001) with no significant heterogeneity (I2 = 40.0%, P = 0.134) (Fig. 2).
Secondary outcomes
Ten studies reported the RRs for the secondary outcomes. The pooled analysis indicated that higher AIP increase the risk of cardiovascular death (RR = 1.79; 95% CI, 1.09–2.78; P = 0.02), MI (RR = 2.21; 95% CI, 1.55–3.13; P < 0.001), revascularization (RR = 1.62; 95% CI, 1.34–1.97; P < 0.001), and no-reflow phenomenon (RR = 3.12 95% CI, 1.09–8.96; P = 0.034). However, AIP was not significantly associated with risk of all-causes death (RR = 1.15; 95% CI, 0.56–2.36; P = 0.699) and stroke (RR = 1.03; 95% CI, 0.69–1.52; P = 0.892) (Fig. 3). A significant heterogeneity was found for the no-reflow phenomenon (I2 = 89.7%, P < 0.001). Three studies analyzed AIP as a continuous variable, which reported an of HR 1.21 (95% CI, 0.72–2.02, P = 0.460), 1.61 (95% CI, 1.12–2.32, P = 0.009), 3.77 (95% CI, 1.34–10.60, P = 0.012), and 13.46 (95%CI, 1.39-129.02; P = 0.025) for cardiovascular death [17], MI [17], all-causes death [21], and stent thrombosis [24], respectively.
Subgroup and sensitivity analysis
A subgroup analysis was performed for primary outcome according to the age (< 60 or ≥ 60 years), study design (retrospective or prospective), sample size (< 1,000 or ≥ 1,000), duration of follow-up (< 24 or ≥ 24 months), and LDL-C (< 1.8 or ≥ 1.8 mmol/L) to identify the sources of heterogeneity. A remarkable reduction in heterogeneity was found in prospective studies (I2 = 0.0%) and LCL-C below 1.8 mmol/L (I2 = 0.0%), suggesting that study design and LDL-C level might be factors contributing to heterogeneity. Besides, the analysis revealed no significant association between AIP and MACE in studies with a duration of follow-up below 24 months (RR = 1.56; 95% CI, 0.85, 2.87; P = 0.150) and mean age of over 60 years (RR = 1.43; 95% CI, 0.72, 2.82; P = 0.305) (Table 2).
A sensitivity analyses was performed including studies with a ≥ 2 years of follow-up. Consistent with our primary analysis, we revealed a significant association of AIP with MACE (RR = 1.66; 95% CI, 1.25, 2.20, P = 0.001). The results for other outcomes remained unchanged except for no-reflow phenomenon, which all studies reported a short duration of follow-up, and hence, the further analysis could not perform.
Publication bias
The funnel plots in Fig. 4 demonstrate the relationship between the AIP and the incidence of MACEs in CAD patients. Upon visual examination, the plots seem to be asymmetrical, suggesting a possible risk of publication bias. However, Egger test found no significant publication bias for categorial (P = 0.052) and continues (P = 0.178) analysis.
Discussion
This meta-analysis showed that a higher AIP is associated with an increased risk of MACE, cardiovascular mortality, MI, revascularization, and the no-reflow phenomenon in patients with CAD. Subgroup analysis revealed that AIP may not be an indicator of MACE among patients aged ≥ 60 years and short follow-up times. Besides, AIP was not associated with all-causes mortality and stroke risk.
In this study, CAD patients with a higher AIP level had a < 1.5-fold higher risk for MACE compared with subjects with lower AIP. In line with our results, a fifteen-year cohort study conducted on 6323 healthy adults demonstrated a 1.2-fold greater risk for cardiovascular events among participants with higher AIP [30]. Moreover, a cross-sectional study compromising 7,362 adults showed that the third tertile of AIP had a 1.3-fold higher risk for cardiovascular disease compared to the first tertile [31]. These collective findings underscore the potential of AIP as a valuable biomarker for identifying individuals at higher risk for cardiovascular disease.
Our subgroup analysis did not detect a significant association between AIP and MACE in older patients. In this context, Nansseu et al. [32] enrolled 108 postmenopausal women, and found no significant correlation between AIP and cardiovascular risk evaluated with Framingham risk score. Similarly, there was no significant association between AIP and CAD in elderly females aged ≥ 65 years [33]. Moreover, AIP could not predict the presence of CAD in elderly males who underwent coronary angiography [34]. A possible explanation to address this finding is that AIP level is increased in elderly population [35]. Several studies showed a positive correlation between AIP and age among different populations [36,37,38], which might impact the likelihood of detecting a significant association between AIP level and cardiovascular events in this group. Further studies are warranted to better understand the impact of age on AIP levels and its implications for assessing cardiovascular risk in the elderly.
This meta-analysis included three studies evaluating the association of AIP as a categorial variable with all-causes death, and the results showed that AIP could not predict all-causes death in patients with CAD. However, Refs. [16, 26, 29]) considered AIP as a continuous variable, and found a significant positive correlation between AIP level and risk of all-causes mortality [21]. This discrepancy may be due to low number of studies and the differences in follow-up duration between the studies. The endpoint of et al. was the in-hospital mortality, and hence, their results showed the predictive value of AIP for all-causes mortality in a median follow-up duration three days. To evaluate the association of AIP with risk of short-term mortality, further studies are needed.
The disbalance of these plasma lipids leads to dyslipidemia, which is characterized by high levels of LDL-C, TG, and total cholesterol and low levels of HDL-C [39]. Although reducing LDL-C levels is a treatment goal in CAD, even after attaining this target, a notable residual cardiovascular risk remains present, encouraging the exploration of more accurate risk factors in these patients [40]. Regarding a practical predictor, the AIP strongly predicts cardiovascular events by reflecting the atherogenic lipid profile and providing valuable insights into the residual cardiovascular risk.
Despite the strengths of this study, including a comprehensive search strategy and evaluating several outcomes, there are limitations that should be considered. First, the majority of studies included in this meta-analysis were retrospective, and hence, further studies with prospective design are needed. Second, the presence of significant heterogeneity in some of the analyses suggests potential variations in methodologies and outcome definitions such as MACE, which may have influenced the results. Third, although this study found a significant association of AIP with MI and cardiovascular death, there was considerable variability across studies. As such, additional validation is required to confirm this tenuous relationship. Finally, the included studies were observational, which limits the ability to establish causal relationships between AIP and cardiovascular outcomes.
Conclusion
In conclusion, the findings of this meta-analysis support the notion that AIP is a potential prognostic marker for adverse cardiovascular events in patients with CAD. A higher AIP was consistently associated with an increased risk of MACE, cardiovascular death, MI, revascularization, and the no-reflow phenomenon. Notably, no association was found between AIP and all-causes death or stroke. These results have important implications for risk stratification and management strategies in CAD patients. Further research is needed to validate these findings.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AIP :
-
Atherogenic index of plasma
- CAD :
-
Coronary artery disease
- HDL-C :
-
High-density lipoprotein cholesterol
- HR :
-
Hazard ratio
- LDL-C :
-
Low-density lipoprotein cholesterol
- OR :
-
Odds ratio
- PCI :
-
Percutaneous coronary intervention
- PRISMA :
-
Preferred Reporting Items for Systematic Review and Meta-analyses statement
- RR :
-
Risk ratio
- sdLDL :
-
Small dense low-density lipoprotein
- TG :
-
Triglyceride
References
Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet. 2018;392(10159):1736–88.
Bosco E, Hsueh L, McConeghy KW, Gravenstein S, Saade E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: a systematic review. BMC Med Res Methodol. 2021;21(1):1–18.
Pepine CJ, Kowey PR, Kupfer S, Kolloch RE, Benetos A, Mancia G, et al. Predictors of adverse outcome among patients with hypertension and coronary artery disease. J Am Coll Cardiol. 2006;47(3):547–51.
Kim SH, Cho YK, Kim YJ, Jung CH, Lee WJ, Park JY, et al. Association of the atherogenic index of plasma with cardiovascular risk beyond the traditional risk factors: a nationwide population-based cohort study. Cardiovasc Diabetol. 2022;21(1):81.
Dobiasova M, Frohlich J. The new atherogenic plasma index reflects the triglyceride and HDL-cholesterol ratio, the lipoprotein particle size and the cholesterol esterification rate: changes during lipanor therapy. Vnitr Lek. 2000;46(3):152–6.
Wu J, Zhou Q, Wei Z, Wei J, Cui M. Atherogenic index of plasma and coronary artery disease in the adult population: a meta-analysis. Front Cardiovasc Med. 2021;8:817441.
Zhang Y, Chen S, Tian X, Wang P, Xu Q, Xia X, et al. Association between cumulative atherogenic index of plasma exposure and risk of myocardial infarction in the general population. Cardiovasc Diabetol. 2023;22(1):210.
Fernández-Macías JC, Ochoa-Martínez AC, Varela-Silva JA, Pérez-Maldonado IN. Atherogenic index of plasma: novel predictive biomarker for cardiovascular illnesses. Arch Med Res. 2019;50(5):285–94.
Zheng H, Wu K, Wu W, Chen G, Chen Z, Cai Z, et al. Relationship between the cumulative exposure to atherogenic index of plasma and ischemic stroke: a retrospective cohort study. Cardiovasc Diabetol. 2023;22(1):313.
Fu L, Zhou Y, Sun J, Zhu Z, Xing Z, Zhou S, et al. Atherogenic index of plasma is associated with major adverse cardiovascular events in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2021;20(1):1–11.
Hang F, Chen J, Wang Z, Zheng K, Wu Y. Association between the atherogenic index of plasma and major adverse cardiovascular events among non-diabetic hypertensive older adults. Lipids Health Dis. 2022;21(1):1–7.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group*. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute. 2011;2(1):1–12.
Ma X, Sun Y, Cheng Y, Shen H, Gao F, Qi J, et al. Prognostic impact of the atherogenic index of plasma in type 2 diabetes mellitus patients with acute coronary syndrome undergoing percutaneous coronary intervention. Lipids Health Dis. 2020;19(1):1–13.
Toprak K, Kaplangoray M, Akyol S, İnanır M, Memioğlu T, Taşcanov MB et al. The non-HDL-C/HDL-C ratio is a strong and independent predictor of the no-reflow phenomenon in patients with ST-elevation myocardial infarction. Acta Cardiol. 2023;1–12.
Wang Y, Wang S, Sun S, Li F, Zhao W, Yang H, et al. The predictive value of atherogenic index of plasma for cardiovascular outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention with LDL-C below 1.8 mmol/L. Cardiovasc Diabetol. 2023;22(1):1–10.
Liu Y, Feng X, Yang J, Zhai G, Zhang B, Guo Q, et al. The relation between atherogenic index of plasma and cardiovascular outcomes in prediabetic individuals with unstable angina pectoris. BMC Endocr Disorders. 2023;23(1):187.
Erdoğan A, İnan D, Genç Ö, Yıldız U, Demirtola Aİ, Çetin İ, et al. The triglyceride–glucose index might be a Better Indicator for Predicting Poor Cardiovascular Outcomes in Chronic Coronary Syndrome. J Clin Med. 2023;12(19):6201.
Çelik İE, Öztürk S, Yarlıoğlu M, Barutçu O, Akgün O, Duran M, et al. The Association between Atherogenic Index of plasma and No-Reflow Phenomenon in Acute Coronary Syndrome. Anatol J Cardiol. 2023;27(12):712.
Alifu J, Xiang L, Zhang W, Qi P, Chen H, Liu L, et al. Association between the atherogenic index of plasma and adverse long-term prognosis in patients diagnosed with chronic coronary syndrome. Cardiovasc Diabetol. 2023;22(1):255.
Kasapkara HA, Erdoğan M. Association between atherogenic index of plasma and in-hospital mortality in patients with STEMI undergoing primary percutaneous coronary intervention. J Health Sci Med. 2023;6(1):158–64.
Özen Y, BILAL ÖZBAY M, Yakut İ, Kanal Y, Abdelmottaleb W, Nriagu B et al. Atherogenic index of plasma and triglycerideglucose index to predict more advanced coronary artery diseases in patients with the first diagnosis of acute coronary syndrome. Eur Rev Med Pharmacol Sci. 2023;27(9).
Kan Y, Sun Y, Shen H, Liu X, Liu Y, Shi D, et al. Effect of body Mass Index on the Prognostic Value of Atherogenic Index of Plasma in patients with Acute Coronary Syndrome undergoing percutaneous coronary intervention. J Clin Med. 2023;12(20):6543.
Abacıoğlu ÖÖ, Yıldırım A, Koyunsever NY, Karadeniz M, Kılıç S. Relationship between atherogenic index of plasma and stent thrombosis in patients with acute coronary syndrome. Anatol J Cardiol. 2022;26(2):112.
Qiao-Yu S, Xiao-Teng M, Zhi-Qiang Y, Qiu-Xuan L, Yu-Fei W, Liang J, et al. Prognostic significance of multiple triglycerides-derived metabolic indices in patients with acute coronary syndrome. J Geriatric Cardiology: JGC. 2022;19(6):456.
Zheng Y, Li C, Yang J, Seery S, Qi Y, Wang W, et al. Atherogenic index of plasma for non-diabetic, coronary artery disease patients after percutaneous coronary intervention: a prospective study of the long-term outcomes in China. Cardiovasc Diabetol. 2022;21(1):1–14.
Refaat H, Tantawy A, Gamal AS, Radwan H. Novel predictors and adverse long-term outcomes of No-reflow phenomenon in patients with acute ST elevation myocardial infarction undergoing primary percutaneous coronary intervention. Indian Heart J. 2021;73(1):35–43.
Süleymanoğlu M, Rencüzoğulları İ, Karabağ Y, Çağdaş M, Yesin M, Gümüşdağ A, et al. The relationship between atherogenic index of plasma and no-reflow in patients with acute ST-segment elevation myocardial infarction who underwent primary percutaneous coronary intervention. Int J Cardiovasc Imaging. 2020;36:789–96.
Qin Z, Zhou K, Li Y, Cheng W, Wang Z, Wang J, et al. The atherogenic index of plasma plays an important role in predicting the prognosis of type 2 diabetic subjects undergoing percutaneous coronary intervention: results from an observational cohort study in China. Cardiovasc Diabetol. 2020;19:1–11.
Sadeghi M, Heshmat-Ghahdarijani K, Talaei M, Safaei A, Sarrafzadegan N, Roohafza H. The predictive value of atherogenic index of plasma in the prediction of cardiovascular events; a fifteen-year cohort study. Adv Med Sci. 2021;66(2):418–23.
Hamzeh B, Pasdar Y, Mirzaei N, Faramani RS, Najafi F, Shakiba E, et al. Visceral adiposity index and atherogenic index of plasma as useful predictors of risk of cardiovascular diseases: evidence from a cohort study in Iran. Lipids Health Dis. 2021;20(1):82.
Nansseu JRN, Ama Moor VJ, Nouaga MED, Zing-Awona B, Tchanana G, Ketcha A. Atherogenic index of plasma and risk of cardiovascular disease among Cameroonian postmenopausal women. Lipids Health Dis. 2016;15(1):49.
Huang H, Yu X, Li L, Shi G, Li F, Xiao J, et al. Atherogenic index of plasma is related to coronary atherosclerotic disease in elderly individuals: a cross-sectional study. Lipids Health Dis. 2021;20(1):68.
Hong L, Han Y, Deng C, Chen A. Correlation between atherogenic index of plasma and coronary artery disease in males of different ages: a retrospective study. BMC Cardiovasc Disord. 2022;22(1):440.
Khakurel G, Kayastha R, Chalise S, Karki PK. Atherogenic index of plasma in postmenopausal women. 2018.
Tagoe EA, Dwamena-Akoto E, Nsaful J, Aikins AR, Clegg-Lamptey JN, Quaye O. High atherogenic index of plasma and cardiovascular risk factors among Ghanaian breast cancer patients. Exp Biol Med (Maywood). 2020;245(18):1648–55.
Nwagha U, Ikekpeazu E, Ejezie F, Neboh E, Maduka I. Atherogenic index of plasma as useful predictor of cardiovascular risk among postmenopausal women in Enugu. Nigeria Afr Health Sci. 2010;10(3).
Hegde SN. Low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein b for cardiovascular risk assessment–what is the right choice? APIK J Intern Med. 2020;8(2):48–50.
Hoogeveen RC, Ballantyne CM. Residual cardiovascular risk at low LDL: remnants, lipoprotein (a), and inflammation. Clin Chem. 2021;67(1):143–53.
Ikezaki H, Lim E, Cupples LA, Liu C, Asztalos BF, Schaefer EJ. Small dense low-density lipoprotein cholesterol is the most atherogenic lipoprotein parameter in the prospective Framingham offspring study. J Am Heart Association. 2021;10(5):e019140.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
MRR and GGD designed the research. MRR, RAB, and BD collected data in electronic database. MRR performed statistical analysis. All authors contributed to drafting of the manuscript, had full access to all the data in the study, approved the final version of the manuscript and had final decision to submit for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
Ethical approval was not applicable for this systematic review and meta-analysis.
Consent for publication
N/A.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rabiee Rad, M., Ghasempour Dabaghi, G., Darouei, B. et al. The association of atherogenic index of plasma with cardiovascular outcomes in patients with coronary artery disease: A systematic review and meta-analysis. Cardiovasc Diabetol 23, 119 (2024). https://doi.org/10.1186/s12933-024-02198-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02198-y