Abstract
Objective
This study aimed to evaluate the association of triglyceride-glucose (TyG) index with all-cause and cardiovascular mortality risk among patients with cardiometabolic syndrome (CMS).
Methods
We performed a cohort study of 5754 individuals with CMS from the 2001–2018 National Health and Nutrition Examination Survey. The TyG index was calculated as Ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2]. Multivariate Cox proportional hazards regression models assessed the associations between TyG index and mortality . Non-linear correlations and threshold effects were explored using restricted cubic splines and a two-piecewise Cox proportional hazards model.
Results
Over a median follow-up of 107 months, 1201 all-cause deaths occurred, including 398 cardiovascular disease-related deaths. The multivariate Cox proportional hazards regression model showed a positive association between the TyG index and all-cause and cardiovascular mortality. Each one-unit increase in the TyG index was associated with a 16% risk increase in all-cause mortality (HR: 1.16, 95% CI 1.03, 1.31, P = 0.017) and a 39% risk increase in cardiovascular mortality (HR: 1.39, 95% CI 1.14, 1.71, P = 0.001) after adjusting for confounders. The restricted cubic splines revealed a U-shaped association between the TyG index and all-cause (P for nonlinear < 0.001) and cardiovascular mortality (P for nonlinear = 0.044), identifying threshold values (all-cause mortality: 9.104; cardiovascular mortality: 8.758). A TyG index below these thresholds displayed a negative association with all-cause mortality (HR: 0.58, 95% CI 0.38, 0.90, P = 0.015) but not with cardiovascular mortality (HR: 0.39, 95% CI 0.12, 1.27, P = 0.119). Conversely, a TyG index exceeding these thresholds was positively associated with all-cause and cardiovascular mortality (HR: 1.35, 95% CI 1.17, 1.55, P < 0.001; HR: 1.54, 95% CI 1.25, 1.90, P < 0.001, respectively). Notably, a higher TyG index (≥ threshold values) was significantly associated with increased mortality only among individuals aged under 55 compared to those with a lower TyG index (< threshold values).
Conclusions
The TyG index demonstrated a U-shaped correlation with all-cause and cardiovascular mortality in individuals with CMS. The thresholds of 9.104 and 8.758 for all-cause and cardiovascular mortality, respectively, may be used as intervention targets to reduce the risk of premature death and cardiovascular disease.
Similar content being viewed by others
Background
The prevalence of cardiometabolic syndrome (CMS) shows a tendency to increase, mirroring the increases observed in obesity and type 2 diabetes, attributed to the prevalence of high-calorie, low-fiber diets, decreased physical activity, and prolonged sedentary behavior [1,2,3]. According to the National Health and Nutrition Examination Survey (NHANES) data spanning from 1988–1994 to 2007–2012, the prevalence of CMS among adults in the United States (US) surged from 25.3% to 34.2% [4]. Various prospective studies have highlighted that CMS not only heightens the risk of cardiovascular disease (CVD) and diabetes but also significantly amplifies cardiovascular mortality and all-cause mortality [5,6,7,8,9]. Consequently, CMS presents a substantial global challenge in public health and clinical realms. Thus, an urgent need exists to evaluate the population at high risk of death among CMS patients and formulate clinical strategies to avert adverse events.
Individuals with higher insulin resistance (IR) are prone to various metabolic disorders, such as high blood sugar, abnormal lipid levels, and hypertension. IR has been confirmed as a predictive factor for cardiovascular diseases and adverse cardiovascular events [5]. Additionally, IR serves as the primary pathological mechanism of CMS [11, 12], prevailing in most CMS patients and strongly correlating with CVD risk [13]. To date, there remains a notable absence of clinically feasible and accurate methods for assessing IR. The gold standard for assessing IR, including the hyperinsulinemic-euglycemic clamp and intravenous glucose tolerance test, is characterized by their prohibitively high costs and invasiveness, rendering them less applicable in extensive epidemiological surveys [14]. Presently, the widely employed index for evaluating IR is the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) [15]; however, fasting insulin measurements have not gained widespread usage in clinical settings.
The triglyceride-glucose (TyG) index, a composite marker combined with fasting triglycerides and glucose for assessing IR, effectively substitutes conventional IR markers in diagnosing CMS [16]. The TyG index offers easier accessibility and cost-effectiveness compared to traditional IR indicators. Furthermore, this index has been validated to correlate with adverse cardiovascular and metabolic-related events [17,18,19,20]. However, there has not been research delving into the relationship between the TyG index and all-cause mortality, as well as cardiovascular mortality among patients with CMS.
Our study aims to evaluate whether the TyG index correlates with the risk of all-cause mortality and cardiovascular mortality among individuals with CMS, utilizing data from the NHANES cohort spanning 2001–2018.
Materials and methods
Study population and design
NHANES is a cross-sectional, multistage, stratified, clustered probability survey conducted by the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention [21]. The survey protocol received approval from the NCHS institutional review board, and all respondents provided written informed consent. Accessible NHANES data for this analysis can be found at https://www.cdc.gov/nchs/nhanes.
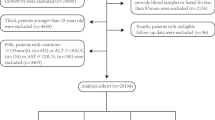
This is a national cohort study of NHANES respondents with CMS from 2001 to 2018, assessed in accordance with the criteria of the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) [22]. CMS was defined as meeting three or more of the following criteria: (1) waist circumference of ≥ 102 cm in men and ≥ 88 cm in women; (2) high circulating triglycerides (TG) ≥ 150 mg/dL; (3) low high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL for men and < 50 mg/dL for women; (4) high fasting blood glucose ≥ 110 mg/dL; (5) diagnosis of arterial hypertension (≥ 130/ ≥ 85 mmHg). After excluding respondents who did not provide blood samples or fasted for less than 8 h (n = 431) and those without valid death data (n = 159), 5,754 individuals from the NHANES dataset with CMS were included in this analysis (Fig. 1).
Measurement of the TyG index
The TyG index, calculated as Ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2], utilized triglycerides and glucose levels from sample persons fasting for at least 8 h but less than 24 h [23]. Fasting blood triglycerides were measured using three different analyzers (Roche Hitachi 717/912, Roche modular P chemistry, and Roche/Hitachi Cobas 6000). Fasting blood glucose (FBG) measurement utilized two instruments (Roche C501 from 2001 to 2015 and Roche C311 from 2015 to 2018). Considering different instruments for these indicators was not necessary under NHANES analysis guidelines. Respondents were categorized into four groups (Q1, Q2, Q3, Q4) based on the TyG index quartiles.
Demographic characteristics and other covariate
Race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other race) were categorized based on the survey design. Education level was simplified into below high school (less than 11th grade), high school graduate or general educational development test (GED) (high school Grad/GED), and some college or above (AA degree or College or above). In addition, marital status was divided into married or living with a partner, never married, widowed/divorced/separated, and never married. The Poverty-Income Ratio (PIR) served as an index of income related to federally established poverty thresholds, accounting for economic inflation and family size. Nicotine exposure, alcohol use, physical activity, history of diabetes, history of CVD, history of hypertension, and history of cancer were obtained via self-report questionnaires. The nicotine exposure has been classified as never smoker, former smoker, or current smoker. The alcohol use was classified into four categories: non-drinker, 1–5 drinks per month, 5–10 drinks per month, and more than 10 drinks per month. Moderate and vigorous physical activity duration was reported by respondents during leisure time. Respondents with physical inactivity if they engaged in moderate-intensity physical activity for < 150 min per week, vigorous-intensity physical activity for < 75 min per week, or an equivalent combination of the two [24]. History of CVD included self-reported angina pectoris, congestive heart failure, coronary heart disease, heart attack, and stroke.
Blood pressure, weight, height, and waist circumference measurements were acquired using standard methods in the mobile examination center. Body mass index (BMI) was calculated as weight/height2 from these measurements. Clinical indicators such as TG, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), HDL-C, FBG, Hemoglobin A1c (HbA1c), Fasting blood insulin (FBI), albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), glutamyl transpeptidase (GGT), lactic dehydrogenase (LDH), total bilirubin (TBIL), serum uric acid (SUA), and serum creatinine concentration (SCR) were measured in the NHANES laboratory. eGFR was calculated using the chronic kidney disease-epidemiology collaboration (CKD-EPI) equation, and chronic kidney disease (CKD) was defined as an eGFR of 15–59 mL/min/1.73 m2 [21].
Ascertainment of mortality
The study encompassed all-cause and cardiovascular mortality as endpoints [25]. All-cause mortality was defined as death from heart diseases, malignant neoplasms, and all other causes. Cardiovascular mortality was defined as the death attributed to heart diseases (ICD-10 codes I00–I09, I11, I13, I20–I51) and cerebrovascular diseases (ICD-10 codes I60–I69), according to the International Classification of Diseases (10th Clinical Modification (ICD-10) system) [26]. Mortality data from NHANES were linked to death certificate data from the National Death Index of the NCHS until December 31, 2019, employing a probability matching algorithm. The helpful website for additional information regarding mortality variables is available at: (https://www.cdc.gov/nchs/data-linkage/mortality.htm).
Statistical analysis
The NHANES is a multistage, stratified, probability-based survey that oversamples certain groups [27]. To account for unequal sampling probability and nonresponses, data for all respondents has been weighted using the recommended NHANES exam weights and fasting subsample weights. Participants were divided into TyG index quartiles (Q1–Q4) for analyses. Mean ± standard deviation (SD) was used to present continuous variables, which were compared using the Wilcoxon rank-sum test based on the study design. Frequency (percentages) was used to present categorical variables, which were compared using Chi-square tests.
Multivariate Cox proportional hazards regression models were utilized to assess the associations between the TyG index and mortality, adjusting for potential confounders. Due to the large number of risk factors investigated in this study, only relevant demographic characteristics and traditional factors associated with the TyG index and deaths were included in the multivariate Cox regression analysis. Hazard ratios (HRs) were calculated across three models: an unadjusted model (Model 1), an age, gender, and race/ethnicity-adjusted model (Model 2), and a comprehensive adjustment for potential confounders (Model 3), encompassing age, gender, race, poverty ratio, marital status, education levels, BMI, nicotine exposure, alcohol use, and physical inactivity.
To explore potential nonlinear relationships between the TyG index and mortality, restricted cubic spline regression for HR was employed. Upon confirmation of a nonlinear relationship, we estimate the threshold value using the maximum likelihood method. A two-piecewise Cox proportional risk model was applied on both sides of the inflection point to further investigate the relationship between the TyG index and the risk of all-cause and cardiovascular mortality.
Missing covariates were addressed through a multilevel imputation approach designed for survey data [28]. The results obtained from this imputation were consistent with analyses that excluded participants with missing covariates. Subsequently, stratification and interaction analyses were performed by gender, age, race, medicine history, physical activity, nicotine exposure, and alcohol use. All analyses were executed using R software (version 4.3.1) and EmpowerStats software (www.empowerstats.com, X&Y solutions, Inc. Boston MA, USA) with a significance level set at a two-tailed alpha of 0.05.
Results
Baseline characteristics of study participants
Based on the quartile of the TyG index within the study, the baseline characteristics of 5,754 respondents are shown in Table 1. Compared with those in the lowest quartile, respondents with a higher TyG index are more likely to be men, non-Hispanic whites, current smokers, and have hypertension, diabetes, and CVD. They generally have a lower BMI, BP, HDL, and LDH but higher HbA1c, FBG, FBI, TG, TC, LDL, ALB, ALT, AST, BUN, GGT, TBIL, and SUA (all P < 0.05). Moreover, respondents with an elevated TyG index have a heightened risk of both all-cause and cardiovascular mortality in contrast to those with a lower TyG index (all-cause mortality: 20.06% vs 15.16%, P = 0.014; cardiovascular mortality: 7.14% vs 4.06%, P = 0.011).
Association of TyG index with mortality
Cox proportional hazard analysis was conducted to assess the association between TyG index levels and mortality risk in respondents with CMS. Over a median follow-up of 107 months, a total of 1201 all-cause deaths and 398 cardiovascular deaths were recorded. Table 2 presents results from three Cox regression model analyses. Models 1 and 2 indicate upward trends between the TyG index and both all-cause and cardiovascular mortality (P for trend < 0.05). After adjusting for age, gender, race, education level, family income-poverty ratio, marital status, physical inactivity, BMI, nicotine exposure, and alcohol use in Model 3, the HRs and 95% confidence intervals (CIs) were 1.00 (reference), 0.92 (0.75, 1.12), 0.95 (0.78, 1.15), and 1.19 (0.98, 1.45) for the Q1, Q2, Q3, and Q4 groups, respectively, for all-cause mortality (P for trend = 0.077). Correspondingly, the HRs and 95% CIs were 1.11 (0.79, 1.56), 1.19 (0.82, 1.73), and 1.68 (1.22, 2.31) for the Q2, Q3, and Q4 groups, respectively, in relation to cardiovascular mortality (P for trend = 0.003) compared with the Q1 group. The continuous models indicate that every one-unit increase in the TyG index was associated with an increased risk of 1.16 (1.03, 1.31) for all-cause mortality and 1.39 (1.14, 1.71) for cardiovascular mortality after adjusting for confounding factors (Table 3).
Utilizing Cox proportional hazards regression models with restricted cubic splines, the association between the TyG index and all-cause and cardiovascular mortality was further examined. A non-linear dose–response relationship was observed between the TyG index and mortality incidence (Fig. 2). Intriguingly, adjusted smoothed plots depicted U-shaped associations between the TyG index and both all-cause (P for non-linear < 0.001, Fig. 2a) as well as cardiovascular mortality (P for non-linear = 0.044, Fig. 2b). Additionally, inflection points for all-cause and cardiovascular mortality were identified as 9.104 and 8.758, respectively (both P values for log-likelihood ratio < 0.001) (Table 3). Following adjustments for various factors, the risk of all-cause mortality decreased by 42% (HR: 0.58, 95% CI 0.38, 0.90) for each unit increase in TyG index below the threshold value and by 35% (HR: 1.35, 95% CI 1.17, 1.55) for each unit increase in TyG index above the threshold value (P = 0.015, P < 0.001, respectively). However, the TyG index below the threshold value did not significantly associate with the risk of cardiovascular mortality but exhibited a 54% (HR: 1.54, 95% CI 1.25, 1.90) increase in risk per unit increase above the threshold value (P = 0.119, P < 0.001, respectively).
Multivariable adjusted spline curves for associations of the TyG index with all-cause (a) and cardiovascular mortality (b) in respondents with cardiometabolic syndrome. Hazard ratios adjusted for age (as a continuous variable), gender and race, poverty ratio (as a continuous variable), education levels, marital status, body mass index (as a continuous variable), nicotine exposure, alcohol use, and physical inactivity. The solid line and red area represent the estimated values and their corresponding 95% CI. HR hazard ratio, CI confidence interval, TyG index triglyceride-glucose index
Stratified analyses
To elucidate the survival advantage of a higher TyG index (all-cause mortality: ≥ 9.104; cardiovascular mortality: ≥ 8.758) compared to a lower TyG index (all-cause mortality: < 9.104; cardiovascular mortality: < 8.758) among respondents with CMS, stratification and interaction analyses were conducted for gender, age, race, medicine history, physical inactivity, nicotine exposure, and alcohol use (Fig. 3). Except for the age subgroup (all-cause mortality: P-interaction = 0.013; cardiovascular mortality: P-interaction < 0.026) and gender subgroup (cardiovascular mortality: P-interaction = 0.018), most subgroups did not exhibit significant interaction (P-interaction > 0.05). A higher TyG index correlated closely with increased all-cause and cardiovascular mortality in patients aged < 55 (all-cause mortality, HR: 1.69, 95% CI 1.10, 2.59, P < 0.05; cardiovascular mortality, HR: 3.49, 95% CI 1.07, 11.42, P < 0.05), while this association was not observed in patients aged ≥ 55 (all-cause mortality, HR: 0.96, 95% CI 0.79, 1.16, P > 0.05; cardiovascular mortality, HR: 0.84, 95% CI 0.57, 1.26, P > 0.05). Furthermore, gender influenced the relationship between the TyG index and cardiovascular mortality but was not found to be statistically significant in the subgroups.
Stratified analyses of the associations between the TyG index and all-cause (a) and cardiovascular mortality (b) among respondents with cardiometabolic syndrome. Hazard ratios were estimated using a two-piecewise Cox proportional risk model on both sides of the inflection point (all-cause mortality: 9.104; cardiovascular mortality: 8.758) and adjusted for confounders. Alcohol use was defined as more than 10 drinks per month. HR hazard ratio, CI confidence interval, TyG index triglyceride-glucose index, NH non-Hispanic
Discussion
To our knowledge, our study represents the first exploration of the association between the TyG index and all-cause mortality and cardiovascular mortality among individuals with CMS. Our research identified a U-shaped correlation between the TyG index and all-cause mortality and cardiovascular mortality, elucidating the threshold points (all-cause mortality: 9.104; CV mortality: 8.758). Specifically, a higher TyG index (≥ threshold values) was significantly associated with increased mortality among individuals aged < 55 compared to those with a lower TyG index. This study highlighted the TyG index as significant and may be helpful in identifying patients with CMS at high risk of mortality and guiding further detections and more aggressive treatments.
Prior studies have already established the significant predictive role of the TyG index for adverse events among healthy individuals and those with CVD [19, 20, 29]. The findings in our study demonstrated that the TyG index was positively associated with higher all-cause and cardiovascular mortality in patients with CMS, which indicated the usefulness of the TyG index in screening individuals who have increased mortality risk in such a population. CMS is acknowledged as an independent risk factor for CVD and diabetes [10], and elevated TyG index levels exhibit a distinct association with an increased likelihood of developing cardiovascular and diabetic conditions [30, 31]. Elevated TyG index levels might escalate the incidence of cardiovascular diseases and diabetes within the CMS population, consequently heightening overall mortality and cardiovascular mortality. Numerous prospective studies utilizing the HOMA-IR index to assess IR demonstrate that IR substantially increases individual diabetes or CVD risk in patients with CMS, suggesting that CMS does not equate to an insulin-resistant phenotype [32,33,34]. The TyG index, an easily accessible surrogate marker of IR [16, 35], is implicated in endothelial dysfunction, impaired cardiac autonomic function, chronic inflammation, and heightened sympathetic nervous system activity, thus accelerating the progression of cardiovascular diseases [36,37,38,39,40]. Recent studies underscore the critical role of IR, chronic inflammation, and neurohormonal activation as pivotal elements in the progression of CMS pathophysiology [10]. Consistent with previous studies, our study also found that the TyG index was positively correlated with traditional cardiovascular risk factors such as HbA1c, FBG, FBI, TG, and TC and negatively correlated with HDL-C [41]. These data support IR as an independent risk factor among CMS patients and suggest increased TyG index measurement could identify individuals at a heightened risk. Compared to the HOMA-IR index, the TyG index, incorporating fasting blood glucose and lipid parameters, is more easily obtainable, with fewer laboratory procedures and lower costs, making it more convenient for clinical application.
Consistent with prior research [20], our study confirmed the association of the TyG index with all-cause mortality and cardiovascular mortality, revealing a U-shaped relationship among patients with CMS. Specifically, a unit increase below the threshold is related to a 42% reduction in all-cause mortality. A cohort analysis concerning statin therapy has suggested elevated triglyceride levels were linked to an increased risk of cardiovascular disease events but a decreased risk of mortality [42]. Similarly, another study affirmed a J-shaped relationship between blood glucose levels and all-cause mortality or cardiovascular events, associating lower fasting blood glucose levels with increased adverse events [43]. Extremely low triglyceride and fasting glucose levels might indicate poor nutritional status. Additionally, hypoglycemia might trigger cardiac arrhythmias, thrombus formation, vascular inflammation, and vasoconstriction, leading to increased cardiovascular events or mortality [44, 45]. Therefore, maintaining an optimal TyG index level is crucial, as excessively high and low levels can lead to detrimental health outcomes. Notably, our study stratified CMS patients according to age, revealing that higher TyG index (all-cause mortality: ≥ 9.104; CV mortality: ≥ 8.758) exhibited a significant association with increased mortality compared to patients with lower TyG index (all-cause mortality: < 9.104; CV mortality: < 8.758), only in individuals aged under 55. This information provides the theoretical foothold for the application of the TyG index in the non-older population. This data might support using the TyG index in the non-older CMS population, emphasising the significance of managing the TyG index at lower levels for their health benefits.
The strengths of the study include its substantial sample size, long follow-up time, and the assessment of the dose–response relationship between the TyG index and mortality, identifying the inflection point in the U-shaped relationship in CMS patients. Nevertheless, we need to consider several potential limitations in our study. Firstly, our analysis involved participants from a single nation, potentially limiting the global applicability of our conclusions. Secondly, Due to the post hoc nature of the study, residual confounding elements may persist despite our efforts to control them. Thirdly, our study did not involve dynamic monitoring of the TyG index, precluding the determination of its long-term status. Additionally, our main exploration centered around the relationship between the TyG index and mortality, without comparing with other non-insulin-based IR indicators. Fourthly, due to the absence of specific information, we did not employ the latest definition to identify CMS patients, hindering the ability to ascertain the robustness of the study results through sensitivity analysis. However, the CMS definition utilized in this study has been thoroughly validated in previous research. Despite these limitations, our findings could extend our understanding of the association between the TyG index and mortality risk and provide new insights and clues for future studies into predicting adverse events among CMS patients.
Conclusions
This cohort study demonstrated the relationship between the TyG index and both all-cause and cardiovascular mortality in individuals diagnosed with CMS. Notably, a U-shaped correlation was observed between the TyG index and all-cause as well as cardiovascular mortality. Adding the TyG index assessment will facilitate a more convenient and effective screening of individuals at high risk in CMS patients. Furthermore, the threshold can serve as an intervention target to mitigate the risk of premature mortality and cardiovascular diseases.
Availability of data and materials
The datasets that were used and evaluated in this study can be obtained from the corresponding author upon making a reasonable request.
Abbreviations
- ALB:
-
Albumin
- ALT:
-
Alanine aminotransferase
- AST III:
-
Aspartate aminotransferase III
- ATP:
-
Adult treatment panel
- BMI:
-
Body mass index
- BUN:
-
Blood urea nitrogen
- CDC:
-
The Centers for Disease Control and Prevention
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- CKD-EPI:
-
Chronic kidney disease-epidemiology collaboration
- CV:
-
Cardiovascular
- CVD:
-
Cardiovascular disease
- DBP:
-
Diastolic blood pressure
- eGFR:
-
Estimated glomerular filtration rate
- FBG:
-
Fasting blood glucose
- FBI:
-
Fasting blood insulin
- GED:
-
General educational development
- GGT:
-
Glutamyl transpeptidase
- HbA1c:
-
Glycosylated hemoglobin A1c
- HDL-C:
-
High-density lipoprotein cholesterol
- HR:
-
Hazard ratio
- IR:
-
Insulin resistance
- LDL-C:
-
Low-density lipoprotein cholesterol
- LDH:
-
Lactic dehydrogenase
- CMS:
-
Cardiometabolic syndrome
- NCHS:
-
National Center for Health Statistics
- NCEP:
-
National Cholesterol Education Program
- NDI:
-
National Death Index
- NHANES:
-
National Health and Nutrition Examination Survey
- PIR:
-
Poverty-income ratio
- SBP:
-
Systolic blood pressure
- SCR:
-
Serum creatinine
- SD:
-
Standard deviation
- SUA:
-
Serum uric acid
- TBIL:
-
Total bilirubin
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- TyG:
-
Triglyceride-glucose index
- US:
-
United States
References
Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12.
Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. The Lancet. 2005;365:1415–28.
Wang Y, Mi J, Shan X-Y, Wang QJ, Ge K-Y. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes (Lond). 2007;31:177–88.
Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev Chronic Dis. 2017;16(14):E24.
Tune JD, Goodwill AG, Sassoon DJ, Mather KJ. Cardiovascular consequences of metabolic syndrome. Transl Res. 2017;183:57–70.
Haffner SM. Relationship of metabolic risk factors and development of cardiovascular disease and diabetes. Obesity (Silver Spring). 2006;14(Suppl 3):121S-127S.
Silveira Rossi JL, Barbalho SM, Reverete de Araujo R, Bechara MD, Sloan KP, Sloan LA. Metabolic syndrome and cardiovascular diseases: going beyond traditional risk factors. Diabetes/Metab Res Rev. 2022;38:e3502.
Guembe MJ, Fernandez-Lazaro CI, Sayon-Orea C, Toledo E, Moreno-Iribas C, RIVANA Study Investigators. Risk for cardiovascular disease associated with metabolic syndrome and its components: a 13-year prospective study in the RIVANA cohort. Cardiovasc Diabetol. 2020;19:195.
Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–32.
Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, Bouferraa Y, et al. Metabolic syndrome: updates on pathophysiology and management in 2021. Int J Mol Sci. 2022;23:786.
Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol. 2013;3:1–58.
Gluvic Z, Zaric B, Resanovic I, Obradovic M, Mitrovic A, Radak D, et al. Link between metabolic syndrome and insulin resistance. Curr Vasc Pharmacol. 2017;15:30–9.
Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119: 154766.
Park SE, Park CY, Sweeney G. Biomarkers of insulin sensitivity and insulin resistance: past, present and future. Crit Rev Clin Lab Sci. 2015;52(4):180–90.
Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95.
Son D-H, Lee HS, Lee Y-J, Lee J-H, Han J-H. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2022;32:596–604.
Tao L-C, Xu J-N, Wang T-T, Hua F, Li J-J. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21:68.
Tao S, Yu L, Li J, Xie Z, Huang L, Yang D, et al. Prognostic value of triglyceride-glucose index in patients with chronic coronary syndrome undergoing percutaneous coronary intervention. Cardiovasc Diabetol. 2023;22:322.
Tao S, Yu L, Li J, Huang L, Huang X, Zhang W, et al. Association between the triglyceride-glucose index and 1-year major adverse cardiovascular events in patients with coronary heart disease and hypertension. Cardiovasc Diabetol. 2023;22(1):305.
Zhang Q, Xiao S, Jiao X, Shen Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001–2018. Cardiovasc Diabetol. 2023;22(1):279.
Liu Q, Bai B, Liu F, Chen Y, Wang Y, Wang H, et al. Long-term trends in risk factor management in respondents with chronic kidney disease in the USA. Am J Nephrol. 2022;53:614–23.
Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C, American Heart Association, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8.
Alizargar J, Bai C-H, Hsieh N-C, Wu SF-V. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol. 2020;19:8.
Navaneethan SD, Zoungas S, Caramori ML, Chan JCN, Heerspink HJL, Hurst C, et al. Diabetes management in chronic kidney disease: synopsis of the KDIGO 2022 clinical practice guideline update. Ann Intern Med. 2023;176:381–7.
Zhang Y-B, Chen C, Pan X-F, Guo J, Li Y, Franco OH, et al. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: two prospective cohort studies. BMJ. 2021;373: n604.
Tian Q, Huang D. Association between urinary IPM3 and the presence of cardio-cerebrovascular diseases: a cross-sectional study. Environ Sci Pollut Res Int. 2023;30:75817–22.
Saint-Maurice PF, Graubard BI, Troiano RP, Berrigan D, Galuska DA, Fulton JE, et al. Estimated number of deaths prevented through increased physical activity among US adults. JAMA Intern Med. 2022;182:349–52.
Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, et al. Trends in blood pressure control among US adults with hypertension, 1999–2000 to 2017–2018. JAMA. 2020;324:1190–200.
Chen J, Wu K, Lin Y, Huang M, Xie S. Association of triglyceride glucose index with all-cause and cardiovascular mortality in the general population. Cardiovasc Diabetol. 2023;22:320.
Li J, Dong Z, Wu H, Liu Y, Chen Y, Li S, et al. The triglyceride-glucose index is associated with atherosclerosis in patients with symptomatic coronary artery disease, regardless of diabetes mellitus and hyperlipidaemia. Cardiovasc Diabetol. 2023;22:224.
Lopez-Jaramillo P, Gomez-Arbelaez D, Martinez-Bello D, Abat MEM, Alhabib KF, Avezum Á, et al. Association of the triglyceride glucose index as a measure of insulin resistance with mortality and cardiovascular disease in populations from five continents (PURE study): a prospective cohort study. Lancet Healthy Longev. 2023;4:e23-33.
Meigs JB, Rutter MK, Sullivan LM, Fox CS, D’Agostino RB Sr, Wilson PWF. Impact of insulin resistance on risk of type 2 diabetes and cardiovascular disease in people with metabolic syndrome. Diabetes Care. 2007;30:1219–25.
Onat A, Hergenç G, Türkmen S, Yazici M, Sari I, Can G. Discordance between insulin resistance and metabolic syndrome: features and associated cardiovascular risk in adults with normal glucose regulation. Metabolism. 2006;55:445–52.
Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp-Pedersen C, Madsbad S. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease: a population-based study. J Am Coll Cardiol. 2007;49:2112–9.
Tahapary DL, Pratisthita LB, Fitri NA, Marcella C, Wafa S, Kurniawan F, et al. Challenges in the diagnosis of insulin resistance: focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr. 2022;16: 102581.
Molina MN, Ferder L, Manucha W. Emerging role of nitric oxide and heat shock proteins in insulin resistance. Curr Hypertens Rep. 2016;18:1.
Poon AK, Whitsel EA, Heiss G, Soliman EZ, Wagenknecht LE, Suzuki T, et al. Insulin resistance and reduced cardiac autonomic function in older adults: the atherosclerosis risk in communities study. BMC Cardiovasc Disord. 2020;20:217.
Wheatcroft SB, Williams IL, Shah AM, Kearney MT. Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med. 2003;20:255–68.
Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409.
Godsland IF, Lecamwasam K, Johnston DG. A systematic evaluation of the insulin resistance syndrome as an independent risk factor for cardiovascular disease mortality and derivation of a clinical index. Metabolism. 2011;60:1442–8.
US Preventive Services Task Force, Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, et al. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US preventive services task force recommendation statement. JAMA. 2020;324:2069–75.
Ambrosy AP, Yang J, Sung SH, Allen AR, Fitzpatrick JK, Rana JS, et al. Triglyceride levels and residual risk of atherosclerotic cardiovascular disease events and death in adults receiving statin therapy for primary or secondary prevention: insights from the KP REACH study. J Am Heart Assoc. 2021;10: e020377.
Lee JH, Han K, Huh JH. The sweet spot: fasting glucose, cardiovascular disease, and mortality in older adults with diabetes: a nationwide population-based study. Cardiovasc Diabetol. 2020;19:44.
Li G, Zhong S, Wang X, Zhuge F. Association of hypoglycaemia with the risks of arrhythmia and mortality in individuals with diabetes—a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023;14:1222409.
Wright RJ, Frier BM. Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab Res Rev. 2008;24:353–63.
Acknowledgements
We are grateful to all participants and project teams in the NHANES study.
Funding
This research was funded by the National Natural Science Foundation of China (82270519, 81970252, 81870352), and the Natural Science Foundation of Hunan Province (2023JJ30838).
Author information
Authors and Affiliations
Contributions
QL, YZ, HL, and ZZ conceived and designed the study. QL and YZ organized the data, conducted the analyses, and drafted the manuscript. SC, HX, HL, JZ, ZZ, and JO reviewed and edited the manuscript. YC, PG, XZ, JF, and XZ contributed to data collection. Each author critically revised successive drafts of the paper and approved the final version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
NHANES is conducted by the Centers for Disease Control and Prevention (CDC) and NCHS. The NCHS Research Ethics Review Committee reviewed and approved the NHANES study protocol. All participants signed written informed consent.
Consent for publication
All the authors have read and agreed to the published version of the manuscript.
Competing interests
The authors declare no competing interests that pertain to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Q., Zhang, Y., Chen, S. et al. Association of the triglyceride-glucose index with all-cause and cardiovascular mortality in patients with cardiometabolic syndrome: a national cohort study. Cardiovasc Diabetol 23, 80 (2024). https://doi.org/10.1186/s12933-024-02152-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02152-y