Abstract
Background
Type 1 diabetes (T1D) is a significant risk factor for a range of cardiovascular diseases. Nonetheless, the causal relationship between T1D and non-ischemic cardiomyopathy (NICM) remains to be elucidated. Furthermore, the mechanisms responsible for the progression from T1D to NICM have not been definitively characterized.
Objective
The aim of this study was to conduct a Mendelian randomization (MR) study to investigate the causal effects of T1D and its complications on the development of NICM. Additionally, this study aimed to conduct a mediation analysis to identify potential mediators within this correlation.
Methods
Genetic variants were used as instrumental variables for T1D. The summary data for T1D were obtained from two genome-wide association study datasets. The summary data for T1D with complications and NICM were obtained from the Finnish database. Two-sample MR, multivariable MR and mediation MR were conducted in this study.
Results
The study revealed a causal association between T1D, T1D with complications, and NICM (with odds ratios of 1.02, 95% CI 1.01–1.04, p = 1.17e-04 and 1.03, 95% CI 1.01–1.05, p = 3.15e-3). Even after adjusting for confounding factors such as body mass index and hypertension, T1D remained statistically significant (with odds ratio of 1.02, 95% CI 1.01–1.04, p = 1.35e-4). Mediation analysis indicated that monokine induced by gamma interferon may play a mediating role in the pathogenesis of T1D-NICM (mediation effect indicated by odds ratio of 1.005, 95% CI 1.001–1.01, p = 4.9e-2).
Conclusion
The study demonstrates a causal relationship between T1D, its complications, and NICM. Additionally, monokine induced by gamma interferon may act as a potential mediator in the pathogenesis of T1D-NICM.
Key points
Question: 1 Does type 1 diabetes(T1D) have an independent causal relationship with non-ischemic cardiomyopathy (NICM)?
2 Which inflammatory factors or diseases mediate the development of NICM in T1D?
The following findings were identified in a Mendelian randomization study:
Primary findings: 1 There is an independent causal relationship between T1D and NICM. Additionally, the causal relationship between T1D with complications and NICM is demonstrated. 2 Monokine induced by IFN-γ (MIG) mediates the progression from T1D to NICM.
Secondary findings: BMI, hypertension, glomerular diseases, and MIG are causally associated with NICM.
Meaning: All the findings are first-time discoveries in Mendelian randomization studies. The study confirms the causal relationship between T1D and NICM, while accounting for confounding factors. The MIG serves as a potential target for new preventive measure and therapy.
Similar content being viewed by others
Introduction
Type 1 diabetes (T1D) is a chronic disease associated with poor cardiac outcomes and an increased risk of premature mortality [1,2,3]. It accounts for approximately 5–15% of diabetes cases in high-income countries and about 2% in low- and middle-income countries [4]. The prevalence of T1D is increasing worldwide, showing variations across different countries and areas, potentially influenced by environmental variables [5,6,7,8].
Cardiovascular diseases are the leading cause of mortality in individuals with T1D [9]. Previous cohort studies have also suggested that T1D can increase the risk of cardiovascular diseases [10,11,12]. For example, a recently published Mendelian randomized(MR) study indicated that T1D increases the risk of atherosclerosis [13]. Furthermore, Marcus Lind et al. showed that heart failure (HF) is a common complication in T1D patients [14]. However, there is an ongoing debate regarding the specific phenotype of HF associated with T1D. Most studies have suggested that T1D mainly affects the diastolic function, while effects on systolic function remain controversial [15,16,17,18,19]. Most cases in these studies were accompanied by confounding factors such as coronary artery disease and hypertension. According to the research on T1D conducted by Konduracka et al., it was found that the occurrence of HF and myocardial dysfunction was observed only in those who developed hypertension or coronary heart disease [20]. In a recent study, no significant differences in echocardiographic findings were observed between patients with T1D and healthy individuals, despite the presence of microvascular damage [21]. Therefore, the influence of T1D on HF, especially non-ischemic cardiomyopathy (NICM), remains incompletely understood based on human studies. Diabetic cardiomyopathy has been proposed as an explanation for the residual risk of HF in diabetic patients after accounting for coronary heart disease, hypertension and other factors [11]. However, most of the studies on T1D-induced diabetic cardiomyopathy have focused mainly on animal and cellular experiments [22,23,24,25]. Although diabetic cardiomyopathy is classified as a NICM resulting from diabetes mellitus, it is noteworthy that the myocardial pathologic phenotypes of T1D and type 2 diabetes (T2D) cardiomyopathy differ. Additionally, conducting real-world studies on T1D-induced NICM presents challenges in controlling for confounding factors. To address these gaps, it is essential to assess the causal relationship between T1D and NICM by MR method. In addition, another noteworthy consideration pertains to identify factors that mediate T1D-induced NICM. Previous observational studies have identified several inflammatory factors, such as interleukin-6, tumor necrosis factor α, and C-reactive protein (CRP), that are associated with HF [26, 27]. However, conflicting results have also been reported in some studies [28, 29]. Additionally, a observational study has shown that factors like renal disease and anemia are associated with the risk of HF [30]. Thus, we aim to investigate whether inflammatory cytokines and certain diseases have mediating roles in the development of T1D-induced NICM.
Conventional observational studies are susceptible to confounding factors and reverse causation bias. To overcome these limitations, MR utilizes genetic variants as instrumental variables (IVs) to infer causal relationships [31]. MR can not only overcome the limitations of observational studies by mimicking a randomized controlled trial but also provide evidence beyond clinical studies to establish the causal association between T1D and NICM. In this study, we performed two-sample MR analyses and multivariable MR (MVMR) to investigate the independent causal effect of T1D and its complications on NCIM. Furthermore, we conducted mediation analysis to explore the mediators in the association between T1D and NICM.
Methods
Two sample MR and MVMR
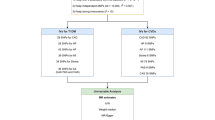
Figure 1 presents the study design. We used two-sample MR to investigate the causal effects of T1D and its complications on NICM [32]. To obtain the necessary data, we collected summary statistics from publicly available databases, as outlined in Table 1. Our single nucleotide polymorphisms (SNPs) selection process focused on SNPs strongly associated with T1D and randomly allocated at conception, ensuring minimal influence from environmental factors [33]. We followed three assumptions for MR analysis: (1) the selected IVs must be strongly associated with T1D; (2) the selected IVs should not be associated with potential confounders; (3) the selected IVs could only influence the NICM through T1D, but not other pathways. In the primary analysis, we conducted MR analysis using data from two T1D datasets and used the conventional random effect inverse variance weighted (IVW) method to estimate the causal effect of T1D on NICM. In addition, we also performed four complementary methods, including the weighted median method, the weighted mode method, simple mode, MR Egger. To ensure the robustness of the outcomes, we performed a meta-analysis of the results from two T1D datasets. We also conducted MVMR to mitigate potential pleiotropy by accounting for confounding factors such as body mass index (BMI) and hypertension. The analytic process adhered to the STROBE-MR guidelines [34].
Mediation MR/Two-step MR analysis
In the mediation analysis, we included glomerular disease, anemia, BMI, and hypertension. Furthermore, we included glycated hemoglobin, HOMA-IR, fasting insulin, blood lipids, CRP, and 41 other inflammatory factors in the mediation analysis. The three-step method provides evidence of a mediating role for a variable in the exposure-outcome effect. The indirect effect of each mediator was derived using the two-step MR method [35]. In the first step, we estimated the causal impact of T1D on a hypothesized mediator using IVs for T1D. In the second step, we established the causal impact of the mediators on NICM using IVs for the mediator. For all mediators individually, we quantified the proportion mediated by dividing the indirect effect by the total effect. Confidence intervals were estimated using the delta method [31].
The data source and the selection of instrumental variables
We extracted summary-level data for the associations of SNPs with T1D from two Genome-Wide Association Studies (GWASs). One is a meta-analysis including 9,266 T1D cases and 15,574 non-cases from 12 European cohorts [36]. The other dataset is derived from the Finnish database and UKB data, consisting of 6,447 cases and 451,248 controls [37]. T1D with complications dataset obtained from Finnish database [38]. The NICM dataset comes from a Finnish database and contains 11,400 cases and 175,752 controls. For inflammatory cytokines, the data was from the study providing genome variant associations with 41 cytokines and growth factors in 8,293 individuals. This study combined the results from The Cardiovascular Risk in Young Finns Study (YFS) and FINRISK surveys [39]. The average participant ages are 37 years for YFS study and 60 years for FINRISK survey. Diseases in the Finnish database were diagnosed using ICD coding. The age distribution of patients and the inclusion process in the Finnish database can be accessed online through the link https://r9.risteys.finngen.fi/endpoints/+ID, such as ID E4_DM1PERIPH. Detailed information about the data sources can be found in Table 1 and Table S1. Table S1 includes information on all datasets and the available diagnostic codes.
We used strict selection criteria to select valid and reliable IVs for T1D. First, we searched for the largest GWAS summary statistics for the genetic proxies of T1D. We extracted SNPs strongly associated with T1D as candidate IVs (p < 5e-8). Second, we eliminated SNPs that were in linkage disequilibrium (r2 < 0.01) or palindromic with intermediate allele frequencies. Third, we excluded SNPs that were not available in the outcome GWAS or had proxy SNPs. In this study, we identified BMI and hypertension as confounding factors for NICM. We calculated the F statistics to measure the strength between IVs and T1D. We only considered SNPs with an F statistic > 10 as valid and reliable IVs for T1D. Finally, we included the 50 qualified SNPs as IVs to conduct the MR analysis. We extracted IVs of complications of T1D using the same method. Detailed information on those IVs is shown in Supplementary Excel 1. Since only few SNPs were identified for part of mediators when they were as the exposure, a higher cutoff (p < 5e-6) was chosen (p < 5e-6, Supplementary Excel 2).
Statistical analysis
The MR estimates were represented by odds ratios (OR) with 95% confidence intervals (CIs). We performed the MR-Egger regression method, the leave-one-out method, and the MR-PRESSO method as sensitivity analysis. We used the MR-egger regression and MR-PRESSO method to test and correct the potential horizontal pleiotropy of the selected IVs. The MR-egger intercept and zero difference could indicate directional pleiotropy. The MR-PRESSO could detect and remove outliers in the IVs. We employed Cochrane’s Q statistic to evaluate the variability of SNPs estimates within each MR association. We used the p-value of the intercept test from MR-Egger regression to assess the horizontal pleiotropy [40]. By using MVMR analysis to adjust for confounding risk factors, we reduced the impact of confounding factors on the causal relationship. We performed all tests using the Two Sample MR [41], MR-PRESSO [42] and Mendelian Randomization [43] packages in the R software (version 4.0.2).
Result
Univariable MR analysis supported a causal role for liability to T1D in the development of NICM. (IVW: GCST010681: OR 1.02; 95% CI 1.01–1.04; p = 1.17e-4; GCST90018925: OR 1.06; 95% CI 1.03–1.09; p = 0.02; Meta-analysis: OR 1.03; 95% CI 1.01–1.04; p<1e-4). Additionally, under sensitivity analyses, the other three methods, including MR-Egger, weighted median, and weighted mode, also revealed significant associations between T1D and NICM in GCST010681 and meta-analysis. Only the simple mode was attenuated (GCST010681: OR 1.02; 95% CI 0.99–1.06; p = 0.17; GCST90018925: OR 0.99; 95% CI 0.92–1.06; p = 0.68; Meta-analysis: OR 1.02; 95% CI 0.99–1.05; p = 0.29).
No heterogeneity or pleiotropy was observed in the associations between T1D (GCST010681) and NICM (p for heterogeneity = 0.35, p for pleiotropy = 0.28, respectively). For GCST90018925, heterogeneity exists but there is no evidence of pleiotropy (p for heterogeneity = 0.01, p for pleiotropy = 0.34, respectively). The results were robust in the leave-one-out and MR-PRESSO tests. To further rule out the influence of confounding factor level pleiotropy, we conducted MVMR. After matching for BMI, hypertension or both, statistical significance remained between T1D and NICM (Fig. 3). For additional information and visual representations of the data analysis, please refer to Supplementary Fig. 1, which includes scatter plots for the pleiotropy analysis, forest plots using the leave-one-out method, and funnel plots.
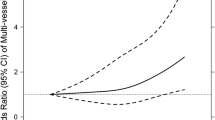
To understand the relationship between different subgroups of T1D and NICM, we analyzed data from the Finnish database, which is the most comprehensive for T1D complications. Both T1D without complications and T1D with complications showed causal correlations with NICM (IVW: OR 1.02; 95% CI 1.004–1.04; p = 1.42e-02; OR 1.03; 95% CI 1.01–1.05; p = 3.15e-3, respectively). T1D with complications encompasses a range of diseases. These subgroup analyses also revealed significant causal correlations with NICM. The ORs of NICM were 1.02 (95% CI 1.01–1.03; p = 7.90e-03) for T1D with renal complications, 1.01 (95% CI 1.00-1.02; p = 8.75e-02) for T1D with ketoacidosis, 1.02 (95% CI 1.02–1.03; p = 4.17e-03) for T1D with coma, 1.03 (95% CI 1.01–1.05; p = 1.39e-02) for T1D with ophthalmic complications, 1.03 (95% CI 1.01–1.05; p = 5.19e-03) for T1D with peripheral circulatory complications, 1.02 (95% CI 1.01–1.04; p = 9.61e-03) for T1D with coma. Except for the analysis for T1D without complications, where heterogeneity was observed, all other subgroup analyses showed no significant heterogeneity or pleiotropy (Fig. 2). The results were robust in the leave-one-out and MR-PRESSO tests. We also conducted MVMR for T1D with complications. After matching for BMI, statistical significance remained (OR1.03, 95% CI 1.002–1.06, p = 3.66e-02) (Fig. 3). However, after adjusting for hypertension, the statistical correlation disappeared. In the subgroup analysis, the exposure and outcome datasets were from the same database. Therefore, there is a significant overlap in the control group. We used https://sb452.shinyapps.io/overlap to estimate the potential for Type I errors. After evaluation, even if the samples completely overlapped, the type I errors rate still be maintained at 0.05 in all subgroup analyses.
Genetically predicted type 1 diabetes and its complications: associations with the non-ischemic cardiomyopathy. IVW (Inverse Variance Weighted), H (Heterogeneity), P (Pleiotropy), CI (Confidence Interval), OR (Odds Ratio), p < 0.05 was considered statistically significant. The FDR-corrected results of the p-values (IVW) in each sub-group remained consistent with the uncorrected results
We then performed mediation analysis involving potential mediators, including anemia, glomerular disease, BMI, hypertension, glycated hemoglobin, HOMA-IR, fasting insulin, low-density lipoprotein cholesterol, triglyceride, intermediate-density lipoprotein and very-low-density lipoprotein. However, none of these factors demonstrated a mediating effect (Supplementary Excel.2). Among analyzed CRP and 41 inflammatory cytokines, a causal relationship with NICM was only found for Nerve Growth Factor and MIG. As Nerve Growth Factor had only 4 SNP instrumental variables, thus further analysis was not performed. Conversely, MIG mediated the relationship between T1D and NICM with an OR of 1.005 (95% CI 1.001–1.01) and accounted for 20% of the mediation effect (See Fig. 4). During the MR process, multiple tests were performed, hence the p-value was adjusted using false discovery rate (FDR) correction. The significance of the p-value for MIG disappears after correction (Supplementary Excel. 1).
Discussion
The study provided genetic evidence supporting the causal association between NICM and T1D in univariable MR and MVMR analyses. Furthermore, the study demonstrated the causal relationship between T1D complications and NICM. Notably, there was no significant difference in the OR of NICM between T1D alone and T1D with complications.
Clinical observational studies have suggested an association between diabetes mellitus and HF [21, 44,45,46]. However, these studies primarily focus on T2D and are influenced by numerous confounding factors. For most diabetic patients who develop HF, their HF is related to coronary artery disease [18]. Therefore, it is necessary to elucidate the isolated impact of T1D on NICM. The impact of T1D on the myocardium is primarily focused on animal studies. In recent studies, it has been suggested that factors such as oxidative stress, inflammatory response, calcium ion imbalance, and energy dysregulation are involved in the impact of diabetes mellitus on the myocardium [47, 48]. Our previous study demonstrated impaired diastolic function in T1D SD rats [49]. Clinical research on the relationship between T1D and NICM is limited due to challenges in conducting prospective clinical studies, including cost and confounding biases. To address these challenges, we used an MR study to establish a causal connection between T1D and NICM, providing valuable evidence.
Anemia and nephropathy are relatively common concurrent diseases in patients with HF. Both of these conditions increase the risk factors for poor prognosis in patients with HF [50, 51]. Additionally, iron deficiency anemia and chronic kidney disease have been identified as risk factors for HF [30, 52]. However, it is worth noting that most research findings in the existing literature are derived from developed countries and largely focus on cases of ischemic cardiomyopathy. In a study from a developing country, the authors observed a significantly lower prevalence of anemia and nephropathy in individuals with NICM compared to studies conducted in Western countries [53]. An MR study suggested bidirectional causality between anemia and chronic HF [54]. Glomerular disease is a common complication of T1D. The correlation between T1D and anemia is unclear, but its complication, diabetic nephropathy, can cause anemia. The current study confirmed a causal association between genetically predicted T1D and genetically predicted glomerular disease as well as anemia. However, the causal relationship between anemia and NICM showed pleiotropy in the MR analysis. Although there is a causal relationship between glomerular disease and NICM, the mediating effect did not reach statistical significance. Therefore, further research is needed to analyze this potential mediating effect.
The association between inflammation and HF is currently a topic of great interest. An observational study conducted in 1990 found that patients with HF had elevated level of pro-inflammatory cytokines compared to healthy individuals [55]. Subsequent experimental and clinical research has highlighted the activation of the innate and adaptive immune systems as important factors in acute and chronic HF, leading to the exploration of potential immunotherapy for HF [56]. However, the outcomes of immunotherapy for HF have been less than satisfactory [57,58,59]. The CANTOS trial, a double-blind, randomized, placebo-controlled outcomes trial involving 10,061 patients with myocardial infarction and inflammatory atherosclerosis characterized by high-sensitivity CRP levels ≥ 2 mg/l, demonstrated a 15% reduction in the risk of the composite endpoint of non-fatal myocardial infarction, non-fatal stroke, or cardiovascular death compared to placebo [60]. Further exploration of this study revealed that patients with evidence of clonal hematopoiesis of indeterminate potential owing to mutations in TET2 had an improved response to canakinumab treatment compared with patients without the mutations [61]. This study provides inspiration that immunotherapy may not be universally effective for all cases of HF, and thus, it is important to explore which specific types of HF may respond positively to immunization. In an MR study, it was proposed that genetically predicted 10 inflammatory biomarkers (not including MIG) did not show a significant association with HF [28]. In current study, we investigated the causal relationship between 42 inflammatory biomarkers and discovered that MIG has a suggestive causal relationship with NICM and may plays a mediating role in the process of T1D causing NICM. Previous studies have also found that MIG is involved in immune checkpoint inhibitor myocarditis and chronic rejection after heart transplantation [62, 63]. Further exploration is warranted to determine the role of MIG in NICM.
Strengths and limitations
To our knowledge, this is the first study to investigate the causal associations between T1D and NICM using univariable MR and MVMR analysis. The study fills a gap in the current human-level research on the causal relationship between T1D and NICM. Additionally, by investigating potential mediators, we can improve our understanding of the potential mechanisms underlying NICM, paving the way toward the development of preventative and therapeutic solutions. The application of the MR method helped to reduce confounding biases and derive robust causal effect estimates. Multiple sensitivity analyses and IV strength evaluations were conducted to ensure the reliability of the results. However, this study has certain limitations. Firstly, most of the data used in this study comes from individuals of European ancestry, which may limit the generalizability of our findings. Secondly, while subgroup MR analysis of T1D with complications can offer us a comprehensive insight into the association between various complications and NICM, it is important to acknowledge the considerable sample overlap between participants in the exposure and outcome datasets. In fact, these are single-sample analysis. This might increase the risk of type I errors, so caution should be exercised when interpreting the results in this section. We used https://sb452.shinyapps.io/overlap to estimate the potential for Type I errors. After evaluation, even if the samples completely overlapped, the type I error rate could be maintained at 0.05 in all subgroup analyses. Thirdly, even with the assurance of F statistic > 10, the explanatory power of IVs on potential mediating variables is limited. Therefore, even though many inflammatory cytokines have not been found to have a mediating effect, further research is still warranted in this area. In addition, the correlation p-value of MIG becomes non-significant after FDR correction. This suggests that it may play a mediating role, but more evidence is needed to confirm this.
Conclusion
In conclusion, the study suggests that genetically predicted T1D and its complications play an independent causal role in the development of NICM. MIG may mediate the progression from T1D to NICM.
Abbreviations
- IVW:
-
Inverse variance weighted
- IVs:
-
Instrumental variables
- SNPs:
-
Single Nucleotide Polymorphisms
- FDR:
-
False Discovery Rate
- MVMR:
-
Multivariable MR
- T1D:
-
Type 1 diabetes
- T2D:
-
Type 2 diabetic
- NICM:
-
Non-ischemic cardiomyopathy
- HF:
-
Heart failure
- OR:
-
Odds ratio
- MIG:
-
Monokine Induced by Gamma Interferon
- HOMA-IR:
-
Homeostatic Model Assessment of Insulin Resistance
- GWAS:
-
Genome-Wide Association Study
- CRP:
-
C-reactive protein
- UKB:
-
UK Biobank
References
Zimmet P, Alberti KGMM, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7.
Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. 2012;8:228–36.
Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, et al. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017;3:17016.
Green A, Hede SM, Patterson CC, Wild SH, Imperatore G, Roglic G, et al. Type 1 diabetes in 2017: global estimates of incident and prevalent cases in children and adults. Diabetologia. 2021;64:2741–50.
Lawrence JM, Divers J, Isom S, Saydah S, Imperatore G, Pihoker C, et al. Trends in Prevalence of Type 1 and type 2 diabetes in children and adolescents in the US, 2001–2017. JAMA. 2021;326:717.
Patterson CC, Gyürüs E, Rosenbauer J, Cinek O, Neu A, Schober E, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989–2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55:2142–7.
Luk AOY, Ke C, Lau ESH, Wu H, Goggins W, Ma RCW et al. Secular trends in incidence of type 1 and type 2 diabetes in Hong Kong: A retrospective cohort study. Basu S, editor. PLoS Med. 2020;17:e1003052.
Egro FM. Why is type 1 diabetes increasing? J Mol Endocrinol. 2013;51:R1–13.
Laing SP, Swerdlow AJ, Slater SD, Botha JL, Burden AC, Waugh NR, et al. The British Diabetic Association Cohort Study, I: all-cause mortality in patients with insulin-treated diabetes mellitus. Diabet Med. 1999;16:459–65.
Rönnback M, Fagerudd J, Forsblom C, Pettersson-Fernholm K, Reunanen A, Groop P-H. Altered age-related blood pressure pattern in type 1 diabetes. Circulation. 2004;110:1076–82.
Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–8.
Amadid H, Clemmensen KKB, Vistisen D, Persson F, Jørgensen ME. Time trends of cardiovascular risk management in type 1 diabetes - nationwide analyses of real-life data. Cardiovasc Diabetol. 2022;21:255.
Liu Z, Wang H, Yang Z, Lu Y, Zou C. Causal associations between type 1 diabetes mellitus and cardiovascular diseases: a mendelian randomization study. Cardiovasc Diabetol. 2023;22:236.
Lind M, Bounias I, Olsson M, Gudbjörnsdottir S, Svensson A-M, Rosengren A. Glycaemic control and incidence of heart failure in 20 985 patients with type 1 diabetes: an observational study. The Lancet. 2011;378:140–6.
Raev DC. Which left ventricular function is impaired earlier in the evolution of Diabetic Cardiomyopathy? An echocardiographic study of young type I diabetic patients. Diabetes Care. 1994;17:633–9.
Berková M, Opavský J, Berka Z, Škraňka V, Salinger J, LEFT VENTRICULAR DIASTOLIC, FILLING IN YOUNG PERSONS WITH TYPE 1 DIABETES MELLITUS. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2003;147:57–61.
Schannwell CM, Schneppenheim M, Perings S, Plehn G, Strauer BE. Left ventricular diastolic dysfunction as an early manifestation of Diabetic Cardiomyopathy. Cardiology. 2002;98:33–9.
Christiansen E. Increased left ventricular systolic function in insulin dependent diabetic patients with normal albumin excretion. Eur Heart J. 1998;19:1735–9.
Di Cori A, Di Bello V, Miccoli R, Talini E, Palagi C, Delle Donne MG, et al. Left ventricular function in Normotensive Young adults with Well-controlled type 1 diabetes Mellitus. Am J Cardiol. 2007;99:84–90.
Konduracka E, Cieslik G, Galicka-Latala D, Rostoff P, Pietrucha A, Latacz P, et al. Myocardial dysfunction and chronic heart failure in patients with long-lasting type 1 diabetes: a 7-year prospective cohort study. Acta Diabetol. 2013;50:597–606.
Brunvand L, Fugelseth D, Stensaeth KH, Dahl-Jørgensen K, Margeirsdottir HD. Early reduced myocardial diastolic function in children and adolescents with type 1 diabetes mellitus a population-based study. Bmc Cardiovasc Disor. 2016;16:103.
Tian C, Shao CH, Moore CJ, Kutty S, Walseth T, DeSouza C, et al. Gain of function of cardiac ryanodine receptor in a rat model of type 1 diabetes. Cardiovasc Res. 2011;91:300–9.
Ni R, Zheng D, Xiong S, Hill DJ, Sun T, Gardiner RB, et al. Mitochondrial Calpain-1 disrupts ATP synthase and induces Superoxide Generation in Type 1 Diabetic hearts: a novel mechanism contributing to Diabetic Cardiomyopathy. Diabetes. 2016;65:255–68.
Chavali V, Nandi SS, Singh SR, Mishra PK. Generating double knockout mice to model genetic intervention for diabetic cardiomyopathy in humans. Methods Mol Biol. 2014;1194:385–400.
Fang Q, Wang J, Wang L, Zhang Y, Yin H, Li Y, et al. Attenuation of inflammatory response by a novel chalcone protects kidney and heart from hyperglycemia-induced injuries in type 1 diabetic mice. Toxicol Appl Pharm. 2015;288:179–91.
Burger PM, Koudstaal S, Mosterd A, Fiolet ATL, Teraa M, van der Meer MG, et al. C-Reactive protein and risk of Incident Heart failure in patients with Cardiovascular Disease. J Am Coll Cardiol. 2023;82:414–26.
Habibi D, Daneshpour MS, Asgarian S, Kohansal K, Hadaegh F, Mansourian M, et al. Effect of C-reactive protein on the risk of Heart failure: a mendelian randomization study. BMC Cardiovasc Disord. 2023;23:112.
Remmelzwaal S, van Oort S, Handoko ML, van Empel V, Heymans SRB, Beulens JWJ. Inflammation and heart failure: a two-sample mendelian randomization study. J Cardiovasc Med (Hagerstown). 2022;23:728–35.
Wehrwein G, Neumeier M, Schäffler A, Kopp A, Weigert J, Abke S, et al. Lipopolysaccharide regulated protein expression is only partly impaired in monocytes from patients with type I diabetes. Cardiovasc Diabetol. 2006;5:5.
Makubi A, Hage C, Lwakatare J, Mmbando B, Kisenge P, Lund LH, et al. Prevalence and prognostic implications of anaemia and iron deficiency in Tanzanian patients with heart failure. Heart. 2015;101:592–9.
Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing mendelian randomization investigations: update for summer 2023. Wellcome Open Res. 2023;4:186.
Rasooly D, Peloso GM. Two-sample multivariable mendelian randomization analysis using R. Curr Protoc. 2021;1:e335.
Hingorani A, Humphries S. Nature’s randomised trials. The Lancet. 2005;366:1906–8.
Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. 2021;375:n2233.
Relton CL, Davey Smith G. Two-step epigenetic mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol. 2012;41:161–76.
Forgetta V, Manousaki D, Istomine R, Ross S, Tessier M-C, Marchand L, et al. Rare genetic variants of large effect influence risk of type 1 diabetes. Diabetes. 2020;69:784–95.
Sakaue S, Kanai M, Tanigawa Y, Karjalainen J, Kurki M, Koshiba S, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. 2021;53:1415–24.
Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613:508–18.
Ahola-Olli AV, Würtz P, Havulinna AS, Aalto K, Pitkänen N, Lehtimäki T, et al. Genome-wide association study identifies 27 loci influencing concentrations of circulating cytokines and growth factors. Am J Hum Genet. 2017;100:40–50.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408.
Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8.
Yavorska OO, Burgess S. MendelianRandomization: an R package for performing mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46:1734–9.
Rawshani A, Sattar N, Franzén S, Rawshani A, Hattersley AT, Svensson A-M, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. The Lancet. 2018;392:477–86.
Mordi IR, Lumbers RT, Palmer CNA, Pearson ER, Sattar N, Holmes MV, et al. Type 2 diabetes, metabolic traits, and risk of Heart failure: a mendelian randomization study. Diabetes Care. 2021;44:1699–705.
Bauters C, Lamblin N, Mc Fadden EP, Van Belle E, Millaire A, de Groote P. Influence of diabetes mellitus on heart failure risk and outcome. Cardiovasc Diabetol. 2003;2:1.
Dillmann WH. Diabetic Cardiomyopathy. Circ Res. 2019;124(8):1160–1162.
Mfarrej B, Keir M, Dada S, Trikudanathan S, Sayegh MH, Sharpe AH, et al. Anti-CD3 mAb treatment cures PDL1-/-.NOD mice of diabetes but precipitates fatal myocarditis. Clin Immunol. 2011;140:47–53.
Zhao Y, Li S, Quan E, Zhang H, Wu Y, Luo Y, et al. Trimetazidine inhibits cardiac fibrosis by reducing reactive oxygen species and downregulating connective tissue growth factor in streptozotocin-induced diabetic rats. Exp Ther Med. 2019;18:1477–85.
Lindenfeld J. Prevalence of anemia and effects on mortality in patients with heart failure. Am Heart J. 2005;149:391–401.
Al-Ahmad A, Rand WM, Manjunath G, Konstam MA, Salem DN, Levey AS, et al. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;38:955–62.
Matsushita K, Ballew SH, Wang AY-M, Kalyesubula R, Schaeffner E, Agarwal R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol. 2022;18:696–707.
Inglis SC, Stewart S, Papachan A, Vaghela V, Libhaber C, Veriava Y, et al. Anaemia and renal function in heart failure due to idiopathic dilated cardiomyopathy. Eur J Heart Fail. 2007;9:384–90.
Gan T, Hu J, Liu W, Li C, Xu Q, Wang Y, et al. Causal Association between Anemia and Cardiovascular Disease: a 2-Sample bidirectional mendelian randomization study. J Am Heart Assoc. 2023;12:e029689.
Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–41.
Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17:269–85.
Mann DL. Innate immunity and the failing heart: the Cytokine Hypothesis Revisited. Circ Res. 2015;116:1254–68.
Mann DL, McMurray JJV, Packer M, Swedberg K, Borer JS, Colucci WS, et al. Targeted anticytokine therapy in patients with Chronic Heart failure: results of the Randomized Etanercept Worldwide evaluation (RENEWAL). Circulation. 2004;109:1594–602.
Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT, Randomized. Double-Blind, Placebo-Controlled, Pilot Trial of Infliximab, a chimeric monoclonal antibody to Tumor Necrosis Factor-α, in patients with moderate-to-severe heart failure: results of the Anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133–40.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31.
Svensson EC, Madar A, Campbell CD, He Y, Sultan M, Healey ML, et al. TET2-Driven clonal hematopoiesis and response to Canakinumab: an exploratory analysis of the CANTOS Randomized Clinical Trial. JAMA Cardiol. 2022;7:521–8.
Ma P, Liu J, Qin J, Lai L, Heo GS, Luehmann H et al. Expansion of pathogenic Cardiac macrophages in Immune checkpoint inhibitor myocarditis. Circulation. 2023.
Yun JJ, Fischbein MP, Whiting D, Irie Y, Fishbein MC, Burdick MD, et al. The role of MIG/CXCL9 in cardiac allograft vasculopathy. Am J Pathol. 2002;161:1307–13.
Acknowledgements
We want to acknowledge the participants and investigators of the FinnGen study, UK Biobank, and other GWASs included in this study. Without the dedication of these organizations and their members, this article would have been difficult to complete.
Funding
This study was supported by the Science and Technology Program of Guangzhou, China (grant nos 202103000060).
Author information
Authors and Affiliations
Contributions
The concept for the project was developed by H C and YT C. And, YY Z designed this study and revised the manuscript. T Z, ZS H, YT L, L P, SH L and Jl L collected data from the public database. YY Z and EX Q wrote the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1:
Supplemental Excel 1. Detailed data for univariate and multivariate mendelian randomization
Supplementary Material 2:
Table S1. Descriptive information for all the datasets included and their corresponding diagnostic codes
Supplementary Material 3:
Supplemental Excel 2. Detailed data for mediation mendelian randomization
Supplementary Material 4:
Supplemental Figure 1. Visualization of Mendelian Randomization for T1D and T1D with Complications: A) Pleiotropy Analysis; B) Stability Analysis Utilizing the Leave-One-Out Method; C) Forest Plot Showing MR Effect Sizes Using MR-Egger and IVW; D) Funnel Plot
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, Y., Quan, E., Zeng, T. et al. Type 1 diabetes, its complications, and non-ischemic cardiomyopathy: a mendelian randomization study of European ancestry. Cardiovasc Diabetol 23, 31 (2024). https://doi.org/10.1186/s12933-023-02117-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-02117-7