Abstract
Background
The triglyceride-glucose (TyG) index has been proposed as a reliable surrogate marker of insulin resistance and an independent predictor of major adverse cardiovascular events (MACEs). Several recent studies have shown the relationship between the TyG index and cardiovascular outcomes; however, the role of the TyG index in chronic coronary syndrome (CCS) progression has not been extensively assessed especially in population after revascularization. This study aimed to investigate the prognostic value of the TyG index in predicting MACEs in CCS patients undergoing percutaneous coronary intervention (PCI).
Methods
The data for the study were taken from the Hospital Information System database in China-Japan Friendship Hospital over the period 2019–2021. Eligible participants were divided into groups according to the TyG index tertiles. The Boruta algorithm was performed for feature selection. Multivariate Cox proportional hazards models and restricted cubic spline (RCS) analysis were applied to examine the dose–response relationship between the TyG index and endpoint, and the results were expressed with hazard ratio (HR) and 95% confidence interval (CI) values. The area under the receiver operating characteristic (ROC) curve (AUC), decision curve analysis (DCA), and clinical impact curve (CIC) were plotted to comprehensively evaluate the predictive accuracy and clinical value of the model. The goodness-of-fit of models was evaluated using the calibration curve and χ2 likelihood ratio test.
Results
After applying inclusion and exclusion criteria, 1353 patients with CCS undergoing PCI were enrolled in the study. After adjusting for all confounders, we found that those with the highest TyG index had a 59.5% increased risk of MACEs over the 1-year follow-up (HR 1.595, 95% CI 1.370 ~ 1.855). Using the lowest TyG index tertile as the reference (T1), the fully adjusted HRs (95% CIs) for endpoints was 1.343 (1.054 ~ 1.711) in the middle (T2) and 2.297 (1.842 ~ 2.864) in highest tertile (T3) (P for trend < 0.001). The TyG index had an excellent predictive performance according to the results of AUC 0.810 (0.786, 0.834) and χ2 likelihood ratio test (χ2 = 7.474, P = 0.486). DCA and CIC analysis also suggested a good overall net benefit and clinical impact of the multivariate model. The results in the subgroup analysis were consistent with the main analyses. RCS model demonstrated that the TyG index was nonlinearly associated with the risk of MACEs within one year (P for nonlinear < 0.001).
Conclusion
The elevated TyG index is associated with an increased risk of cardiovascular events and predicts future MACEs in patients with CCS undergoing PCI independently of known cardiovascular risk factors, indicating that the TyG index may be a potential marker for risk stratification and prognosis in CCS patients undergoing PCI.
Similar content being viewed by others
Introduction
Coronary artery disease (CAD) has affected 244.11 million individuals worldwide and is the leading cause of death, constituting an increasing public health burden worldwide [1, 2]. Chronic coronary syndrome (CCS), previously referred to as stable CAD, characterized by the interruption of the coronary artery intimal layer and intramural hematoma, causing vessel compression, and typically presenting as an acute coronary syndrome (ACS) [3]. CCS contributes to the major population of CAD and encompasses patients with or without previous ACS or revascularization [4].
Observational studies indicate that most conservatively managed patients recover without further intervention [5,6,7]. However, in patients with ongoing ischemia, vessel occlusion, or patient instability, selective revascularization may be necessary [4, 8]. Percutaneous coronary intervention (PCI) with drug-eluting stent (DES) is one of the most conventional revascularization strategies performed in CCS patients. Although the management of PCI-DES in CCS patients has made great advances over the past few decades, the incidence of major adverse cardiovascular events (MACEs) such as repeat revascularization and in-stent restenosis may still exceeds 25% at 5-year follow-up [9, 10]. Therefore, it is critically important to identify high-risk patients of suffering from future MACEs so that intense management can be offered. The identification of rapidly available and reliable markers may have great clinical significance in optimizing the risk stratification of recurrent cardiovascular risk.
Insulin resistance (IR) is a clinical state of impaired insulin sensitivity which may lead to cardiometabolic alterations such as hyperglycemia, dyslipidemia, and hypertension [11, 12]. Previous evidence indicated that IR was a strong predictor of increased cardiovascular morbidity and mortality [13,14,15]. The triglyceride-glucose (TyG) index, combined with fasting glucose and triglycerides, has been proposed as a reliable surrogate marker for IR [16,17,18]. Growing studies demonstrated that the TyG index may independently predict adverse cardiovascular outcomes among CAD cohorts with different clinical manifestations [19,20,21]. However, there are limited clinical studies assessing the prognostic value of the TyG index in CCS patients undergoing PCI.
This study aimed to examine the association between the TyG index and the risk of MACEs and to determine the prognostic value of the TyG index in CCS patients undergoing PCI through accessible real-world data. The findings of this study may help identify high-risk individuals and develop clinical strategies to improve outcomes in these specific populations at the earliest, and provide crucial new insights into the research of TyG in predicting outcomes in the field of CAD.
Materials and methods
Study design and participants
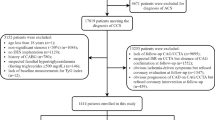
This was a single-center, retrospective, observational cohort study. Admission data of consecutive CCS patients undergoing PCI were collected and assessed from the Hospital Information System database in China-Japan Friendship Hospital between January 2019 and December 2021. The definition of CCS complied with the current guideline of the European Society of Cardiology [3]. Among the 8141 patients, 6388 patients were excluded based on the study exclusion criteria, which included (1) younger than 18 or older than 80; (2) not first admission or lack of data at admission; (3) no DES implantation; (4) severe complications such as advanced cancer, severe hepatic and renal dysfunction, severe hematological and endocrine system diseases; and (5) history of coronary artery bypass grafting. Eventually, 1353 eligible patients were enrolled in this study and classified into three groups according to the TyG index tertiles (Fig. 1). This retrospective study was performed in line with the Declaration of Helsinki. Because of the retrospective design of this study, the need for informed consent was waived by the institutional review board, and information related to patient identity was concealed.
Data collection and definitions
Clinical data, including demographic characteristics, the clinical history, laboratory indicators, echocardiography and peripheral arterial disease features, and number of coronary lesions and stent implantation, were collected using a standardized questionnaire by trained clinicians who were blinded to the purpose of the study. Demographic characteristics included weight, height [to calculate body mass index (BMI)], age, gender, baseline systolic blood pressure (SBP) and diastolic blood pressure (DBP), heart rate (HR), smoking history and drinking history. Clinical history included established diabetes mellitus, hypertension, stroke, old myocardial infarction (OMI), dyslipidemia, and history of cardiovascular diseases (CVDs). Laboratory tests consisting of neutrophil (Neu), lymphocyte (Lym), platelets (PLT), hemoglobin (Hb), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglyceride (TG), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), lipoprotein (a) [Lp (a)], homocysteine (HCY), hypersensitive C-reactive protein (Hs-CRP), serum creatinine (Scr), fasting blood glucose (FBG) and glycated hemoglobin A1c (HbA1c) were performed under standardized instructions and assaying system. All blood samples were collected after overnight fasting before coronary angiography. Echocardiography features consisting of left atrial diameter (LAD), left ventricular end-diastolic diameter (LVDd), interventricular septal thickness (IVST), left ventricular posterior wall thickness (PWT), and left ventricular ejection fraction (LVEF) were analyzed and recorded by two independent echocardiographers. The angiographic data was obtained from the cardiac catheterization laboratory records. Peripheral arterial disease indicators included brachial-ankle pulse wave velocity (baPWV), ankle-brachial index (ABI), and brachial artery flow-mediated vasodilatation (FMD) value. The TyG index was calculated as Ln [fasting TG (mg/dL) × FBG (mg/dL)/2] [17].
Feature selection
Boruta algorithm, characterized by an extension of the random forest algorithm and the creation of “shadow features” by shuffling the real features, was utilized to identify the most critical features related to the risk of MACEs and to establish the radiomics signatures. A replicated feature called the shadow feature was created by Boruta-based method from the original dataset to compare the Z-score between the genuine features and the shadow feature generated by the random forest classifier in each iteration of the model development. The Z-value of each feature is evaluated based on its importance in the random forest model, and the maximum Z-value of the shadow features is recorded. A real feature is considered important if its Z-value is greater than the maximum Z-value of the shadow features; otherwise, it was eliminated [22].
Follow-up and endpoints
Clinical follow-up was carried out by skilled clinicians in outpatient or telephone contact at the time points of one year, and standard computerized case report forms were filled out. The endpoint events were independently categorized by three cardiovascular specialists who were not aware of the baseline information. When there were disagreements regarding event identification, the three experts came to a decision together after talking.
The primary endpoint of this clinical trial was defined as a compound endpoint of the first occurrence of total MACEs within one-year follow up. The total MACEs was defined as follows: (1) unplanned revascularization, which means that the patient underwent revascularization again due to unexpected internal cardiac causes; (2) in-stent restenosis, which was defined as 50% or more of the target vessel stenosis within 5 mm from the edge of the stent or both ends of the stent after percutaneous coronary intervention as shown by coronary angiography; (3) non-fatal myocardial infarction, referring to myocardial necrosis but no death, accompanied by ischemia symptoms, abnormal myocardial markers, ST segment changes or pathological Q wave changes; (4) other cardiovascular events such as cardiac death, stroke, unplanned rehospitalization for cardiac causes.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) and median [interquartile range (IQR)] for those with normal and skewed distributions, respectively. Categorical variables were expressed as number (percentage). All participants were stratified into three groups: T1 (TyG index < 8.59, n = 438), T2 (8.59 ≤ TyG index < 9.06, n = 458), and T3 (TyG index ≥ 9.06, n = 457) in accordance with the TyG index tertiles. Continuous data were compared using one-way analysis of variance or the Kruskal-Wallis test among the three groups according to the presence or absence of normal distribution, and the chi-squared test or Fisher’s exact test was performed for categorical variables.
The Boruta-based feature selection method was performed to determine the potential markers associated with endpoints. The cumulative incidence of endpoints was described by the Kaplan-Meier method and compared between groups using the log-rank test. Multivariate Cox proportional hazards models were applied to test the associations of the TyG index with endpoints, and the results were expressed as hazard ratio (HR) and 95% confidence interval (CI) values. Risk factors that were statistically significant in the univariate analysis (P < 0.05), screened by Boruta algorithm, or clinically significant were selected as candidates for the multivariate model. Besides the Model 1 without any other adjustments for confounding factors, two other models were fitted. In Model 2, age, gender, BMI, SBP, and DBP were modified. Model 3 was a completely adjusted model that took feature selection results and clinical experience adjustments into account. The linear trends across TyG tertiles were evaluated by a median value within each tertile as a continuous variable. Additionally, multivariate restricted cubic spline (RCS) analysis was used to assess any potential nonlinear relationships between the TyG index and endpoints. The area under the receiver operating characteristic (ROC) curve (AUC), decision curve analysis (DCA), and clinical impact curve (CIC) were plotted to comprehensively evaluate the predictive accuracy and clinical value of the model. The goodness-of-fit of models was evaluated using the calibration curve and χ2 likelihood ratio test. Furthermore, we performed subgroup and interaction analyses based on age(< 65 years or ≥ 65 years), gender (male or female), BMI (< 24 kg/m2 or ≥ 24 kg/m2), diabetes (yes or no), hypertension (yes or no), familial CVDs (yes or no), LDL-C (yes or no), multi-vessel disease (yes or no), and multi-DES implantation (yes or no), to identify whether the relationships between the TyG index and endpoints varied according to the status of the potential covariates.
All statistical analyses were performed using IBM-SPSS (version 26.0, Chicago, IL, USA) and R (version 4.1.2, Vienna, Austria). A two-sides P value of less than 0.05 was considered to indicate statistical significance.
Results
Baseline characteristics
Overall, a total of 1353 CCS patients undergoing PCI from the Hospital Information System database were included in the analysis with a median TyG index of 8.83 (8.47, 9.18). The median follow-up time was 1.7 years. Among the 1353 eligible patients, 542 (40.06%) suffered from MACEs within one year while 811 did not. The baseline characteristics of the study population are summarized in Table 1 and Additional file 1: Table S1.
The average age of the participants was 62 years, and 72.36% of the participants were male. Patients with higher baseline TyG index had a greater prevalence of MACEs and comorbidities (including diabetes, hypertension, stroke, and dyslipidemia) and higher ratios of patients with familial CVDs and a history of multi-DES implantation. They also had higher BMI, SBP, HR, Neu, Lym, PLT, ALT, TC, TG, LDL-C, HCY, Hs-CRP, FBG, and HbA1c (all P < 0.05). Furthermore, the highest TyG index tertile were more likely to have higher baPWV, lower ABI and FMD, and higher LAD, LVDd, IVST, and PWT (all P < 0.05), indicating a worse peripheral artery condition and poorer cardiac performance. Additionally, the proportions of individuals with the use of antiplatelet medication such as aspirin, nitrates were significantly lower in this group, as well as the HDL-C level. Collinearity diagnostics showed that no potentially significant collinearity was observed among variables (Additional file 1: Table S2).
Feature selection
Thirty-nine variables that were the most associated with the risk of MACEs were identified important after 100 iterations using the Boruta-based feature selection method, while 4 attributes were identified as unimportant and 6 tentative attributes were left (Fig. 2). Although several important characteristics, such as gender, smoking, a history of diabetes and stroke and medication situation, such as clopidogrel/ticagrelor, β-blockers and statins use, were disregarded because of the low Z-value in comparison to the shadow feature, they were nonetheless included in the analysis based on prior research and clinical experience. Factors were chosen for the final complete adjustment model when in the Boruta analysis, their Z-scores were higher than the shadow features or when added to the model, they had the largest matched effect (odds ratio or hazard ratio) among a group of biomarkers (max, mean and min) or they were based on previous findings and clinical constraints.
Feature selection for the potential markers associated with endpoints using the Boruta algorithm. A. The process of feature selection. B. The value evolution of Z-score in the screening process. The horizontal axis shows the name of variables and the number of iterations in Fig. 2-A and -B, respectively. While the vertical axis represents the Z-value of each variable, and the blue boxes and lines corresponds to the minimum, average, and maximum Z-scores for a shadow feature. The green boxes and lines represent confirmed variables, the yellow ones represent tentative attributes, and the red ones represent rejected variables in the model calculation. TyG, triglyceride-glucose; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; OMI, old myocardial infarction; CVD, cardiovascular disease; DES, drug-eluting stent; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blockers; Neu, neutrophil; Lym, lymphocyte; PLT, platelets; Hb, hemoglobin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; Lp (a), lipoprotein (a); HCY, homocysteine; Hs-CRP, hypersensitive C-reactive protein; Scr, serum creatinine; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; PAD, peripheral artery disease; baPWV, brachial-ankle pulse wave velocity; ABI, ankle-brachial index; FMD, brachial artery flow-mediated vasodilatation; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; LVDd, left ventricular end-diastolic diameter; IVST, interventricular septal thickness; PWT, left ventricular posterior wall thickness
Association between the TyG index and endpoints
A multivariate RCS analysis was conducted to determine whether there was a potential linear or nonlinear association between the TyG index and the endpoints in CCS patients undergoing PCI. As can be seen in Fig. 3, We recorded that the TyG index was proved to have a nonlinear relationship with the probability of the risk of MACEs within one year according to the RCS mode (P for non-linearity < 0.001). Furthermore, we defined three categories of included patients based on the TyG index tertiles. The Kaplan-Meier analysis for endpoints grouped by the TyG index tertiles were shown in Fig. 4. The probability of cumulative incidences of MACEs such as unplanned revascularization, in-stent restenosis, non-fatal myocardial infarction and other cardiovascular events was significantly higher in patients with a higher TyG index than in those with a lower TyG index (all Log rank P < 0.05).
Table 2 describes the results of the multivariate Cox proportional hazards regression analysis, revealing an association between the TyG index score and an elevated risk of one-year endpoints (HR 1.595, 95% CI 1.370 ~ 1.855, P < 0.001). The unadjusted model 1 indicated that the TyG index was statistically significantly associated with one-year endpoints, and the T3 was at elevated risk for an endpoint event. After adjusting for age, gender, BMI, SBP, and DBP in model 2, the TyG index as a continuous variable was an independent predictor for the endpoints (HR 1.742, 95% CI 1.504 ~ 2.016, P < 0.001). After further adjusting for diabetes, hypertension, familial CVDs, smoking and drinking history, TC, LDL-C, HCY, Hs-CRP, number of coronary lesions and stent implantation in model 3, the TyG index still remained independently associated with one-year endpoints (P < 0.001). Moreover, taking the T1 in as a reference, the risks of the primary endpoint were 2.522-fold higher (HR 2.522, 95% CI 2.032 ~ 3.128, P < 0.001) and 2.297-fold higher (HR 2.297, 95% CI 1.842 ~ 2.864, P < 0.001) in the T3 of Model 2 and Model 3, respectively. The trend analyses from T1 to T3 for the three models were all statistically significant (all P for trend < 0.001).
Kaplan-Meier curves for endpoints grouped by the TyG index tertiles. The cumulative incidence of (A) major adverse cardiovascular events (MACEs), (B) unplanned revascularization, (C) in-stent restenosis, (D) non-fatal myocardial infarction, and (E) other cardiovascular events during follow-up grouped according to the TyG index tertiles was analyzed by Kaplan-Meier analysis. The P value was calculated with the log-rank test. TyG, triglyceride-glucose; MACE, major adverse cardiovascular event 185B208
Predictive ability test
The ROC curve, calibration curve, DCA, and CIC were performed to comprehensively identify the predictive power of the TyG index for endpoints (Fig. 5). After adjustment for all confounders, the ROC curve demonstrated a high predictive ability of the TyG index for endpoints with an AUC of 0.810 (0.786, 0.834) and the calibration curve indicated an excellent goodness-of-fit of the multivariate model using the χ2 likelihood ratio test (χ2 = 7.474, P = 0.486). In addition, DCA and CIC analysis were conducted to assess the clinical utility of the model, suggesting that the model had a good overall net benefit and clinical impact within most reasonable threshold probability.
Performance evaluation of the TyG index for predicting endpoints. After adjustment for all confounders, predictive ability of the TyG index for endpoints was assessed using (A) the receiver operating characteristic (ROC) curve, (B) calibration curve, (C) decision curve analysis (DCA), and (D) clinical impact curve (CIC) analysis. AUC, the area under the ROC curve; MACE, major adverse cardiovascular event
Subgroup analysis
To confirm the relationship between the TyG index and the risk of MACEs stratified by age, gender, BMI, diabetes, hypertension, familial CVDs, LDL-C, multi-vessel disease and multi-DES implantation, subgroup analyses were carried out. As shown in Table 3, the results of subgroup analyses were almost consistent with the major findings of the study. Patients in higher TyG index tertile group tended to have a higher incidence of MACEs within one year across all subgroups. Meanwhile, LDL-C level and the number multi-DES implantation were found to interact with the relationship between the TyG index and endpoints (P < 0.05).
Discussion
In our population-based study, we recorded an association between the TyG index and the risk of MACEs within one year in CCS patients undergoing PCI, and this relationship also remained significant even after adjusting for all confounding factors. Simultaneously, the results implied that a higher TyG index indicated a greater prevalence of MACEs within a certain range, and the highest TyG index values enhanced the risk by 59.5% over the 1-year follow-up in the population. Moreover, the TyG index had an excellent predictive performance according to the results of AUC and χ2 likelihood ratio test after adjustment for potential confounders. DCA and CIC analysis also revealed a good overall net benefit and clinical impact of the multivariate model. The multivariate RCS model showed that the TyG index had a nonlinear relationship with the probability of the risk of MACEs within one year. These findings revealed the prognostic value of the TyG index for MACEs in CCS patients undergoing PCI. Most importantly, this study suggests that a simple method of estimating IR may optimize the risk stratification of recurrent cardiovascular risk in CCS patients undergoing PCI.
IR is defined as a decrease in the efficiency of insulin in promoting glucose uptake and utilization, which reflects the disorder of the metabolic balance [23]. According to previous studies, IR is believed as an important risk factor for CVDs can lead to poor clinical outcomes in various ways, such as inducing endothelial dysfunction, causing systemic glucose-lipid metabolism disorders, and triggering oxidative stress and inflammatory response [24,25,26,27]. Conventional approaches for detecting IR mainly include the hyperinsulinemic-euglycemic clamp technique and the homeostasis model assessment for IR (HOMA-IR) [28]. Given the limitations of traditional assessment methods such as time-consuming nature, high cost and complexity, and instability of the results, it is difficult to apply them in practical clinical settings and large-scale studies.
The triglyceride-glucose (TyG) index was firstly proposed by Unger G et al. in 2013 as an alternative predictor of IR [29]. Recently, substantial studies have confirmed that the TyG index is not only strongly correlated with IR [30], even the risk of CVDs in general population [31], but also could be considered as a prognostic surrogate indicator of hypertension [32], heart failure [11], CAD [33], and other CVDs [34, 35]. Furthermore, the TyG index is strongly associated with HOMA-IR and HIEC, could be used to identify IR with high sensitivity (96.5%) and specificity (85.0%), even outperforming the HOMA-IR in evaluating IR [17, 36, 37]. At present, numerous clinical studies demonstrated that the TyG index has been considered as a comparatively extensive method in clinical research with regard to CVDs. Data from a study [38] of 30,291 subjects screened from the China National Diabetes and Metabolic Disorders Study proposed that the TyG index was simpler and more suitable for the identification of metabolically unhealthy individuals as well as who have high risk of cardiometabolic diseases among the Chinese adult population, comparing with other surrogate indices of IR. A study [39] investigated 5014 patients of the Vascular Metabolic CUN cohort with a 10-year follow-up and supported that the TyG index might be useful to early identify individuals at a high risk of developing CVDs, including coronary heart disease, cerebrovascular disease, and peripheral arterial disease. Besides, a significantly improvement of the predictive ability was recorded when the TyG index was added to the Framingham model, with AUCs ranging from 0.708 (0.68, 0.73) to 0.71 (0.70, 0.74) (P = 0.014). Similarly, two cohort studies [40, 41] showed that healthy participants with elevated TyG index may have a higher risk of cardiovascular events. Previous studies also confirmed that the TyG index was associated with cardiovascular risk factors and could be used as a useful predictive marker for CVDs both in diabetic [42] and non-diabetic population [43]. Further researches showed that the TyG index was substantially associated with arterial stiffness [44], coronary artery calcification [45], and carotid atherosclerosis [36], and could be regarded as a better predictor of cardiovascular risk than FPG or HbA1c in ACS patients undergoing PCI [46]. Among TG-derived metabolic indices such as the atherogenic index of plasma and TG to HDL-C ratio, the TyG index showed a better ability to predict the risk of MACEs in ACS patients [47,48,49].
In the current study, we found that a higher TyG index was associated with a greater prevalence of adverse cardiovascular events, which is consistent with the previous reports [39, 50]. Although some adverse cardiovascular events such as repeat revascularization was a controversial outcome in clinical trials for its subjective and biased nature, previous studies supported that it was significantly corrected with elevated risk for mortality and morbidity in the short term and composite safety events in the long term [51]. Meanwhile, the TyG index was determined positively associated with ischemia-driven revascularization and target vessel revascularization in ACS patients [52, 53]. In-stent restenosis is a delayed complication of stenting [54]. Observational studies discovered that patients with in-stent restenosis were more prone to develop ACS and adverse cardiovascular events at follow-up [55]. In addition, the TyG index was identified as an independent predictor of in-stent restenosis in ACS patients, indicating a prospect for the TyG index in in-stent restenosis assessment [56]. In this context, this study was designed to focus on the adverse cardiovascular events such as repeat revascularization and in-stent restenosis in the CCS population, which reflects not only the target lesion failure but also the progression of non-target lesions. Consistently, our study suggested that the TyG index was significantly associated with MACEs in a nonlinear relationship among CCS patients undergoing PCI after adjustment for all confounding factors and the highest TyG index values enhanced the risk by 59.5% over the 1-year follow-up in the population. Consequently, the present study powered by adverse cardiovascular events among the CCS population has extended the association between the TyG index and CAD, indicating that the TyG index could serve as a potent prognostic indicator for risk stratification in CCS patients undergoing DES-PCI.
In the analysis of the association between the TyG index and endpoints, we used three unadjusted and adjusted models to develop the multivariate Cox proportional hazards regression analysis. Model 2 only adjusted for demographics (age, gender, and BMI) and blood pressure, which can be ascertained easily, showed great usefulness and generality in clinical practice. While the almost fully adjusted model (Model 3) provided the most specific risk prediction model and showed the independent prediction value of the TyG index for the incidence of MACEs within one year which cannot be explained by other covariates. Meanwhile, consistent with the major findings of the study, the results of subgroup analyses implied that age, gender, BMI, diabetes, hypertension, familial CVDs, LDL-C, multi-vessel disease and multi-DES implantation were the crucial risk factors of adverse cardiovascular events in CCS patients undergoing PCI. Additionally, our findings indicated that patients with higher baseline TyG index were more likely to have altered cardiac structure and worse peripheral artery conditions, which was manifested by higher LAD, LVDd, IVST, PWT and lower FMD, ABI, respectively. In general, the innovative finding of this study was that the cardiac structure and peripheral artery function were valuable factors for cardiovascular prognosis. Furthermore, population-based studies indicated that the TyG index be identified to be independently associated with aortic intima-media thickness [57], left ventricular functional impairment and structure abnormality [58], and cardiac hemodynamics [59], and an increased risk of incident PAD [60].
Nevertheless, some limitations must be acknowledged. Firstly, this was a single-center, retrospective study based on Chinese patients, selection bias may be introduced and the generalizability of our results need to be further demonstrated externally. Secondly, only baseline measurements at admission were available, and data collected at different time points during the follow-up period were lacking, which may lead to deviations in the analysis results. Thirdly, although some confounders were adjusted, our research results would still be affected by residual confounding factors. In addition, due to the limited clinical information collected, the differences between the TyG index and other predictors for the prognosis of CCS patients undergoing PCI still need to be investigated. Thus, further prospective, multicenter studies with larger sample sizes and multi-time node information need to be conducted to make our findings more reliable.
Conclusion
Overall, our research indicated that the TyG index could be considered as a prognostic indicator of adverse cardiovascular events such as unplanned revascularization, in-stent restenosis, non-fatal myocardial infarction and other cardiovascular outcomes in CCS patients undergoing PCI. In the high-risk group, TyG might be a valuable tool for risk categorization and management. Further studies are needed to confirm our findings and examine the potential mechanisms between TyG and the population.
Data Availability
The data of the study population were extracted from the Hospital Information System. The datasets are not publicly available because the individual privacy of the participants should be protected. Data are however available from the corresponding author on reasonable request.
Abbreviations
- TyG:
-
Triglyceride-glucose
- CCS:
-
Chronic coronary syndrome
- PCI:
-
Percutaneous coronary intervention
- MACE:
-
Major adverse cardiovascular event
- CAD:
-
Coronary artery disease
- CVD:
-
Cardiovascular disease
- DES:
-
Drug-eluting stent
- ACS:
-
Acute coronary syndrome
- CABG:
-
Coronary artery bypass grafting
- IR:
-
Insulin resistance
- RCS:
-
Restricted cubic spline
- ROC:
-
Receiver operating characteristic curve
- AUC:
-
Area under curve
- DCA:
-
Decision curve analysis
- CIC:
-
Clinical impact curve
- BMI:
-
Body mass index
- OMI:
-
Old myocardial infarction
- ACEI:
-
Angiotensin converting enzyme inhibitor
- ARB:
-
Angiotensin receptor blocker
- Neu:
-
Neutrophil
- Lym:
-
Lymphocyte
- PLT:
-
Platelets
- Hb:
-
Hemoglobin
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- Lp (a):
-
Lipoprotein (a)
- HCY:
-
Homocysteine
- Hs-CRP:
-
Hypersensitive C-reactive protein
- Scr:
-
Serum creatinine
- FBG:
-
Fasting blood glucose
- HbA1c:
-
Glycosylated hemoglobin
- PAD:
-
Peripheral artery disease
- baPWV:
-
Brachial-ankle pulse wave velocity
- ABI:
-
Ankle-brachial index
- FMD:
-
Brachial artery flow-mediated vasodilatation
- LAD:
-
Left atrial diameter
- LVEF:
-
Left ventricular ejection fraction
- LVDd:
-
Left ventricular end-diastolic diameter
- IVST:
-
Interventricular septal thickness
- PWT:
-
Left ventricular posterior wall thickness
References
Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart Disease and Stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–e639. https://doi.org/10.1161/CIR.0000000000001052. Epub 2022 Jan 26. Erratum in: Circulation. 2022;145(8):e153-e639. doi:10.1161/CIR.0000000000001052.
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 Diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22. https://doi.org/10.1016/S0140-6736(20)30925-9.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–77. https://doi.org/10.1093/eurheartj/ehz425.
Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, Demeter SH, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary Disease: a report of the American Heart Association/American College of Cardiology Joint Committee on clinical practice guidelines. Circulation. 2023;148(9):e9–e119. https://doi.org/10.1161/CIR.0000000000001168.
Lettieri C, Zavalloni D, Rossini R, Morici N, Ettori F, Leonzi O, et al. Management and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 2015;116(1):66–73. https://doi.org/10.1016/j.amjcard.2015.03.039.
Tweet MS, Eleid MF, Best PJ, Lennon RJ, Lerman A, Rihal CS, et al. Spontaneous coronary artery dissection: revascularization versus Conservative therapy. Circ Cardiovasc Interv. 2014;7(6):777–86. https://doi.org/10.1161/CIRCINTERVENTIONS.114.001659.
Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7(5):645–55. https://doi.org/10.1161/CIRCINTERVENTIONS.114.001760.
Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2022;145(3):e18–14. https://doi.org/10.1161/CIR.0000000000001038.
Tam DY, Dharma C, Rocha R, Farkouh ME, Abdel-Qadir H, Sun LY, et al. Long-term survival after surgical or percutaneous revascularization in patients with Diabetes and multivessel coronary Disease. J Am Coll Cardiol. 2020;76(10):1153–64. https://doi.org/10.1016/j.jacc.2020.06.052.
Parasca CA, Head SJ, Milojevic M, Mack MJ, Serruys PW, Morice MC, et al. Incidence, characteristics, predictors, and outcomes of repeat revascularization after percutaneous coronary intervention and coronary artery bypass grafting: the SYNTAX trial at 5 years. JACC Cardiovasc Interv. 2016;9(24):2493–507. https://doi.org/10.1016/j.jcin.2016.09.044.
Huang R, Wang Z, Chen J, Bao X, Xu N, Guo S, et al. Prognostic value of triglyceride glucose (TyG) index in patients with acute decompensated Heart Failure. Cardiovasc Diabetol. 2022;21(1):88. https://doi.org/10.1186/s12933-022-01507-7.
Di Pino A, DeFronzo RA. Insulin resistance and Atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev. 2019;40(6):1447–67. https://doi.org/10.1210/er.2018-00141.
Louie JZ, Shiffman D, McPhaul MJ, Melander O. Insulin resistance probability score and incident Cardiovascular Disease. J Intern Med. 2023;294(4):531–5. https://doi.org/10.1111/joim.13687.
Echouffo-Tcheugui JB, Zhang S, McEvoy JW, Juraschek SP, Fang M, Ndumele CE, et al. Insulin resistance and N-Terminal Pro-B-Type Natriuretic peptide among healthy adults. JAMA Cardiol. 2023;8(10):989–95. https://doi.org/10.1001/jamacardio.2023.2758.
Lee JH, Jeon S, Joung B, Lee HS, Kwon YJ. Associations of homeostatic model assessment for insulin resistance trajectories with Cardiovascular Disease incidence and mortality. Arterioscler Thromb Vasc Biol. 2023;43(9):1719–28. https://doi.org/10.1161/ATVBAHA.123.319200.
Guerrero-Romero F, Villalobos-Molina R, Jiménez-Flores JR, Simental-Mendia LE, Méndez-Cruz R, Murguía-Romero M, et al. Fasting triglycerides and glucose index as a diagnostic test for insulin resistance in young adults. Arch Med Res. 2016;47(5):382–7. https://doi.org/10.1016/j.arcmed.2016.08.012.
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51. https://doi.org/10.1210/jc.2010-0288.
Brito ADM, Hermsdorff HHM, Filgueiras MS, Suhett LG, Vieira-Ribeiro SA, Franceschini SDCC, et al. Predictive capacity of triglyceride-glucose (TyG) index for insulin resistance and cardiometabolic risk in children and adolescents: a systematic review. Crit Rev Food Sci Nutr. 2021;61(16):2783–92. https://doi.org/10.1080/10408398.2020.1788501.
Barzegar N, Tohidi M, Hasheminia M, Azizi F, Hadaegh F. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: Tehran lipid and glucose study. Cardiovasc Diabetol. 2020;19(1):155. https://doi.org/10.1186/s12933-020-01121-5.
Ma X, Dong L, Shao Q, Cheng Y, Lv S, Sun Y, et al. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 Diabetes Mellitus and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):31. https://doi.org/10.1186/s12933-020-01006-7.
Luo E, Wang D, Yan G, Qiao Y, Liu B, Hou J, et al. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation Myocardial Infarction after percutaneous coronary intervention. Cardiovasc Diabetol. 2019;18(1):150. https://doi.org/10.1186/s12933-019-0957-3.
Degenhardt F, Seifert S, Szymczak S. Evaluation of variable selection methods for random forests and omics data sets. Brief Bioinform. 2019;20(2):492–503. https://doi.org/10.1093/bib/bbx124.
Robinson MR, Scheuermann-Freestone M, Leeson P, Channon KM, Clarke K, Neubauer S, et al. Uncomplicated obesity is associated with abnormal aortic function assessed by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10(1):10. https://doi.org/10.1186/1532-429X-10-10.
Vlachopoulos C, Dima I, Aznaouridis K, et al. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112(14):2193–200. https://doi.org/10.1161/CIRCULATIONAHA.105.535435.
Perticone M, Maio R, Gigliotti S, Shehaj E, Toscani AF, Capomolla A, et al. Mutual effect modification between insulin resistance and endothelial dysfunction in predicting incident Heart Failure in hypertensives. Biomedicines. 2023;11(8):2188. https://doi.org/10.3390/biomedicines11082188.
Jelenik T, Flögel U, Álvarez-Hernández E, Scheiber D, Zweck E, Ding Z, Rothe M, et al. Insulin resistance and vulnerability to cardiac ischemia. Diabetes. 2018;67(12):2695–702. https://doi.org/10.2337/db18-0449.
Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in Cardiovascular Disease development. Nat Rev Endocrinol. 2014;10(5):293–302. https://doi.org/10.1038/nrendo.2014.29.
Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–E26. https://doi.org/10.1152/ajpendo.00645.2007.
Unger G, Benozzi SF, Perruzza F, Pennacchiotti GL. Triglycerides and glucose index: a useful indicator of insulin resistance. Endocrinol Nutr. 2014;61(10):533–40. https://doi.org/10.1016/j.endonu.2014.06.009.
Mohd Nor NS, Lee S, Bacha F, Tfayli H, Arslanian S. Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 Diabetes Mellitus: comparison with the hyperinsulinemic-euglycemic clamp. Pediatr Diabetes. 2016;17(6):458–65. https://doi.org/10.1111/pedi.12303.
Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, et al. Relationship between the triglyceride-glucose index and risk of Cardiovascular Diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1):124. https://doi.org/10.1186/s12933-022-01546-0.
Zheng R, Mao Y. Triglyceride and glucose (TyG) index as a predictor of incident Hypertension: a 9-year longitudinal population-based study. Lipids Health Dis. 2017;16(1):175. https://doi.org/10.1186/s12944-017-0562-y.
Wu Z, Cheng C, Sun X, Wang J, Guo D, Chen S, et al. The synergistic effect of the triglyceride-glucose index and serum uric acid on the prediction of major adverse cardiovascular events after coronary artery bypass grafting: a multicenter retrospective cohort study. Cardiovasc Diabetol. 2023;22(1):103. https://doi.org/10.1186/s12933-023-01838-z.
Thai PV, Tien HA, Van Minh H, Valensi P. Triglyceride glucose index for the detection of asymptomatic coronary artery stenosis in patients with type 2 Diabetes. Cardiovasc Diabetol. 2020;19(1):137. https://doi.org/10.1186/s12933-020-01108-2.
Huang R, Xu X, Xu C, Zhang S, Xiong Z, Liu M, et al. Association between the insulin resistance and all-cause mortality in patients with moderate and severe aortic stenosis: a retrospective cohort study. Cardiovasc Diabetol. 2023;22(1):238. https://doi.org/10.1186/s12933-023-01975-5.
Irace I, Carallo C, Scavelli C, De Franceschi FB, Esposito MS, Tripolino T. Markers of insulin resistance and carotid Atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract. 2013;67(7):665–72. https://doi.org/10.1111/ijcp.12124.
Vasques AC, Novaes FS, de Oliveira Mda S, Souza JR, Yamanaka A, Pareja JC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–e100. https://doi.org/10.1016/j.diabres.2011.05.030.
Yu X, Wang L, Zhang W, Ming J, Jia A, Xu S, et al. Fasting triglycerides and glucose index is more suitable for the identification of metabolically unhealthy individuals in the Chinese adult population: a nationwide study. J Diabetes Investig. 2019;10(4):1050–8. https://doi.org/10.1111/jdi.12975.
Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–97. https://doi.org/10.1111/eci.12583.
Li S, Guo B, Chen H, Shi Z, Li Y, Tian Q, et al. The role of the triglyceride (triacylglycerol) glucose index in the development of cardiovascular events: a retrospective cohort analysis. Sci Rep. 2019;9(1):7320. https://doi.org/10.1038/s41598-019-43776-5.
Jafari A, Najafipour H, Shadkam M, Aminizadeh S. Evaluation of the novel three lipid indices for predicting five- and ten-year incidence of Cardiovascular Disease: findings from Kerman coronary artery Disease risk factors study (KERCADRS). Lipids Health Dis. 2023;22(1):169. https://doi.org/10.1186/s12944-023-01932-x.
Lertsakulbunlue S, Mungthin M, Rangsin R, Kantiwong A, Sakboonyarat B. Trends in baseline triglyceride-glucose index and association with predicted 10-year Cardiovascular Disease risk among type 2 Diabetes patients in Thailand. Sci Rep. 2023;13(1):12960. https://doi.org/10.1038/s41598-023-40299-y.
Liu L, Wu Z, Zhuang Y, Zhang Y, Cui H, Lu F, et al. Association of triglyceride-glucose index and traditional risk factors with Cardiovascular Disease among non-diabetic population: a 10-year prospective cohort study. Cardiovasc Diabetol. 2022;21(1):256. https://doi.org/10.1186/s12933-022-01694-3.
Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, et al. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17(1):41. https://doi.org/10.1186/s12933-018-0692-1.
Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol. 2017;16(1):108. https://doi.org/10.1186/s12933-017-0589-4.
Hu C, Zhang J, Liu J, Liu Y, Gao A, Zhu Y, et al. Discordance between the triglyceride glucose index and fasting plasma glucose or HbA1C in patients with acute coronary syndrome undergoing percutaneous coronary intervention predicts cardiovascular events: a cohort study from China. Cardiovasc Diabetol. 2020;19(1):116. https://doi.org/10.1186/s12933-020-01091-8.
Shao QY, Ma XT, Yang ZQ, Li QX, Wang YF, Liang J, et al. Prognostic significance of multiple triglycerides-derived metabolic indices in patients with acute coronary syndrome. J Geriatr Cardiol. 2022;19(6):456–68. https://doi.org/10.11909/j.issn.1671-5411.2022.06.001.
Wan GX, Xia WB, Ji LH, Qin HL, Zhang YG. Triglyceride to high density lipoprotein cholesterol ratio may serve as a useful predictor of major adverse coronary event in female revascularized ST-elevation Myocardial Infarction. Clin Chim Acta. 2018;485:166–72. https://doi.org/10.1016/j.cca.2018.06.049.
Askin L, Tanrıverdi O. Is the atherogenic index of plasma (AIP) a Cardiovascular Disease marker? Cor et Vasa. 2023;65(1):100–3. https://doi.org/10.33678/cor.2022.085.
Liu Q, Cui H, Ma Y, Han X, Cao Z, Wu Y. Triglyceride-glucose index associated with the risk of Cardiovascular Disease: the Kailuan study. Endocrine. 2022;75(2):392–9. https://doi.org/10.1007/s12020-021-02862-3.
Palmerini T, Della Riva D, Biondi-Zoccai G, et al. Mortality following nonemergent, uncomplicated target lesion revascularization after percutaneous coronary intervention: an individual patient data pooled analysis of 21 randomized trials and 32,524 patients. JACC Cardiovasc Interv. 2018;11(9):892–902. https://doi.org/10.1016/j.jcin.2018.01.277.
Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Impacts of triglyceride-glucose index on prognosis of patients with type 2 Diabetes Mellitus and non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Cardiovasc Diabetol. 2020;19(1):108. https://doi.org/10.1186/s12933-020-01086-5.
Mao Q, Zhou D, Li Y, Wang Y, Xu SC, Zhao XH. The triglyceride-glucose index predicts coronary artery Disease severity and cardiovascular outcomes in patients with non-ST-segment elevation acute coronary syndrome. Dis Markers. 2019;2019:6891537. https://doi.org/10.1155/2019/6891537.
Moussa ID, Mohananey D, Saucedo J, Stone GW, Yeh RW, Kennedy KF, et al. Trends and outcomes of restenosis after coronary stent implantation in the United States. J Am Coll Cardiol. 2020;76(13):1521–31. https://doi.org/10.1016/j.jacc.2020.08.002.
Assali AR, Moustapha A, Sdringola S, Denktas AE, Willerson JT, Holmes DR Jr, et al. Acute coronary syndrome may occur with in-stent restenosis and is associated with adverse outcomes (the PRESTO trial). Am J Cardiol. 2006;98(6):729–33. https://doi.org/10.1016/j.amjcard.2006.04.007.
Zhu Y, Liu K, Chen M, Liu Y, Gao A, Hu C, et al. Triglyceride-glucose index is associated with in-stent restenosis in patients with acute coronary syndrome after percutaneous coronary intervention with drug-eluting stents. Cardiovasc Diabetol. 2021;20(1):137. https://doi.org/10.1186/s12933-021-01332-4.
Özbiçer S, Yüksel G, Deniz Urgun Ö. Triglyceride glucose index is independently associated with aortic intima-media thickness in patients without known atherosclerotic Cardiovascular Disease or Diabetes. Diab Vasc Dis Res. 2022;19(5):14791641221136203. https://doi.org/10.1177/14791641221136203.
Cetin Sanlialp S, Sanlialp M, Nar G, Malcok A. Triglyceride glucose index reflects the unfavorable changes of left ventricular diastolic functions and structure in uncomplicated newly diagnosed hypertensive patients. Clin Exp Hypertens. 2022;44(3):215–22. https://doi.org/10.1080/10641963.2021.2018599.
Wang C, Zhao Z, Deng X, Cai Z, Gu T, Li L, et al. Association of triglyceride-glucose with cardiac hemodynamics in type 2 Diabetes. Diab Vasc Dis Res. 2022;19(1):14791641221083396. https://doi.org/10.1177/14791641221083396.
Gao JW, Hao QY, Gao M, Zhang K, Li XZ, Wang JF, et al. Triglyceride-glucose index in the development of peripheral artery Disease: findings from the Atherosclerosis risk in communities (ARIC) Study. Cardiovasc Diabetol. 2021;20(1):126. https://doi.org/10.1186/s12933-021-01319-1.
Acknowledgements
We would like to gratefully acknowledge all of the investigators and patients participating in this work.
Funding
This work was supported by the Central High-level Hospital of Traditional Chinese Medicine Clinical Research and Achievement Transformation Capacity Improvement Project (No. HLCMHPP2023065), the National Key Research and Development Program of China (No. 2022YFC3500102), the National Natural Science Foundation of China (No. 81973836) and the Fundamental Research Funds for the Central Public Welfare Research Institutes (No. ZZ15-XY-CT-08).
Author information
Authors and Affiliations
Contributions
JL and ST contributed to the study concept and design. ST, LY, ZX, and LH performed the data analyses and wrote the manuscript. ST, DY, YT, and WZ were responsible for the case collection and database establishment. ST, LY, JL, XH, and TX contributed to interpreted the results. All authors made critical revision of the manuscript for important intellectual content and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki. We analyzed a dataset which has been collected for the previous studies and information related to patient identity was concealed. Thus, ethics approval and consent for participation is not applicable for this study.
Consent for publication
All authors have consent for publication.
Competing interests
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tao, S., Yu, L., Li, J. et al. Prognostic value of triglyceride-glucose index in patients with chronic coronary syndrome undergoing percutaneous coronary intervention. Cardiovasc Diabetol 22, 322 (2023). https://doi.org/10.1186/s12933-023-02060-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-02060-7