Abstract
Background
Type 2 diabetes and non-alcoholic fatty liver disease (NAFLD) commonly coexist. However, NAFLD’s effect on mortality in Asian patients with type 2 diabetes awaits full elucidation. Therefore, we examined NAFLD-related all-cause and cause-specific mortality in a nationwide Asian population with type 2 diabetes.
Methods
We included patients who had undergone general health checkups between 2009 and 2012 using the National Health Insurance Service database linked to death-certificate data. Hepatic steatosis was defined as a fatty liver index (FLI) ≥ 60, and advanced hepatic fibrosis was determined using the BARD score.
Findings
During the follow-up period of 8.1 years, 222,242 deaths occurred, with a mortality rate of 14.3/1000 person-years. An FLI ≥ 60 was significantly associated with increased risks of all-cause and cause-specific mortality including cardiovascular disease (CVD)-, cancer-, and liver disease (FLI ≥ 60: hazard ratio [HR] = 1.02, 95% confidence interval [CI] 1.01–1.03 for all-cause; 1.07, 1.04–1.10 for CVD; 1.12, 1.09–1.14 for cancer; and 2.63, 2.50–2.77 for liver disease). Those with an FLI ≥ 60 and fibrosis (BARD ≥ 2) exhibited increased risks of all-cause (HR, 95% CI 1.11, 1.10–1.12), CVD- (HR, 95% CI 1.11, 1.09–1.14), cancer- (HR, 95% CI 1.17, 1.15–1.19), and liver disease-related (HR, 95% CI 2.38, 2.29–2.49) mortality.
Conclusion
Hepatic steatosis and advanced fibrosis were significantly associated with risks of overall and cause-specific mortality in patients with type 2 diabetes. Our results provide evidence that determining the presence of hepatic steatosis and/or fibrosis potentially plays a role in risk stratification of mortality outcomes in patients with type 2 diabetes mellitus.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus is prevalent worldwide, and health-related burden has increased over the last few decades [1]. As insulin resistance and obesity are common pathogenic factors for type 2 diabetes mellitus and non-alcoholic fatty liver disease (NAFLD), these two diseases commonly coexist, exhibiting a strong relationship [2, 3]. The prevalence of NAFLD in patients with type 2 diabetes mellitus is estimated to be up to 75%, which is more than twice that in the general population [4, 5]. NAFLD and type 2 diabetes mellitus not only coexist but may act synergistically to induce related adverse outcomes due to their shared metabolic risk factors [6,7,8]. Metabolic syndrome, NAFLD, and imaging biomarkers predicted long-term risk of cardiac events [9]. Subjects with genetic NAFLD and without metabolic disturbances do not have increased cardiovascular risk, whereas those with metabolic disease such as diabetes, have high cardiometabolic risks [10].
The presence of type 2 diabetes mellitus potentially accelerates the risk of advanced hepatic fibrosis [11]. However, the association of NAFLD and fibrosis with the risk of clinical outcomes in specific causes of death among Asian patients with type 2 diabetes mellitus remains unclear. Diabetes in Asian is characterized by early β-cell dysfunction and develops at a younger age, requiring early insulin treatment and posing a higher risk of cardiovascular complications than that in Westerners [12]. Therefore, elucidating the effects of NAFLD and fibrosis on mortality-related outcomes in Asian patients with type 2 diabetes mellitus is of paramount importance.
Although there is controversy regarding routine screening for NAFLD in patients with type 2 diabetes mellitus [13], a previous study reported the cost-effectiveness of NAFLD screening in patients with type 2 diabetes mellitus using ultrasonography plus liver enzymes followed by transient elastography [14]. NAFLD assessed by computed tomography could be an useful tool for identifying type 2 diabetes mellitus patients at higher risk of cardiovascular events [15]. However, imaging modalities have limitations due to technological difficulties, a relatively high cost, and unsuitability for population-based mass screening. Thus, noninvasive biomarkers have been used to predict hepatic steatosis and fibrosis in large populations [16]. The fatty liver index (FLI) and BARD score are easily applicable, as each individual component is a common measurement in clinical practice with acceptable performance in the general population [17,18,19] and in patients with type 2 diabetes mellitus [20, 21]. In this study, we aimed to investigate the association of the FLI and BARD score with all-cause and cause-specific mortality in patients with type 2 diabetes mellitus based on a population-based, nationwide Korean cohort.
Methods
Data source and study setting
This retrospective population-based study was based on the National Health Insurance Service (NHIS) in Korea. Approximately 97% of the Korean population are subscribers to the NHIS and the remaining 3% are receiving medical aid program. The NHIS database contains information about claims submitted by the health care providers for reimbursement, including demographics, medical treatments and procedures, and disease diagnoses based on the International Classification of Diseases, 10th revision (ICD-10). The National Health Screening Program (NHSP) is offered to all insured persons every two years. The NHSP includes self-reported questionnaires for lifestyle behaviors, anthropometric data, and laboratory tests [22].
Study population
Patients with type 2 diabetes mellitus were defined as follows i) at least one claim per year for the prescription of an antidiabetic medication under ICD-10 codes E11–14 in the insurance claims data or ii) fasting plasma glucose level ≥ 126 mg/dL without insulin and/or a prescription of at least one oral hypoglycemic agent (OHA) [23]. OHAs include sulfonylurea, metformin, meglitinide, a dipeptidyl peptidase-4 inhibitor, an α-glucosidase inhibitor and thiazolidinedione [24].
Among a total of 2,745,689 patients with type 2 diabetes mellitus (age 20 years and older) who participated in health screening between 2009 and 2012, people who met the following criteria were excluded from the study: previous diagnosis of liver cirrhosis (K74, n = 19,645), hepatitis (B15–B19, n = 417,798) before the index year, heavy alcohol consumption (≥ 30 g for men and ≥ 20 g for women of alcohol/day, n = 188,879), or missing information (n = 93,978). Since we confirmed the outcome events after a delay of one year, those with outcome events within one year were excluded (n = 17,614). Finally, 2,007,775 patients with type 2 diabetes mellitus were included in this study. They were followed up to 31 December, one year after the NHSP day, or the date of death, whichever occurred first.
This study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and approved by the Institutional Review Board of Soongsil University approved this study (SSU-202007-HR-236-01). The requirement for written informed consent was waived because anonymized and de-identified data were used.
Calculation of the FLI and determination of advanced hepatic fibrosis
We used the FLI to predict fatty liver based on the following components: triglyceride (TG), body mass index (BMI), gamma-glutamyl transferase (GGT) and waist circumference (WC).
The FLI was calculated using the following formula:
A previous study suggested that an FLI score < 30 rules out fatty liver, while that ≥ 60 corresponds to fatty liver with favorable accuracy of diagnosis [25]. In this study, the participants were categorized into three FLI-based groups (< 30 [reference], 30–59, and ≥ 60).
Among the patients with type 2 diabetes mellitus with an FLI ≥ 60, advanced hepatic fibrosis was determined using the BARD score, which is derived from summating the following points: aspartate aminotransferase (AST)/ alanine transaminase (ALT) ratio ≥ 0.8 (two points), BMI ≥ 28 kg/m2 (one point), and type 2 diabetes mellitus (one point). A total score of 2 to 4 indicates advanced hepatic fibrosis [19].
Outcome
The NHIS database was linked to the death certificates from Statistics Korea regarding cause of death and date. Using Korean Standard Classification of Diseases, the cause of death was identified based on ICD-10 codes and specific causes of death were classified as cardiovascular disease (CVD, I00-I99), cancer (including hepatocellular carcinoma, C00-C97), respiratory disease (J00-J99), and liver disease (excluding hepatocellular carcinoma, K70-76) [26].
Covariates
During the health examination, self-reported, standardized questionnaires were administered to obtain data including alcohol consumption habits, smoking status, and physical activity. Alcohol consumption was classified as non- or mild-to-moderate drinker (< 30 g for men and < 20 g for women of alcohol per day). Smoking status was classified as nonsmoker, former smoker, or current smoker. Regular physical activity was defined as high-intensity exercise 3 or more times a week or moderate-intensity exercise 5 or more times a week. The lowest 20% income proportion was dichotomized into low-income status.
Comorbidities were defined according to the data from the NHSP and each ICD-10 code with a prescription history of related medication. For example, hypertension was defined using ICD-10 codes (I10–13 and I15) with antihypertensive medications, a systolic blood pressure ≥ 140 mmHg, or a diastolic blood pressure ≥ 90 mmHg. Dyslipidemia was defined by ICD-10 code (E78), lipid-lowering medications, or a total cholesterol level greater than 240 mg/dL. The Charlson comorbidity index (CCI) was determined using ICD-10 codes [27]. The severity of type 2 diabetes mellitus was assessed based on the presence of type 2 diabetes complications (retinopathy, end-stage renal disease, stroke, and ischemic heart disease), duration of type 2 diabetes mellitus (new-onset, < 5 years, and ≥ 5 years), number of OHAs administered, or amount of insulin used [28].
On the day of the health examination, anthropometric measurements, including height, weight and WC were measured, and BMI was calculated as follows: weight (kg) divided by square of the height (m2). Laboratory tests were performed to assess the serum levels of fasting glucose, total cholesterol, TGs, high density lipoprotein cholesterol, ALT, AST, and GGT. Estimated glomerular filtration rates (eGFR) were calculated from serum creatinine levels using the Modification of Diet in Renal Disease Study Group [29].
Statistical analysis
Continuous and categorical variables were expressed as mean ± standard deviation and numbers (%). Analysis of variance for continuous variables and chi-square tests for categorical variables were used for evaluating FLI-based differences. For skewed distributed continuous variables, geometric mean values with 95% confidence interval (CIs) were used.
We performed Cox proportional hazards analysis to evaluate the association between the FLI and/or BARD score and mortality and obtain hazard ratios (HRs). Multivariable adjusted models were adjusted for age and sex (model 1); lifestyle habits (smoking status, alcohol consumption, and physical activity), income level, comorbidities (hypertension and dyslipidemia), ALT, CCI score, presence of type 2 diabetes complications, and diabetes duration in addition to age and sex (model 2); Furthermore, stratification analysis was performed according to sex, age (< 40, 40–64, and ≥ 65 years), and BMI in kg/m2 (< 18.5 [underweight], 18.5–23 [normal], 23–25 [overweight], and ≥ 25 [obesity]). Statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC, USA). Two-tailed p-values < 0.05 were considered statistically significant.
Results
Baseline characteristics
Table 1 shows the baseline characteristics of the study population according to FLI score: 40.5%, 33.8%, and 25.7% of the participants were in the < 30, 30–59, ≥ 60 FLI groups, respectively.
The mean age in the FLI ≥ 60 group was lower than that in the other groups (54.2 vs. 58.8 and 59.2 years). The FLI ≥ 60 group predominantly comprised men (72.2%), whereas only 45.9% of the FLI < 30 group were men. People with an FLI ≥ 60 were more likely to be former or current smokers and alcohol consumers than other groups of people. Hypertension and dyslipidemia were more prevalent in the FLI ≥ 60 group. The FLI < 30 group had a higher proportion of patients with a diabetes duration ≥ 5 years, number of OHAs ≥ 2, and insulin use than the other groups. Higher BMI and WC values; systolic/diastolic blood pressure; serum levels of fasting glucose, total cholesterol, TG, ALT, AST and GGT were found in the FLI ≥ 60 group (P < 0.001).
Association between fatty liver index and all-cause/cause-specific mortality
In the total population, 222,242 deaths occurred over a median follow-up period of 8.1 years with a mortality rate of 14.3 per 1000 person-years.
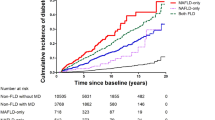
Table 2 shows the different HR trends by FLI category for all-cause and cause-specific mortality. In the multivariate analysis, patients with an FLI ≥ 60 exhibited slightly high risk of all-cause mortality (FLI ≥ 60: HR, 95% CI = 1.02, 1.01–1.03 and FLI 30–59: 0.88, 0.87–0.89) compared to those in the reference group (FLI < 30). The risks of CVD- and cancer-related mortality increased in patients with an FLI ≥ 60 (HR, 95% CI = 1.07, 1.04–1.10, and 1.12, 1.09–1.14, respectively). The risks of liver disease-related mortality linearly increased with higher FLI scores (FLI ≥ 60: HR, 95% CI = 2.63, 2.50–2.77 and FLI 30–59: 1.46, 1.39–1.53, Fig. 1). When we included kidney function as a covariate, consistent results were found Additional file 1: Table S1). The associations of an FLI ≥ 60 with cause-specific mortality were similarly observed in both male and females while the positive association between FLI and all-cause mortality maintained only in female Additional file 1: Table S2).
Association between the BARD score and all-cause/cause-specific mortality
Among 516,035 patients with type 2 diabetes mellitus with FLI scores ≥ 60, 404,610 (78.4%) had advanced fibrosis (BARD score ≥ 2). Compared with those with FLI scores < 60, patients with an FLI ≥ 60 and fibrosis (BARD score ≥ 2) exhibited increasing risks of all-cause (HR, 95% CI = 1.11,1.10–1.12), CVD- (HR, 95% CI = 1.11, 1.09–1.14), cancer- (HR, 95% CI = 1.17, 1.15–1.19), and liver disease-related mortality (HR, 95% CI = 2.38, 2.29–2.49), while decreased respiratory disease- related mortality (HR, 95% CI = 0.95, 0.91–0.99) in the multivariate model (Table 3, Fig. 2).
Next, we considered AST/ALT ratio (AAR) alone as surrogate biomarker of fibrosis because all individuals had diabetes (all had 1 point in BARD score). Consistently, patients with an FLI ≥ 60 and fibrosis (AAR ≥ 0.8) showed increasing risks of all-cause, CVD-, cancer-, and liver disease-related mortality compared with those with FLI < 60, Additional file 1: Table S3).
Stratification analysis by age and BMI
We conducted subgroup analyses stratified by age and BMI to confirm different subgroup associations. On performing stratified analysis by age group, a stronger relationship was noted in middle-aged groups (40–64 years) than in other age groups (FLI ≥ 60: HR, 95% CI = 1.05, 0.94–1.19 for ages < 40; 1.12, 1.10–1.15 for ages 40–64; and 0.98, 0.91–0.99 for ages ≥ 65) (P for interaction < 0.001). We stratified the participants according to BMI categories (< 18.5, 18.5–23, 23–25, and ≥ 25), and there was an increased risk of all-cause mortality with FLI score in all BMI groups. A greater association was observed in those with underweight group (BMI < 18.5) than in the other BMI groups (FLI ≥ 60: HR, 95% CI = 2.19, 1.83–2.63 for BMI < 18.5; 1.97, 1.90–2.04 for BMI of 18.5–23; 1.61, 1.57–1.66 for BMI of 23–25; and 1.38, 1.35–1.41 for BMI ≥ 25) (P for interaction < 0.001) Additional file 1.
Discussion
This is, to the best of our knowledge, the first study to investigate the association of all-cause and cause-specific mortality with FLI scores in patients with type 2 diabetes mellitus. All-cause, CVD-, cancer-, and liver disease-related mortality increased with an FLI ≥ 60 in patients with type 2 diabetes mellitus. Moreover, advanced hepatic fibrosis assessed using the BARD score was significantly associated with an increased risk of mortality in patients with type 2 diabetes mellitus. Our findings suggest that determining the presence of hepatic steatosis and/or fibrosis potentially plays a role in risk stratification of mortality outcomes in patients with type 2 diabetes mellitus.
Association of fatty liver index with mortality
Consistent with our results, previous studies have demonstrated a significant association between all-cause mortality and FLI, and it has not been limited to people with type 2 diabetes mellitus [30, 31]. Regarding cause-specific mortality, including that related to CVD, cancer, and liver diseases, similar trends have been observed in previous studies based on the general population [32]. [26] However, the presence of NAFLD in patients with type 2 diabetes mellitus aggravates the complications of diabetes, rendering it difficult to achieve proper glycemic goals [33, 34]. The coexistence of NAFLD and type 2 diabetes mellitus potentially amplifies the risk of mortality. Although limited evidence exists for the association between NAFLD and mortality or cause-of-death in people with type 2 diabetes mellitus, a previous study reported that NAFLD was associated with increased risks of CVD (HR [95% CI]: 1.70 [1.52–1.90]), hepatocellular carcinoma (HCC) (19.12 [11.71–31.2]), non-HCC cancer (1.10 [0.94–1.29]), and all-cause (1.60 [1.40–1.83]) mortality among people with type 2 diabetes mellitus [8]. In this study, the HR of liver disease-related mortality was the greatest (HR = 2.6) in the FLI ≥ 60 group among cause-specific mortalities. The conflicting results from the two studies may be related to the heterogeneous criteria used to categorize causes of death and diagnostic criteria for NAFLD. There was an inverse association with high FLI and respiratory disease-mortality in this study. Although there are few studies regarding NAFLD and respiratory mortality, Lin et al. reported an inverse relationship between NAFLD and respiratory disease-related mortality, consistent with our findings [35].
Association of hepatic fibrosis with mortality
In this study, advanced hepatic fibrosis was associated with all-cause, CVD-, cancer-, and liver disease-related mortality. Regarding the increased risk of CVD-related mortality, a recent study consistently revealed that advanced hepatic fibrosis was significantly associated with the risks of CVD events and mortality [36]. Advanced liver fibrosis, measured by hepatic transient elastography, was a risk marker while severe steatosis was a protective factor for cardiovascular complications and mortality in individuals with type 2 diabetes and NAFLD [37]. Collectively, these results suggest that advanced hepatic fibrosis is potentially useful as a screening tool for predicting both hepatic and extrahepatic adverse outcomes in patients with type 2 diabetes mellitus. Accordingly, the appropriate assessment of fibrosis stage is recommended in patients with type 2 diabetes mellitus and NAFLD [13, 38].
Stratification analysis
On performing a stratification analysis by age, the increased risk of all-cause mortality in the high-FLI group was highest among middle aged patients (40–64 years). We further stratified the participants by BMI, and there was an increased risk of all-cause mortality with high FLI in all BMI groups. In the high FLI group, the increased risk of all-cause death was greatest in the underweight group. These findings indicate that the prognosis of lean NAFLD may be worse in patients with type 2 diabetes mellitus, thus exhibiting consistency with the findings of previous studies that were not limited to people with type 2 diabetes mellitus [39]. Thus, providing intensive NAFLD management may be helpful, especially to middle-aged and lean patients with type 2 diabetes mellitus and NAFLD.
Liver disease-related mortality
Among the risks of cause-specific mortality in this study, the HR of liver disease-related mortality was the greatest in patients with high FLI scores and/or advanced fibrosis. This finding may be related to the association between hepatic lipid accumulation and an increased risk of type 2 diabetes mellitus as well as adipose tissue and insulin resistance [40]. Recently, the association between obesity and the risk of type 2 diabetes mellitus has been reported to be mediated by the presence of NAFLD [41]. This association may be bidirectional, and the presence of type 2 diabetes mellitus in patients with excessive fatty liver infiltration potentially contributes to an increased risk of all-cause and liver-related mortality [42].
Clinical implication and limitations
Emerging evidence supports that some antidiabetic agents may improve NAFLD or hepatic fibrosis when added to lifestyle changes in patients with type 2 diabetes mellitus [38]. Thiazolidinedione treatment has been reported to improve the histologic features of hepatic steatosis, inflammation, and ballooning and reduce hepatic fibrosis progression in patients with prediabetes or type 2 diabetes mellitus [43]. Treatment with sodium-glucose cotransporter 2 inhibitors reportedly leads to reduced liver fat content [44] and has been associated with a lower risk of major hepatic events in type 2 diabetes mellitus [45]. Thus, proactive pharmacologic treatment has been recommended in patients with diabetes and concomitant advanced liver disease or in those at high risk of liver disease [46].
In this study, all-cause and cause-specific mortality increased in patients with high FLI and BARD scores, suggesting the prognostic potential of these serum markers in the risk stratification of mortality-related outcomes in patients with type 2 diabetes mellitus. In the absence of data on effective primary screening tools for NAFLD in type 2 diabetes mellitus, identifying high-risk groups and providing interventions, including lifestyle changes and medication, may be helpful.
Notwithstanding, this study has several limitations. First, imaging studies or pathology are mandatory for the diagnosis of hepatic steatosis; however, these methods are expensive and generally not feasible for screening fatty liver in a large population-based cohort. Although FLI cannot distinguish simple steatosis from steatohepatitis and fibrosis, FLI was used to define NAFLD in several previous large-population studies using claims data [47, 48]. Nevertheless, the biomarkers for detecting hepatic steatosis are not as accurate in patients with diabetes as they are in the general population since these markers were not developed for the population with diabetes or only included a minority of patients with type 2 diabetes mellitus. In addition, some of these biomarkers rely on the diabetic status and impaired blood glucose levels, thus not allowing for the accurate estimation of the prevalence of NAFLD [49]. In addition, the BARD score, which was used as a surrogate marker of advanced fibrosis in this study, is less specific than other biomarkers, such as the NAFLD fibrosis or fibrosis-4 scores. Because the Korean NHIS database does not include information regarding platelet count or albumin level, we could not assess other liver-fibrosis prediction scores. Second, since this was an observational study, unmeasured variables, such as insulin resistance, family history of type 2 diabetes mellitus, or hemoglobin A1C levels, could have influenced the results; thus, we could not ascertain the causality of the associations. However, we attempted to thoroughly adjust for possible confounding factors. Third, since mortality data were obtained through linkage to the NHIS, deaths that occurred outside Korea were not captured, and the possibility of a potential misclassification of some causes of death cannot be excluded. Fourth, since antidiabetic therapy employing sodium glucose co transporter 2 inhibitors was initiated in 2013, the conclusions of the study could be outdated. Finally, the study population consisted of Korean subjects; therefore, the results of this study cannot be generalized to other ethnic groups. More research is needed to validate our results and elucidate the mechanisms underlying our findings.
In conclusion, hepatic steatosis and/or advanced fibrosis, as assessed using the FLI and BARD scores, were significantly associated with the risks of overall and cause-specific mortality in patients with type 2 diabetes mellitus.
Availability of data and materials
We used the claim data provided by the Korean National Health Insurance Service (NHIS) database. Data can only be accessed by visiting the NHIS datacenter, after approval from data access committee of NHIS (https://nhiss.nhis.or.kr/bd/ab/bdaba001cv.do).
References
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98.
Kosmalski M, Ziółkowska S, Czarny P, Szemraj J, Pietras T. The coexistence of nonalcoholic fatty liver disease and type 2 Diabetes mellitus. J Clin Med. 2022;11(5):1375.
Lomonaco R, Bril F, Portillo-Sanchez P, Ortiz-Lopez C, Orsak B, Biernacki D, Lo M, Suman A, Weber MH, Cusi K. Metabolic Impact of nonalcoholic steatohepatitis in obese patients with type 2 diabetes. Diabetes Care. 2016;39(4):632–8.
Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801.
Shabbirhussain BV, Singh S, Dixit VK, Verma A, Singh SK. Carotid intima media as predictor of liver fibrosis in type 2 diabetes mellitus with NAFLD. Diabetes Metab Syndr. 2022;16(7): 102560.
Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, Arcaro G. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30(8):2119–21.
Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18(9):599–612.
Wild SH, Walker JJ, Morling JR, McAllister DA, Colhoun HM, Farran B, McGurnaghan S, McCrimmon R, Read SH, Sattar N, et al. Cardiovascular disease, cancer, and mortality among people with type 2 diabetes and alcoholic or nonalcoholic fatty liver disease hospital admission. Diabetes Care. 2018;41(2):341–7.
Lin A, Wong ND, Razipour A, McElhinney PA, Commandeur F, Cadet SJ, Gransar H, Chen X, Cantu S, Miller RJH, et al. Metabolic syndrome, fatty liver, and artificial intelligence-based epicardial adipose tissue measures predict long-term risk of cardiac events: a prospective study. Cardiovasc Diabetol. 2021;20(1):27.
Baratta F, D’Erasmo L, Bini S, Pastori D, Angelico F, Del Ben M, Arca M, Di Costanzo A. Heterogeneity of non-alcoholic fatty liver disease (NAFLD): Implication for cardiovascular risk stratification. Atherosclerosis. 2022;357:51–9.
Barb D, Repetto EM, Stokes ME, Shankar SS, Cusi K. Type 2 diabetes mellitus increases the risk of hepatic fibrosis in individuals with obesity and nonalcoholic fatty liver disease. Obesity. 2021;29(11):1950–60.
Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281(1):64–91.
American Diabetes Association Professional Practice, Draznin C, Aroda B, Bakris VR, Benson G, Brown G, Freeman FM, Green R, Huang JE, et al. 4 Comprehensive medical evaluation and assessment of comorbidities standards of medical care in diabetes. Diabetes Care. 2022;45:46–59.
Noureddin M, Jones C, Alkhouri N, Gomez EV, Dieterich DT, Rinella ME. Nashnet: screening for nonalcoholic fatty liver disease in persons with type 2 diabetes in the United states is cost-effective: a comprehensive cost-utility analysis. Gastroenterology. 2020;159(5):1985–7.
Ichikawa K, Miyoshi T, Osawa K, Miki T, Toda H, Ejiri K, Yoshida M, Nanba Y, Yoshida M, Nakamura K, et al. Prognostic value of non-alcoholic fatty liver disease for predicting cardiovascular events in patients with diabetes mellitus with suspected coronary artery disease: a prospective cohort study. Cardiovasc Diabetol. 2021;20(1):8.
Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68(2):305–15.
Kim JH, Kwon SY, Lee SW, Lee CH. Validation of fatty liver index and lipid accumulation product for predicting fatty liver in Korean population. Liver Int. 2011;31(10):1600–1.
Lee YH, Bang H, Park YM, Bae JC, Lee BW, Kang ES, Cha BS, Lee HC, Balkau B, Lee WY, et al. Non-laboratory-based self-assessment screening score for non-alcoholic fatty liver disease: development, validation and comparison with other scores. PLoS ONE. 2014;9(9): e107584.
Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57(10):1441–7.
Cuthbertson DJ, Weickert MO, Lythgoe D, Sprung VS, Dobson R, Shoajee-Moradie F, Umpleby M, Pfeiffer AF, Thomas EL, Bell JD, et al. External validation of the fatty liver index and lipid accumulation product indices, using 1H-magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin-resistant individuals. Eur J Endocrinol. 2014;171(5):561–9.
Ciardullo S, Muraca E, Perra S, Bianconi E, Zerbini F, Oltolini A, Cannistraci R, Parmeggiani P, Manzoni G, Gastaldelli A, et al. Screening for non-alcoholic fatty liver disease in type 2 diabetes using non-invasive scores and association with diabetic complications. BMJ Open Diabetes Res Care. 2020;8(1):00904.
Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, Do CH, Song JS, Hyon Bang J, Ha S, et al. Data resource profile: the national health information database of the national health insurance service in South Korea. Int J Epidemiol. 2017;46(3):799–800.
Lee HJ, Choi EK, Han KD, Kim DH, Lee E, Lee SR, Oh S, Lip GYH. High variability in bodyweight is associated with an increased risk of atrial fibrillation in patients with type 2 diabetes mellitus: a nationwide cohort study. Cardiovasc Diabetol. 2020;19(1):78.
Choi YJ, Han KD, Choi EK, Jung JH, Lee SR, Oh S, Lip GYH. Alcohol abstinence and the risk of atrial fibrillation in patients with Newly diagnosed type 2 diabetes mellitus: a nationwide population-based study. Diabetes Care. 2021;44(6):1393–401.
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33.
Chung GE, Jeong SM, Cho EJ, Yoo JJ, Cho Y, Lee KN, Shin DW, Kim YJ, Yoon JH, Han K, et al. Association of fatty liver index with all-cause and disease-specific mortality: A nationwide cohort study. Metabolism. 2022;133: 155222.
Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–82.
Hui Bae E, Yeob Lim S, Kim B, Ryom OhT, Hyun Song S, Heon Suh S, Sang Choi H, Mi Yang E, Seong Kim C, Kwon Ma S, et al. Effects of blood pressure according to age on end-stage renal disease development in patients with patients with diabetes: a nationwide population-based cohort study. Hypertension. 2022;79(8):1765–76.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Calori G, Lattuada G, Ragogna F, Garancini MP, Crosignani P, Villa M, Bosi E, Ruotolo G, Piemonti L, Perseghin G. Fatty liver index and mortality: the cremona study in the 15th year of follow-up. Hepatology. 2011;54(1):145–52.
Unalp-Arida A, Ruhl CE. Liver fat scores predict liver disease mortality in the United States population. Aliment Pharmacol Ther. 2018;48(9):1003–16.
Hwang YC, Ahn HY, Park SW, Park CY. Nonalcoholic fatty liver disease associates with increased overall mortality and death from cancer, cardiovascular disease, and liver disease in women but not men. Clin Gastroenterol Hepatol. 2018;16(7):1131–7.
Targher G, Bertolini L, Rodella S, Zoppini G, Lippi G, Day C, Muggeo M. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51(3):444–50.
Afolabi BI, Ibitoye BO, Ikem RT, Omisore AD, Idowu BM, Soyoye DO. The relationship between glycaemic control and non-alcoholic fatty liver disease in Nigerian type 2 diabetic patients. J Natl Med Assoc. 2018;110(3):256–64.
Lin Y, Gong X, Li X, Shao C, Wu T, Li M, Li F, Ma Q, Ye J, Zhong B. Distinct cause of death profiles of hospitalized non-alcoholic fatty liver disease: a 10 years’ cross-sectional multicenter study in China. Front Med (Lausanne). 2020;7: 584396.
Park J, Kim G, Kim BS, Han KD, Kwon SY, Park SH, Lee YB, Jin SM, Kim JH. The associations of hepatic steatosis and fibrosis using fatty liver index and BARD score with cardiovascular outcomes and mortality in patients with new-onset type 2 diabetes: a nationwide cohort study. Cardiovasc Diabetol. 2022;21(1):53.
Cardoso CRL, Villela-Nogueira CA, Leite NC, Salles GF. Prognostic impact of liver fibrosis and steatosis by transient elastography for cardiovascular and mortality outcomes in individuals with nonalcoholic fatty liver disease and type 2 diabetes: the Rio de Janeiro cohort study. Cardiovasc Diabetol. 2021;20(1):193.
Lee BW, Lee YH, Park CY, Rhee EJ, Lee WY, Kim NH, Choi KM, Park KG, Choi YK, Cha BS, et al. Non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: a position statement of the fatty liver research group of the Korean diabetes association. Diabetes Metab J. 2020;44(3):382–401.
Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, Yang H, Liu C, Kam LY, Tan XXE, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(8):739–52.
Sung KC, Jeong WS, Wild SH, Byrne CD. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care. 2012;35(4):717–22.
Rodriguez LA, Kanaya AM, Shiboski SC, Fernandez A, Herrington D, Ding J, Bradshaw PT. Does NAFLD mediate the relationship between obesity and type 2 diabetes risk? evidence from the multi-ethnic study of atherosclerosis (MESA). Ann Epidemiol. 2021;63:15–21.
Stepanova M, Rafiq N, Makhlouf H, Agrawal R, Kaur I, Younoszai Z, McCullough A, Goodman Z, Younossi ZM. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD). Dig Dis Sci. 2013;58(10):3017–23.
Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, et al. Long- 2 Diabetes Mellitus: A term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type randomized trial. Ann Intern Med. 2016;165(5):305–15.
Latva-Rasku A, Honka MJ, Kullberg J, Mononen N, Lehtimäki T, Saltevo J, Kirjavainen AK, Saunavaara V, Iozzo P, Johansson L, et al. The SGLT2 inhibitor dapagliflozin reduces liver fat but does not affect tissue insulin sensitivity: a randomized, double-blind, placebo-controlled study with 8 week treatment in type 2 diabetes patients. Diabetes Care. 2019;42(5):931–7.
Bea S, Jeong HE, Park S, Yu OHY, Chang Y, Cho J, Sinn DH, Cho YM, Shin JY. Hepatic events associated with sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes: a nationwide cohort study. Gut. 2022. https://doi.org/10.1136/gutjnl-2022-327504.
Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care. 2017;40(3):419–30.
Park JH, Han K, Hong JY, Park YS, Hur KY, Kang G, Park JO. Changes in metabolic syndrome status are associated with altered risk of pancreatic cancer: a nationwide cohort study. Gastroenterology. 2021;162(2):509–20.
Chung GE, Cho EJ, Yoon JW, Yoo JJ, Chang Y, Cho Y, Park SH, Han K, Shin DW, Yu SJ. Nonalcoholic fatty liver disease increases the risk of diabetes in young adults: a nationwide population-based study in Korea. Metabolism. 2021;123: 154866.
Alhinai A, Patel K, Fonseca VA, Sebastiani G. Non-invasive diagnosis of nonalcoholic fatty liver disease in patients with type 2 diabetes. J Diabetes Complications. 2021;35(9): 107978.
Acknowledgements
This study was performed using a database from the Korean National Health Insurance System (NHIS).
Funding
This work was supported by grants from the Seoul National University Hospital Research Fund (06-2020-4150) and from Liver Research Foundation of Korea as part of the Bio Future Strategies Research Project.
Author information
Authors and Affiliations
Contributions
The corresponding authors (KH and SJY) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: KH, Su JY. Provision of study materials or patients: EJC, SMJ, GEC, JWY, JJY, YC, DWS, YJK, JHY, SJY. Collection and assembly of data: KNL, KH. Data analysis and interpretation: KNL, EJC, JWY, SMJ, GEC, KH, SJY. Manuscript writing: GEC, SMJ. Final approval of manuscript: All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethics approval and consent to participate: This study was approved by the Institutional Review Board of Soongsil University (IRB No. SSU-202003-HR-201-01) and performed in accordance with the relevant guidelines and regulations. The requirement for written informed consent was waived because anonymized and de-identified data were used.
Consent for publication
Not applicable.
Competing interests
None declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1 Table S1.
All-cause and cause-specific mortality by fatty liver index with additional adjustment for kidney function. Table S2. Hazard ratios (95% confidence interval) for all-cause mortality and cause-specific mortality according to fatty liver index by sex. Table S3. All-cause and cause-specific mortality according to advanced fibrosis using aspartate aminotransferase/alanine transaminase ratio. Table S4. Stratified analysis: hazard ratios (95% confidence interval) for all-cause mortality according to fatty liver index by age groups and body mass index.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chung, G.E., Jeong, SM., Cho, E.J. et al. The association of fatty liver index and BARD score with all-cause and cause-specific mortality in patients with type 2 diabetes mellitus: a nationwide population-based study. Cardiovasc Diabetol 21, 273 (2022). https://doi.org/10.1186/s12933-022-01691-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01691-6