Abstract
Background
Triglyceride glucose (TyG) index is a new marker associated with atherosclerosis. This study aimed to assess the association between TyG index and the severity of coronary artery disease (CAD) in patients with coronary heart disease (CHD) and further explore the association between TyG index and CAD severity in different glucose metabolic states.
Methods
This multi-centre retrospective study included 731 patients with CHD between January 1, 2014 and September 30, 2020 in China. All patients were stratified into groups based on the tertiles of TyG index (T1: 5.48 ≤ TyG index ≤ 7.17; T2: 7.18 ≤ TyG index ≤ 7.76; T3: 7.77 ≤ TyG index ≤ 10.82). The number of diseased vessels [single-vessel and multi-vessel CAD (≥ 50% stenosis in ≥ 2 large vessels)] represented the severity of CAD, which was measured using coronary angiography (CAG). Glucose metabolic states were defined by the American Diabetes Association as normal glucose regulation (NGR), prediabetes mellitus (Pre-DM), and diabetes mellitus (DM).
Results
The baseline analysis results showed significant differences in the clinical and biological characteristics of CHD patients according to TyG index tertiles (P < 0.05 to < 0.001). Logistic regression analysis showed that the TyG index was significantly related to the risk of multi-vessel CAD (odds ratio [OR]: 1.715; 95% confidence interval [CI] 1.339–2.197; P < 0.001). The OR for multi-vessel CAD in TyG index T3 compared to that of T1 was 2.280 (95% CI 1.530–3.398; P < 0.001). Receiver operating characteristic (ROC) curve was generated to evaluate the accuracy of the TyG index in detecting the CAD severity, and the area under the curve (AUC) of the ROC plots was 0.601 (95% CI 0.559–0.643). The association between TyG index and multi-vessel CAD was significant in patients with DM, achieving the highest OR among the different glucose metabolic states (OR: 1.717; 95% CI 1.161–2.539; P < 0.05).
Conclusion
TyG index was associated with CAD severity in patients with CHD, and an increased TyG index could identify patients with a high risk of multi-vessel CAD. There was an association between TyG index and CAD severity for the condition of DM.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Coronary heart disease (CHD) is a chronic cardiovascular disease (CVD) caused by the hardening of the coronary arteries. The morbidity and mortality of CHD are on the rise due to aging, urbanization, obesity, and unhealthy lifestyle habits. Therefore, CHD is becoming a serious public health problem [1, 2]. Type 2 diabetes mellitus (T2DM) is widely considered a prevalent comorbidity that affects the progression of CHD and the treatment choices for these patients [3]. Patients with CHD with a combined abnormal glucose metabolic state have a higher risk of adverse cardiovascular events [4,5,6]. Specifically, T2DM increases the probability of developing CHD and the risk of death from CHD by at least two-fold [7, 8].
In patients with CHD, the severity of coronary artery disease (CAD) varies and is assessed by the patient’s clinical symptoms and ancillary investigations. Previous studies have found that 42.4% of individuals who experienced sudden cardiac deaths caused by CHD had no clinical symptoms; however, on autopsy, these individuals were found to have severe CAD and myocardial infarction [9, 10]. Coronary angiography (CAG) is a common and accurate imaging test known as the “gold standard” for diagnosing CAD in China. CAG may accurately detect the number and degree of arterial stenosis in patients. Based on CAG results, CHD is defined when there is lumen stenosis of ≥ 50% in at least one major coronary artery. The number of diseased vessels infers the severity of the CAD. However, many patients are unable to undergo CAG testing due to its high cost or invasive nature and, consequently, miss out on the right medical assessments. Therefore, it is necessary to develop a simple, valid, and widely applicable biomarker to detect the severity of CAD in patients with CHD.
Triglyceride glucose (TyG) index, a combination of fasting plasma glucose (FPG) and triglyceride (TG) calculations, is considered to be a representative biomarker of insulin resistance (IR) [11, 12]. Among the traditional risk factors, FPG and TG levels have been associated with an increased risk of T2DM [13, 14]. In addition, a large-scale Korean study indicated that the TyG index was associated with atherosclerotic CVD and has recently attracted considerable attention as a simple and low-cost biomarker of atherosclerosis [15]. TyG index is also associated with carotid arterial stenosis, and our previous study revealed that the relationship between TyG index and carotid artery plaques was significant in patients with CHD [16, 17]. Numerous studies have shown that an elevated TyG index is an independent risk factor for atherosclerosis [18,19,20]. Additionally, elevated TyG indexes play a potentially valuable role in the early recognition of individuals at high risk of CVD [21]. In the meantime, the severity of CAD plays a crucial role in the prognosis of CHD and the incidence of CVD. It is necessary to identify people at high risk of multi-vessel CAD among patients with CHD, especially those with abnormal glucose metabolic states are at an even higher risk. However, the ability of TyG index to detect CAD severity in patients with CHD has not been proven, and the influence of glucose metabolic states requires further study.
This study aimed to investigate the association between TyG index and CAD severity in patients with CHD and the effects of different glucose metabolic states on this association. Furthermore, this study determined the accuracy of the TyG index for detecting disease severity in patients with CHD.
Methods
Subjects
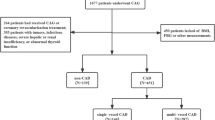
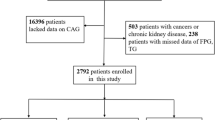
This large-scale multi-center retrospective study population comprised 107,301 CHD patients hospitalized in Tianjin between January 1, 2014, and September 30, 2020. The following patients were excluded: (1) those younger than 35 years or older than 75 years; (2) those with tumors, infectious or severe liver or kidney disease; and (3) those who lacked data on glycated haemoglobin (HbA1c), FPG, TG, and CAG. Ultimately, 731 subjects were enrolled in the final analysis. The inclusion process is illustrated in Fig. 1. Approval for the study was obtained from the Ethics Committee of Tianjin University of Traditional Chinese Medicine (TJUTCM-EC20190008) and registered with the China Clinical Trials Registry (ChiCTR-1900024535) and with Clinical Trials. gov (NCT04026724).
Data collection
The following data for the study analysis were acquired from medical records: clinical history, anthropometric data, blood analysis, and medical imaging data. Anthropometric data such as weight, height, body mass index (BMI), and blood pressure, and personal information such as age, sex, smoking history, and alcohol consumption history were recorded. The blood samples tested were fasting venous blood collected by specialist medical staff from all participants early in the morning. FPG, HbA1c, TG, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels were measured using an automatic hematology analyzer. CAG was performed using a percutaneous femoral arteriography technique with an angiography machine with sufficient right and left anterior oblique views to diagnose all coronary artery lesions.
Definitions
TyG index is derived arithmetically as follows: Ln[TG (mg/dL) × FPG (mg/dL)/2] [22]. BMI was calculated by dividing weight by square height and was expressed in kg/m2. CHD was defined as a lumen stenosis of ≥ 50% in at least one major coronary artery (left anterior descending, left circumflex, and right coronary arteries). The number of diseased vessels with ≥ 50% stenosis indicated the severity of CAD in patients with CHD. Patients with only one major coronary artery affected were considered to have single-vessel CAD, and those with two or more major coronary arteries affected were considered to have multi-vessel CAD. No other vascular lesions but stenosis of ≥ 50% in the left main coronary artery was regarded as two lesions. The participants were divided into the following two groups: single-vessel and multi-vessel CAD. Coronary stenoses (CS) was considered significant when one or more major branches of the coronary artery had ≥ 50% stenosis, moderate stenosis if 50–69%, and severe stenosis if ≥ 70%. According to the American Diabetes Association’s Standards for the Medical Treatment of Diabetes [23], diabetes mellitus (DM) is defined as FPG ≥ 7.0 mmol/L or HbA1c ≥ 6.5%, prediabetes mellitus (Pre-DM) is defined as 5.6 mmol/L ≤ FPG ≤ 6.9 mmol/L or 5.7% ≤ HbA1c ≤ 6.4%, and normal glucose regulation (NGR) is defined as FPG < 5.6 mmol/L or HbA1c < 5.7%.
Statistical analysis
Continuous data are presented as mean ± standard deviation (SD) or median and interquartile range (IQR). Categorical variables are expressed as percentages. Differences between the groups were calculated using the χ2 test for categorical variables and the t-test, Mann–Whitney test or Kruskal–Wallis test for continuous variables. Odds ratios (OR) and 95% confidence intervals (CI) were calculated using logistic regression analysis to test the association between TyG index and CAD severity in patients with CHD. The area under the curve (AUC) and 95% CI of the receiver operating characteristic (ROC) curve were calculated to determine the accuracy of the TyG index in detecting CAD severity in patients with CHD. A P-value of < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS 24.0 (IBM Corp, New York, NY, USA).
Results
Clinical and biological characteristics
Of the 731 participants involved in this study, the average age was 63 (IQR, 58–68) years, 58.7% were male, and 64.4% had multi-vessel CAD. The patients were divided into three groups based on TyG index tertiles: T1 (5.48 ≤ TyG index ≤ 7.17), T2 (7.18 ≤ TyG index ≤ 7.76) and T3 (7.77 ≤ TyG index ≤ 10.82) in Table 1. Compared to participants in the lowest TyG index tertile (T1), participants in the highest tertile (T3) were associated with higher BMI, TC, TG, LDL-C, FPG, and HbA1c but lower HDL-C (P < 0.001). Furthermore, patients in T3 were more likely to have abnormal glucose metabolism, more diseased vessels, and more severe degree of CS than those in T1 (P < 0.001). The effect of age, sex, systolic blood pressure (SBP), diastolic blood pressure (DBP), and history of smoking and drinking did not show significant differences between the groups (P > 0.05).
The patients' baseline characteristics are listed in Table 2. The multi-vessel CAD group comprised more participants who were male and had abnormal glucose metabolism (P < 0.001). TyG index, FPG, TG, HbA1c, and LDL-C were significantly positively associated with multi-vessel CAD, while HDL-C was negatively associated (P < 0.05).
Association between TyG index and CAD severity
Binary logistic regressions were used to assess the associations between CAD severity and various risk factors. Multi-vessel CAD was used as the dependent variable (single-vessel CAD was used as a reference). There was no multicollinearity between the independent variables. Male, TC, LDL-C, FPG, and HbA1c were significantly associated with multi-vessel CAD (P < 0.05) when compared to patients with single-vessel CAD (Table 3). TyG index was positively associated with multi-vessel CAD in patients with higher ORs (OR: 1.715, 95% CI 1.349–2.179; P < 0.001).
As shown in Table 4, logistic regression models were constructed to show that the TyG index was significantly related to CAD severity before and after multivariate adjustment (P < 0.001). When the TyG index was analyzed as a continuous variable, it was significantly associated with multi-vessel CAD (OR: 1.715; 95% CI 1.339–2.197). When the TyG index served as a classified variable, the risk of multi-vessel CAD for patients in T3 was 2.280 times greater than the risk for patients in T1 (95% CI 1.530–3.398, P < 0.001) after adjusting for confounding factors. An elevated TyG index was significantly associated with an increased risk of multi-vessel CAD.
The ROC curve for multi-vessel CAD and TyG index is shown in Fig. 2. The results show that a TyG index of > 8.0 indicated that the number of affected coronary arteries was ≥ 2. The AUC for TyG index was 0.601 (95% CI 0.559–0.643; P < 0.001).
Associations between TyG index and CAD severity in different glucose metabolism states
Binary logistic regressions were used to assess the impact of glucose metabolic states on the relationship between TyG index and CAD severity (Table 5). There was no statistically significant association between TyG index and the presence of multi-vessel CAD for NGR and Pre-DM (P > 0.05). However, there was a statistically significant association for DM, which had the highest observed OR value (OR: 1.717; 95% CI 1.161–2.539, P < 0.001). Taking T1 as a reference, T3 was significantly associated with a 2.105 times increased risk of multi-vessel CAD for patients with CHD and DM (OR: 2.105; 95% CI 1.069–4.144, P < 0.05).
Discussion
This is the first study to reveal a significant association between TyG index and CAD severity in patients with CHD and assess this association according to different states of glucose metabolism. In the present study, the TyG index was significantly associated with CAD severity. Among the different states of glucose metabolism, the association between TyG index and multi-vessel CAD was significant in patients with DM.
In this retrospective cohort study, we observed that the prevalence of abnormalities in clinical and biological characteristics was higher than in the general population. The results showed that an elevated TyG index was also associated with severe dyslipidemia, progressively impaired glucose metabolic status, and coronary stenosis progression. This may be because our study was hospital-based, and participants had CHD with more risk factors. Epidemiological studies have identified multiple important risk factors responsible for the progression of CAD, such as IR, hypertension, hyperlipidemia, and obesity [24, 25]. Our results corroborated that patients with CHD have abnormal biochemical parameters and glucose metabolic statuses when experiencing progressive CAD.
Previous literature has suggested that the TyG index is related to the degree of coronary artery stenosis and could identify asymptomatic patients with T2DM who are at high risk of coronary artery stenosis [26]. In line with this study, our research supports this notion by more thoroughly demonstrating that the TyG index is associated with CAD severity in patients with CHD. TyG index has become a simple surrogate marker for IR, which is a fundamental clinical feature of severe metabolic syndrome and a marker for a collection of pathological conditions associated with systemic inflammation, endothelial dysfunction, oxidative stress, and prothrombotic states [27]. A 13-year follow-up study reported that metabolic syndrome was a critical CAD risk factor that leads to approximately twice the amount of CAD mortality [28]. Patients with IR result in the disappearance of the normal coordinated hypoglycemic response and the loss of, for example, inhibition of endogenous glucose production and lipolysis, cellular uptake of available blood glucose, and net glycogen synthesis [29]. During IR, TG stored in adipose tissue undergoes lipolysis, which produces fatty acids before raising blood glucose levels [30]. Abnormal blood glucose and lipid metabolism are risk factors that lead to poor CVD prognosis [31]. Therefore, IR is considered a pivotal risk factor for cardiometabolic diseases [32]. IR plays an essential role in the atherosclerotic process by increasing the risk of atherogenic damage, causing myocardium damage and atherosclerotic plaque generation [33]. TyG index performs better than the homeostasis model assessment of insulin resistance in predicting subclinical atherosclerosis [34, 35]. Consistent with the above literature, multi-vessel CAD was associated with an elevated TyG index in patients with CHD.
It is well known that multi-vessel CAD is a type of CHD with a high risk of adverse events and death and is a clinical indication for coronary artery bypass grafting. This study showed that an increased TyG index was significantly associated with the risk of multi-vessel CAD regardless of confounders, such as age, sex, SBP, DBP, history of smoking and alcohol consumption, and drug therapy, further emphasizing the strength of this association. Additionally, the study’s findings showed that the incidence of multi-vessel CAD was higher in patients with DM than in those with Pre-DM and NGR (72.7% > 57.4% > 51.6%) and that the TyG indexes of these patients were significantly related to multi-vessel CAD. Studies have indicated that DM presents with dyslipidemia which increases CHD risk [3, 36]. Compared to individuals with NGR and Pre-DM, patients with DM have a higher prevalence and severity of CAD [37]. DM may increase cardiovascular risk by accelerating the development of atherosclerosis [38, 39]. The pathogenesis of diabetic atherosclerosis involves hyperglycemia, dyslipidemia, changes in secretion of hormone other than insulin, and a proinflammatory state. Oxidative stress and inflammation interact in the development of abnormal glucose metabolic states to accelerate atherosclerosis [40].
IR is a critical pathophysiological pathway leading to DM and is present for an extended period before diabetes is diagnosed [41]. Previous studies have stated that the TyG index can indicate IR in individuals without diabetes [42]. However, we found no association between TyG index and CAD severity in patients with NGR or Pre-DM. In contrast to the theory above, we found that the TyG index has some limitations for detecting CAD in patients with CHD with NGR or Pre-DM. TyG index may only be optimal for detecting multi-vessel CAD in patients with DM and not for patients with NGR or Pre-DM. Further investigation is required to assess the exact parameters of this index in the detection of multi-vessel CAD in patients with different metabolic states.
Strengths and limitations
A strength of this study is that we explored the association between TyG index and the severity of CAD in different glucose metabolic states, which to our knowledge, is the first to be conducted. In addition, the data were recorded and described by professional doctors, and the results were reconfirmed using sensitivity analysis. This may increase their reliability in determining whether the TyG index can provide a new basis for predicting and diagnosing CAD severity. This study also had some limitations. First, we could not determine the existence of a causal relationship due to the inherent limitations of retrospective studies. Second, the study population was relatively small. Third, the participants were inpatients; thus, there may be some limitations to the generalizability of the results to other populations.
Conclusion
In conclusion, this study demonstrated that the TyG index was associated with the CAD severity in patients with CHD using a cohort dataset. An elevated TyG index could identify patients with CHD with a high risk of multi-vessel CAD. There was an association between TyG index and CAD severity among patients with DM, while no association was observed in patients without DM. Testing TyG index can be used as a risk management strategy for patients with CHD with different glucose metabolic states to assist in determining the severity of CAD and in making more appropriate healthcare decisions.
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- TyG:
-
Triglyceride glucose
- CAD:
-
Coronary artery disease
- CVD:
-
Cardiovascular disease
- CHD:
-
Coronary heart disease
- CS:
-
Coronary stenoses
- CAG:
-
Coronary angiography
- NGR:
-
Normal glucose regulation
- Pre-DM:
-
Prediabetes mellitus
- DM:
-
Diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- BMI:
-
Body mass index
- FPG:
-
Fasting plasma glucose
- HbA1c:
-
Glycated haemoglobin
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- IR:
-
Insulin resistance
- OR:
-
Odds ratios
- CI:
-
Confdence interval
- β:
-
Regression coefficient
References
Virani SS, Alonso A, Aparicio HJ, et al. American Heart Association council on epidemiology and prevention statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–743.
Report on cardiovascular health and diseases in China 2020. China Cardiovasc Dis Res. 2021;19(07):582–90.
Arnold SV, Bhatt DL, Barsness GW, Beatty AL, Deedwania PC, Inzucchi SE, Kosiborod M, Leiter LA, Lipska KJ, Newman JD, Welty FK, American Heart Association Council on Lifestyle and Cardiometabolic Health and Council on Clinical Cardiology. Clinical management of stable coronary artery disease in patients with type 2 diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2020;141(19):e779–806.
Jin JL, Cao YX, Zhang HW, Sun D, Hua Q, Li YF, Guo YL, Wu NQ, Zhu CG, Gao Y, Dong QT, Liu HH, Dong Q, Li JJ. Lipoprotein (a) and cardiovascular outcomes in patients with coronary artery disease and prediabetes or diabetes. Diabetes Care. 2019;42(7):1312–8.
Liu SL, Wu NQ, Shi HW, Dong Q, Dong QT, Gao Y, Guo YL, Li JJ. Fibrinogen is associated with glucose metabolism and cardiovascular outcomes in patients with coronary artery disease. Cardiovasc Diabetol. 2020;19(1):36.
Jin JL, Zhang HW, Cao YX, Liu HH, Hua Q, Li YF, Zhang Y, Guo YL, Wu NQ, Zhu CG, Xu RX, Gao Y, Li XL, Cui CJ, Liu G, Sun J, Dong Q, Santos R, Li JJ. Long-term prognostic utility of low-density lipoprotein (LDL) triglyceride in real-world patients with coronary artery disease and diabetes or prediabetes. Cardiovasc Diabetol. 2020;19(1):152.
Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njølstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J, Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–41.
Bragg F, Holmes MV, Iona A, Guo Y, Du H, Chen Y, Bian Z, Yang L, Herrington W, Bennett D, Turnbull I, Liu Y, Feng S, Chen J, Clarke R, Collins R, Peto R, Li L, Chen Z, China Kadoorie Biobank Collaborative Group. Association between diabetes and cause-specific mortality in rural and urban areas of China. JAMA. 2017;317(3):280–9.
Vähätalo JH, Huikuri HV, Holmström LTA, Kenttä TV, Haukilahti MAE, Pakanen L, Kaikkonen KS, Tikkanen J, Perkiömäki JS, Myerburg RJ, Junttila MJ. Association of silent myocardial infarction and sudden cardiac death. JAMA Cardiol. 2019;4(8):796–802.
Cohn PF. Prognosis and treatment of asymptomatic coronary artery disease. J Am Coll Cardiol. 1983;1(3):959–64.
Khan SH, Sobia F, Niazi NK, Manzoor SM, Fazal N, Ahmad F. Metabolic clustering of risk factors: evaluation of triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr. 2018;10:74.
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–51.
Rhee SY, Woo JT. The prediabetic period: review of clinical aspects. Diabetes Metab J. 2011;35:107–16.
Janghorbani M, Amini M. Normal fasting plasma glucose and risk of prediabetes and type 2 diabetes: the Isfahan diabetes prevention study. Rev Diabet Stud. 2011;8:490.
Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18(1):361.
Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, Kim JH, Park JS. Elevated TyG index predicts progression of coronary artery calcifcation. Diabetes Care. 2019;42(8):1569–73.
Li Z, He Y, Wang S, Li L, Yang R, Liu Y, Cheng Q, Yu L, Zheng Y, Zheng H, Gao S, Yu C. Association between triglyceride glucose index and carotid artery plaque in different glucose metabolic states in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol. 2022;21(1):38.
Wu S, Xu L, Wu M, Chen S, Wang Y, Tian Y. Association between triglyceride-glucose index and risk of arterial stiffness: a cohort study. Cardiovasc Diabetol. 2021;20(1):146.
Mao Q, Zhou D, Li Y, Wang Y, Xu SC, Zhao XH. The triglyceride-glucose index predicts coronary artery disease severity and cardiovascular outcomes in patients with non-ST-segment elevation acute coronary syndrome. Dis Markers. 2019;11(2019):6891537.
Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46:189–97.
Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. 2021;20(1):76.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes care. 2014;37(Suppl 1):S81-90.
Mahmood SS, et al. The Framingham heart study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):999–1008.
Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18(3):109–14.
Thai PV, Tien HA, Van Minh H, Valensi P. Triglyceride glucose index for the detection of asymptomatic coronary artery stenosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19(1):137.
Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuniga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122.
Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–50.
Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuniga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122.
Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133–223.
Li X, Zhai Y, Zhao J, He H, Li Y, Liu Y, Feng A, Li L, Huang T, Xu A, Lyu J. Impact of metabolic syndrome and it’s components on prognosis in patients with cardiovascular diseases: a meta-analysis. Front Cardiovasc Med. 2021;15(8): 704145.
Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293–302.
Aboonabi A, Meyer RR, Singh I. The association between metabolic syndrome components and the development of atherosclerosis. J Hum Hypertens. 2019;33(12):844–55.
Vasques AC, Novaes FS, de Oliveira MS, Souza JR, Yamanaka A, Pareja JC, Tambascia MA, Saad MJ, Geloneze B. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–100.
Irace C, Carallo C, Scavelli FB, De Franceschi MS, Esposito T, Tripolino C, Gnasso A. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract. 2013;67(7):665–72.
Zhang MY, Zhang JH, Chua HZ, Feng R, Lu MJ, Tian Y. Core outcome set for stable angina pectoris in traditional Chinese medicine (COS-SAP-TCM). Acupuncture Herb Med. 2021;1(1):39–48.
Rana JS, Dunning A, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng VY, Chinnaiyan K, Chow BJ, Cury R, Delago A, Feuchtner G, Hadamitzky M, Hausleiter J, Kaufmann P, Karlsberg RP, Kim YJ, Leipsic J, Labounty TM, Lin FY, Maffei E, Raff G, Villines TC, Shaw LJ, Berman DS, Min JK. Differences in prevalence, extent, severity, and prognosis of coronary artery disease among patients with and without diabetes undergoing coronary computed tomography angiography: results from 10,110 individuals from the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes): an InteRnational Multicenter Registry. Diabetes Care. 2012;35(8):1787–94.
Lu T, Forgetta V, Yu OHY, et al. Polygenic risk for coronary heart disease acts through atherosclerosis in type 2 diabetes. Cardiovasc Diabetol. 2020;19(1):12.
Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22.
Yuan T, Yang T, Chen H, Fu D, Hu Y, Wang J, Yuan Q, Yu H, Xu W, Xie X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019;20:247–60.
Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133–223.
Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, Ko YL. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE. 2016;11(3): e0149731.
Acknowledgements
We thank all the participants in the study, the members of the survey teams, and the groups providing financial support.
Funding
This study was supported by the National Basic Research Program of China (973 Project, Grant Number: 2014CB542902).
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to this work. CY, LL, CL, and JS participated in the study design and statistical analysis. JS, ZL, and MH analyzed the data and drafted the manuscript. YW, TY, GP, MM, YN, and ZL participated in data collection. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Tianjin University of Traditional Chinese Medicine (TJUTCM-EC20190008) and was registered in the Chinese Clinical Trial Registry (ChiCTR-1900024535) and on Clinical Trials. gov (NCT04026724).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Association between the TyG index and the number of vessels with stenosis ≥ 50%. Table S2. Association between the TyG index and degree of coronary stenoses.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Su, J., Li, Z., Huang, M. et al. Triglyceride glucose index for the detection of the severity of coronary artery disease in different glucose metabolic states in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol 21, 96 (2022). https://doi.org/10.1186/s12933-022-01523-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01523-7