Abstract
Background
Diabetes is associated with adverse outcomes after percutaneous coronary intervention with drug-eluting stents (DES), but for prediabetes this association has not been definitely established. Furthermore, in patients with prediabetes treated with contemporary stents, bleeding data are lacking. We assessed 3-year ischemic and bleeding outcomes following treatment with new-generation DES in patients with prediabetes and diabetes as compared to normoglycemia.
Methods
For this post-hoc analysis, we pooled patient-level data of the BIO-RESORT and BIONYX stent trials which both stratified for diabetes at randomization. Both trials were multicenter studies performed in tertiary cardiac centers. Study participants were patients of whom glycemic state was known based on hemoglobin A1c, fasting plasma glucose, or medically treated diabetes. Three-year follow-up was available in 4212/4330 (97.3 %) patients. The main endpoint was target vessel failure, a composite of cardiac death, target vessel myocardial infarction, or target vessel revascularization.
Results
Baseline cardiovascular risk profiles were progressively abnormal in patients with normoglycemia, prediabetes, and diabetes. The main endpoint occurred in 54/489 patients with prediabetes (11.2 %) and 197/1488 with diabetes (13.7 %), as compared to 142/2,353 with normoglycemia (6.1 %) (HR: 1.89, 95 %-CI 1.38–2.58, p < 0.001, and HR: 2.30, 95 %-CI 1.85–2.86, p < 0.001, respectively). In patients with prediabetes, cardiac death and target vessel revascularization rates were significantly higher (HR: 2.81, 95 %-CI 1.49–5.30, p = 0.001, and HR: 1.92, 95 %-CI 1.29–2.87, p = 0.001), and in patients with diabetes all individual components of the main endpoint were significantly higher than in patients with normoglycemia (all p ≤ 0.001). Results were consistent after adjustment for confounders. Major bleeding rates were significantly higher in patients with prediabetes and diabetes, as compared to normoglycemia (3.9 % and 4.1 % vs. 2.3 %; HR:1.73, 95 %-CI 1.03–2.92, p = 0.040, and HR:1.78, 95 %-CI 1.23–2.57, p = 0.002). However, after adjustment for confounders, differences were no longer significant.
Conclusions
Not only patients with diabetes but also patients with prediabetes represent a high-risk population. After treatment with new-generation DES, both patient groups had higher risks of ischemic and bleeding events. Differences in major bleeding were mainly attributable to dissimilarities in baseline characteristics. Routine assessment of glycemic state may help to identify patients with prediabetes for intensified management of cardiovascular risk factors.
Trial registration: BIO-RESORT ClinicalTrials.gov: NCT01674803, registered 29-08-2012; BIONYX ClinicalTrials.gov: NCT02508714, registered 27-7-2015.
Similar content being viewed by others
Background
The presence of diabetes is a well-known risk factor for coronary artery disease and has been associated with an increased adverse event risk after percutaneous coronary intervention (PCI) with drug-eluting stents (DES) [1,2,3,4,5]. Refinements in stent technology and concomitant medical therapy have improved outcomes, yet, diabetic patients still show higher adverse event rates [1,2,3,4,5]. The increased risk of ischemic events has been linked to the presence of a prothrombotic state due to platelet hyperactivity, increased platelet aggregation, and endothelial dysfunction [6,7,8]. While ischemic outcomes of diabetic patients following DES implantation have been evaluated, such data for patients with prediabetes are scarce. Furthermore, conflicting data have been reported regarding bleeding risk in diabetic patients undergoing PCI [9,10,11], and there is a lack of data on bleeding in patients with prediabetes.
BIO-RESORT and BIONYX, two large-scale randomized clinical trials in all-comer patients undergoing PCI for obstructive coronary artery disease, have established non-inferiority of several new-generation DES versus contemporary reference DES [12, 13]. Furthermore, in patients with diabetes no difference in outcome was seen between DES groups [14]. For the present analysis at 3 years, we examined pooled patient-level data of trial participants with known glycemic state, based on hemoglobin A1c (HbA1c) and/or fasting plasma glucose (FPG) testing, or medically treated diabetes. Subsequently, we assessed potential differences in the incidence of ischemic and bleeding events after PCI with new-generation DES in patients with prediabetes and diabetes as compared to normoglycemia.
Methods
Study design and participants
For the current analysis, we pooled patient-level data of two randomized trials of which design and details have been published [12, 13]. In brief, BIO-RESORT (Comparison of biodegradable polymer and durable polymer drug-eluting stents in an all-comers population; ClinicalTrials.gov NCT01674803) is a 3-arm, patient- and assessor-blinded trial, performed at four cardiac centers in the Netherlands. From December 2012 to August 2015, patients were randomized (1:1:1) to treatment with the ultrathin strut biodegradable polymer sirolimus-eluting Orsiro stent (Biotronik, Bülach, Switzerland), the very thin strut biodegradable polymer everolimus-eluting Synergy stent (Boston Scientific, Marlborough, MA) or the thin strut durable polymer zotarolimus-eluting Resolute Integrity stent (Medtronic, Santa Rosa, CA) [12]. BIONYX (Bioresorbable polymer-coated Orsiro versus durable polymer-coated Resolute ONYX stents; ClinicalTrials.gov NCT025087140) is a patient- and assessor-blinded trial that was performed in seven cardiac centers in Israel, Belgium, and the Netherlands. From October 2015 to December 2016, patients were randomized (1:1) to treatment with the thin strut durable polymer zotarolimus-eluting Resolute Onyx stent (Medtronic) or the ultrathin strut biodegradable polymer sirolimus-eluting stent 13.
For both trials, patients were eligible for enrollment if they were 18 years or older, capable of providing informed consent, and required PCI. Exclusion criteria were very limited, and included intolerance to dual antiplatelet therapy (DAPT), known pregnancy, and life expectancy of < 1 year. There was no restriction for clinical syndrome, target lesion type, lesion length, reference vessel size, and number of lesions or vessels to be treated. Web-based randomization was performed with the use of a custom designed computer program in random block sizes of 6 and 3, stratified according to the presence of diabetes mellitus. Follow-up will be extended up to 5-years. Figure 1 shows the study flow diagram. The trials complied with the Declaration of Helsinki and were approved by the Medical Ethics Committee Twente and the Institutional Review Boards of all centers. All patients provided written informed consent.
Procedures, follow-up, and monitoring
All coronary interventions were performed according to international medical guidelines and the operator’s judgment. Detailed information on the contemporary DES that were used have been published [12, 13]. DAPT was generally prescribed for 12 months in patients with acute coronary syndromes (ACS) and for 6 months in patients with stable angina, as guidelines recommended during the study periods. All study sites were encouraged to measure FPG and HbA1c shortly before or after index procedure. Clinical follow-up was obtained at visits to the outpatient clinic, by telephone, or by paper-based questionnaire. The trials were monitored (Diagram, Zwolle, Netherlands), and events were adjudicated by independent committees that were blinded for treatment (Diagram, Zwolle, the Netherlands, or a committee of cardiologists of University of Amsterdam, The Netherlands).
Clinical endpoints and definitions
Clinical endpoints were prespecified according to the Academic Research Consortium [15, 16]. The main endpoint was target vessel failure (TVF), a composite of safety and efficacy, consisting of cardiac death, target vessel-related myocardial infarction (MI), or clinically indicated target vessel revascularization. Secondary composite endpoints included target lesion failure (cardiac death, target vessel MI, or clinically indicated target lesion revascularization) and major adverse cardiac events (all-cause death, any MI, or clinically-indicated target lesion revascularization). Other secondary endpoints were all-cause mortality, the individual components of TVF, target lesion revascularization, bleeding, and both definite and definite-or-probable stent thrombosis. The endpoint any MI included peri-procedural MI and was based on definitions from the Academic Research Consortium [16]. Major bleeding was defined as class 3 or 5 of the Bleeding Academic Research Consortium (BARC 3a, 3b, 3c, 5a, 5b) and/or all Thrombolysis in Myocardial Infarction (TIMI) major bleedings [17, 18].
Definitions of glycemic state were based on the World Health Organization definition and diagnosis of diabetes mellitus and intermediate hyperglycemia statement [19] and the International Expert Committee 2009 criteria for HbA1c with FPG [20]. Normoglycemia was defined as FPG < 6.1 mmol/l and/or HbA1c ≤ 41 mmol/mol, prediabetes as FPG 6.1–6.9mmol/l and/or HbA1c 42–47 mmol/mol, and diabetes as FPG ≥ 7.0 mmol/l and/or HbA1c ≥ 48 mmol/mol.
Statistical analysis
Differences in categorical variables were examined with Pearson’s χ2 or Fisher’s exact test, and differences in continuous variables with Mann-Whitney U or t test, as appropriate. Kaplan–Meier methods were used to assess time-to-endpoints. Hazard ratios (HR) with 2-sided confidence intervals (CI) were computed by Cox proportional hazards analysis. A two-sided p-value < 0.05 was considered significant. All analyses were performed according to the intention-to-treat principle. A multivariable model was constructed, including all baseline variables that showed a between-group difference and an association with TVF (p < 0.15). The final model was made using step-wise backward selection. It included: clinical presentation; number of diseased vessels; at least one severely calcified lesion treated. In the same manner, a multivariable model was constructed for bleeding endpoints. Based on stepwise backward selection, age, sex, hypercholesterolemia, vitamin K antagonist use at 3-year follow-up, and multivessel treatment were included, and based on literature, hypertension, renal insufficiency, and previous stroke. Statistical analyses were done with SPSS version-24.0 (IBM, Armonk, NY).
Results
Three-year follow-up was available in 4212/4330 (97.3 %) patients. Consent withdrawal was balanced between groups (n = 63). Slightly more patients with diabetes were lost to follow-up: normoglycemia 0.8 %, prediabetes 1.3 %, diabetes 2.2 % (total n = 55). As may be expected, baseline patient, lesion, and procedural characteristics showed significant between-group differences (Table 1). Baseline cardiovascular risk profiles were progressively abnormal in patients with normoglycemia, prediabetes, and diabetes, with the exception of smoking which was more common among patients with normoglycemia. In addition, patients with prediabetes or diabetes had smaller vessels, more severely calcified lesions and more bypass grafts treated. At 3-years, DAPT was used by 5.7 % of patients with normoglycemia, 9.2 % with prediabetes, and 12.1 % with diabetes (p < 0.001; Additional file 1: Table S1). Vitamin K antagonist use differed across groups (normoglycemia 7.5 %, prediabetes 10.0 %, diabetes 10.7 %; p = 0.003), while direct oral anticoagulant use was similar (5.2 %, 6.8 %, 5.9 %, respectively; p = 0.35).
Prediabetes versus normoglycemia
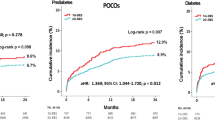
The 3-year rate of the main composite endpoint TVF was significantly higher in patients with prediabetes as compared to patients with normoglycemia (11.2 % vs.6.1 %, HR: 1.89, 95 %-CI 1.38–2.58, p < 0.001) (Table 2). The rates of cardiac death and target vessel revascularization were also higher in patients with prediabetes (3.1 % vs. 1.1 %, HR: 2.81, 95 %-CI 1.49–5.30, p = 0.001, and 7.0 % vs. 3.7 %, HR: 1.92, 95 %-CI 1.29–2.87, p = 0.001). Target vessel MI did not differ between groups (3.6 % vs. 2.5 %, HR: 1.45, 95 %-CI 0.84–2.49, p = 0.18). Figure 2 displays Kaplan–Meier event curves for TVF and components. All-cause mortality was higher in patients with prediabetes (5.4 % vs. 3.4 %, HR:1.59, 95 %-CI 1.02–2.47, p = 0.041). There was no significant between-group difference in definite stent thrombosis (HR: 1.08, 95 %-CI 0.23–4.99, p = 0.92). The rate of any bleeding did not differ between groups (5.9 % vs. 4.2 %; HR: 1.43, 95 %-CI 0.94–2.18, p = 0.09), while the major bleeding rate was higher in patients with prediabetes (3.9 % vs. 2.3 %, HR: 1.73, 95 %-CI 1.03–2.92, p = 0.040; Fig. 3). The incidence of TVF was significantly higher in patients with prediabetes, irrespective of whether patients presented with ACS at index procedure (Additional file 1: Table S2). Among patients with ACS, the incidence of major bleeding was higher in the presence of prediabetes, while among patients with stable angina the major bleeding rate was similar in both patients with prediabetes and normoglycemia (Additional file 1: Table S2).
Kaplan–Meier event curves for target vessel failure and its individual components at 3-year follow-up. Kaplan–Meier event curves for target vessel failure and its individual components up to 3-year follow-up showing higher rates of the main endpoint in patients with prediabetes and diabetes as compared to patients with normoglycemia
Kaplan–Meier event curves for major bleeding and stent thrombosis at 3-year follow-up. Kaplan–Meier event curves for major bleeding and stent thrombosis up to 3-year follow-up showing higher rates of major bleeding in patients with prediabetes and diabetes, and higher rates of definite-or-probable stent thrombosis in patients with diabetes as compared to patients with normoglycemia
After adjustment for confounders, multivariable analysis still showed a significantly higher TVF risk for patients with prediabetes as compared to patients with normoglycemia (Table 2). This was also the case for cardiac death and target vessel revascularization, while all-cause mortality risk was no longer significantly higher. The risk of any bleeding and major bleeding showed no independent association with prediabetes (Table 2).
Diabetes versus normoglycemia
Patients with diabetes, as compared to patients with normoglycemia, had significantly higher rates of TVF (13.7 % vs. 6.1 %, HR:2.30, 95 %-CI 1.85–2.86, p < 0.001) and its components (Table 2). Figure 2 displays Kaplan–Meier curves for TVF and its components. The incidence of TVF was significantly higher in patients with diabetes, irrespective of whether they presented with ACS at index procedure (Additional file 1: Table S2). Furthermore, definite stent thrombosis occurred more frequently in patients with diabetes (1.0 % vs.0.4 %, HR:2.52, 95 %-CI 1.09–5.82, p = 0.030). The rates of other individual and composite endpoints were also significantly higher in patients with diabetes (Table 2), including any bleeding (6.5 % vs. 4.2 %, HR:1.59, 95 %-CI 1.19–2.11, p = 0.001), as well as major bleeding (4.1 % vs. 2.3 %, HR: 1.78, 95 %-CI 1.23–2.57, p = 0.002; Fig. 3). When separately assessing patients with acute and stable coronary syndromes, patients with diabetes showed a higher major bleeding rate only in the ACS but not in stable coronary syndrome group (Table S2).
Multivariable analysis showed that the risk of TVF remained significantly higher for patients with diabetes as compared to patients with normoglycemia (Table 2). Similarly, patients with diabetes had significantly higher risks of other endpoints. Yet, the risk of any and major bleeding showed no independent association with diabetes.
Discussion
This analysis of pooled patient-level data from two large-scale randomized clinical trials which assessed new-generation DES, showed that not only patients with diabetes but also patients with prediabetes represent a high-risk PCI population. There was a higher 3-year rate of the main safety and efficacy endpoint for both patient groups as compared to patients with normoglycemia. In patients with prediabetes, this difference was driven by cardiac death and target vessel revascularization, while in patients with diabetes it was also based on target vessel-related MI.
In patients with prediabetes, only the risks of repeated revascularization and cardiac death remained significantly higher after adjustment for confounders. Patients with diabetes showed higher risks of all ischemic clinical endpoints, including definite-or-probable stent thrombosis, which was consistent after adjustment. Furthermore, the major bleeding risk was higher in both, patients with prediabetes and diabetes. However, after adjustment for the potential confounders the two glycemic states were not independently associated with major bleeding. In other words, the higher bleeding risk in patients with prediabetes and diabetes can largely be explained by comorbidities and demographics that are related to an increased bleeding risk.
Ischemic outcomes
Several studies assessed patients with diabetes who were treated with new-generation DES, and showed a higher risk of adverse events following PCI, including ischemic outcomes [4, 5, 21, 22]. Yet, data on PCI patients with prediabetes are scarce. The South Korean KAMIR registry examined patients with acute MI treated with contemporary DES, and compared 3709 patients with prediabetes and 5173 patients with diabetes to 3080 patients with normoglycemia [23]. The findings of the present analysis corroborate that study which showed in patients with prediabetes and diabetes higher 2-year rates of revascularization and cardiac death, and in patients with diabetes also higher rates of all-cause mortality and MI. A previous subgroup analysis of BIO-RESORT assessed 324 patients with prediabetes, 793 with diabetes, and 1869 with normoglycemia and showed that patients with prediabetes, similar to patients with diabetes, had higher 1-year risks of mortality and repeat revascularization after treatment with contemporary DES [4]. Furthermore, the BIO-RESORT Silent Diabetes study [24] previously reported that patients with prediabetes and silent diabetes—assessed by oral glucose tolerance testing and HbA1c at the time of the index procedure—had a higher 3-year risk of TVF. Yet, this was mainly driven by events during the first 48 h. Those previously reported data and the findings of the present study suggest that patients with prediabetes have an increased risk of ischemic events. Therefore, patients with prediabetes should be considered high-risk, and routine assessment of glycemic state in PCI patients may help to target this group for intensified management of cardiovascular risk factors.
Bleeding outcomes
While in the current analysis patients with prediabetes and diabetes experienced more ischemic events, both groups also showed higher rates of major bleeding (Fig. 3). At baseline, patients with diabetes had higher rates of several risk factors for bleeding, such as hypertension, renal insufficiency, previous stroke, and older age. In addition, at 3-year follow-up they more often used anticoagulant therapy. After adjustment for such potential confounders, the differences in major bleeding were no longer significant.
So far, bleeding risk in patients with prediabetes treated with DES had not been assessed. Previous studies that assessed bleeding in diabetic patients showed conflicting results. In the randomized GLOBAL LEADERS trial, long-term ticagrelor monotherapy (after 1-month DAPT) was compared to conventional DAPT in 15,968 all-comer patients who underwent PCI with DES [10]. The study found no statistically significant difference in major bleeding in 4038 patients with diabetes as compared to patients without diabetes. Another study that evaluated DAPT strategies and examined bleeding risk was PLATO which assessed DAPT with ticagrelor versus clopidogrel in 11,289 patients with acute coronary syndrome, irrespective of treatment strategy (conservative treatment included). A substudy [9] in diabetic patients showed a higher rate of major bleeding in 2520 patients with diabetes, which was still apparent after adjustment for confounders. Considering all of the above, it is questionable whether hyperglycemia on its own increases bleeding risk, but it is quite clear that the cardiovascular risk profiles of patients with diabetes and prediabetes do increase that risk.
DAPT strategies
The balance between ischemic and bleeding events in patients with diabetes is delicate, and can be challenging to manage. Several studies have investigated alternative antiplatelet strategies for these patients. The THEMIS trial assessed 19,220 patients with diabetes and stable coronary artery disease, who were randomized to treatment with aspirin only or DAPT with ticagrelor [25]. The patients on DAPT had lower rates of ischemic events, but this was offset by an increase in major bleedings. In our study, diabetic patients treated for stable angina did not show a higher incidence of major bleeding than patients with normoglycemia. Yet, this may be related to the modest sample size of the subgroup with stable angina. A meta-analysis that compared short-term (≤ 6 months) and long-term (12 months) DAPT following PCI in patients with diabetes (40 % ACS) found no difference in major adverse cardiac events, but a higher rate of major bleeding in patients on long-term DAPT [21]. However, in patients with diabetes, but not in those without diabetes, the definite-or-probable stent thrombosis rate was lower after long-term DAPT. In 7119 high-risk PCI patients, the TWILIGHT study assessed ticagrelor monotherapy following 3 months of DAPT versus conventional DAPT and found in the ticagrelor monotherapy group a lower incidence of bleeding without increase in ischemic events [26]. These results were consistent in patients with diabetes. These findings are certainly promising, yet the optimal DAPT strategy for patients with diabetes (or prediabetes) is a matter of ongoing discussion and warrants further research.
Stent thrombosis
In patients with prediabetes and normoglycemia, definite-or-probable stent thrombosis occurred at comparable rates, but in patients with diabetes it occurred more often. While findings of such infrequent events are no more than hypothesis generating, it may be of interest that a similar between-group distribution of stent thromboses was seen in the acute MI patients of the KAMIR registry. That study also found a higher 2-year incidence of stent thromboses in patients with diabetes (1.0 %), but not with prediabetes (0.6 %), as compared to patients with normoglycemia (0.5 %) [23]. A meta-analysis that assessed stent thrombosis following PCI with DES showed that 5123 patients with diabetes had a higher late stent thrombosis rate than 13,775 normoglycemic patients [27]. The increased stent thrombosis risk in patients with diabetes may in part be related to their hypercoagulable state. The prothrombotic setting is promoted via several pathways, such as platelet hyperactivity, increased platelet aggregation, endothelial dysfunction, and elevated levels of multiple clotting factors [6,7,8]. Another mechanism that may increase stent thrombosis risk in diabetic patients is a reduction of early arterial healing, which leads to more uncovered stent struts [28] that were found to be associated with coronary stent thrombosis [29]. Furthermore, stent sizing and apposition may be suboptimal in patients with diabetes due to more diffuse and calcified coronary artery disease. If there is diffuse coronary artery disease, true vessel dimensions may be underestimated, as ‘reference segments’ may also be diseased. Suboptimal stent sizing and apposition, prothrombotic setting, and delayed arterial healing may all contribute to the increased stent thrombosis risk of patients with diabetes, which in the present study was most pronounced during the acute and subacute phase. The risk of MI was also higher in patients with diabetes. As discussed above, there are several factors that increase the risk of stent thrombosis in diabetic patients, and these factors are also relevant to the occurrence of MI. Furthermore, in patients with diabetes who may have a higher plaque burden, the risk of micro-embolization of atherothrombotic debris is increased which can lead to peri-procedural MI.
Strengths and limitations
The present study analyzed patient-level pooled data of two large-scale randomized trials that primarily evaluated PCI with new-generation DES and stratified for diabetes at randomization. We examined 4330 patients in whom information on their glycemic state was available. Both trials applied the same definitions of baseline characteristics and clinical endpoints, assessed a relatively long follow-up, underwent independent monitoring, and reported clinical events following assessment by independent clinical event committees. In addition, for a PCI study, the groups of patients with diabetes and prediabetes are sizable. Nevertheless, the study also has several limitations. This analysis was not prespecified and not powered to provide definite conclusions regarding subpopulations with (pre)diabetes. Therefore, the findings are hypothesis generating. Furthermore, we cannot exclude unmeasured confounders. Information on the use of antidiabetic medication and glycemic control during follow-up is not available. Therefore, we do not know how many patients with prediabetes progressed to diabetes during follow-up, and it is unknown whether good or poor metabolic control affected the outcome of patients. Yet, the purpose of this study was to evaluate whether information on the glycemic state at the time of index PCI can be used to assess ischemic and bleeding risks of patients with (pre)diabetes in order to identify a high-risk group. We used four different new-generation DES, which theoretically could have affected clinical outcome. However, stent type was found to have no significant association with TVF in patients with known glycemic state, and therefore DES type was not included as potential confounder in the multivariable model. Multivessel disease differed between metabolic groups and was included in the multivariable model, but incomplete revascularization for clinically relevant (based on ischemia detection or invasive measurements) and treatable lesions was not recorded in our database. Nevertheless, in the participating centres, it is common practice to discuss patients with multivessel disease in a Heartteam, aiming at optimal revascularization. Therefore, it may be assumed that there was a low rate of patients with incomplete revascularization of clinically relevant and treatable lesions.
Conclusions
Not only patients with diabetes but also patients with prediabetes represent a high-risk population. After treatment with new-generation DES, both patient groups had higher risks of ischemic and bleeding events. Differences in major bleeding were mainly attributable to between-group dissimilarities in patient characteristics. Routine assessment of glycemic state may help to identify PCI patients with prediabetes for intensified management of cardiovascular risk factors in order to improve outcome.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to privacy restrictions for pseudo-anonymized data under European law, but can be made available from the corresponding author on reasonable request after signing a data-sharing agreement.
Abbreviations
- ACS:
-
Acute coronary syndrome
- BARC:
-
Bleeding Academic Research Consortium
- CI:
-
Confidence interval
- DAPT:
-
Dual antiplatelet therapy
- DES:
-
Drug-eluting stent
- FPG:
-
Fasting plasma glucose
- HbA1c:
-
Hemoglobin A1c
- HR:
-
Hazard ratio
- MI:
-
Myocardial infarction
- PCI:
-
Percutaneous coronary intervention
- TIMI:
-
Thrombolysis in myocardial infarction
- TVF:
-
Target vessel failure
References
Kereiakes DJ, Smits PC, Kedhi E, et al. Predictors of death or myocardial infarction, ischaemic-driven revascularisation, and major adverse cardiovascular events following everolimus-eluting or paclitaxel-eluting stent deployment: pooled analysis from the SPIRIT II, III, IV and COMPARE trials. EuroIntervention. 2011;7(1):74–83.
Kedhi E, Généreux P, Palmerini T, et al. Impact of coronary lesion complexity on drug-eluting stent outcomes in patients with and without diabetes mellitus: analysis from 18 pooled randomized trials. J Am Coll Cardiol. 2014;63(20):2111–8.
Koskinas KC, Sionitis GCM, Piccolo R, et al. Impact of diabetic status on outcomes after revascularization with drug-eluting stents in relation to coronary artery disease complexity: patient-level pooled analysis of 6081 patients. Circ Cardiovasc Interv. 2016;9(2):e003255.
Kok MM, von Birgelen C, Sattar N, et al. Prediabetes and its impact on clinical outcome after coronary intervention in a broad patient population. EuroIntervention. 2018;14(9):e1049-56.
Konigstein M, Ben-Yehuda O, Smits PC, et al. Outcomes among diabetic patients undergoing percutaneous coronary intervention with contemporary drug-eluting stents: analysis from the BIONICS randomized trial. J Am Coll Cardiol Intv. 2018;11:2467–76.
Li Y, Woo V, Bose R. Platelet hyperactivity and abnormal Ca2+ homeostasis in diabetes mellitus. Am J Physiol Heart Circ Physiol. 2001;280(4):H1480-9.
Pomero F, Di Minno MN, Fenoglio L, Gianni M, Ageno W, Dentali F. Is diabetes a hypercoagulable state? A critical appraisal. Acta Diabetol. 2015;52(6):1007–16.
Keating FK, Sobel BE, Schneider DJ. Effects of increased concentrations of glucose on platelet reactivity in healthy subjects and in patients with and without diabetes mellitus. Am J Cardiol. 2003;92(11):1362–5.
James S, Angiolillo DJ, Cornel JH, PLATO Study Group, et al. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2010;31(24):3006–16.
Chichareon P, Modolo R, Kogame N, et al. Association of diabetes with outcomes in patients undergoing contemporary percutaneous coronary intervention: pre-specified subgroup analysis from the randomized GLOBAL LEADERS study. Atherosclerosis. 2020;295:45–53.
Faggioni M, Baber U, Sartori S, et al. Incidence, patterns, and associations between dual-antiplatelet therapy cessation and risk for adverse events among patients with and without diabetes mellitus receiving drug-eluting stents: results from the PARIS Registry. JACC Cardiovasc Interv. 2017;10(7):645–54.
von Birgelen C, Kok MM, van der Heijden LC, et al. Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT): a three-arm, randomised, non-inferiority trial. Lancet. 2016;388(10060):2607–17.
von Birgelen C, Zocca P, Buiten RA, et al. Thin composite wire strut, durable polymer-coated (Resolute Onyx) versus ultrathin cobalt-chromium strut, bioresorbable polymer-coated (Orsiro) drug-eluting stents in allcomers with coronary artery disease (BIONYX): an international, single-blind, randomised non-inferiority trial. Lancet. 2018;392(10154):1235–45.
Ploumen EH, Buiten RA, Kok MM, et al. Treating diabetic all-comers with contemporary drug-eluting stents: prespecified comparisons from the BIO-RESORT and the BIONYX randomized trials. Int J Cardiol. 2021;325:37–44.
Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–51.
Vranckx P, Cutlip DE, Mehran R, et al. Myocardial infarction adjudication in contemporary all-comer stent trials: balancing sensitivity and specificity. Addendum to the historical MI definitions used in stent studies. EuroIntervention. 2010;5(7):871–4.
Bovill EG, Terrin ML, Stump DC, et al. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. Results of the thrombolysis in myocardial infarction (TIMI), phase II trial. Ann Intern Med. 1991;115(4):256–65.
Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. 2011;123(23):2736–47.
World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Geneva: World Health Organization; 1999.
International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–34.
Gargiulo G, Windecker S, da Costa BR, et al. Short term versus long term dual antiplatelet therapy after implantation of drug eluting stent in patients with or without diabetes: systematic review and meta-analysis of individual participant data from randomised trials. BMJ. 2016;355:i5483.
Iglesias JF, Heg D, Roffi M, et al. Five-year outcomes in patients with diabetes mellitus treated with biodegradable polymer sirolimus-eluting stents versus durable polymer everolimus-eluting stents. J Am Heart Assoc. 2019;8(22):e013607.
Kim YH, Her AY, Jeong MH, et al. Effects of prediabetes on long-term clinical outcomes of patients with acute myocardial infarction who underwent PCI using new-generation drug-eluting stents. Diabetes Res Clin Pract. 2020;160:107994.
Ploumen EH, Buiten RA, Kok MM, et al. Three-year clinical outcome in all-comers with “silent” diabetes, prediabetes, or normoglycemia, treated with contemporary coronary drug-eluting stents: from the BIO-RESORT silent diabetes study. Catheter Cardiovasc Interv. 2020;96(2):E110-8.
Steg PG, Bhatt DL, Simon T, THEMIS Steering Committee and Investigators, et al. Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med. 2019;381(14):1309–20.
Mehran R, Baber U, Sharma SK, et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. 2019;381(21):2032–42.
Yuan J, Xu GM. Early and late stent thrombosis in patients with versus without diabetes mellitus following percutaneous coronary intervention with drug-eluting stents: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2018;18(6):483–92.
Ishihara T, Sotomi Y, Tsujimura T, et al. Impact of diabetes mellitus on the early-phase arterial healing after drug-eluting stent implantation. Cardiovasc Diabetol. 2020;19(1):203.
Adriaenssens T, Joner M, Godschalk TC, Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort (PRESTIGE) Investigators, et al. Optical coherence tomography findings in patients with coronary stent thrombosis: a report of the PRESTIGE Consortium (Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort). Circulation. 2017;136(11):1007–21.
Acknowledgements
Not applicable.
Funding
The BIO-RESORT trial was equally funded by Biotronik, Boston Scientific, and Medtronic. The BIONYX trial was equally funded by Biotronik, and Medtronic. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
EHP and MMK had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors fulfill the criteria of authorship and agree to be accountable for all aspects of the work. CvB, MMK, CJMD, AR, RLA, CES, EB, AA, MH, KGvH, PWD, MS, GCML have contributed to the conception and design of the trials. EHP and MMK wrote the first draft of the manuscript. CvB participated in drafting. RAB, THP CJMD, AR, RLA, CES, EB, AA, MH, KGvH, PWD, MS, GCML, and PZ revised the manuscript for important intellectual content. EHP, MMK and CvB interpreted the data. EHP and CJMD performed the statistical analyses. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The trials complied with the Declaration of Helsinki and were approved by the Medical Ethics Committee Twente (BIO-RESORT reference number P12-22; BIONYX reference number P15-19) and the Institutional Review Boards of all centers. All patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
CvB reports that the research department of Thoraxcentrum Twente has received institutional research grants provided by Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. RLA reports a teaching grant from Biotronic, a lincense from Sanovi, a speaking fee from Abiomed and support from Amgen for attending a meeting, all outside the submitted work. All other authors declared that they have no potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Use of antiplatelet and oral anticoagulant therapy at 3-year follow-up. Table S2. Subgroup analysis of patients with acute coronary syndrome.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ploumen, E.H., Pinxterhuis, T.H., Zocca, P. et al. Impact of prediabetes and diabetes on 3-year outcome of patients treated with new-generation drug-eluting stents in two large-scale randomized clinical trials. Cardiovasc Diabetol 20, 217 (2021). https://doi.org/10.1186/s12933-021-01405-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-021-01405-4