Abstract
Background
Small airway dysfunction not only affects asthma control, but also has adverse effects on the psychological and/or social activities of asthma patients. However, few long-term observational studies have explored the complex relationship between small airway dysfunction and asthma control and health-related quality of life in patients with asthma exacerbations.
Methods
The study recruited 223 patients with exacerbations of asthma (i.e. those with at least one asthma attack over the past year) and 228 patients without exacerbations of asthma (i.e. those without asthma attacks over the past year). We evaluated SAD in patients with asthma exacerbations using impulse oscillometry method. At each evaluation time point within one year of follow-up, the attending physician conducts a case investigation of the patients. We analyzed the correlation between SAD and general characteristics (age, obesity, smoking history), type 2 inflammation (blood eosinophils, exhaled nitric oxide), FEV1, as well as asthma control (ACT) and health-related quality of life (mini-AQLQ) in patients with asthma exacerbations, and constructed a structural equation model to evaluate the causality of these clinical variables.
Results
The SAD prevalence in patients with asthma exacerbation is as high as 75%. SAD is connected with poor asthma control and poor health-related quality of life. The structural equation model indicates that age, obesity, FeNO, and FEV1 are independent predictive factors of SAD. SAD is the main determinant factor of asthma control, which in turn affected health-related quality of life. FEV1 and age directly affect asthma control and affect health-related quality of life through asthma control. In addition, there is a bidirectional relationship between FEV1 and small airway dysfunction and between asthma control and health-related quality of life.
Conclusions
Small airways are involved from an early stage in asthma. Abnormal function of the small airways can significantly increase airway resistance in asthma patients, while worsening their clinical symptoms. In addition, aging is also a key risk factor for asthma control. Especially, small airway dysfunction links asthma control with health-related quality of life.
Similar content being viewed by others
Introduction
Asthma is a common chronic non communicable diseases with heterogeneous clinical phenotypes and characteristics, affecting patients of all ages. Different clinical features, such as fixed airway obstruction, small airway dysfunction, airway inflammation, obesity, etc., can cause frequent and life-threatening exacerbation and extreme symptoms in asthma patients, which have a serious adverse impact on the psychological health, family and medical economy, as well as health-related quality of life (HR-QoL) of millions of asthma patients worldwide.
Small airway dysfunction (SAD) is reported to be quite prevalent in asthma. The ATLANTIS study [1] found that in a large sample of asthma patients, the prevalence of SAD defined by any abnormal physiological indicators is as high as 90.7%. However, this “silent zone of the lung” is often overlooked in clinical practice. Multiple risk factors are closely related to SAD, including age, gender, smoking history, high PM2.5 exposure, elevated BMI, and history of respiratory diseases, all of which affect small airway function to varying degrees [2]. In recent years, there has been accumulated evidence that alterations in the structure and function of small airways can increase airway hyperresponsiveness, which is closely related to the disease progression, frequency of exacerbation, and poor control of asthma [3,4,5]. However, it is not entirely clear whether type 2 inflammatory markers, FEV1 and other clinical features related to the clinical outcomes of asthma directly affect asthma control or mediate the influence of SAD on asthma control.
Health-related quality of life assessment is important when it comes to asthma management and is influenced by asthma exacerbations, asthma control, disease-specific factors such as allergens, and patient self-management of the disease [6, 7]. Identifying the risk factors that affect asthma HR-QoL is crucial for improving patients’ mental health. Although the relationship between asthma triggers and asthma exacerbations has been well confirmed [8], few studies quantified the correlation between small airway function and its related variables with quality of life in patients with exacerbations of asthma.
In view of the multiple factors that influence asthmatic patients’ HR-QoL, the assessment of quality of life should not be limited to a single dimension, such as the stability of symptoms, but should also include subjective factors and general characteristics of patients. This study combines numerous clinical variables with structural equation modeling (SEM) to elucidate the multi-directional associations and potential causal pathways between risk factors such as asthma control, SAD, and HR-QoL in patients with exacerbations of asthma during the observation period. We hypothesize that SAD is the key to linking the clinical characteristics of asthma, type 2 inflammation, symptom control, and HR-QoL.
Methods
Study design
The study adopts prospective observational study. The participants in the study were patients who received treatment in the outpatient and inpatient departments of Henan Provincial People’s Hospital. The Ethics Committee of Henan Provincial People’s Hospital authorized this study (No.2022 − 158). Before the start of this study, all patients thoroughly understood the study and provided written consent.
Participates
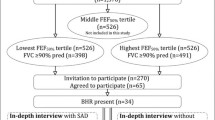
This study recruited 516 asthma patients who received treatment in the outpatient and inpatient departments of Henan Provincial People’s Hospital from October 2021 to December 2022. After completing one year of observation and follow-up, a total of 451 patients completed the case investigation at all research nodes (Fig. 1). All participants were considered eligible to participate in this study after meeting all of the following criteria:⑴ Chinese citizens aged 18 or above,⑵ according to the guidelines in GINA [9], asthma was diagnosed by a professional respiratory physician based on the history of patient’s characteristic symptom patterns and evidence of variable expiratory airflow limitation. Variable expiratory airflow limitation includes (any of the following is sufficient): ①positive bronchodilator test: FEV1 improvement rate after inhalation of bronchodilator is greater than 12% and the absolute value is greater than 200 mL; ②positive bronchial provocation test: FEV1 decreases by ≥ 20% compared with baseline when using standard dose of acetylcholine; ③evidence of excessive variability of PEF twice daily within 2 weeks: average daily diurnal PEF variability is greater than 10%; and ⑶ filled out the patient questionnaire after informed consent is obtained. Patients were excluded if they:⑴ respiratory disease patients such as such as chronic obstructive pulmonary disease, pulmonary fibrosis, and thorax deformities,⑵ patients with coronary heart disease, arrhythmia, uncontrolled malignant hypertension, untreated hypothyroidism, and pregnancy,⑶ merge malignant tumors,⑷ previous history of lobectomy surgery,⑸ patients with cognitive impairment and mental illness.
After providing informed consent, patients would cease to participate in this study if any of these criteria were met: ⑴ the patient withdraws consent,⑵ incomplete questionnaire responses or loss of follow-up,⑶ the investigators believe that patients are no longer suitable to participate in this study.
To avoid selectivity bias, smokers with pack-year ≥ 10 and had no obvious characteristics of chronic obstructive pulmonary disease (FEV1/FVC of at least 0.70 after bronchodilator use) were also included in the study [10,11,12]. Within 4 weeks before the start of the study, we ensured that the patient had a stable disease state and no acute exacerbations or respiratory infection.
Measurements
Measures of small airway function
Lung function and small airway function were performed using MasterScreen® (Viasys Healthcare GmbH, Hoechberg, Germany) using standard procedures according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines and reference values of the Global Lung Function Initiative (GLI) [2, 13,14,15,16]. Indicators of small airway function include difference of resistance between 5 Hz and 20 Hz (R5-R20), pulmonary elastic resistance (X5) measured by IOS, maximal mid-expiratory flow (FEF25-75) measured by pulmonary ventilation function, as well as expiratory flow at forced exhalation of 50% and 75% vital capacity (FEF50 and FEF75). R5-R20 reflects the frequency dependence of resistance, have been shown to be the strongest correlated with small airway disease among several indicators of small airway function, and have the best diagnostic value for respiratory diseases [1, 2]. Therefore, we used R5-R20 to evaluated the correlation between SAD and asthma control and HR-QoL, and applied it to SEM. We divided patients with asthma exacerbations into SAD and non-SAD groups based on whether R5-R20 was higher than 0.07 kPa×s×L− 1.
Health-related quality of life
Health-related quality of life data were gathered at month 12 using the empirically validated Mini Asthma Quality of Life Questionnaire (mini-AQLQ), which is a simple, short, easy to apply test with specificity for asthma. It is divided into four dimensions: symptoms, limitation of activities, emotional function, and environmental stimuli. The closer the average score is to 7 points, the more satisfied the quality of life; The closer the average score is to 1 point, the worse the quality of life [17].
Asthma control
The asthma control test (ACT) questionnaire was used to access the level of symptom control in asthma patients, which contained 5 items: the impact of asthma on life and work, the number of dyspnea, asthma symptoms, the use of emergency medicine, and the self-evaluation of asthma control. The ACT score ranges from 5 to 25, with higher scores indicating better asthma control. A score of 20–25 is considered to be well controlled, 16–19 is poorly controlled, and 5–15 is very poorly controlled.
Asthma exacerbations
According to the 2021 GINA guidelines [9], after diagnosis of asthma and under standardized treatment, patients with the following conditions during follow-up are considered to have exacerbation: patients with a progressive increase in symptoms of shortness of breath, cough, wheezing, or chest tightness and progressive decrease in lung function require additional reliever medications or changes to conventional treatment regimens to control symptoms, including increasing the frequency and dosage of inhaled corticosteroids, oral corticosteroids, or seeking medical attention.
Inflammatory markers
By measuring the blood eosinophil count and exhaled nitric oxide (FeNO) as biomarkers for studying type 2 inflammation. Collected the patient’s elbow venous blood and the blood eosinophil count was measured by Sysmex XN-9100 hematology analyzer. FeNO was measured using NIOX VERO® (Cricassia AB, Uppsala, Sweden) using standard procedures according to the ATS/ERS guidelines [18, 19].
Statistical analyses
Descriptive statistics were conducted using SPSS 26.0.0.0, and the differences in clinical variables between the two study groups were analyzed using independent samples t-test, Wilcoxon U test, and chi-square test and Fisher exact method. The correlation between two variables was completed using Pearson and Spearman. The independent influencing factors of the dependent variable were explored using multivariate regression analysis. The structural equation model was analyzed in Amos 22.0. According to established guidelines, several fitting indices were used to evaluate the goodness of fit of the model(χ2/df, RMSEA, GFI, CFI, NFI).
Result
Study population and patients’ characteristics
This study included 451 participants, of whom 228 patients belonged to the “without exacerbation group” and 223 patients belonged to the “with exacerbation group”. Table 1 summarizes the detailed clinical characteristics of 451 patients. Patients in the “with exacerbation group” are older, with a higher BMI, longer smoking time, more severe ventilation dysfunction, and poorer asthma control scores. Compared with patients without exacerbation, patients with exacerbation had significantly higher exhaled nitric oxide and blood eosinophil count.
There was a significant difference in small airway function between patients with and without asthma exacerbation (Table 2). Small airway resistance, elastic resistance, area of reactance and forced expiratory flow are all associated with whether asthma patients experience exacerbation (Table 3).
Small airway dysfunction: risk factors and outcomes
According to the R5-R20 measurements, Asthma patients with exacerbation were divided into two groups, with 167 in the SAD group and 56 No SAD. Table 4 lists the clinical characteristics, health-related quality of life, and small airway function of each group. Exacerbation asthma patients with SAD are older, have higher body mass index, and have a longer history of smoking. Compared to exacerbation asthma patients without SAD, exacerbation asthma patients with SAD have higher exhaled nitric oxide, but there is no significant difference in blood eosinophil count between the two groups. According to the mini-AQLQ, exacerbation asthma patients with SAD have poorer HR-QoL. Small airway dysfunction is also associated with poorer asthma control scores and a higher number of exacerbation (Fig. 2). In addition, we evaluated the correlation between clinical characteristics, respiratory function, HR-QoL, asthma control and SAD. The results showed that the correlation between blood eosinophil count and SAD was statistically significant, while there was no significant correlation between FVC and FEV1/FVC and SAD (Table 5). We used multivariate logistic regression analysis to evaluate the causal relationship between clinical characteristics and respiratory function and SAD. The results showed that age, BMI and FEV1 were independent influencing factors of SAD. (Figure S1).
Structural equation model
Clinical variables associated with asthma exacerbations or the occurrence of SAD are interrelated in structural equation model. Predictors of SAD include age, BMI, blood eosinophil count, FeNO, smoking, and FEV1, with the expected outcome being asthma control and HR-QoL. The structural equation model (Fig. 3) shows that age, BMI, FeNO, and FEV1 have a significant direct impact on R5-R20. In addition, there is a mutual influence between FEV1 and R5-R20, as well as age and FEV1. However, blood eosinophil count and smoking did not show a statistically significant regression to R5-R20, so it cannot be proven that blood eosinophil count and smoking have a direct impact on the increase of R5-R20. Besides, R5-R20 is a major determinant of asthma control, followed by FEV1 and age. The model also indicates that there is a mutual relationship between asthma control and quality of life, and that the negative effect of R5-R20 on quality of life is mainly mediated by the impact of R5-R20 on asthma control. The full mediation model fit well with the data (χ2/df = 2.331, RMSEA = 0.077 [90% CI, 0.036–0.119], GFI = 0.942, CFI = 0.934, NFI = 0.902) proving that the hypothetical model of quality of life for patients with asthma exacerbations designed in this study is very appropriate.
Discussion
This study shows that SAD is prevalent in patients with asthma exacerbations, involving a range of distal small airway function limitations, linking multiple risk factors for asthma exacerbations with asthma control and quality of life. The structural equation model indicates that SAD negatively impacts quality of life mainly through asthma control. In addition, the model also emphasizes the bidirectional correlation between quality of life and asthma control.
The prevalence of SAD is positively correlated with asthma exacerbations. In this study, 75% of patients with asthma exacerbations had small airway dysfunction, poorer ACT scores, higher frequency of exacerbation, and poorer quality of life. This is consistent with the results of the ATLANTIS cohort study, which showed that the prevalence of SAD ranged from 54 to 91%, and that the prevalence of small airway disease measured using impulse oscillometry correlated with the severity of asthma [3]. In previous epidemiological and clinical observations, obesity has been identified as a key risk factor for asthma pathogenesis, promoting its occurrence and affecting its control through inflammation, oxidative stress, metabolic responses, and changes in the gut microbiome [20, 21]. There are research results shown that obese patients with asthma have a tendency to small airway closure during acetylcholine stimulation, which improves with weight loss; In a nationwide cross-sectional study on the prevalence of SAD in China, it was found that the risk of SAD increases by 6% for every 5 Kg/m2 increase in BMI [22, 23]. These support our research findings that obesity is an independent influencing factor of SAD. The accumulation of chest fat in obese patients leads to a decrease in lung contour compliance, terminal airway compression and limited dilation, as well as small airways and alveolar closure and collapse are all possible causes of SAD.
A positive correlation between the prevalence of small airway dysfunction and increasing age has been found to be positive [23, 24]. Our model confirms this view, and the structural equation shows that age is a direct factor affecting the decline of small airway function in patients with asthma exacerbations. This can be explained by the fact that elderly asthma patients often have more comorbidities such as chronic metabolic diseases, restricted fixed airflow, and emphysema. At the same time, aging itself can lead to alveolar enlargement, bronchial thickening, reduced gas exchange area, and weakened alveolar elastic resilience. Meanwhile, in this model, age can directly and significantly affect asthma control, and part of the effect is mediated by a decrease in FEV1. This finding is consistent with findings from other previous studies [25]. The most common reasons include poor adherence to treatment and improper use of asthma inhalation devices in elderly asthma patients, in addition to the synergistic effect between pathological changes in the airways of asthma patients and age-related deterioration of lung structure and function.
Eosinophils and exhaled nitric oxide, as the main biomarkers of asthma type 2 inflammation, may have different effects on asthma severity, symptom control, and pulmonary function impairment. Studies have shown that airway eosinophilic inflammation is associated with more severe SAD, poorer asthma control, and more frequent severe exacerbation [26]. But our results did not find a statistically significant correlation between eosinophils and R5-R20, which may be due to the following reasons: ⑴ blood eosinophil count was used in our study, which was not as sensitive and accurate as sputum eosinophil count in exploring local airway inflammation; ⑵ Airway eosinophilic inflammation was associated with various clinical indicators, which may indirectly affect R5-R20 by affecting the decrease of FEV1. Although the increase of FeNO level is also one of the predictors of asthma attack [27, 28], a single FeNO index cannot objectively and accurately predict the occurrence of SAD, and it needs to be combined with other conventional control parameters. Therefore, although anti-T2 biotherapy has been proved to improve SAD [29], but there is still no evidence that peripheral airway diseases are directly related to eosinophilic airway inflammation, which needs further exploration.
Smoking has been shown to be one of the major preventable risk factors for small airway dysfunction in most previous studies, with smokers at a 1.16-fold risk of developing small airway dysfunction compared with nonsmokers [23, 30]. In our study, the proportion of smokers with a pack-year great than 10 in the SAD group was significantly higher than the No SAD group, but there was no significant correlation between smoking and R5-R20 in the structural equation model. We analyze this in relation to the widespread adoption of healthy lifestyles at present. People’s health awareness has increased, both the number of smokers, the duration of smoking and the amounts of cigarettes smoked are significantly reduced. Therefore, current amount of smoking does not induce the occurrence of R5-R20. In addition, smoking may indirectly affect R5-R20 by affecting other measures of small airway function, such as FEF25 − 75.
At present, the main goal of asthma treatment is to achieve symptom control, aiming to maintain optimal lung function in its long-term management. Our model suggests that FEV1 interacts with SAD, demonstrating a direct relationship between SAD and airflow obstruction in the large airway. Chronic airway inflammation affects both large and small airways, and is the main cause of asthma development and airflow limitation. Airway inflammation exhibits heterogeneity, with varying degrees of impact on the large and small airways, as well as differences in the degree and process of airway obstruction. Inflammatory cell penetration sites are mainly concentrated in the small airways and progressively involve the large airways and alveoli, and damage the elastic tissues of the airways through the secretion of proteases, leading to airway dysfunction and airway remodeling; Small airway dysfunction can alter airway pressure and flow, leading to changes in lung mechanics, such as decreased lung compliance, which in turn affects the pressure in the large airways [31, 32]. The interaction between SAD and FEV1 jointly promotes airway injury, and each affects asthma control as independent risk factors. Therefore, the management of pulmonary function in asthma control needs to simultaneously consider both large and small airway functions.
HR-QoL, as a measure of outcome, there are individual differences in the extent to which different symptoms and/or functional limitations impair patients. For effective disease management, self-perception of disease severity in patients with asthma needs to be assessed to determine the individual impact of asthma on health-related quality of life in different patients. It is generally believed that the higher the severity of symptoms and the frequency of exacerbation, the greater the impairment of physical or emotional functioning, which leads to the hypothesis that the impact of asthma on an individual’s quality of life can be determined only by assessing these areas. However, the extent to which a patient’s quality of life is impaired by any particular level of symptoms and/or functional limitations varies and may also change with disease-specific variables or potential relationships between variables. The model in this study confirms that asthma control is an influencing factor of health-related quality of life for asthma patients, and the relationship between the two is bidirectional, suggesting that improving the quality of life of asthma patients can also alleviate asthma control. Notably, small airway dysfunction is more prevalent in the early stages of asthma, so it is often overlooked because airflow limitation is insufficient to impact quality of life. Ben et al. showed that people with small airway obstruction are more likely to have a worse quality of life [33]. This is consistent with our results, and we further demonstrate that the negative effects of SAD on quality of life in people with asthma are mediated by poorly asthma control.
Our research still has some limitations. Firstly, at the beginning of the study, we ignored the confounding factor of patients’ therapies which could have biased our results even if we ensured that patients were in a stable state for the 4 weeks before enrollment. Secondly, our study may have underestimated the correlation between type 2 inflammation and SAD, as anti-T2 biotherapy significantly improves the condition of severe SAD patients [29]. This may be related to the use of different statistical methods, and more rigorous prospective studies are still needed to explore the relationship between blood eosinophils, FeNO and SAD. In term of methodology, there may be some deviation in evaluating the impact of SAD on asthma control and HR-QoL by using a single R5-R20 index. In the follow-up, FEF25 − 75 combined with LCI can be added as evaluation indicators of SAD and establish an adjustment model to ensure the robustness of the model. In conclusion, our study explained the risk factors and influencing outcomes of small airway dysfunction through the establishment of structural equation models, and understood the relationship between the impact of SAD and asthma control on health-related quality of life for asthma patients, which is the basis for improving the management of patients with asthma exacerbations.
Conclusion
Small airway dysfunction is an important disease feature in patients with asthma exacerbations. We linked the small airway dysfunction with the health-related quality of life in patients with asthma exacerbations, and proved that the risk factors associated with asthma exacerbations directly affect patients’ quality of life or indirectly by mediating small airway dysfunction. In addition, there is a correlation between asthma control and health-related quality of life.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACT:
-
Asthma control test
- AX:
-
Area of reactance
- BMI:
-
Body mass index
- FEF25-75 :
-
Forced expiratory flow from 25 to 75% of FVC
- FEF25 :
-
Forced expiratory flow at 25% of FVC
- FEF50 :
-
Forced expiratory flow at 50% of FVC
- FEF75 :
-
Forced expiratory flow at 75% of FVC
- FeNO:
-
Fractional exhaled nitric oxide
- FEV1/FVC:
-
Forced expiratory volume in one second/forced vital capacity
- FEV1 :
-
Forced expiratory volume in one second
- FVC:
-
Forced vital capacity
- HR-QoL:
-
Health-related quality of life
- Mini-AQLQ:
-
Mini asthma quality of life questionnaire
- R20:
-
Resistance at 20 Hz
- R5-R20:
-
The difference between the total airway viscosity resistance and the central airway viscosity resistance
- R5:
-
Resistance at 5 Hz
- X5:
-
Pulmonary elastic resistance
References
Postma DS, Brightling C, Baldi S, Van den Berge M, Fabbri LM, Gagnatelli A, Papi A, Van der Molen T, Rabe KF, Siddiqui S, et al. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): baseline data from a prospective cohort study. Lancet Respiratory Med. 2019;7:402–16.
Chiu H-Y, Hsiao Y-H, Su K-C, Lee Y-C, Ko H-K, Perng D-W. Small airway dysfunction by impulse oscillometry in symptomatic patients with preserved pulmonary function. J Allergy Clin Immunol Pract. 2020;8.
Kraft M, Richardson M, Hallmark B, Billheimer D, Van den Berge M, Fabbri LM, Van der Molen T, Nicolini G, Papi A, Rabe KF, et al. The role of small airway dysfunction in asthma control and exacerbations: a longitudinal, observational analysis using data from the ATLANTIS study. Lancet Respiratory Med. 2022;10:661–8.
Kole TM, Vanden Berghe E, Kraft M, Vonk JM, Nawijn MC, Siddiqui S, Sun K, Fabbri LM, Rabe KF, Chung KF, et al. Predictors and associations of the persistent airflow limitation phenotype in asthma: a post-hoc analysis of the ATLANTIS study. Lancet Respiratory Med. 2023;11:55–64.
van den Bosch WB, James AL, Tiddens HAWM. Structure and function of small airways in asthma patients revisited. Eur Respiratory Review: Official J Eur Respiratory Soc. 2021;30.
Kansen HM, Le T-M, Meijer Y, Uiterwaal CSPM, Knulst AC, van der Ent CK, van Erp FC. Perceived triggers of asthma impair quality of life in children with asthma. Clin Experimental Allergy: J Br Soc Allergy Clin Immunol. 2019;49:980–9.
Matsumoto-Sasaki M, Suzuki M, Kimura H, Shimizu K, Makita H, Nishimura M, Konno S. Association of longitudinal changes in quality of life with comorbidities and exacerbations in patients with severe asthma. Allergology International: Official J Japanese Soc Allergology. 2022;71:481–9.
Luskin AT, Chipps BE, Rasouliyan L, Miller DP, Haselkorn T, Dorenbaum A. Impact of asthma exacerbations and asthma triggers on asthma-related quality of life in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol Pract. 2014;2.
Global Initiative for Asthma. Global Stratrgy for Asthma Management and Prevention, 2021. Updated April 2021. Available from: www.ginasthma.org.
Abdo M, Trinkmann F, Kirsten A-M, Pedersen F, Herzmann C, von Mutius E, Kopp MV, Hansen G, Waschki B, Rabe KF et al. Small airway dysfunction links asthma severity with physical activity and symptom control. J Allergy Clin Immunol Pract. 2021;9.
Tommola M, Ilmarinen P, Tuomisto LE, Haanpää J, Kankaanranta T, Niemelä O, Kankaanranta H. The effect of smoking on lung function: a clinical study of adult-onset asthma. Eur Respir J. 2016;48:1298–306.
Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, Gouskova NA, Hansel NN, Hoffman EA, Kanner RE, et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–21.
Bhakta NR, McGowan A, Ramsey KA, Borg B, Kivastik J, Knight SL, Sylvester K, Burgos F, Swenson ER, McCarthy K et al. European Respiratory Society/American Thoracic Society technical statement: standardisation of the measurement of lung volumes, 2023 update. Eur Respir J. 2023;62.
Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic I, Cooper BG, Culver B, Derom E, Hall GL et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60.
Bickel S, Popler J, Lesnick B, Eid N. Impulse oscillometry: interpretation and practical applications. Chest. 2014;146:841–7.
Hall GL, Filipow N, Ruppel G, Okitika T, Thompson B, Kirkby J, Steenbruggen I, Cooper BG, Stanojevic S. Official ERS technical standard: global lung function Initiative reference values for static lung volumes in individuals of European ancestry. Eur Respir J. 2021;57.
Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the mini asthma quality of life questionnaire. Eur Respir J. 1999;14:32–8.
Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin A-C, Plummer AL, Taylor DR. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–15.
ATS/ERS recommendations for standardized procedures for the. Online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30.
Miethe S, Karsonova A, Karaulov A, Renz H. Obesity and asthma. J Allergy Clin Immunol. 2020;146:685–93.
Barros R, Moreira P, Padrão P, Teixeira VH, Carvalho P, Delgado L, Moreira A. Obesity increases the prevalence and the incidence of asthma and worsens asthma severity. Clin Nutr. 2017;36:1068–74.
Peters U, Subramanian M, Chapman DG, Kaminsky DA, Irvin CG, Wise RA, Skloot GS, Bates JHT, Dixon AE. BMI but not central obesity predisposes to airway closure during bronchoconstriction. Respirol (Carlton Vic). 2019;24:543–50.
Xiao D, Chen Z, Wu S, Huang K, Xu J, Yang L, Xu Y, Zhang X, Bai C, Kang J, et al. Prevalence and risk factors of small airway dysfunction, and association with smoking, in China: findings from a national cross-sectional study. Lancet Respiratory Med. 2020;8:1081–93.
Dai C, Wu F, Wang Z, Peng J, Yang H, Zheng Y, Lu L, Zhao N, Deng Z, Xiao S, et al. The association between small airway dysfunction and aging: a cross-sectional analysis from the ECOPD cohort. Respir Res. 2022;23:229.
Wang J, Zhang X, Zhang L, Liu Y, Wang G, Zhang HP, Wang L, Kang DY, Oliver BG, Wan HJ et al. Age-related clinical characteristics, inflammatory features, phenotypes, and treatment response in asthma. J Allergy Clin Immunol Pract. 2023;11.
Abdo M, Pedersen F, Kirsten A-M, Veith V, Biller H, Trinkmann F, von Mutius E, Kopp M, Hansen G, Rabe KF et al. Longitudinal impact of sputum inflammatory phenotypes on small airway dysfunction and disease outcomes in asthma. J Allergy Clin Immunol Pract. 2022;10.
Menzies-Gow A, Mansur AH, Brightling CE. Clinical utility of fractional exhaled nitric oxide in severe asthma management. Eur Respir J. 2020;55.
Busse WW, Wenzel SE, Casale TB, FitzGerald JM, Rice MS, Daizadeh N, Deniz Y, Patel N, Harel S, Rowe PJ, et al. Baseline FeNO as a prognostic biomarker for subsequent severe asthma exacerbations in patients with uncontrolled, moderate-to-severe asthma receiving placebo in the LIBERTY ASTHMA QUEST study: a post-hoc analysis. Lancet Respiratory Med. 2021;9:1165–73.
Abdo M, Watz H, Veith V, Kirsten A-M, Biller H, Pedersen F, von Mutius E, Kopp MV, Hansen G, Waschki B, et al. Small airway dysfunction as predictor and marker for clinical response to biological therapy in severe eosinophilic asthma: a longitudinal observational study. Respir Res. 2020;21:278.
Verbanck S, Schuermans D, Paiva M, Meysman M, Vincken W. Small airway function improvement after smoking cessation in smokers without airway obstruction. Am J Respir Crit Care Med. 2006;174:853–7.
Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet (London England). 2004;364:709–21.
Aoshiba K, Nagai A. Differences in airway remodeling between asthma and chronic obstructive pulmonary disease. Clin Rev Allergy Immunol. 2004;27:35–43.
Knox-Brown B, Patel J, Potts J, Ahmed R, Aquart-Stewart A, Barbara C, Buist AS, Cherkaski HH, Denguezli M, Elbiaze M, et al. The association of spirometric small airways obstruction with respiratory symptoms, cardiometabolic diseases, and quality of life: results from the burden of obstructive lung disease (BOLD) study. Respir Res. 2023;24:137.
Acknowledgements
We would like to thank Yue Liu for his suggestions when we had difficulties in conducting this study, and Guihua Zhao for her help in reviewing the data.
Funding
This study is supported by the Provincial Science and Technology Development Plan Joint Fund ( 222103810053) and Henan Province Medical Science and Technology Research and Development Plan Key Projects Jointly Constructed by the Provincial and Ministerial Departments ( SBGJ202302003).
Author information
Authors and Affiliations
Contributions
FG conceived and designed the study, FG drafted the manuscript, HZ assisted with the preparation of tables, and JHL assisted with statistical analysis. LMZ critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The project was approved by the Ethics Committee of Henan Provincial People’s Hospital, and the clinical study was carried out in accordance with the ethical guidelines, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Author contributions
We would like to thank Yue Liu for his suggestions when we had difficulties in conducting this study, and Guihua Zhao for her help in reviewing the data.
Data sharing statement
No additional data are available.
Additional file.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Figure S1
Forest diagram of Multivariate logistic regression analysis. Square depicts the point estimate. Horizontal bars depicts 95% confidence interval (CI)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, F., Lei, J., Zhu, H. et al. Small airway dysfunction links asthma exacerbations with asthma control and health-related quality of life. Respir Res 25, 306 (2024). https://doi.org/10.1186/s12931-024-02937-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-024-02937-5