Abstract
Objectives
Non-tuberculous mycobacteria (NTM) infection is an increasing health problem due to delaying an effective treatment. However, there are few data on 18F-FDG PET/CT for evaluating the status of NTM patients. The aim of this study was to investigate the potential value of 18F-FDG PET/CT in guiding the treatment strategy of NTM patients.
Methods
We retrospectively analyzed the cases of 23 NTM patients who underwent 18F-FDG PET/CT. The clinical data, including immune status and severity of NTM pulmonary disease (NTM-PD), were reviewed. The metabolic parameters of 18F-FDG included maximum standardized uptake value (SUVmax), SUVmax of the most FDG-avid lesion (SUVTop), SUVTop/SUVmax of the liver (SURLiver), SUVTop/SUVmax of the blood (SURBlood), metabolic lesion volume (MLV), and total lesion glycolysis (TLG). The optimal cut-off values of these parameters were determined using receiver operating characteristic curves.
Results
There were 6 patients (26.09%) with localized pulmonary diseases and 17 patients (73.91%) with disseminated diseases. The NTM lesions had high or moderate 18F-FDG uptake (median SUVTop: 8.2 ± 5.7). As for immune status, the median SUVTop in immunocompromised and immunocompetent patients were 5.2 ± 2.5 and 10.0 ± 6.4, respectively, with a significant difference (P = 0.038). As for extent of lesion involvement, SURLiver and SURBlood in localized pulmonary and disseminated diseases were 1.9 ± 1.1 vs. 3.8 ± 1.6, and 2.7 ± 1.8 vs. 5.5 ± 2.6, respectively, with a significant difference (P = 0.016 and 0.026). Moreover, for disease severity, SUVmax of the lung lesion (SUVI−lung) and SUVmax of the marrow (SUVMarrow) in the severe group were 7.7 ± 4.3 and 4.4 ± 2.7, respectively, significantly higher than those in the non-severe group (4.4 ± 2.0 and 2.4 ± 0.8, respectively) (P = 0.027 and 0.036). The ROC curves showed that SUVTop, SURLiver, SURBlood, SUVI−lung, and SUVMarrow had a high sensitivity and specificity for the identification of immune status, lesion extent, and severity of disease in NTM patients.
Conclusion
18F-FDG PET/CT is a useful tool in the diagnosis, evaluation of disease activity, immune status, and extent of lesion involvement in NTM patients, and can contribute to planning the appropriate treatment for NTM.
Similar content being viewed by others
Introduction
Non-tuberculous mycobacteria (NTM) are Mycobacterium (M.) species other than M. leprae and M. tuberculous. NTM are prevalent in the environment, such as soil, dust, plants, water sources, foods, and animals, and are more likely to infect immunocompromised than immunocompetent individuals [1]. The prevalence of diseases caused by NTM has increased in recent years; for instance, in the United States, the number of people affected rose from 40 to 85 per 100,000 individuals between 2010 and 2014 [2]. In China, NTM-related diseases have also gradually become more common over the last 20 years [3], with more than 170 NTM species reported [4, 5].
NTM can cause a variety of clinical syndromes ranging from lymphadenopathy to aseptic meningitis [6, 7]. The most common clinical manifestation of NTM infection is NTM pulmonary disease (NTM-PD). Furthermore, extra-pulmonary NTM diseases, such as infections of soft tissues, lymph nodes or bone, can emerge as a result of NTM exposure [5]. The primary diagnostic methods are culture of clinical samples, and molecular and genetic detection (including DNA PCR assay, metagenomic next-generation sequencing (mNGS) from body fluid or histopathological tissue) [9]. However, these methods are invasive, requiring biopsies from patients. In addition, the sensitivity and accuracy of these procedures are limited due to sampling errors or contamination, as well as NTM heterogeneity within biopsy samples [8, 9].
Computed tomography (CT) has been widely used in the diagnosis of pulmonary diseases. However, NTM-PD have poor specific radiological manifestations. NTM can also infect organs and tissues besides lungs, such as the skin, bones and soft tissues, also known as disseminated NTM infections due to immune status [5]. The current guidelines for NMT treatment, such as the Official ATS/ERS/JRS/ALAT Clinical Practice Guideline for Treatment of NTM in 2020 [10] and the British Thoracic Society guidelines for the management of NTM pulmonary disease in 2017 [11], strongly recommend that individuals may require a period of longitudinal assessment (including immune status, the severity and risk of progressive NTM infection, and the extent of lesion involvement) before a decision is made to start treatment. Therefore, it is critical to develop a noninvasive imaging tool for evaluating active lesion, immune status, and the extent of lesion involvement [12].
18F-FDG (2-[18F]-fluoro-2-deoxy-D-glucose) is a non-physiological glucose analogue, which is metabolized by the same physiological processes as glucose [13]. Inflammatory cells with multiple mechanistic similarities to malignant cells, such as macrophages, demonstrate greater FDG uptake [13]. 18F-FDG PET/CT has been used for detecting inflammation and infection such as fever of unknown origin (FUO), sarcoidosis, IgG4-related systemic disease, and human immunodeficiency virus (HIV)-related infections, among others. Its roles in diagnosis include mapping extent of disease, assessing therapy response based on whole-body imaging, and changes in FDG metabolic parameters ahead of morphological changes by conventional imaging techniques (CT and MRI) [14,15,16]. Moreover, 18F-FDG PET/CT has been suggested as a potential tool for evaluating the responsiveness of tuberculosis (TB) infection to anti-tuberculosis therapy [17, 18]. A few case reports have indicated a possible value of 18F-FDG PET/CT for patients with NTM [19,20,21,22]. However, available data concerning the findings of 18F-FDG PET/CT in NTM has been limited. Furthermore, according to the guidelines for the management of non-tuberculosis mycobacterial pulmonary disease by the British Thoracic Society [11], the choice of therapeutic strategy relies strongly on the immune status, extent of lesion involvement, and disease severity in NTM patients. Meanwhile, there are limited data on the use of 18F-FDG PET/CT for evaluating the status of NTM patients. Therefore, this retrospective study was performed to investigate and confirm the potential value of 18F-FDG PET/CT in guiding the treatment strategy in NTM patients.
Materials and methods
Patient information
We retrospectively reviewed 23 patients with a confirmed diagnosis of NTM infection who underwent 18F-FDG PET/CT between December 2016 and October 2021 in the First Affiliated Hospital, Zhejiang University School of Medicine. The flow diagram of the study design is presented in Fig. 1. The clinical information (age, gender, risk factor, and syndrome) and laboratory test results (serum cytokines, immune cell, and mycobacterium species) were obtained by reviewing the available medical records. A total of 16 patients underwent 18F-FDG PET/CT imaging before anti-NTM therapy, while the other 7 patients underwent 18F-FDG PET/CT imaging after anti-NTM treatment (within less than 6 months).
Based on the risk factors of immune status, the 23 enrolled patients were divided into two groups: immunocompromised patients and immunocompetent patients. According to a previous article [23], immunocompromised patients were defined as follows: those with primary immune deficiency disease; active malignancy; receiving cancer chemotherapy; HIV infection with a CD4 T-lymphocyte count < 200 cells/mL; solid organ transplantation; hematopoietic stem cell transplantation; receiving corticosteroid therapy with a dose 20 mg prednisone or equivalent daily for ≥ 14 days or a cumulative dose > 700 mg of prednisone; receiving biologic immune modulators (such as azathioprine, corticosteroids and mesalazine etc.); receiving disease-modifying anti-rheumatic or other immunosuppressive drugs. Based on the extent of lesion involvement in NTM patients [24, 25], these 23 patients classified into two groups: localized pulmonary diseases group and disseminated diseases group. The patients with NTM disease involvement only in lungs were defined as localized pulmonary diseases, while the patients with NTM disease involvement both in lungs and other tissues or organs (such as skin, lymph nodes, bone and joint, soft tissue, urinary system, etc.) were defined as disseminated diseases. According to the disease severity, these patients were further classified into severe and non-severe disease groups based on the British Thoracic Society guidelines for the management of NTM-PD in 2017 [5, 11]. Non-severe disease included: (a) mild-moderate symptoms, (b) no signs of systemic illness, (c) absence of lung cavitation and extensive lung disease, (d) acid-fast bacilli (AFB) smear-negative in the pulmonary specimens. Severe disease included: (a) presence of severe symptoms and signs of systemic illness, (b) presence of lung cavitation and extensive lung involvement, (c) pulmonary specimens positive for acid-fast bacilli (AFB) smear.
18F-FDG PET/CT imaging
18F-FDG was synthesized using a Siemens Eclipse cyclotron (Siemens Medical Solutions, Knoxville, TN 37,932, USA) and a Siemens Explora FDG4 chemical synthesis module. The patients were fasted for at least 4 h before being injected with 18F-FDG at a dose of 4.44 to 5.55 MBq/kg. Images were acquired using a PET/CT instrument (Biograph 16 and Version, Siemens Company, Germany). Two experienced nuclear medicine physicians independently reviewed PET/CT images until consensus was reached. Semiquantitative analysis was performed using the maximum standardized uptake value (SUVmax). Furthermore, 10 metabolic parameters were also recorded: SUVTop (SUVmax of the most FDG-avid lesion), SUVI−Lung (SUVmax of intra-pulmonary lesions), SUVE−Lung (SUVmax of extra-pulmonary lesions), SUVLiver (SUVmax of the liver), SUVSpleen (SUVmax of the spleen), SUVMarrow (SUVmax of the marrow), SURLiver (the most FDG-avid lesion-to-liver SUVmax ratio) and SURBlood (the most FDG-avid lesion-to-blood pool SUVmax ratio), metabolic lesion volume (MLV) and total lesion glycolysis (TLG). The MLV of each lesion was calculated using an automated region-growing algorithm with SUV thresholds of 2.5. The TLG was measured as MTV multiplied by the average SUV of each lesion.
Statistical analysis
Quantitative data were presented as mean and standard deviation. All statistical analyses were performed using GraphPad Prism, version 8.0 (GraphPad Software, San Diego, CA, USA). The differences in immune status, extent of lesion involvement and severity of NTM-PD were analyzed using the chi-squared test. A p-value of less than 0.05 was considered statistically significant. ROC curves were used to determine the optimal cut-off values.
Results
Patients’ clinical characteristics
The demographics and clinical features of the 23 patients with NTM infection were summarized in Table 1. These patients included 19 (82.6%) men and 4 (17.4%) women, with an age range between 14 and 84 years (median, 60.0 years). The diagnosis of these patients was confirmed by tissue culture (n = 6), non-tuberculous mycobacteria DNA PCR assay (NTM-DNA-PCR) (n = 3), and metagenomic next-generation sequencing (mNGS) (n = 14). Specimens were collected from blood or bronchoalveolar lavage fluid (BALF), pus, sputum, tissue of lung lesions, and bone lesions. The most frequent NTM species detected was M. intracellulare (12 cases, 52.1%), and 1 patient had mixed infection with M. intracellulare and M. kansasii.
In terms of the risk factors of immune status, among the 23 patients, 9 (39.1%) were immunocompromised, including during chemotherapy (6 cases, 26.1%), due to lymphoma (2 cases), leukemia (one case), MDS (one case), lung cancer (one case), pancreatic cancer (one case), acquired immune deficiency syndrome (AIDS) (2 cases, 8.7%), and monocytopenia and mycobacterial infection syndrome (MonoMAc syndrome) (1 case, 4.3%). The other 14 patients were immunocompetent, of which 5 patients (37.8%) presented with underlying diseases, such as splenectomy (2 case, 8.6%), hepatitis B cirrhosis (1 case, 4.3%), tuberculosis (1 case, 4.3%), and early-stage lung cancer (1 case, 4.3%). Six patients exhibited a mild chronic obstructive pulmonary disease (COPD) because of long-term smoking, whereas 3 patients had no risk factors or underlying diseases.
Laboratory characteristics
The laboratory characteristics of the 23 patients with NTM infection were presented in Supplementary Table 1. The median white cell count was 7.6 × 109/L (range: 2.3–22.8 × 109/L), and 10 patients (43.5%) had leukocytosis (10 cases, 43.5%). The median platelet count was 226 × 109/L (range: 85–492 × 109/L), and 8 patients (34.8%) had thrombocytosis. The majority of patients showed varying levels of increase in the C-reactive protein (CRP) (16 cases, 69.6%), erythrocyte sedimentation rate (ESR) (15 cases, 65.2%) and interleukin 6 (IL-6) level, (20 cases, 87%). The other laboratory test results were almost within the normal range, including procalcitonin (PCT), serum cytokines, and cellular immune function in the peripheral blood.
18F-FDG PET/CT imaging characteristics
The 18F-FDG PET/CT imaging characteristics of the 23 patients with NTM infection were summarized in Table 2.
Intra-pulmonary lesions
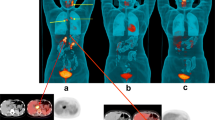
18F-FDG PET/CT revealed NTM-PD in 22 patients (95.6%) with lung involvement, including the involvement of a single lobe (5 cases) and two lobes (17 cases). Among these patients, the common CT feature was the presence of patchy infiltrate (18 cases, 81.8%) and bronchiectasis (8 cases, 36.4%). Lesions with high FDG-uptake (SUVI−Lung > 2.5) were observed in 19 patients (Fig. 2A, blue arrows) and those with low FDG-uptake (SUVI−Lung ≤ 2.5) were observed in 3 patients (Fig. 2B, white arrows).
Patient 1 (No.1): A 38-year-old male patient presented with cough, phlegm and intermittent fever for two months. The pathology of the pulmonary puncture suggested chronic granulomatous inflammation of the lungs. Finally, metagenomics next-generation sequencing (mNGS) of alveolar lavage fluid confirmed M. abscessus infection. Multiple patchy shadows (white arrows, SUVmax = 11.3) and consolidation (blue arrows, SUVmax = 3.7) in both lungs on the axial slices of 18F-FDG PET/CT scan images.Patient 2 (No.10): A 60-year-old female patient with a history of acute lymphocyte leukemia. After one cycle of chemotherapy, opportunistic intracellular mycobacterial infection appeared in both lungs and was confirmed by TB-DNA-PCR of the sputum. The patient was given active anti-infection treatment (oral azithromycin + ethambutol + rifampicin for 5 months). To assess the tumor activity after treatment, an 18F-FDG PET/CT scan was performed, which revealed multiple FDG-unavid nodules (white arrows, SUVmax = 2.2) in the right lung. The pathology of the wedge resection of the right lung nodule indicated pulmonary granulomatosis
Extra-pulmonary lesions
Extra-pulmonary lesions with abnormally high 18F-FDG FDG uptake were found in 17 patients (73.8%). Among them, 18F-FDG PET/CT showed increased 18F-FDG accumulation in the lymph node (14 cases), bone (6 cases), subcutaneous tissue (1 case), the spleen (1 case), and adrenal gland (1 case). Lesions with high FDG-uptake (SUVE−Lung > 2.5) were observed in 15 patients, and those with low FDG-uptake (SUVE−Lung ≤ 2.5) were observed in 2 patients.
Extent of lesion involvement
Disseminated NTM infection was observed in 17 patients using 18F-FDG PET/CT. Among them, involvements of the common multi-system were found in the lung and lymph nodes (9 cases), and lung and bone (3 cases) (Fig. 3). Other multi-system involvements included lung, lymph node, bone and subcutaneous tissue (1 case), lung, lymph node and bone (1 case), lung, lymph node and spleen (1 case), lung, lymph node and adrenal gland (1 case), and lymph node and bone (1 case). Localized pulmonary NTM infection was identified in 6 patients.
A 47-year-old man (No.2) presented with right calf and chest pain, fever, and sweating for one month. Laboratory examination revealed significant increases in leukocyte count (19 × 109/L), erythrocyte sedimentation rate (77 mm/hour), and C-reactive protein level (87 mg/L). 18F-FDG PET/CT showed diffuse bony destruction with increased high FDG uptake (SUVmax = 14.8) and mild-uptake small nodule in the upper lobe of the right lung (white arrows, SUVmax = 2.0). Finally, M. intracellulare was confirmed by sputum culture of a rib lesion after removal in orthopedics
Metabolic parameters
The median SUVTop in 23 patients with lung involvement was 8.2 ± 5.7 (range: 1.2–28.1); the median SUVI−Lung in 22 patients with lung involvement was 6.6 ± 3.9 (range: 1.2–13.9); the median SUVE−Lung in 17 patients was 6.3 ± 6.3 (range: 1.8–28.1); the median SUVLiver, SUVMarrow and SUVSpleen were 2.7 ± 0.6 (range: 1.7–3.8), 3.7 ± 2.0 (range: 1.7–6.7), and 2.5 ± 0.9 (range: 1.6-6.0), respectively; the median SURLiver and SURBlood were 3.7 ± 2.3 (range: 0.5–9.1) and 5.9 ± 3.9 (range: 0.6–16.4), respectively; the median MLV and TLG were 122.2 ± 211.7 (range: 0-972.6) and 526.9 ± 992.1 (range: 0-4628.6), respectively.
18F-FDG PET/CT characteristics in different immune states, extent of lesion involvement, and severity of NTM-PD
The comparison of clinical and 18FDG PET/CT characteristics in patients with different immune states, extent of lesion involvement, and severity of NTM-PD were shown in Table 3; Fig. 4. As for the immune state, SUVTop in immunocompetent patients was significantly higher than that in immunocompromised patients (median SUVTop, 10.0 ± 6.4 vs. 5.2 ± 2.5, P = 0.038, Fig. 4A). As for extent of lesion involvement, SURLiver and SURBlood in disseminated disease were significantly higher than those in localized pulmonary disease (median SURLiver, 3.8 ± 1.6 vs. 1.9 ± 1.1, P = 0.016, Fig. 4B; median SURBlood, 5.5 ± 2.6 vs. 2.7 ± 1.8, P = 0.026, Fig. 4C). As for the severity of NTM-PD, the median SUVMarrow, and SUVI−Lung in patients with severe NTM-PD were significantly higher than those in patients with non-severe NTM-PD (median SUVMarrow, 4.4 ± 2.0 vs. 2.4 ± 0.8, P = 0.027, Fig. 4D; median SUVI−Lung, 7.7 ± 4.3 vs. 4.3 ± 2.3, P = 0.036). There were no significant differences in the metabolic parameters of MLV and TLG between the localized pulmonary disease group and the disseminated disease group, the severe and non-severe NTM-PD group, and the immunocompetent and immunocompromised patients.
(A). Comparison of SUVTop between immunocompromised and immunocompetent patients. (B). Comparison of SURLiver between the localized pulmonary diseases group and the disseminated diseases group. (C). Comparison of SURBlood between the localized pulmonary diseases group and the disseminated diseases group. (D). Comparison of SUVMarrow between severe NTM-PD and non-severe NTM-PD. (E). Comparison of SUVI−Lung between severe NTM-PD and non-severe NTM-PD. (F). Comparison of platelet count between severe NTM-PD and non-severe NTM-PD. (G). ROC curve analysis for predicting immunocompromised status in NTM patients using SUVTop values of 18F-FDG-PET/CT scans. (H). ROC curve analysis for predicting disseminated NTM infection using SURLiver and SURBlood values of 18F-FDG-PET/CT scans. (I). Receiver operating characteristic curve (ROC) analysis for predicting severe NTM-PD using platelet count, and SUVMarrow, and SUVI−Lung values of 18F-FDG-PET/CT scans
Receiver operating characteristic (ROC) curves were drawn to evaluate the consistency between the 18F-FDG PET/CT metabolic parameters and clinical features (Fig. 4). The area under the curve (AUC) values in immunocompromised and immunocompetent groups were 0.794 for SUVTop (P = 0.02) (Fig. 4G). The cut-off value of SUVTop was 7.1, and the sensitivity and specificity of 18F-FDG PET/CT for immunocompromised states in NTM patients were 88.9% and 64.3%, respectively. The AUC in group of the localized pulmonary NTM infection and the disseminated NTM infection group were 0.819 for SURLiver (P = 0.023) and 0.814 for SURBlood (P = 0.025) (Fig. 4I). When the cut-off values of SURLiver and SURBlood were 2.8 and 4.4, respectively, the sensitivity and specificity of 18F-FDG PET/CT for disseminated NTM infection were 83.3% and 70.6%, and 83.3% and 52.9%, respectively. The AUC in the severe and non-severe groups were 0.897 for SUVMarrow (P = 0.001) and 0.688 for SUVI−Lung (P = 0.158), respectively (Fig. 4H). When the cut-off values of SUVMarrow and SUVI−Lung were 3.1 and 4.6, respectively, the sensitivity and specificity of 18F-FDG PET/CT for the identification severity of NTM-PD were 76.9% and 88.9%, and 76.9% and 55.9%, respectively.
Correlations between the metabolic parameters of 18F-FDG PET/CT and the clinical laboratory results
Spearman’s correlation analysis (Fig. 5) revealed a significant positive correlation between serum PCT, IL-6 and SUVMarrow (r = 0.507 and 0.530, p < 0.05). Furthermore, serum PCT and ferritin were significantly positively related to SUVSpleen (r = 0.577 and 0.558, respectively, p < 0.05).
Discussion
Non-tuberculous mycobacteria (NTM) are ubiquitously present in the environment and include opportunistic pathogens that can cause various diseases in multiple organs in both immunocompromised and immunocompetent individuals. Recently, the prevalence of NTM diseases have increased globally, which are now recognized to represent an emerging public health problem [1]. According to established criteria for NTM disease treatment [10, 11], it strongly recommended that individuals receive a period of longitudinal assessment (including immune status, the severity and risk of progressive NTM infection, and the extent of lesion involvement) before a decision is made to start treatment. Therefore, it is critical to develop noninvasive imaging tools for evaluating active lesions, immune status, and the extent of lesion involvement [12]. Previous studies have revealed the usefulness of 18F-FDG PET in differentiating latent from active TB and evaluating early therapeutic response to anti-TB. Moreover, high FDG-avid lesions in a patient with TB may also reflect a host immune system response that will ultimately prevail [26].
18F-FDG PET/CT has revolutionized oncology care owing to its mechanism of action, including being taken up by cell surface transporters (mainly the glucose transporter-1 and 3, GLUT-1 and 3) and being transformed by the rate-limiting glycolytic enzyme hexokinase into FDG-6-phosphate [13]. Infection and inflammation contribute to enhancing vascular permeability and the release of inflammatory mediator expressing increased levels of GLUT-1 and 3 (macrophages, neutrophils), as well as increased HK activity, resulting in greater 18F-FDG delivery to affected sites [13]. Furthermore, 18F-FDG PET/CT has been used for diagnosing tumor, infection, fever of unknown origin and assessing therapy response due to a whole-body imaging and changes in FDG metabolic parameters ahead of morphological changes by conventional imaging techniques (CT and MRI). Therefore, 18F-FDG PET/CT has been widely used not only for the diagnosis of infectious and inflammatory diseases but also in the evaluation active stage of systemic disease [13]. SUVmax and SUR are the most commonly used imaging biomarkers to assess FDG uptake levels. Besson FL et al. [27] indicated that there was a significant improvement in specificity by using SURLiver > 1 compared to visual analysis. In the present study, we collected 18F-FDG PET/CT scanning data from 22 patients with NTM-PD with a median SUVmax of 6.6 (range: 1.2–13.9), which was higher than that reported previously (median SUVmax of 4.2) [28]. It may possible be more patients with disseminated NTM (17/23) in our study. Moreover, 18F-FDG PET/CT showed an intense or moderate 18F-FDG uptake in both intra-pulmonary and extra-pulmonary lesions in 21 patients, and high FDG-uptake (SUVmax > 2.5) was observed in 19 patients with intra-pulmonary lesions and 15 patients with extra-pulmonary lesions, suggesting an active stage of the disease. Sinner V et al. [29] reported that the activity of the chorioretinal lesions with NTM infection corresponded to disease progression. The above evidences highlight that 18F-FDG PET/CT can be of great value to assess the extent of active NTM lesion, particularly for extra-pulmonary lesions, since obtaining tissue or fluid for culture or mNGS may be not always possible or too invasive.
NTM are opportunistic pathogens to humans. Exposure to NTM organisms in the environment in common in day-to-day life, while NTM diseases occur infrequently due to their lower virulence than Mycobacterium tuberculosis. However, to date, it has become apparent that some individuals are prone to NTM diseases, particularly immunosuppressed patients with reduced cell-mediated immunity [3,4,5]. The rate of glucose utilization is accentuated by immune cell activation during inflammation and infection. 18F-FDG PET/CT has provided the most robust evidence regarding this phenomenon in the initial and treatment response assessment of IFD (invasive fungal disease) in immunocompromised patients [30]. The cases selected for our study included 9 (39.1%) immunosuppressed patients and 14 (60.9%) immunocompetent patients. SUVTop in immunocompetent patients was significantly higher than that in immunocompromised patients. Moreover, the cut-off value of SUVTop was 7.1, and the sensitivity and specificity of 18F-FDG PET/CT for compromised immune status in NTM patients were 88.9% and 64.3%, respectively. These results indicated that SUVTop was significantly positively related to immune cell activation due to the upregulation of GLUT-1 and 3, along with increased hexokinase activity [13], whereas immunosuppressed patients exhibit the disruption or depletion of cell-mediated immunity and immune cell activation in immunocompromised patients is lower than that in immunocompetent patients. NTM guidelines [10, 11] strongly recommend that immunocompromised patients with NTM infection require a longer duration and dose of treatment. In future work, we aim to expand the sample size and conduct prospective experiments to further explore the relationship of immune function and metabolic parameters of FDG PET/CT in NTM patients.
NTM can affect any organ in the body, and the main clinical manifestations in adults are localized NTM pulmonary disease and/or other disseminated diseases. Disseminated NTM diseases (defined as the involvement of two or more non-contiguous body organs) often occur in patients with an immune defect, and 2–8 per cent of patients with disseminated diseases may have concurrent pulmonary involvement [5]. The Mycobacterium avium complex (MAC) and rapidly growing mycobacteria (RGM) have been identified as important pathogens contributing disseminated diseases [31]. Thus, early diagnosis and treatment is essential to minimize morbidity and costs, as well as prevent long-term disability [5]. In this study, extra-pulmonary lesions with abnormally high 18F-FDG FDG uptake were found in 17 patients (73.8%) with disseminated diseases. In these patients, 18F-FDG PET/CT showed increased 18F-FDG accumulation in the lymph node (14 cases), bone (6 cases), subcutaneous tissue (1 case), spleen (1 case), and adrenal gland (1 case). The metabolic parameters SURLiver and SURBlood in disseminated diseases were significantly higher than those in localized pulmonary diseases. When the cut-off values of SURLiver and SURBlood were 2.8 and 4.4, respectively, the sensitivity and specificity of 18F-FDG PET/CT for disseminated diseases were 83.3% and 70.6%, and 83.3% and 52.9%, respectively. These results showed that an active NTM lesion readily takes up 18F-FDG, both in pulmonary and extra-pulmonary lesions. Moreover, NTM patients with high SURLiver and SURBlood level were related to disseminated diseases. This result needs to be confirmed in the next prospective study.
According to the guidelines for the management of non-tuberculosis mycobacterial pulmonary disease by the British Thoracic Society [11], as the treatment of NTM pulmonary disease is arduous, lengthy and costly, several factors should be considered before a decision is made to begin treatment, such as the severity of NTM-PD, the risk of progressive NTM-PD, the presence of any comorbidities, and the goals of treatment [9, 12]. 18F-FDG PET/CT is a functional imaging technique that detects metabolically active areas by measuring the accumulation of 18F-FDG, and may help to establish NTM-PD diagnoses [32]. In the present study, when the cut-off values of SUVMarrow, and SUVI−lung were 3.1 and 4.6, respectively, the sensitivity and specificity of 18F-FDG PET/CT for the identification of NTM-PD severity were 76.9% and 88.9%, and 76.9% and 55.9%, respectively. A high FDG uptake of NTM-PD lesion indicates inflammatory activity, significant mycobacterial pathogenicity, or an elicited systemic response (such as in Patient 1 in Fig. 2), while normal or low FDG uptake shows inflammation regression or scar formation (such as in Patient 2 in Fig. 2). Therefore, the indices SUVI−Lung and SUVMarrow can both suggest systemic inflammatory response, either directly after NTM infection or indirectly by reflecting the virulence of the bacteria. NTM-PD patients with SUVMarrow ≥ 3.1 and SUVI−Lung ≥ 4.6 may likely be severe NTM if effective treatment is delayed. Therefore, these metabolic parameters can contribute to the planning of appropriate treatment for NTM. Furthermore, the Spearman’s correlation analysis revealed a significant positive correlation between serum IL-6 and SUVMarrow, ferritin and SUVSpleen. The PET metabolic parameters of bone marrow and spleen may reflect the development and progression of a cytokine storm [33]. To this end, the value of 18F-FDG PET/CT in predicting immune cell activation and evaluating treatment response of NTM patients deserves further investigation.
This study inevitably has some limitations. First, it was conducted at a single center, and the sample size was limited (n = 23). All images were acquired on the same PET/CT scanner model (Biograph version; Siemens, Germany), imaging protocol, and reconstruction. Second, because this was a retrospective study, the use of 18F-FDG PET/CT in the assessment of patients potentially introduced a bias toward a disseminated NTM infection cohort. Third, in our study, each patient underwent an 18F-FDG PET/CT scan only once (baseline assessment in 16 patients and anti-NTM pre-PET in 7 patients) and lacked post-treatment reassessment. Fourth, 10 patients were misdiagnosed malignancies (multiple myeloma, lung cancer and lymphadenoma, etc.) by 18F-FDG PET/CT (Table 1). So, 18F-FDG PET/CT has a limited role in differentiating disseminated NTM diseases from malignancies. Lastly, metabolic measurements reflected the FDG uptake by inflammatory cells rather than NTM. Thus, NTM-specific radiolabeled compounds and imaging approaches urgently need to be developed. In addition, a multicenter, prospective analysis should be designed in a future study to attain a better representation of NTM-infected patients.
Conclusion
18F-FDG PET/CT is a useful tool in the diagnosis, evaluation of disease activity, immune status, and extent of lesion involvement in NTM patients, and can contribute to planning the appropriate treatment for NTM.
Abbreviations
- NTM:
-
Non-tuberculous mycobacteria
- NTM-PD:
-
Non-tuberculous mycobacterial pulmonary diseases
- MDS:
-
Myelodysplastic syndromes
- AIDS:
-
Acquired immunodeficiency syndrome
- COPD:
-
Chronic obstructive pulmonary disease
- BALF:
-
Bronchoalveolar lavage fluid
- NTM-DNA-PCR:
-
PCR assay for non-tuberculous mycobacteria DNA
- IIP:
-
Idiopathic interstitial pneumonia
- mNGS:
-
Metagenomic next-generation sequencing
- 18F-FDG PET/CT:
-
18F-fluorodeoxyglucose positron emission tomography/computed tomography
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- SUVTop :
-
The most FDG-avid lesion in the whole body
- SUVI−Lung :
-
SUVmax of intra-pulmonary lesions
- SUVE−Lung :
-
SUVmax of extra-pulmonary lesions
- SUVLiver :
-
SUVmax of the liver
- SUVSpleen :
-
SUVmax of the spleen
- SUVMarrow :
-
SUVmax of the marrow
- SURLiver :
-
SUVTop/SUVmax of the liver (SURLiver)
- SURBlood :
-
SUVTop/SUVmax of the blood (SURBlood)
- MLV:
-
Metabolic lesion volume
- TLG:
-
Total lesion glycolysis
- ROC:
-
Receiver operating characteristic curve
- AUCs:
-
Areas under the curve
References
Johansen MD, Herrmann JL, Kremer L, Non-tuberculous mycobacteria and. rise Mycobacterium abscessus Nat Rev Microbiol. 2020;18(7):392–407.
Meoli A, Deolmi M, Iannarella R, Esposito S. Non-Tuberculous Mycobacteri- al Dis Child Pathogens. 2020;9(7):553–65.
Zhou L, Xu D, Liu H, Wan K, Wang R, Yang Z. Trends in the prevalence and antibiotic resistance of non-tuberculous mycobacteria in Mainland China, 2000–2019: systematic review and Meta-analysis. Front Public Health. 2020;8:295–304.
Ahmed I, Tiberi S, Farooqi J, Jabeen K, Yeboah-Manu D, Migliori GB, Hasan R. Non-tuberculous mycobacterial infections-A neglected and emerging problem. Int J Infect Dis. 2020;92S:S46–50.
Sharma SK, Upadhyay V. Epidemiology, diagnosis & treatment of non-tubercu- lous mycobacterial diseases. Indian J Med Res. 2020;152(3):185–226.
Ratnatunga CN, Lutzky VP, Kupz A, Doolan DL, Reid DW, Field M, Bell SC, Thomson RM, Miles JJ. The rise of Non-tuberculosis Mycobacterial Lung. Disease Front Immunol. 2020;11:303–15.
Eisenberg I, Yasin A, Fuks L, Stein N, Saliba W, Kramer MR, Adir Y, Shteinberg M. Radiologic Characteristics Non-tuberculous Mycobact Infect Patients Bronchiectasis Lung. 2020;198(4):715–22.
Chanda-Kapata P, Kapata N, Klinkenberg E, Mulenga L, Tembo M, Katemangwe P, Sunkutu V, Mwaba P, Grobusch MP. Non-tuberculous mycobacteria (NTM) in Zambia: prevalence, clinical, radiological and microbiological characteristics. BMC Infect Dis. 2015;15:500–7.
Porvaznik I, Solovič I, Mokrý J. Non-tuberculous mycobacteria: classification, Diagnostics, and Therapy. Adv Exp Med Biol. 2017;944:19–25.
Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, Huitt GA, Knight SL, Leitman P, Marras TK, Olivier KN, Santin M, Stout JE, Tortoli E, van Ingen J, Wagner D, Winthrop KL. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020;56(1):2000535.
Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, Leitch A, Loebinger MR, Milburn HJ, Nightingale M, Ormerod P, Shingadia D, Smith D, Whitehead N, Wilson R, Floto RA. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax. 2017;72(Suppl 2):ii1–64.
Henkle E, Aksamit T, Barker A, Daley CL, Griffith D, Leitman P, Leitman A, Malanga E, Marras TK, Olivier KN, Prevots DR, Prieto D, Quittner AL, Skach W, Walsh JW, Winthrop KL, NTMRC Patient Advisory Panel. Patient-centered Research priorities for Pulmonary Nontuberculous Mycobacteria (NTM) infection. An NTM Research Consortium Workshop Report. Ann Am Thorac Soc. 2016;13(9):S379–84.
Mochizuki T, Tsukamoto E, Kuge Y, Kanegae K, Zhao S, Hikosaka K, Hosokawa M, Kohanawa M, Tamaki N. FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J Nucl Med. 2001 Oct;42(10):1551–5.
Vaidyanathan S, Patel CN, Scarsbrook AF, Chowdhury FU. FDG PET/CT in infection and inflammation–current and emerging clinical applications. Clin Radiol. 2015;70(7):787–800.
O’Brien DP, Currie BJ, Krause VL. Nontuberculous mycobacterial disease in northern Australia: a case series and review of the literature. Clin Infect Dis. 2000;31(4):958–67.
Polverosi R, Guarise A, Balestro E, Carloni A, Dalpiaz G, Feragalli B. High- resolution CT of nontuberculous mycobacteria pulmonary infection in immunoc- ompetent, non-HIV-positive patients. Radiol Med. 2010;115(2):191–204.
Sathekge M, Maes A, D’Asseler Y, Vorster M, Gongxeka H, Van de Wiele C. Tuberculous lymphadenitis: FDG PET and CT findings in responsive and nonresponsive disease. Eur J Nucl Med Mol Imaging. 2012;39:1184–90.
Kim IJ, Lee JS, Kim SJ, Kim YK, Jeong YJ, Jun S, Nam HY, Kim JS. Double-phase 18F-FDG PET-CT for determination of pulmonary tuberculoma activity. Eur J Nucl Med Mol Imaging. 2008;35(4):808–14.
Namkoong H, Fujiwara H, Ishii M, Yagi K, Haraguchi M, Matsusaka M, Suzuki S, Asakura T, Asami T, Saito F, Fukunaga K, Tasaka S, Betsuyaku T, Hasegawa N. Immune reconstitution inflammatory syndrome due to Mycobacterium avium complex successfully followed up using 18 F-fluorodeoxyglucose positron emission tomography-computed tomography in a patient with human immunodeficiency virus infection: a case report. BMC Med Imaging. 2015;15:24.
Lin KH, Wang JH, Peng NJ. Disseminated nontuberculous mycobacterial infection mimic metastases on PET/CT scan. Clin Nucl Med. 2008;33(4):276–7.
Ishii T, Tamura A, Matsui H, Nagai H, Akagawa S, Hebisawa A, Ohta K. Disseminated Mycobacterium avium complex infection in a patient carrying autoantibody to interferon-γ. J Infect Chemother. 2013;19(6):1152–7.
Tanaka S, Hoshino Y, Sakagami T, Fukano H, Matsui Y, Hiranuma O. Pathogenicity of Mycolicibacterium Phlei, a non-pathogenic nontuberculous mycobacterium in an immunocompetent host carrying anti-interferon gamma autoantibodies: a case report. BMC Infect Dis.
Ramirez JA, Musher DM, Evans SE, Dela Cruz C, Crothers KA, Hage CA et al. Treatment of Community-Acquired Pneumonia in Immunocompro- mised Adults: A Consensus Statement Regarding Initial Strategies. Chest.2020, 158: 1896–1911.
Sesin GP, Manzi SF, Pacheco R. New trends in the drug therapy of localized and disseminated Mycobacterium avium complex infection. Am J Health Syst Pharm. 1996;53(21):2585–90.
Ricotta EE, Adjemian J, Blakney RA, Lai YL, Kadri SS, Prevots DR. Extrapulmonary Nontuberculous Mycobacteria infections in hospitalized patients, United States, 2009–2014. Emerg Infect Dis. 2021;27(3):845–52.
Ankrah AO, van der Werf TS, de Vries EF, Dierckx RA, Sathekge MM, Glaudemans AW. PET/CT imaging of Mycobacterium tuberculosis infection. Clin Transl Imaging. 2016;4:131–44.
Besson FL, Parienti JJ, Bienvenu B, Prior JO, Costo S, Bouvard G, Agostini D. Diagnostic performance of 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2011;38(9):1764–72.
Stroffolini G, Gaviraghi A, Penna D, Piccioni P, Venuti F, Botto C, Trezzi M, Betti M, Sestini S, Erba PA, Lupia T, Di Perri G, Aliberti S, Calcagno A. 18-fluorodeoxyglucose positron emission tomography (FDG-PET) in patients with non-tuberculous mycobacterial infections. J Infect. 2023;87(5):462–4.
Sinner V, Rüesch R, Valmaggia C, Todorova M. Choroidal manifestation of systemic non-tuberculous mycobacterial infection: a Case Series. Klin Monbl Augenheilkd. 2020;237(4):493–6.
Lawal IO, Mokoala KMG, Kgatle MM, Dierckx RAJO, Glaudemans AWJM, Sathekge MM, Ankrah AO. Radionuclide Imaging of Invasive Fungal Disease in Immunocomprom- ised Hosts.Diagnostics (Basel). 2021;11(11):2057.
Henkle E, Winthrop KL. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015;36(1):91–9.
Del Giudice G, Bianco A, Cennamo A, Santoro G, Bifulco M, Marzo C, Mazzarella G. Lung and nodal involvement in Nontuberculous Mycobacterial Disease: PET/CT role. Biomed Res Int. 2015;2015:353202.
Signore A, Lauri C, Bianchi MP, et al. [< sup > 18 F]FDG PET/CT in patients affected by SARS-CoV-2 and Lymphoproliferative Disorders and treated with Tocilizumab. J Personalized Med. 2022;12(11):1839.
Acknowledgements
We gratefully acknowledge our colleagues for their comments on this study.
Funding
This work was received by grants from the National Natural Science Foundation of China (NSFC) (82071965) and Huadong Medicine Joint Funds of the Zhejiang Provincial Natural Science Foundation of China (LHDMZ22H300010).
Author information
Authors and Affiliations
Contributions
Authors’ contributionsDC and XS: Study conception and design; drafting of manuscript; critical revision; review, analysis and interpretation of scientific literature; YC was involved in the management of the specimen samples of the patient; drafting of manuscript; KZ, KL, ZW, SY and GW: Drafting of manuscript; review, analysis and interpretation of scientific literature. All authors have contributed to the manuscript and approved its final version.
Corresponding authors
Ethics declarations
Informed consent
Written informed consent was not required because of the retrospective nature for this study.
Ethical approval
This retrospective study protocol was reviewed and approved by the Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (No. IIT20220576A). The requirement for informed consent was waived by the Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine due to retrospective study.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, D., Chen, Y., Yang, S. et al. The additional value of 18F-FDG PET/CT imaging in guiding the treatment strategy of non-tuberculous mycobacterial patients. Respir Res 25, 132 (2024). https://doi.org/10.1186/s12931-024-02757-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-024-02757-7