Abstract
Background
Diaphragmatic dysfunction is a major factor responsible for weaning failure in patients that underwent prolonged invasive mechanical ventilation for acute severe respiratory failure from COVID-19. This study hypothesizes that ultrasound measured diaphragmatic thickening fraction (DTF) could provide corroborating information for weaning COVID-19 patients from mechanical ventilation.
Methods
This was an observational, pragmatic, cross-section, multicenter study in 6 Italian intensive care units. DTF was assessed in COVID-19 patients undergoing weaning from mechanical ventilation from 1st March 2020 to 30th June 2021. Primary aim was to evaluate whether DTF is a predictive factor for weaning failure.
Results
Fifty-seven patients were enrolled, 25 patients failed spontaneous breathing trial (44%). Median length of invasive ventilation was 14 days (IQR 7–22). Median DTF within 24 h since the start of weaning was 28% (IQR 22–39%), RASS score (− 2 vs − 2; p = 0.031); Kelly-Matthay score (2 vs 1; p = 0.002); inspiratory oxygen fraction (0.45 vs 0.40; p = 0.033). PaO2/FiO2 ratio was lower (176 vs 241; p = 0.032) and length of intensive care stay was longer (27 vs 16.5 days; p = 0.025) in patients who failed weaning. The generalized linear regression model did not select any variables that could predict weaning failure. DTF was correlated with pH (RR 1.56 × 1027; p = 0.002); Kelly-Matthay score (RR 353; p < 0.001); RASS (RR 2.11; p = 0.003); PaO2/FiO2 ratio (RR 1.03; p = 0.05); SAPS2 (RR 0.71; p = 0.005); hospital and ICU length of stay (RR 1.22 and 0.79, respectively; p < 0.001 and p = 0.004).
Conclusions
DTF in COVID-19 patients was not predictive of weaning failure from mechanical ventilation, and larger studies are needed to evaluate it in clinical practice further.
Registered: ClinicalTrial.gov (NCT05019313, 24 August 2021).
Similar content being viewed by others
Background
Interstitial pneumonia and adult respiratory distress syndrome (ARDS) are severe COVID-19 complications that lead to intensive care unit (ICU) admission. Mortality remains higher than 30%, especially in non-vaccinated individuals that develop severe ARDS [1,2,3]. Initial COVID-19 interstitial pneumonia primarily shows alveolar-capillary shunting without clinically relevant respiratory signs (silent hypoxemia). However, as the infection progresses, so does the involvement of lung parenchyma, outlining a more severe clinical picture (manifest hypoxemia). It includes increasing elastance, right-left shunting and lung de-recruitment ultimately requiring intensive care admission, sedation, pronation, and muscle relaxation to reach adequate gas exchanges [4]. These patients require prolonged mechanical ventilation, often longer than 2–3 weeks, potentially inducing diaphragmatic dysfunction that could impact ventilatory support duration and weaning outcome [5]. Ultrasounds represent a non-invasive bedside approach to evaluate diaphragmatic thickening. This field has rapidly grown in recent years, with many publications concerning non-COVID-19 patients. The expression of transdiaphragmatic pressure, in terms of diaphragmatic thickening fraction (DTF), indicates muscular contractility. Goligher et al. first described the relationship between changes in transdiaphragmatic pressure and DTF in healthy subjects [6]. Diaphragmatic dysfunction had already been documented before a possible correlation between diaphragmatic dysfunction and weaning failure from mechanical ventilation emerged in patients with COVID-19 pneumonia. Although it is known that diaphragmatic force is mainly preserved or improved in the first phases of COVID-19 infection, some patients have shown early dysfunction in spontaneous breathing [7,8,9]. However, only few studies focused on diaphragmatic function during weaning from mechanical ventilation in these patients. This study evaluates the role of diaphragmatic dysfunction, as assessed by DTF at the beginning of weaning from mechanical ventilation, and weaning failure in COVID-19 patients. The aim of this study was that the DTF value could be a potential predictive factor of weaning success.
Methods
This was an observational, pragmatic, cross-section, multicenter study. The study was conducted in 6 Italian ICUs as Udine, Ferrara, Ravenna, Pisa, Turin and Milan, from 1st March 2020 to 30th June 2021. Every center’s Ethical Committee Board approved the study following local guidelines for ethical standards and good clinical practice. Each principal investigator was responsible for obtaining informed consent, in accordance with hospital privacy protocols during the emergency, local rules, and institutional regulations. All ethic committees’ approvals are added in the dedicated section at the end of the article. The study was registered at ClinicalTrial.gov (NCT05019313, 24 August 2021) and observed the STROBE guidelines for an observational study.
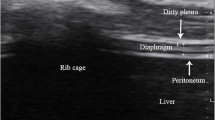
Inclusion criteria were as follows: age > 18 years, admission to a COVID-19 ICU with hypoxemic respiratory failure (defined as PaO2 < 70 mmHg or PaO2/FiO2 < 150), start of weaning from mechanical ventilation, defined as the ability to maintain respiratory stability (no dyspnea, respiratory rate < 25/min, percutaneous oxygen saturation (SpO2) ≥ 96%) with mechanical ventilation in pressure support mode at standardized settings (pressure support of 8 cmH2O, PEEP 5 cmH2O, and inspired fraction of oxygen (FiO2) < 0.5). Exclusion criteria were: being non-cooperative (Kelly-Matthay score ≥ 5) or Richmond Agitation-Sedation Scale (RASS) ≥ + 2, participation refusal, tracheostomy, unstable clinical conditions or multiple organ failure (> 2 organs). Weaning failure was defined as failure to pass the spontaneous-breathing trial or the need for re-intubation within 48 h following extubation. [10, 11] DTF was evaluated within 24 h since the start of weaning. DTF was assessed for the right hemidiaphragm, using a linear probe to identify a muscular layer between two hyperechoic lines, the pleura and peritoneum, superficial to the liver, with patients in supine position with the trunk elevated 10°–15°. In all patients, DTF was calculated while patients were ventilated with pressure support ventilation, with standardized settings (8 cmH2O pressure support, 5 cmH2O positive end-expiratory pressure (PEEP), FiO2 < 0.5) and hemodynamic stability. Measurements were obtained by employing instrumentations available in the respective ICUs. DTF was calculated using M-mode imaging, maximal diaphragm thickness during inspiration (Tdi, pi), minus diaphragm thickness at end-expiration (Tdi,ee), divided by Tdi,ee and multiplied by 100. Three consecutive measures were obtained, and the mean value was recorded. We also collected Simplified Acute Physiology Score 2 (SAPS2); RASS; Kelly-Matthay score; the systolic and diastolic arterial pressure; the heart rate; the respiratory rate; the body temperature; the base excess; non-invasive ventilation trial before invasive mechanical ventilation and its duration; invasive mechanical ventilation duration; ICU and hospital length of stay.
End-points
The primary end-point was to verify if the DTF predicts weaning success/failure from mechanical ventilation. The secondary end-point was to analyze the co-variables correlated with DTF values.
Sample size estimation
We assumed a priori a prevalence of diaphragmatic dysfunction of 42.5% [12]. To achieve the adequate prevalence within this study with a two-sided 95% confidence interval and a maximum accuracy error of 15%, 38 patients will be needed. However, considering a drop out of 20% due to the COVID-19 environment problem in data acquisition, we estimate to enrol a minimum of 45 subjects.
Statistical analysis
Described variables were recorded as median [IQR] for continuous variables and frequencies (%) for categorical variables. Study population was divided according to weaning success or failure. After data distribution was verified, group comparison was executed using Mann-Withney test. Chi-squared test or Fisher's exact test were used for categorical variables. A Benjamini & Hochberg correction for the multiplicity of the p-value was considered. P-values ≤ 0.05 were considered statistically significant. A generalized linear regression model was adopted to detect variables correlated with weaning failure. A multivariable linear regression model was applied to identify variables related to DTF. Predictive variables were selected by carrying out a stepwise backward method, which eliminated less predictive variables until the most predictive model was achieved. Further post-hoc sub-analyses were carried out to analyse the behaviour of certain subgroups within the population. Statistical calculations were performed using the R-CRAN platform.
Results
Fifty-seven patients admitted to participating ICUs from March 2020 to June 2021 were enrolled in the study. The main characteristics of the study population are illustrated in Table 1.
Median age was 65 years (IQR 56–71); 42 patients (74%) were male. Forty-six (81%) patients underwent a trial of non-invasive ventilation (NIV) before intubation (duration: 15 h; IQR 3–36). Median length of invasive ventilation was 14 days (IQR 7–22). Median DTF at weaning start (or at SBT start) was 28.0 (IQR 21.7–38.9).
Comparison between weaning success and failure
DTF was not different between weaning success and weaning failure groups (Fig. 1).
The following parameters were statistically different between groups: RASS (− 2 vs − 2; p = 0.031); Kelly-Matthay score (2 vs 1; p = 0.002); inspiratory oxygen fraction (0.45 vs 0.40; p = 0.033), PaO2/FiO2 ratio (176 vs 241; p = 0.032) and length of ICU stay (27 vs 16.5 days; p = 0.025) (Table 1). The generalized linear regression model did not identify any variable independently associated with weaning failure. DTF distribution according to PaO2/FiO2 values in patients who failed weaning showed a rough U-shaped trend, in which extreme DTF values were associated with the lowest PaO2/FiO2 (Fig. 2).
DTF distribution, with respect to PaO2/FiO2, in successfully weaned patients (in blue) and unsuccessfully (in red). The trend was obtained through a mixed linear regression model, which demonstrates a U-shaped trend in DTF values, lowest PaO2/FiO2 values at both extremes, for patients who failed weaning
In the regression model performed to identify variables correlated to DTF, pH (RR 1.56 × 1027; p = 0.002); Kelly-Matthay score (RR 353; p < 0.001); RASS (RR 2.11; p = 0.003); PaO2/FiO2 ratio (RR 1.03; p = 0.05); SAPS2 (RR 0.71; p = 0.005); hospital and ICU length of stay (RR 1.22 and 0.79, respectively; p < 0.001 and p = 0.004) were all significantly correlated. Subgroup post-hoc analysis showed that male patients had greater DTF values than female patients, at equal disease severity (SAPS2 score) (Fig. 3).
Comparison of SAPS-2 scores (x-axis) and DTF values (y-axis), divided by gender (females on the left and males on the right) and PaO2/FiO2 ratio subclasses (highest values in green, lowest in red). As shown in the lower right quadrants of the graphs (highest SAPS-2 scores) DTF values are higher in males, compared to females, for each PaO2/FiO2 subclass
Discussion
The study evaluated a possible correlation between weaning failure and DTF values and other potential predictors, in critically ill COVID-19 patients. DTF was found to be unrelated to weaning outcome. In agreement with current literature, the present work highlighted a very high rate of weaning failure, around 40%, requiring longer ICU stay and sedation. Observing gathered data, COVID-19 is likely implicated in determining an initial thickening diaphragm and ultimately lung parenchyma fibrosis [13]. Poulard et al. have stressed how, although DTF ultrasound evaluation has recently become popular, its correlation with transdiaphragmatic pressure (measured by invasive catheters to record esophageal and gastric pressure) is widely variable, especially in patients subjected to mechanical ventilation [14]. It is reasonable to hypothesize that extremes—hypertrophy and atrophy—correlate with a more pronounced respiratory dysfunction [15, 16]. Similarly, ventilatory support, either above or below patient’s needs, might lead to diaphragmatic dysfunction [17]. In addition, patient’s basal diaphragmatic strength could play a determining role in the development of dysfunction, further complicating achievement of a standardized ultrasound method [18, 19].
Other factors related to DTF are degree of sedation (RASS score) and length of hospital stay, which are both linearly implicated. On the contrary, PaO2/FiO2 shows a very weak correlation. Even though pathophysiological mechanisms of parenchymal damage could be involved, the study data seems to reflect the U-shaped pattern previously highlighted: PaO2/FiO2 values show a linear correlation with the diaphragm thickening capacity within a range, beyond which at the low end of PaO2/FiO2, this diaphragm thickening capacity can be almost completely abolished or, on the contrary, excessively expressed (the result of an excess of respiratory effort).
A contributing mechanism to diaphragmatic dysfunction is pronounced respiratory acidosis, which could both be cause and consequence of diaphragmatic thinning; namely, depletion of energy substrates favors a hyperlactatemia that may not be sufficient to support required muscular effort [20]. However, the diaphragm may be the major contributor to lactate production, making the patient prone to acidosis. In our study, pH directly correlated with DTF, a possible expression of respiratory alkalosis. Conversely, the reduction in pH could result from the diaphragmatic metabolic debt condition.
Fever due to viral infection can determine diaphragmatic dysfunction [21] through a “cytokine storm”, which in turn may contribute to muscle catabolism [22]. In a cluster analysis of 55 patients undergoing mechanical ventilation, Vivier et al. detected a linear correlation between PaCO2 and DTF [23]. The authors hypothesize that increased DTF results in increased respiratory workload in spontaneously breathing patients. Conversely, the use of sedation, as occurs in patients admitted to ICUs, could lead to muscle deconditioning—similarly to global ICU-acquired weakness—and may be responsible for increased hospital LOS and weaning failure. However, special attention should be paid when labeling diaphragmatic dysfunction as a predictor of ICU-acquired weakness. Jung et al. have shown that, although diaphragmatic dysfunction is frequent in patients with ICU-acquired weakness, about half of patients with the latter condition undergo successful extubation [24].
A recent study by Cammarota et al. found that prone position in spontaneously breathing patients, though supported by non-invasive ventilation, caused an increase in DTF [25]. Authors suggest that the correlation between DTF and ventilation efficacy is more complex than previously thought. The mere evaluation of DTF per se does not account for improvements in ventilation. How far DTF is to be pursued as an expression of greater muscle strength or, vice versa, as a negative expression of increased respiratory workload, has yet to be established. A recent meta-analysis of the diagnostic accuracy of diaphragmatic ultrasound to predict weaning outcome showed low sensitivity [26]. Furthermore, authors highlighted a wide heterogeneity of the studies considered which suggests the extent of the effect of other variables, not directly measured in different subpopulations, can contribute to weaning outcome [27].
Lastly, as hypothesis-generator, the present study shows that male patients, compared to female patients, have higher DTF values for the same severity of disease (SAPS2 score and PaO2/FiO2 ratio). However, what makes females prone to particularly severe forms of diaphragmatic dysfunction in the course of COVID-19 is yet to be determined.
Limitations
Study limitations were mainly related to conditions in which it was performed: patient enrollment, data collection and practical methods reflect, in fact, the course of the pandemic. Standardized ventilation criteria before weaning were not established amongst participating centers. Though operators involved in the study are considered experts, different levels of proficiency may result in data collection discrepancies. In order to maximize the inter-observer reproducibility, we have disseminated video tutorials prior the enrollment [28]. Given the pragmatic nature of the study the adopted definition of weaning failure was restricted to an easily applicable and contextualizable definition in all participating centers.
Conclusions
DTF ultrasound evaluation is a technique that can be performed at the patient’s bedside, but it does not predict weaning outcome in COVID-19 patients. Further studies with specific endpoints need to be conducted before the role of DTF in weaning from ventilation can be defined, even in patients without COVID-19 respiratory failure.
Availability of data and materials
The datasets from this study are available from the corresponding author upon request.
Abbreviations
- DTF:
-
Diaphragmatic thickening fraction
- ARDS:
-
Adult respiratory distress syndrome
- RASS:
-
Richmond Agitation-Sedation Scale
- PEEP:
-
Positive end-expiratory pressure
- Tdi, Pi:
-
Thickness during inspiration
- Tdi, Ee:
-
Thickness at end-expiration
- SAPS2:
-
Simplified Acute Physiology Score
References
Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–81. https://doi.org/10.1001/jama.2020.5394.
Ashish A, Unsworth A, Martindale J, Sundar R, Kavuri K, Sedda L, et al. CPAP management of COVID-19 respiratory failure: a first quantitative analysis from an inpatient service evaluation. BMJ Open Respir Res. 2020;7(1): e000692. https://doi.org/10.1136/bmjresp-2020-000692.
Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in-hospital mortality in a US National Sample of Patients with COVID-19. JAMA Netw Open. 2020;3(12): e2029058. https://doi.org/10.1001/jamanetworkopen.2020.29058.
Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–102. https://doi.org/10.1007/s00134-020-06033-2.
Corradi F, Vetrugno L, Orso D, Bove T, Schreiber A, Boero E, et al. Diaphragmatic thickening fraction as a potential predictor of response to continuous positive airway pressure ventilation in COVID-19 pneumonia: a single-center pilot study. Respir Physiol Neurobiol. 2021;284: 103585. https://doi.org/10.1016/j.resp.2020.103585.
Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, et al. Mechanical ventilation–induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care. 2018;197(2):204–13. https://doi.org/10.1164/rccm.201703-0536OC.
van Steveninck AL, Imming LM. Diaphragm dysfunction prior to intubation in a patient with COVID-19 pneumonia; assessment by point of care ultrasound and potential implications for patient monitoring. Respir Med Case Rep. 2020;31: 101284. https://doi.org/10.1016/j.rmcr.2020.101284.
Pivetta E, Cara I, Paglietta G, Scategni V, Labarile G, Tizzani M, et al. Diaphragmatic point-of-care ultrasound in COVID-19 patients in the emergency department—a proof-of-concept study. J Clin Med. 2021;10(22):5291. https://doi.org/10.3390/jcm10225291.
Corradi F, Isirdi A, Malacarne P, Santori G, Barbieri G, Romei C, et al. Low diaphragm muscle mass predicts adverse outcome in patients hospitalized for COVID-19 pneumonia: an exploratory pilot study. Minerva Anestesiol. 2021;87:432–8. https://doi.org/10.23736/S0375-9393.21.15129-6.
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–56. https://doi.org/10.1183/09031936.00010206.
Schmidt GA, Girard TD, Kress JP, Morris PE, Ouellette DR, Alhazzani W, et al. ATS/CHEST Ad Hoc Committee on Liberation from Mechanical Ventilation in Adults. Official Executive Summary of an American Thoracic Society/American College of Chest Physicians Clinical Practice Guideline: Liberation from Mechanical Ventilation in Critically Ill Adults. Am J Respir Crit Care Med. 2017;195:115–9. https://doi.org/10.1164/rccm.201610-2076ST.
Barbariol F, Deana C, Guadagnin GM, Cammarota G, Vetrugno L, Bassi F. Ultrasound diaphragmatic excursion during non-invasive ventilation in ICU: a prospective observational study. Acta Biomed. 2021;92(3): e2021269. https://doi.org/10.23750/abm.v92i3.11609.
Roozeman JP, Mazzinari G, Serpa Neto A, Hollmann MW, Paulus F, Schultz MJ, et al. Prognostication using SpO2/FiO2 in invasively ventilated ICU patients with ARDS due to COVID-19—insights from the PRoVENT-COVID study. J Crit Care. 2021;68:31–7. https://doi.org/10.1016/j.jcrc.2021.11.009.
Poulard T, Bachasson D, Fossé Q, Niérat MC, Hogrel JY, Demoule A, et al. Poor correlation between diaphragm thickening fraction and transdiaphragmatic pressure in mechanically ventilated patients and healthy subjects. Anesthesiology. 2022;136:162–75. https://doi.org/10.1097/ALN.0000000000004042.
Dres M, Goligher EC, Dubé BP, Morawiec E, Dangers L, Reuter D, et al. Diaphragm function and weaning from mechanical ventilation: an ultrasound and phrenic nerve stimulation clinical study. Ann Intensive Care. 2018;8(1):53. https://doi.org/10.1186/s13613-018-0401-y.
Zambon M, Greco M, Bocchino S, Cabrini L, Beccaria PF, Zangrillo A. Assessment of diaphragmatic dysfunction in the critically ill patient with ultrasound: a systematic review. Intensive Care Med. 2017;43:29–38. https://doi.org/10.1007/s00134-016-4524-z.
Somhorst P, Gommers D, Endeman H. Advanced respiratory monitoring in mechanically ventilated patients with coronavirus 2019-associated acute respiratory distress syndrome. Curr Opin Crit Care. 2021. https://doi.org/10.1097/MCC.0000000000000905.
Spadaro S, Grasso S, Mauri T, Dalla Corte F, Alvisi V, Ragazzi R, Cricca V, et al. Can diaphragmatic ultrasonography performed during the T-tube trial predict weaning failure? The role of diaphragmatic rapid shallow breathing index. Crit Care. 2016;20(1):305. https://doi.org/10.1186/s13054-016-1479-y.
Spiesshoefer J, Herkenrath S, Henke C, Langenbruch L, Schneppe M, Randerath W, et al. Evaluation of respiratory muscle strength and diaphragm ultrasound: normative values, theoretical considerations, and practical recommendations. Respiration. 2020;99:369–81. https://doi.org/10.1159/000506016.
Goligher EC, Dres M, Patel BK, Sahetya SK, Beitler JR, Telias I, et al. Lung- and Diaphragm-Protective Ventilation. Am J Respir Crit Care Med. 2020;202(7):950–61. https://doi.org/10.1164/rccm.202003-0655CP.
McCool FD, Tzelepis GE. Dysfunction of the diaphragm. N Engl J Med. 2012;366:932–42. https://doi.org/10.1056/NEJMra1007236.
Lyu Q, Wen Y, Zhang X, Addinsall AB, Cacciani N, Larsson L. Multi-omics reveals age-related differences in the diaphragm response to mechanical ventilation: a pilot study. Skelet Muscle. 2021;11(1):11. https://doi.org/10.1186/s13395-021-00267-4.
Vivier E, Roche-Campo F, Brochard L, Mekontso DA. Determinants of diaphragm thickening fraction during mechanical ventilation: an ancillary study of a randomized trial. Eur Respir J. 2017;50(3):1700783. https://doi.org/10.1183/13993003.00783-2017.
Jung B, Moury PH, Mahul M, de Jong A, Galia F, Prades A, et al. Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med. 2016;42:853–61. https://doi.org/10.1007/s00134-015-4125-2.
Cammarota G, Rossi E, Vitali L, Simone R, Sannipoli T, Anniciello F, et al. Effect of awake prone position on diaphragmatic thickening fraction in patients assisted by non-invasive ventilation for hypoxemic acute respiratory failure related to novel coronavirus disease. Crit Care. 2021;25(1):305. https://doi.org/10.1186/s13054-021-03735-x.
Le Neindre A, Philippart F, Luperto M, Wormser J, Morel-Sapene J, Aho SL, et al. Diagnostic accuracy of diaphragm ultrasound to predict weaning outcome: a systematic review and meta-analysis. Int J Nurs Stud. 2021;117: 103890. https://doi.org/10.1016/j.ijnurstu.2021.103890.
Llamas-Álvarez AM, Tenza-Lozano EM, Latour-Pérez J. Diaphragm and lung ultrasound to predict weaning outcome: systematic review and meta-analysis. Chest. 2017;152:1140–50. https://doi.org/10.1016/j.chest.2017.08.028.
Dugar S, Mehkri O, Li M, Hastings A, Siuba MT, Vashisht R, et al. Validation of a web-based platform for online training in point-of-care diaphragm ultrasound. ATS Scholar. 2022. https://doi.org/10.34197/ats-scholar.2021-0057BR(webaccessed12/01/2022).
Acknowledgements
None.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
LV, DO, FC, GZ, and SS designed the concept of the study. FM, ND, TB, GC, EDR, SF, MG, MA, MF, EB, GZ, SS, FC, and CD conducted the study and performed the data acquisition. LV, SMM, FF, SS, DLG, EDR and FF assessed the quality of the study and performed the analysis interpretation. LV, DO, FC, SS, GC, SMM and FF wrote the manuscript, and the other authors made substantial revisions and edits. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The institutional review board of each participating hospital approved the study. Regional Ethics Committee of Udine (Protocol no. approval CEUR-2020-Os-087), Ethics Committee of ASL “Città di Torino” (Protocol no. approval 0000594/A.08/2020), Ethics Committee of Milano AREA 3 (Protocol no. approval 279-27052020), Regional Ethics Committee for Clinical Sperimentation of Tuscany Region (Protocol no. approval 19161), Ethics Committee of Emilia-Romagna (Protocol no. approval C.E.ROM 1449-2021), Ethics Committee of Ferrara (Protocol no. approval AOU_FE 0018894). Written informed consent was obtained following the local rules in Udine, Ferrara, Milano and Pisa. Informed consent was waived in Ravenna and Torino. The study was performed in accordance with the principles of the Declaration of Helsinki.
Consent for publication
All co-authors have provided consent for publications.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Vetrugno, L., Orso, D., Corradi, F. et al. Diaphragm ultrasound evaluation during weaning from mechanical ventilation in COVID-19 patients: a pragmatic, cross-section, multicenter study. Respir Res 23, 210 (2022). https://doi.org/10.1186/s12931-022-02138-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-022-02138-y