Abstract
Background
The assessment of small airways obstruction (SAO) using spirometry is practiced in population-based studies. However, it is not clear what are the most used parameters and cut-offs to define abnormal results.
Methods
We searched three databases (Medline, Web of Science, Google Scholar) for population-based studies, published by 1 May 2021, that used spirometry parameters to identify SAO and/or provided criteria for defining SAO. We systematically reviewed these studies and summarised evidence to determine the most widely used spirometry parameter and criteria for defining SAO. In addition, we extracted prevalence estimates and identified associated risk factors. To estimate a pooled prevalence of SAO, we conducted a meta-analysis and explored heterogeneity across studies using meta regression.
Results
Twenty-five studies used spirometry to identify SAO. The most widely utilised parameter (15 studies) was FEF25–75, either alone or in combination with other measurements. Ten studies provided criteria for the definition of SAO, of which percent predicted cut-offs were the most common (5 studies). However, there was no agreement on which cut-off value to use. Prevalence of SAO ranged from 7.5% to 45.9%. As a result of high heterogeneity across studies (I2 = 99.3%), explained by choice of spirometry parameter and WHO region, we do not present a pooled prevalence estimate.
Conclusion
There is a lack of consensus regarding the best spirometry parameter or defining criteria for identification of SAO. The value of continuing to measure SAO using spirometry is unclear without further research using large longitudinal data.
PROSPERO registration number CRD42021250206
Similar content being viewed by others
Introduction
Around 7% of the world population is estimated to be living with a chronic respiratory illness, with chronic obstructive pulmonary disease (COPD) and asthma being the most prevalent [1]. Since the late 1960s, the small airways of the lungs have been investigated as a site of interest in early obstructive lung disease [2]. The small airways are those with a diameter of less than 2 mm and have been described as a silent zone, where disease states can go unnoticed for many years [3]. Studies have shown that the small airways contribute significantly to airflow obstruction in both COPD [4] and asthma [5]. Characterised by inflammation, hypersecretion of mucus and airway remodelling [6], small airways obstruction (SAO) has been shown to precede both emphysematous changes and reduction in traditional spirometric parameters in COPD [7, 8].
In the absence of a non-invasive gold standard method to assess SAO, spirometry is the most widely used on account of its relatively easy performance and simple measurement devices [9]. In 1972, the mid-maximal expiratory flow rate (MMEF) was proposed as the best spirometric parameter to identify SAO [10]. MMEF, widely known as FEF25-75, corresponds to the mean forced expiratory flow between 25 and 75% of the forced vital capacity (FVC). Its use is based on the hypothesis that the mid-late portion of the FVC reflects the airflow through the small airways, which are prone to expiratory collapse due to their lack of cartilaginous support. In more recent years, other spirometry parameters have been used to identify SAO, including forced expiratory flow rates at 25%, 50% and 75% of the FVC (FEF25, FEF50, FEF75), and forced expiratory ratios such as the forced expiratory volume in three seconds (FEV3) as a ratio of the FVC (FEV3/FVC) [11]. The lack of consensus over which spirometry parameter is best to identify SAO is compounded by the wide range of definitions of an abnormal result, with lower limits of normal (LLN) [12], percent predicted [13] and other arbitrary cut-offs [14], being used to diagnose isolated SAO in the presence of otherwise normal spirometry.
Unsurprisingly, there is debate in the scientific community as to the clinical significance of isolated SAO [15, 16]. Despite this, population-based studies have attempted to provide estimates of prevalence and associated risk factors [13, 17], as well as demonstrate its usefulness as a prognostic marker for future development of chronic respiratory disease, such as COPD [18]. There is, therefore, an argument that identification of those with isolated SAO in the general population is important, and may highlight an unrecognised potentially symptomatic, clinical population who are at risk of further lung function decline, and in whom intervention may be warranted. We conducted a systematic review to evaluate the consensus in the literature regarding the spirometry parameters used to identify SAO and the cut-offs used to define abnormal results. In addition, we evaluated the prevalence estimates and risk factors for SAO identified in population-based studies.
Methods
Search strategy and selection criteria
We adhered to our published study protocol [19]. We included population-based studies that were cross-sectional, cohort or case-cohort in design. Selection was restricted to adult populations (≥ 18 years), where at least one spirometry parameter was used to define SAO. Effort was made to translate all articles that were not in English. Studies were excluded if they were not based in the general population (for example occupational or hospital based) or if longitudinal studies had less than 1-year follow-up. We searched Medline (PubMed) and Web of Science from database inception to 1 May 2021. We also searched for grey literature using Google Scholar and reviewed reference lists of included studies. Literature search strategies for Medline (PubMed) and Web of Science used medical subject headings (MeSH) and text words related to selected spirometry parameters and derivations of the phrase SAO. The search strategy is fully described in Additional file 1: Table S1. Publications returned by the search were imported into the Covidence web-based software (www.covidence.org), which automatically removed duplicates. When two studies reported on the same group of subjects, full texts were reviewed and the study with the most complete data relating to our study outcomes was included. For example, if one study measured SAO but did not report prevalence and one measured SAO and reported prevalence, then the latter study was given preference. We used a complete dual review approach in which title and abstract screening as well as full-text screening were independently done by two reviewers (BKB and OM). Disagreements were resolved after discussion with a third reviewer (AFSA). If abstracts contained insufficient information, they were included in the full text screening.
Quality assessment
Study quality was evaluated independently by two reviewers (BKB and OM) using the Newcastle–Ottawa scale for observational studies [20]. Cross-sectional studies received a score from 0 to 8 and cohort studies from 0 to 9. They were rated on selection, comparability and outcome, and assigned a rating of good, fair or poor according to predefined quality criteria (Additional file 1: Tables S2, S5 and S6). Disagreements were resolved after discussion with author AFSA.
Data extraction and analysis
We created and completed data extraction forms using the Covidence software. Data extraction was conducted by two reviewers (BKB and OM), and discrepancies were resolved after re-reviewing the full texts and discussion with a third reviewer (AFSA). Data extraction included study characteristics, primary outcomes (spirometry parameter used to measure SAO and definition of an abnormal result), secondary outcomes (prevalence estimates for SAO and odds ratios for risk factors), and for longitudinal studies, we extracted the number of years of follow-up and data on lung function decline.
Pooled prevalence estimates for SAO
Where not explicitly stated, prevalence estimates for SAO were calculated from the number of cases and total size of the study population. When studies used multiple parameters or criterion to identify SAO, we used a hierarchy of evidence table to decide which parameters to include (Additional file 1: Table S3). To pool the prevalence estimates from several studies, we conducted a meta-analysis using Stata, version 17 (StataCorp LLC, TX, USA), and the metaprop command. Prior to meta-analysis, prevalence estimates were transformed using the Freeman-Tukey double arcsine method to account for overestimation of result weight, which could occur due to the presence of prevalence estimates at either extreme (0 or 1). To conduct the meta-analysis, we used a DerSimonian-Laird random effects model, which incorporates a measure of heterogeneity across studies. We tested for heterogeneity by using the I2 statistic, with a value > 75% indicating considerable heterogeneity. To explore potential sources of heterogeneity, we built a random effects meta regression model that included spirometry parameter, definition of an abnormal result, WHO region and gross national income (GNI). We used a backward elimination procedure and kept the variables that were significant in the final model. Due to significant heterogeneity across studies, pooled estimates were suppressed to avoid reporting inaccurate estimates of prevalence of SAO. Meta-analysis on risk factors and lung function decline was not possible due to insufficient data available for extraction.
Results
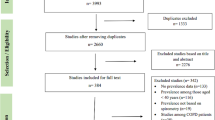
The search returned 1800 articles (full selection process is shown in Fig. 1) of which we identified 38 for full text review. One was in French and one was in Japanese, both of which were translated. We excluded 13 full texts, leaving 25 for inclusion. Publication dates ranged from 1979 to 2020. There were 18 cross-sectional, 6 cohort and 1 nested case–control study. All 25 studies used at least one parameter to identify SAO—these are described in full in Additional file 1: Table S4. Ten studies provided estimates for prevalence of SAO or contained data from which it could be calculated.
Spirometry parameters used to measure SAO
Across the 25 studies, 16 different spirometry parameters were used to identify SAO (Fig. 2). The majority (60%) used either FEF25–75 alone [21,22,23,24,25,26,27,28] or in combination with other parameters [13, 29,30,31,32,33,34]. Three studies used FEF25–75/FVC [35,36,37]. Two studies used FEF25, FEF50 or FEF75 either alone [38] or in combination [39]. One study used FEV3/FVC [40] and another used both FEV3/FVC and FEV3/FEV6. [12] Three studies used parameters that are not widely documented in the literature, forced expiratory time between 25 and 75% of the FVC (FET25–75) [41], concavity index [42] and forced expiratory flow at 50% of the FVC as a ratio of the forced expiratory flow with 25% of the FVC remaining (FEF50/FEF25) [14]. Twenty-one studies were of fair quality and 4 studies were of good quality (Additional file 1: Table S2).
Definitions of an abnormal result
Ten studies presented criteria defining an abnormal result (Table 1), utilising eight separate methods. Three used percent predicted cut offs including: FEF25–75 < 75% [29], FEF25–75 < 67% [23], and FEF50 < 70% predicted [38]. Two used LLN for FEV3/FVC [12, 40]. One used concavity index above the upper limit of normal (ULN) [42], and two used other methods: FEF50/FEF25 > 4 [14]; and FEF25–75/VC < FEV1/VC [35]. A further two studies used a combination of criteria, one used 2 out of 3 of FEF25–75, FEF50 or FEF75 < 65% predicted [13], and one used one of FEF25–75 < 60% or FEF25 < 65% predicted.
Prevalence of small airways obstruction
Nine studies provided prevalence estimates for pre-bronchodilator SAO and are summarised in Table 1. Sex was split evenly across studies, except for two which recruited only males [32, 40]. In most studies, age ranged from 18 to 60 years, the smallest contained 74 participants [23] and the largest 50,479 participants [13]. Two studies were European [32, 35], 2 were from the Americas [12, 29], 4 from the Western Pacific [13, 14, 38, 40] and 1 from South-East Asia [23]. Five studies were from high-income countries [12, 14, 29, 32, 35] and 4 were from low- and middle-income countries (LMICs) [13, 23, 38, 40]. Estimates of SAO prevalence ranged from 7.5% [38] to 49.5% [32]. In studies that measured forced expiratory flow rates (FEF25–75, FEF25, FEF50 and FEF75), prevalence of SAO ranged from 7.5% [38] to 45.9% [32], and in studies that used forced expiratory ratios (FEF25–75/VC, FEF50/FEF25, FEV3/FVC and FEV3/FEV6) estimates ranged from 16.3% [12] to 36.5% [14]. When a percent predicted cut-off was used to define an abnormal result, prevalence estimates ranged from 7.5% [38] to 45.9% [32], and when LLN was used it ranged from 16.3% [12] to 26.9% [13]. In high-income countries, prevalence ranged from 16.3% [12] to 45.9% [32] and in LMICs, prevalence was between 7.5% [38] and 26.9% [13]. In males, prevalence ranged from 9.5% [38] to 45.9% [28] and in females from 7.5% [38] to 49.8% [35]. In those < 40 years old, prevalence of SAO ranged from 10.1% [12] to 45.9% [32], between 41 and 59 years old it was 15% [40] to 42.2% [35] and in those > 60 years old prevalence was between 25.3% [40] and 32.5% [29]. In never smokers, prevalence ranged from 19.8% [29] to 39.1% [32] and in ever smokers 24.3% [13] to 53.9% [32]. Two studies reported SAO prevalence post-bronchodilator, giving estimates of 5.4% [42] and 13.3% [13], respectively. Raw data for prevalence of SAO by subgroup can be found in Additional file 2.
The prevalence of SAO across the nine studies with pre-bronchodilator measurements are displayed in Fig. 3. We did not report a pooled estimate due to marked heterogeneity across studies (I2 = 99.3%). The meta regression showed a significant effect of choice of spirometry parameter and WHO region on SAO prevalence. Results of the meta-regression are summarised in Table 2. These two variables accounted for 100% of the between-study variation. Using the Newcastle–Ottawa scale, 8 of the studies were scored as fair quality and one as good quality (Table 1).
Risk factors for small airways obstruction
Two Chinese studies reported adjusted odds ratios (OR) for the association of pre-bronchodilator SAO with risk factors (Table 3) [13, 38]. Both studies found that smoking was a significant risk factor for SAO, with Xiao et al. [13] showing that ever smokers were more likely to have SAO than never smokers (OR: 1.13, 95% CI 1.04–1.22), and Chen et al. [38] showing the same for heavy smokers (OR: 4.04, 95% CI 2.14–7.77). Chen et al. also found that exposure to second-hand smoke was associated with SAO (OR: 1.53, 95% CI 1.06–2.22) [38]. Only one of these studies found age and sex to be significant risk factors for SAO, with being > 70 years old associated with greater odds of having SAO compared to being 20–29 years old (OR: 2.41, 95% CI 2.13–2.72), and being female associated with increased odds of SAO (OR: 1.56, 95% CI 1.48–1.54) [13]. Both elevated body mass index (OR: 1.54, 95% CI 1.02–2.31) [38] and waist circumference (OR: 1.29, 95% CI 1.22–1.37) [13] were shown to be significant risk factors for SAO. Chen et al. [38] also reported a protective effect for exercising at least 30 min per day (OR: 0.31, 95% CI 0.20–0.48). Xiao et al. [13] found that biomass use (OR: 1.08, 95% CI 1.02–1.13) and high PM2.5 exposure (OR: 1.14, 95% CI 1.04–1.24) were associated with pre-bronchodilator SAO. Using the Newcastle–Ottawa scale, Chen et al. [38] was rated as fair, and Xiao et al. [13] rated as good quality (Additional file 1: Table S2).
Five studies assessed decline in FEF25-75 over time (Table 4). Two were from the UCLA cohort with follow-up after 5 years, [21, 30] two were from the SAPALDIA Study cohort with 11 years follow-up [25, 26], and one Italian study had 6 years of follow-up [32]. Taskin et al. [30] demonstrated that males who continued to smoke at follow-up had significantly greater decline in FEF25–75 (− 0.61 L/s) compared to those who quit prior to follow-up (− 0.39 L/s, p = 0.004). Three studies looked at the impact of pollution on FEF25–75 decline. Detels et al. [21] found that mean annual decline in FEF25–75 was significantly greater in the higher pollution Glendora region (− 93 ml/s) compared to the lower pollution Lancaster region of California (− 50 ml/s, p < 0.05). While the two studies from the SAPALIDA cohort showed that reduced exposure to PM10 was associated with a significant decrease (16%) in the annual rate of decline of FEF25–75, [26] and that certain gene polymorphisms attenuated annual rate of decline further [25]. Study quality was assessed as fair for two studies [30, 32], and good for three studies [21, 25, 26] (Additional file 1: Table S2).
Discussion
We found 25 population-based studies that used spirometry parameters to assess outcomes relating to SAO. There was significant variation in diagnostic methods across studies, with 16 different spirometry parameters used. The most widely utilised parameter was FEF25–75 either alone, or in combination with other parameters. Only 10 studies (50%) gave diagnostic criteria for SAO, with 8 different methods being used. The most popular criterion used was a result being below a percent predicted cut-off. Arbitrary cut-offs between 60 and 75% predicted were selected without justification [32], or justified by referencing studies in clinical populations that themselves did not provide justification [13, 38]. Knudson and Lebowitz [43] showed in 1978 that the normal 95th percentile for FEF25–75 is actually closer to 56% predicted in those over 36 years old, and the benefits of using LLN as opposed to percent predicted cut-offs is well documented [44]. The methodological variation seen in this review is in agreement with a systematic review of 15 studies in adults with asthma, which reported 5 different spirometry parameters for diagnosing of SAO. [45].
We found 9 studies that provided pre-bronchodilator estimates for prevalence of SAO, ranging from 7.5% to 45.9%. Due to significant heterogeneity, we were unable to provide pooled estimates. From the meta-regression, we identified choice of spirometry parameter and WHO region as causes of this heterogeneity. The contribution of WHO region to heterogeneity across studies is not unexpected as the prevalence of obstructive lung disease varies across world regions [46]. We found that prevalence estimates where a percent predicted cut-off was used had a wider range than estimates where LLN was used. Similarly, FEV3/FVC was shown to have a narrower prevalence range than FEF25–75. In the study by Xiao et al. [13] the prevalence of SAO was 26.9% using FEF25–75 < LLN and 13.9% using FEV3/FVC < LLN, demonstrating the impact that choice of parameter can have on prevalence estimates. FEF25–75 has been shown to lack sensitivity, have large between subject variability in healthy populations, and potentially misestimate the prevalence of mild obstructive lung disease depending on the diagnostic criterion used [15, 47]. This may explain why some prevalence estimates are very high. In contrast, FEV3/FVC < LLN has been shown to be a sensitive measure of expiratory obstruction [47], associated with several indicators of mild lung injury [48]. Both methods rely on the accuracy of the measurement of the FVC, and provide overestimates of SAO when the FVC manoeuvre is terminated early. Even with these methodological limitations, spirometric measures of SAO have been shown to correlate well with markers of SAO taken from computed tomography (CT) scans in COPD [16]. However, spirometry has a significant advantage over other diagnostic methods such as thoracic CT scans and impulse oscillometry because devices are cheaper, more portable, and a wealth of potential data already exists in established population based studies.
Risk factors for SAO were found to be very similar to those for chronic airflow obstruction (CAO) [13, 38, 49], and included increasing age, previous smoking, passive smoke exposure, low education, and history of tuberculosis. Biomass fuel use for cooking and heating was found not to be a risk factor for post-bronchodilator SAO [13] in keeping with previous research on CAO [50], although available data are limited. However, in a single study, exposure to high annual levels of PM2.5 was associated with increased risk of post-bronchodilator SAO [13], contradicting previous research in populations with CAO [51]. In support of an association between SAO, smoking and PM2.5, the five included cohort studies reported a greater decline in FEF25–75 among current smokers, compared to never or former smokers [30], and a lesser decline in FEF25–75 in individuals exposed to lower levels of air particulates. A recent hospital-based study in adults, aged 40 years and above, has suggested that SAO might be an important predictor of COPD diagnosis several years later [18]. Therefore, the importance of isolated SAO at population level is its potential to be a modifiable precursor to future CAO.
The main limitation in the literature is related to the lack of agreement between studies as to which spirometry parameter or definition of an abnormal result to use to assess SAO. In addition, the observational design of included studies increases risk of bias. The overall quality of evidence included in this review was fair, this was largely due to selection bias caused by choice of study population [14, 23, 32, 40], and unsatisfactory response rate [12, 14, 23, 29, 38]. Additionally, in several studies assessment of SAO was not a primary outcome, meaning they were likely not sufficiently powered to draw conclusions. There was potential information bias in 6 studies [12, 14, 23, 29, 38, 40], which did not specify whether FEV1 and FEV1/FVC were normal in the populations diagnosed with SAO. In the three studies that did specify [13, 32, 35], arbitrary percent predicted cut-offs were used. This makes it hard to discern whether SAO was present due to existing obstructive lung disease or as its own clinical entity. There was also considerable variation in the spirometry reference equations used. Most studies used locally derived regression equations, while highly applicable to the population being studied, they are not transferable across regions. Additionally, only 50% of studies followed American Thoracic Society and European Respiratory Society (ATS/ERS) spirometry performance guidelines [52], meaning the quality of spirometry measurements in some studies could be questioned. Furthermore, most recent studies were based in the western pacific region, whereas studies providing prevalence estimates for SAO in Europe and the Americas were pre-2000 and likely not applicable to modern day. At review level, every effort was made to limit risk of bias. However, as the primary outcome of this study was which spirometry parameters are used to identify SAO, we may have missed publications which contained relevant data for prevalence estimates because they did not explicitly state that they were assessing SAO. In addition, as per the study protocol, we intended to assess certainty of evidence using the GRADE methodology for prevalence and risk factors for SAO [53]. However, as we could not present pooled estimates for either of these outcomes, and therefore draw firm conclusions, we could not comment on the certainty of evidence.
Conclusions
This review highlights significant methodological inconsistencies in the measurement of SAO in population-based studies. Importantly, it highlights that researchers and clinicians should be cautious about continuing to use spirometry to identify SAO until a consensus is reached. Prevalence estimates derived from spirometry should not be used to inform policy while so many different diagnostic methods are being used. The significant association of SAO with risk factors such as smoking, air pollution, education level and age should not be ignored, but further examined in larger population-based studies. Future research should use LLN to estimate prevalence and risk factors for SAO with longitudinal follow-up, to determine whether those with SAO at baseline go on to develop obstructive lung conditions later in life.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its additional information files].
Abbreviations
- COPD:
-
Chronic obstructive pulmonary disease
- CAO:
-
Chronic airflow obstruction
- SAO:
-
Small airways obstruction
- MMEF/FEF25–75 :
-
Mid-maximal expiratory flow rate
- FEV1 :
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- FEV3:
-
Forced expiratory volume in 3 s
- FEV1/FVC:
-
Forced expiratory volume in 1 s as a ratio of the forced vital capacity
- FEV3/FVC:
-
Forced expiratory volume in 3 s as a ratio of the forced vital capacity
- FEV3/FEV6 :
-
Forced expiratory volume in 3 s as a ratio of the forced expiratory volume in 6 s
- FEF25, 50, & 75 :
-
Forced expiratory flow rate at 25%, 50% and 75% of the forced vital capacity
- LLN:
-
Lower limit of normal
- ULN:
-
Upper limit of normal
- MeSH:
-
Medical subject heading
- FET25–75 :
-
Forced expiratory time between 25 and 75% of the forced vital capacity
- FEF50/FEF75 :
-
Forced expiratory flow rate at 50% of the forced vital capacity as a ratio of the forced expiratory flow rate at 75% of the forced vital capacity
- IQR:
-
Inter quartile range
- CI:
-
Confidence interval
- SD:
-
Standard deviation
- NOS:
-
Newcastle Ottawa scale
- GRADE:
-
Grading of recommendations, assessment, development and evaluation
- WHO:
-
World Health Organisation
- CT:
-
Computed tomography
- Pre-BD:
-
Pre bronchodilator
- Post-BD:
-
Post bronchodilator
- BMI:
-
Body mass index
- ATS:
-
American thoracic society
- ERS:
-
European respiratory society
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- LMIC’s:
-
Low/middle income countries
References
Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–96. https://doi.org/10.1016/s2213-2600(20)30105-3.
Macklem PT, Mead J. Resistance of central and peripheral airways measured by a retrograde catheter. J Appl Physiol. 1967;22(3):395–401. https://doi.org/10.1152/jappl.1967.22.3.395.
Mead J. The lung’s “quiet zone.” N Engl J Med. 1970;282(23):1318–9. https://doi.org/10.1056/nejm197006042822311.
Baraldo S, Turato G, Saetta M. Pathophysiology of the small airways in chronic obstructive pulmonary disease. Respiration. 2012;84(2):89–97. https://doi.org/10.1159/000341382.
Postma DS, Brightling C, Baldi S, et al. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): baseline data from a prospective cohort study. Lancet Respir Med. 2019;7(5):402–16. https://doi.org/10.1016/s2213-2600(19)30049-9.
Higham A, Quinn AM, Cancado JED, et al. The pathology of small airways disease in COPD: historical aspects and future directions. Respir Res. 2019;20(1):49. https://doi.org/10.1186/s12931-019-1017-y.
Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–53. https://doi.org/10.1056/NEJMoa032158.
Pompe E, Moore CM, Mohamed Hoesein FAA, et al. Progression of emphysema and small airways disease in cigarette smokers. Chronic Obstr Pulm Dis. 2021;8(2):198–212. https://doi.org/10.15326/jcopdf.2020.0140.
Konstantinos Katsoulis K, Kostikas K, Kontakiotis T. Techniques for assessing small airways function: possible applications in asthma and COPD. Respir Med. 2016;119:e2–9. https://doi.org/10.1016/j.rmed.2013.05.003.
McFadden ER Jr, Linden DA. A reduction in maximum mid-expiratory flow rate. A spirographic manifestation of small airway disease. Am J Med. 1972;52(6):725–37. https://doi.org/10.1016/0002-9343(72)90078-2.
Hoesterey D, Das N, Janssens W, et al. Spirometric indices of early airflow impairment in individuals at risk of developing COPD: spirometry beyond FEV(1)/FVC. Respir Med. 2019;156:58–68. https://doi.org/10.1016/j.rmed.2019.08.004.
Hansen JE, Porszasz J, Casaburi R, et al. Re-defining lower limit of normal for FEV1/FEV6, FEV1/FVC, FEV3/FEV6 and FEV3/FVC to improve detection of airway obstruction. Chronic Obstr Pulm Dis-J Copd Foundation. 2015;2(2):94–102. https://doi.org/10.15326/jcopdf.2.2.2014.0144.
Xiao D, Chen Z, Wu S, et al. Prevalence and risk factors of small airway dysfunction, and association with smoking, in China: findings from a national cross-sectional study. Lancet Respir Med. 2020;8(11):1081–93. https://doi.org/10.1016/s2213-2600(20)30155-7.
Nemoto T, Shibata Y, Osaka D, et al. Impact of cigarette smoking on maximal expiratory flows in a general population: the Takahata study. Intern Med. 2011;50(21):2547–55. https://doi.org/10.2169/internalmedicine.50.5948.
Quanjer PH, Weiner DJ, Pretto JJ, et al. Measurement of FEF25-75% and FEF75% does not contribute to clinical decision making. Eur Respir J. 2014;43(4):1051–8. https://doi.org/10.1183/09031936.00128113.
Gove K, Wilkinson T, Jack S, et al. Systematic review of evidence for relationships between physiological and CT indices of small airways and clinical outcomes in COPD. Respir Med. 2018;139:117–25. https://doi.org/10.1016/j.rmed.2018.05.005.
Chen YS, Li XQ, Li HR, et al. Risk factors for small airway obstruction among Chinese island residents: a case-control study. PLoS ONE. 2013;8(7): e68556. https://doi.org/10.1371/journal.pone.0068556.
Kwon DS, Choi YJ, Kim TH, et al. FEF(25–75%) values in patients with normal lung function can predict the development of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2020;15:2913–21. https://doi.org/10.2147/copd.S261732.
Knox-Brown B, Mulhern O, Amaral AFS. Spirometry parameters used to define small airways obstruction in population-based studies: systematic review protocol. BMJ Open. 2021;11(10): e052931. https://doi.org/10.1136/bmjopen-2021-052931.
Wells G SB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp2013.
Detels R, Tashkin DP, Sayre JW, et al. The UCLA population studies of chronic obstructive respiratory disease. 9. Lung function changes associated with chronic exposure to photochemical oxidants; a cohort study among never-smokers. Chest. 1987;92(4):594–603. https://doi.org/10.1378/chest.92.4.594.
Behera D. An analysis of effect of common domestic fuels on respiratory function. Indian J Chest Dis Allied Sci. 1997;39(4):235–43.
Cullinan P, Acquilla S, Dhara VR. Respiratory morbidity 10 years after the Union Carbide gas leak at Bhopal: a cross sectional survey. Bmj-Br Med J. 1997;314(7077):338–42. https://doi.org/10.1136/bmj.314.7077.338.
Matheson MC, Ellis JA, Raven J, et al. Association of ILS, CXCR2 and TNF-alpha polymorphisms and airway disease. J Hum Genet. 2006;51(3):196–203. https://doi.org/10.1007/s10038-005-0344-7.
Curjuric I, Imboden M, Schindler C, et al. HMOX1 and GST variants modify attenuation of FEF25–75% decline due to PM10 reduction. Eur Respir J. 2010;35(3):505–14.
Downs SH, Schindler C, Liu LJ, et al. Reduced exposure to PM10 and attenuated age-related decline in lung function. N Engl J Med. 2007;357(23):2338–47. https://doi.org/10.1056/NEJMoa073625.
Abdel-Hamid HEM. Impulse oscillometry may be of value in detecting early effects of obesity on airway resistance. Egypt J Chest Dis Tuberc. 2019;68(1):96–101. https://doi.org/10.4103/ejcdt.ejcdt_106_18.
Al Khathlan N, Salem AM. The effect of adiposity markers on fractional exhaled nitric oxide (FeNO) and pulmonary function measurements. Int J General Med. 2020;13:955–62. https://doi.org/10.2147/ijgm.S280395.
Detels R, Rokaw SN, Coulson AH, et al. The UCLA population studies of chronic obstructive respiratory disease. I. Methodology and comparison of lung function in areas of high and low pollution. Am J Epidemiol. 1979;109(1):33–58. https://doi.org/10.1093/oxfordjournals.aje.a112658.
Tashkin DP, Clark VA, Coulson AH, et al. The UCLA population studies of chronic obstructive respiratory disease. VIII. Effects of smoking cessation on lung function: a prospective study of a free-living population. Am Rev Respir Dis. 1984;130(5):707–15. https://doi.org/10.1164/arrd.1984.130.5.707.
White JR, Froeb HF. Small-airways dysfunction in nonsmokers chronically exposed to tobacco smoke. N Engl J Med. 1980;302(13):720–3. https://doi.org/10.1056/nejm198003273021304.
Marazzini L, Cavigioli G, Mastropasqua B, et al. FEV1 decline in asymptomatic young adults: relationships with some tests of small airways function. Eur Respir J. 1989;2(9):817–21.
Kiter G, Uçan ES, Ceylan E, et al. Water-pipe smoking and pulmonary functions. Respir Med. 2000;94(9):891–4. https://doi.org/10.1053/rmed.2000.0859.
Havet A, Hulo S, Cuny D, et al. Residential exposure to outdoor air pollution and adult lung function, with focus on small airway obstruction. Environ Res. 2020;183: 109161. https://doi.org/10.1016/j.envres.2020.109161.
Wipf R, Stinghe R, Perrin J. First results of a longitudinal survey upon the small airways disease. Poumon Coeur. 1982;38(2):85–90.
Tager IB, Balmes J, Lurmann F, et al. Chronic exposure to ambient ozone and lung function in young adults. Epidemiology. 2005;16(6):751–9. https://doi.org/10.1097/01.ede.0000183166.68809.b0.
Cox CA, Vonk JM, Kerstjens HAM, et al. Predicted values for the forced expiratory flow adjusted for forced vital capacity, a descriptive study. Erj Open Res. 2020. https://doi.org/10.1183/23120541.00426-2020.
Chen YS, Li XQ, Li HR, et al. Risk factors for small airway obstruction among Chinese island residents: a case-control study. PLoS ONE. 2013. https://doi.org/10.1371/journal.pone.0068556.
Marrero O, Beck GJ, Schachter EN. Discriminating power of measurements from maximum expiratory flow-volume curves. Respiration. 1986;49(4):263–73. https://doi.org/10.1159/000194889.
Lam DCL, Fong DYT, Yu WC, et al. FEV3, FEV6 and their derivatives for detecting airflow obstruction in adult Chinese. Int J Tuberc Lung Dis. 2012;16(5):681–6.
Brown LK, Miller A, Pilipski M, et al. Forced midexpiratory time: reference values and the effect of cigarette smoking. Lung. 1995;173(1):35–46. https://doi.org/10.1007/bf00167599.
Johns DP, Das A, Toelle BG, et al. Improved spirometric detection of small airway narrowing: concavity in the expiratory flow-volume curve in people aged over 40 years. Int J Chron Obstruct Pulmon Dis. 2017;12:3567–77. https://doi.org/10.2147/copd.S150280.
Knudson RJ, Lebowitz MD. Maximal mid-expiratory flow (FEF25-75%): normal limits and assessment of sensitivity. Am Rev Respir Dis. 1978;117(3):609–10. https://doi.org/10.1164/arrd.1978.117.3.609.
Miller MR, Quanjer PH, Swanney MP, et al. Interpreting lung function data using 80% predicted and fixed thresholds misclassifies more than 20% of patients. Chest. 2011;139(1):52–9. https://doi.org/10.1378/chest.10-0189.
Usmani OS, Singh D, Spinola M, et al. The prevalence of small airways disease in adult asthma: a systematic literature review. Respir Med. 2016;116:19–27. https://doi.org/10.1016/j.rmed.2016.05.006.
Aaron SD, Gershon AS, Gao Y, et al. Influence of country-level differences on COPD prevalence. Int J Chron Obstruct Pulmon Dis. 2016;11:2305–13. https://doi.org/10.2147/copd.S113868.
Hansen JE, Sun XG, Wasserman K. Discriminating measures and normal values for expiratory obstruction. Chest. 2006;129(2):369–77. https://doi.org/10.1378/chest.129.2.369.
Morris ZQ, Coz A, Starosta D. An isolated reduction of the FEV3/FVC ratio is an indicator of mild lung injury. Chest. 2013;144(4):1117–23. https://doi.org/10.1378/chest.12-2816.
Burney P, Patel J, Minelli C, et al. Prevalence and population attributable risk for chronic airflow obstruction in a large multinational study. Am J Respir Crit Care Med. 2020. https://doi.org/10.1164/rccm.202005-1990OC.
Amaral AFS, Patel J, Kato BS, et al. Airflow obstruction and use of solid fuels for cooking or heating: BOLD results. Am J Respir Crit Care Med. 2018;197(5):595–610. https://doi.org/10.1164/rccm.201701-0205OC.
Amaral AFS, Burney PGJ, Patel J, et al. Chronic airflow obstruction and ambient particulate air pollution. Thorax. 2021. https://doi.org/10.1136/thoraxjnl-2020-216223.
Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An Official American Thoracic Society and European Respiratory Society Technical statement. Am J Respir Crit Care Med. 2019;200(8):e70–88. https://doi.org/10.1164/rccm.201908-1590ST.
Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. https://doi.org/10.1016/j.jclinepi.2010.04.026.
Acknowledgements
Not applicable.
Funding
This research received no specific Grant from any funding agency in the public, commercial or not-for-profit sectors. Ben Knox-Brown is in receipt of a National Heart and Lung Institute (WHRG-F26289) PhD scholarship, which allows the conduct of this review.
Author information
Authors and Affiliations
Contributions
BKB and AFSA planned the review. BKB and OM performed title and abstract screening, full text review and data extraction. AFSA acted as arbiter at all stages of the review. BKB performed statistical analysis with assistance from AFSA. BKB wrote the initial draft of the manuscript and all tables and figures. BKB, OM JF and AFSA contributed to the writing of the manuscript. All co-authors read and commented on the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
None declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Table S1. Search terms used within MEDLINE (PubMed) and Web of Science. Table S2. Quality assessment scores of selected studies. Table S3. Hierarchy of evidence ranking system. Table S4. Summary of different parameters used to assess small airways obstruction in population-based studies. Table S5. The modified Newcastle Ottawa scale for cross sectional studies. Table S6. The modified Newcastle-Ottawa scale for Cohort studies.
Additional file 2.
Tables displaying raw data for prevalence of SAO by subgroup.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Knox-Brown, B., Mulhern, O., Feary, J. et al. Spirometry parameters used to define small airways obstruction in population-based studies: systematic review. Respir Res 23, 67 (2022). https://doi.org/10.1186/s12931-022-01990-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-022-01990-2