Abstract
Background
RSV can lead to persistent airway inflammation and airway hyperresponsiveness (AHR), and is intimately associated with childhood recurrent wheezing and asthma, but the underlying mechanisms remain unclear. Lipopolysaccharide (LPS) is also implicated in the onset and exacerbation of asthma. However, whether inhalation of LPS can boost airway inflammation induced by RSV is not clear. In this study, we utilized an LPS- and RSV-superinfected mouse model to explore underlying pathogenesis.
Methods
Mice were infected with RSV on day 0 and inoculated with LPS from day 35 to day 41, samples were collected on day 42. Inflammatory cells, lung histopathology and AHR were measured. Cytokines were detected by ELISA and ERK, JNK, p38 was determined by western blot. MMP408, PD98059, SP600125 and SB203580 were used to inhibit MMP-12, ERK, JNK and p38 respectively.
Results
LPS exposure superimposed on RSV-infected lungs could lead to more vigorous cellular influx, lung structures damage, augmented AHR and higher MMP-12 levels. Inhibition of MMP-12 or ERK signaling pathway in vivo both diminished LPS-driven airway inflammation and AHR.
Conclusions
Exposure to LPS in RSV-infected mice is associated with enhanced increases in ERK-MMP-12 expression that translates into increased lung inflammation and AHR. These findings contribute novel information to the field investigating the onset of post-RSV bronchiolitis recurrent wheezing as a result of LPS exposure.
Similar content being viewed by others
Background
Respiratory syncytial virus (RSV) is the most frequent cause of lower respiratory infection (LRTI) in infants worldwide. An estimated 30–70% of infants develop bronchiolitis upon primary RSV infection, 1–3% of whom are hospitalized [1, 2]. Moreover, studies conducted in different parts of the world and with different study designs, have shown that RSV LRTI / bronchiolitis in early life is associated with up to a five-fold increase in risk of developing recurrent wheezing and asthma later in childhood [3]. A birth cohort study that has been ongoing for almost four decades showed that early life RSV LRTI is associated with a persistently low lung function trajectory that represents an important pathway to COPD [4]. Despite the risks of RSV infection being well accepted, the precise mechanisms leading to the onset of these chronic airway diseases are poorly understood.
Co-infection of bacteria and viruses had a synergistic effect, leading to more severe disease and hospitalization [5]. It’s reported that RSV-infected infants are colonized with pathogenic bacteria and have a higher proportion of Gram-negative bacterial colonization compared to healthy age-matched controls [6]. In addition to bacterial co-infection, environmental pollution is also an adverse risk to asthma exacerbation [7]. Lipopolysaccharide (LPS) is a major compound of wall for Gem-negative bacteria, and also acts as a significant immunostimulatory component of air pollution. Both epidemiological studies and animal experiments have demonstrated that exposure to LPS can sensitize or exacerbate COPD and asthma by promoting Th2 responses [8]. We have identified that a Th2 cytokines-dominant airway microenvironment similar to asthma was triggered during the later stage of RSV infection [9]. However, it’s not clear whether inhalation of LPS might boost RSV-associated airway inflammation and AHR by increasing Th2 responses.
In addition to Th2 cytokines, our preliminary results have also demonstrated that matrix metalloproteinases (MMPs), especially MMP-12 are important in RSV pathogenesis [10, 11]. MMP-12 is mainly secreted by macrophages. It regulates the turnover of extracellular matrix (ECM) and is widely involved in the pathogenesis of chronic airway diseases including asthma and COPD, and also delays viral clearance by affecting IFN-α [12]. In vitro studies stated that LPS could induce MMP-12 in mouse liver macrophages [13] and human bronchial epithelial cells [14]. However, whether LPS exposure can affect RSV-associated MMP-12 overproduction is also not clear.
LPS is recognized by Toll-like receptor 4 (TLR4) and MD-2 on host innate immune cells and can signal to activate the MAP kinase proteins (MAPK), finally leading to the increase of multi-inflammatory mediators and co-stimulatory molecules. Among the three MAPK proteins, the ERK1/2 kinase induces gene expression responsible for mucin production via promoting MUC5AC in inflammatory cells resulting in mucus production in asthmatic model. JNK kinase has been reported to be associated with T cell activation and maturation, whereas p38 plays an important role in inducing expression of pro-inflammatory mediators IL-1β, TNF-α, IL-8, IL-6, and IL-3, especially in the macrophages [15]. MMP-12 was induced in the LPS-treated bronchial epithelial cells, which was decreased by ERK inhibition [14]. Moreover, cigarette smoke could activate ERK pathway that mediated the DC-induced Th2 polarization [16]. In this pre-clinical study, we utilized an LPS- and RSV-superinfected mouse model to explore whether LPS can exaggerate airway disorders by boosting the MAPK-Th2 cytokines / MMP-12 pathway.
Methods
Animal
Adult 6-week-old female BALB/c mice were purchased from the Animal Laboratory of Chongqing Medical University and housed in individually filtered cages. All experiments involving animals were in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Committee (IACUC), which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, China and Experimental Animal Committee of the Chongqing Medical University (license numbers: SCXK (Yu) 2012–0001 and SYXK (Yu) 2012–0001).
Virus preparation
RSV A2 (ATCC, VR-1540) strain was propagated in Hep-2 cells (ATCC) with Dulbecco’s Modified Eagle’s Medium (DMEM; GIBCO, California, USA) plus 5% fetal bovine serum (FBS, GIBCO). Viral titer was determined by plaque assay. The supernatants of uninfected Hep-2 cells were generated under the same condition.
LPS preparation
LPS (Escherichia coli 055:B5, Sigma), was assayed using the limulus hemocyanin agglutination assay. Ambient environmental LPS exposure concentrations have been reported up to 10,000 endotoxin units (EU)/m3 although ambient levels are usually below 1000 EU/m3. 1 EU is equivalent to approximately 0.1 ng LPS and so 1000 EU is equivalent to 0.1 mg LPS. Our LPS challenge method with 10 ng was therefore within the level bounds of exposure that would usually occur in the environment. Certainly, the LPS doses that we used are in accordance with previous publications [17].
Inoculation procedure
Mice were anaesthetized with isoflurane and held upright before intranasal inoculation. Mice were divided into four groups:
-
The control group: Mice were mock-infected with 100 μl cell supernatant on day 0 and then inoculated with 50 μl of PBS every 2 days from day 35 to day 41.
-
The LPS group: Mice were mock-infected with 100 μl cell supernatant on day 0 and then inoculated with 10 μg LPS dissolved in 50 μl PBS every 2 days from day 35 to day 41.
-
The RSV group: Mice were infected with RSV (1.8*107 PFU in 100 μl of virus supernatant) on day 0 and then inoculated with 50 μl of PBS every 2 days from day 35 to day 41 post RSV infection.
-
The RSV + LPS group: Mice were infected with RSV on day 0 and then inoculated with 10 μg LPS dissolved in 50 μl PBS every 2 days from day 35 to day 41 post RSV infection.
Disease parameters were assessed on day 42.
Whole lung lavage
Following euthanasia, the trachea was cannulated and bronchoalveolar lavage fluid (BALF) was collected for cytokine concentration measurement and inflammatory cell evaluation. The total number of cells was quantified by automated cell counter (Count Star, China). Cytospin slides were fixed and stained with DiffQuik (Baxter Healthcare Corp, Deerfield, Miami, FL) for leukocyte differential analysis.
Flow cytometry analysis
The single cell suspensions of mouse lung tissue were prepared as described previously [9]. Samples were blocked with rat serum for 20 min, and then immunostained with antibody to mouse CD45, CD3, CD49b, CD19, F4/80, CD11c, CD11b, Ly6G, Ly6C or isotype control conjugated with PerCP-cy7, PerCP-cy5.5, PE, FITC or APC for 30 min on ice. The indicated antibodies were obtained from eBioscience (San Diego, CA) or BD Biosciences or Invitrogen. Next, stained samples were fixed with 1% Formaldehyde in FACS Staining Buffer and measured on a flow cytometer, FACSCalibur (BD Biosciences), which collected data on at least 5000/10,000 events.
Lung histology
For histology studies, left-lung lobes from mice were removed, fixed in 10% formalin, cut into 5 μm sections, and stained with HE solution (Sigma, St. Louis, MO, USA). Images were captured under a Nikon Eclipse E200 microscope connected to a Nikon Coolpix 995 camera (Nikon, Tokyo, Japan).
Airway hyper-responsiveness (AHR)
AHR was measured 24 h after the final LPS challenge by measuring the lung resistance (LR) using an invasive lung function test. Animals were anesthetized with pentobarbital (30 mg/kg, ip) and connected via a tracheostomy tube to a computer-controlled piston ventilator (flexiVent, Scireq). Subsequently, mice were exposed to aerosolized acetyl-β-methylcholine (Sigma-Aldrich, Saint Louis, MO, USA), at increasing doses: 0, 3.125, 6.25, 12.5, 25, and 50 mg/ml. At each dose, LR was calculated using the single-compartment model.
Cytokine analysis
The levels of IL-4, IL-5, IL-13, IFN-γ, IL-10, IL-6, IL-17, IL-21, IL-1β, MMP-9 and MMP-12 in BALF were measured using an enzyme-linked immunosorbent assay (ELISA) with commercial kits (eBioscience, CA, USA)) according to the manufacturer’s instructions. Duplicate wells were run, and the mean values were reported.
RNA extraction, reverse transcription, and quantitative PCR (qPCR)
Total RNA from mouse lung tissues was purified, and cDNA synthesis was performed using a PrimeScript RTReagent Kit according to manufacturer’s recommendations (Takara, Otsu, Japan). Quantitative PCR (qPCR) was performed using standard techniques [18]. GAPDH was used as endogenous controls. The primer sequences of GATA3 were 5′-CTCGGCCATTCGTACATGGAA-3' (forward) and 5'-GGATACCTCTGCACCGTAGC-3' (reverse); ID2 were 5'-GCATCCCACTATCGTCAGCC-3' (forward) and 5'-AAGGGAATTCAGATGCCTGCAA-3' (reverse); MMP-12 were 5'-CGATGTGGAGTGCCCGATGT-3' (forward) and 5′- AGTCTCCGTGAGCTCCAAATGC-3′ (reverse); and GAPDH were 5′-AGCAATGCCTCCTGCACCACCAAC-3′ (forward) and 5′-CCGGAGGGGCCATCCACAGTCT-3′ (reverse).
Western blotting analysis
Total protein of mice lung tissues were obtained and the concentrations were determined as previously reported [11]. Samples were separated on an 8% SDS-PAGE gels, transferred onto PVDF membranes (Millipore, Billerica, MA), bathed in blocking buffer for 1 h at room temperature, and then incubated overnight at 4 °C with primary antibody of ERK (Santa Cruz Biotechnology, 1:500), p-ERK (Santa Cruz Biotechnology, 1:500), JNK (Santa Cruz Biotechnology, 1:500), p-JNK (Santa Cruz Biotechnology, 1:500), p38 (Santa Cruz Biotechnology, 1:1000), p-p38 (Santa Cruz Biotechnology, 1:1000) or GAPDH (Santa Cruz Biotechnology, 1:3000) respectively. An alkaline phosphatase-conjugated goat anti-mouse antibody (MultiSciences, China, 1:5000) was used to detect the presence of the respective protein bands. Densitometry of bands from Western blots was done by ImageJ2x 2.1.4.7 (Wayne Rasband, National Institutes of Health, USA), and the densities of the ERK, p-ERK, JNK, p-JNK, p38, p-p38 proteins relative to GAPDH were measured.
MMP-12 inhibition
Mice were treated with MMP408, a potent and specific MMP-12 inhibitor (CALBIOCHEM, EMD Chemicals, Inc. San Diego, CA 92121) at 5 mg/kg intragastrically twice a day consecutively from day 35 to day 41 post RSV infection. The control animals received sterile PBS similarly.
MAPK pathway inhibition
To assess the effects of ERK, JNK, and p38 pathway on the airway disorders induced by LPS in our model, where indicated, mice were treated with the specific inhibitors of ERK (PD98059, invivogen, 10 mg/kg) or JNK (SP600125, invivogen, 20 mg/kg) or p38 (SB203580, invivogen, 5 mg/kg) respectively. The inhibitor was solubilized in 2% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA). Treatments with inhibitors or DMSO alone were given intraperitoneally 1 h before and 2 h after the first LPS inoculation on day 35, and once a day successively from day 36 to day 41.
Statistical analysis
All statistical tests were performed using Prism GraphPad Software (La Jolla, CA), and the results are expressed as the mean ± sem. Two-way ANOVA with Bonferroni post-tests were used to compare the differences among multiple groups to AHR. Analysis of variance (ANOVA) was used to determine the differences between all groups to other indices. Data lacking normal distribution were evaluated using the nonparametric Kruskal-Wallis test, followed by Dunn’s multiple comparison. Differences were considered to be significant for p-values less than 0.05.
Results
LPS increases lung inflammation and AHR during the later stage of RSV infection
Mice were infected with RSV on day 0 and then inoculated with LPS as described in the materials and methods section. As shown in Fig. 1, inflammatory cells (A), monocytes (B), neutrophils (C) in BALF were dramatically aggravated in the RSV + LPS group as compared to the control group, the RSV group and the LPS group (all P < 0.05). Lung tissue flow cytometric analysis revealed that macrophages (Fig. 2a) were dramatically aggravated in the RSV + LPS group as compared to the control group, the RSV group and the LPS group. Neutrophils (Fig. 2b), NK cells (Fig. 2c), T cells (Fig. 2d) and B cells (Fig. 2e) were all significantly increased in the RSV + LPS group as compared to the control group (all P < 0.05), but showed no significant difference versus the RSV group and the LPS group.
Inflammatory cells in bronchoalveolar lavage fluid (BALF). Mice were infected or mock-infected with RSV or cell supernatant on day 0 and then inoculated with LPS as described in the materials and methods part. BALF was collected and inflammatory cells were counted. a: Total inflammatory cells. b: Monocytes. c: Neutrophils. d: Lymphocytes. e: Eosinophils. Graphs are represented as the mean ± sem. Data are representative of two independent experiments performed on 8 animals per group. *, p < 0.05, **, p < 0.01, ***, p < 0.001, shown comparing the control group with the other groups; #, p < 0.05, ###, p < 0.001, shown comparing the RSV + LPS group with the LPS group; ^, p < 0.05, ^^, p < 0.01, ^^^, p < 0.001, shown comparing the RSV + LPS group with the RSV group
Inflammatory cells in lung tissues. The single cell suspensions of mouse lung tissue were prepared and detected with flow cytometry. a: Macrophages. b: Neutrophils. c: NK cells. d: T cells. e: B cells. Data are representative of two independent experiments performed on 8 animals per group. *, p < 0.05, **, p < 0.01, ***, p < 0.001, shown comparing the control group with the other groups; #, p < 0.05, ###, p < 0.001, shown comparing the RSV + LPS group with the LPS group; ^^, p < 0.01, shown comparing the RSV + LPS group with the RSV group
Next, lung tissue HE staining and histological scores (HPS) were performed to estimate pathological injury. As shown in Fig. 3a, no pulmonary hyper-cellularity or other pathological characteristics were observed in the control group. However, in the other mice groups, a mass of inflammatory cells infiltrated in the interstitial, peribronchiolar and perivascular compartments, with partial destruction of regular tissue structures (Fig. 3b-d). HPS (Fig. 3e) was similar to the morphological changes, which was markedly increased in the RSV + LPS group compared with the other three groups (all P < 0.05).
Pathological damage of lung tissues. Lungs were harvested on day 42 post-RSV infection, stained with hematoxylin-and-eosin (HE), and scored for levels of inflammation as described in the Materials and Methods section. Representative HE staining of mouse lung tissue sections (original Magnification × 200) were shown. a: control group; b: RSV group; c: LPS group; d: RSV + LPS group. Histological scores (HPS) were assessed (e). Graphs are represented as the mean ± sem. Data are representative of two independent experiments performed on 6 animals per group. *, p < 0.05, ***, p < 0.001, shown comparing the control group with the other groups; ###, p < 0.001, shown comparing the RSV + LPS group with the LPS group; ^^, p < 0.01, shown comparing the RSV + LPS group with the RSV group
Analysis for AHR showed that lung resistance (LR) of the RSV + LPS group was much higher than those of the control group, RSV group and the LPS group at methacholine concentrations of 6.25 mg/ml (all P < 0.05), 25 mg/ml (all P < 0.001) and 50 mg/ml (all P < 0.001) (Fig. 4).
Airway hyperresponsiveness (AHR). AHR was assessed by measuring the lung resistance (LR) to increasing doses of methacholine. Graphs are represented as the mean ± sem. Data are representative of two independent experiments performed on 6 animals per group. **, p < 0.01, ***, p < 0.001, shown comparing the control group with the other groups; #, p < 0.05, ###, p < 0.001, shown comparing the RSV + LPS group with the LPS group; ^, p < 0.05, ^^^, p < 0.001, shown comparing the RSV + LPS group with the RSV group
MMP-12 contributes to airway disorders provoked by LPS during the later stage of RSV infection
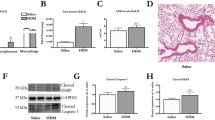
Matrix metalloproteinase 12 (MMP-12) was involved in the pathogenesis of both RSV- and LPS- associated airway disorders. Thus, MMP-12 was detected. As shown in Fig. 5, both mRNA (A) and protein levels (B) of MMP-12 were much higher in the RSV + LPS group versus the other three groups (all P < 0.05). To further elucidate the deleterious role of MMP-12, MMP408, a specific inhibitor of MMP-12 was administrated to mice. As shown in Fig. 6a, MMP-12 was significantly blocked by MMP408 (all P < 0.05 for RSV + LPS group vs. the other groups). Synergistically, BALF inflammatory cells, monocytes, neutrophils, eosinophils (Fig. 6b, P < 0.05 for RSV + LPS group vs. the other groups) were all dramatically reduced in the MMP408-treated mice (RL + i MMP-12 group). Lung tissue damage (Fig. 6c-g), HPS (Fig. 6h, P < 0.001 for RSV + LPS group vs. the other groups) and AHR (Fig. 6i, P < 0.001 for RSV + LPS group vs. the other groups at methacholine concentrations of 25 mg/ml and 50 mg/ml) were all dramatically reduced in the RL + i MMP-12 group in parallel. Taken together, MMP-12 contributed to airway disorders provoked by LPS during the later stage of RSV infection.
MMP-12 was markedly increased by LPS during the later stage of RSV infection. a: mRNA levels of MMP-12. b: MMP-12 protein in BALF. Values are expressed as mean ± sem. Data are representative of two independent experiments performed on 6 animals per group. *, p < 0.05, **, p < 0.01, ***, p < 0.001, shown comparing the control group with the other groups; #, p < 0.05, ##, p < 0.01, shown comparing the RSV + LPS group with the LPS group; ^, p < 0.05, shown comparing the RSV + LPS group with the RSV group
MMP-12 contributed to lung inflammation and AHR provoked by LPS during the later stage of RSV infection. MMP-12 levels (a), inflammatory cells in BALF (b), pathological damage of lung tissues (c-h) and AHR (i) were noteworthy alleviated by MMP-408. Representative HE staining: original Magnification × 200, c: control group; d: RSV group; e: LPS group; f: RSV + LPS group; g: RSV + LPS+ i MMP-12 group; h: Histological scores. Graphs are represented as the mean ± sem. Data are representative of two independent experiments performed on 6 animals per group. *, p < 0.05, **, p < 0.01, ***, p < 0.001, shown comparing the control group with the RSV + LPS group; #, p < 0.05, ##, p < 0.01, ###, p < 0.001, shown comparing the RSV + LPS group with the LPS group; ^, p < 0.05, ^^, p < 0.01, ^^^, p < 0.001, shown comparing the RSV + LPS group with the RSV group; ϕ, p < 0.05, ϕ ϕ, p < 0.01, ϕ ϕ ϕ, p < 0.001, shown comparing the RSV + LPS group with the RL + i MMP-12 group
ERK signaling pathway is involved in MMP-12 production provoked by LPS during the later stage of RSV infection
MAPK signaling pathway has been reported to participate in MMPs production, so ERK, JNK and p38 were detected. As shown in Supplementary data 1, JNK (Supplementary data 1 A, B) and p38 (Supplementary data 1 C, D) were not increased in the RSV + LPS group. When JNK or p38 was inhibited respectively, MMP-12 levels were not decreased (Supplementary data 1 E). As shown in Fig. 7a and b, p-ERK was significantly induced in the RSV + LPS group (all P < 0.05 for RSV + LPS group vs. the other groups as to p-ERK semi-quantitative expression). To further examine the role of ERK pathway in MMP-12 modulation, a specific inhibitor of ERK (PD98059) was used, which truly decreased p-ERK (Fig. 6a-b). In parallel, MMP-12 levels (Fig. 7c, all P < 0.05 for RSV + LPS group vs. the other groups), BALF inflammatory cells, monocytes, neutrophils, lymphocytes, eosinophils (Fig. 7d, all P < 0.05 for RSV + LPS group vs. the other groups), lung tissue damage (Fig. 7e-i) and HPS (Fig. 7j, all P < 0.001 for RSV + LPS group vs. the other groups) and AHR (Fig. 7k, all P < 0.01 for RSV + LPS group vs. the other groups at methacholine concentrations of 25 mg/ml and 50 mg/ml), were all dramatically reduced in the ERK-inhibitor-treated mice (RL + i ERK group). Thus, MMP-12 provoked by LPS during the later stage of RSV infection was regulated by the ERK signaling pathway.
ERK signaling pathway contributed to MMP-12 over-production and the airway disorders provoked by LPS. Mice were treated intraperitoneally with the specific inhibitor of ERK. ERK expression was detected by western blot (a) and was semi-quantitatively assessed (b). MMP-12 levels (c), inflammatory cells (d), pathological damage of lung tissues (e-i), HPS (j) and AHR (k) were noteworthy alleviated by ERK inhibition. Representative HE staining: original Magnification × 200, e: control group; f: RSV group; g: LPS group; h: RSV + LPS group; i: RSV + LPS+ i ERK group; j: Histological scores. Graphs are represented as the mean ± sem. Data are representative of two independent experiments performed on 6 animals per group. *, p < 0.05, **, p < 0.01, ***, p < 0.001, shown comparing the control group with the RSV + LPS group; #, p < 0.05, ##, p < 0.01, ###, p < 0.001, shown comparing the RSV + LPS group with the LPS group; ^, p < 0.05, ^^, p < 0.01, ^^^, p < 0.001, shown comparing the RSV + LPS group with the RSV group; ϕ, p < 0.05, ϕ ϕ, p < 0.01, ϕ ϕ ϕ, p < 0.001, shown comparing the RSV + LPS group with the RL + i ERK group
Discussion
The results of this study clearly demonstrate that LPS exposure superimposed on RSV-infected lungs could lead to more vigorous cellular influx, lung structures damage, augmented AHR and higher MMP-12 levels. Inhibition of MMP-12 or ERK signaling pathway in vivo both diminished LPS-driven lung inflammation and AHR. Therefore, the mouse model we used in the current study shares key features with respiratory virus-induced exacerbation of asthma in humans, and a larger ERK-MMP-12 response observed in RSV-infected mice treated with LPS may account for the robust inflammatory cascade.
LPS is found ubiquitously in the environment, and on many occasions, it can create difficulties by inducing a large amount of inflammatory responses, even to the extent of shock or death of the host [19]. In asthmatic children, a significant correlation was found between levels of LPS and airway neutrophils in bronchoalveolar lavage [20]. In animal models, authors also identified that further LPS application in the airway might induce a state of worsened inflammation with neutrophilia predominant with eosinophilia persistence or abolished [21]. In our study, BALF and lung tissue inflammatory cells infiltration and AHR were markedly aggravated by LPS during the later stage of RSV infection. These results keep well consistent with the previous reports, emphasized the pathogenic roles of LPS. However, several epidemiological studies have contradictorily verified that exposure to environmental LPS in early childhood would reduce the incidence of allergic disorders in later life [22,23,24]. Animal studies also confirmed LPS exposure before or shortly after sensitization protects against the development of allergy [25]. Moreover, normal airway epithelial cells are relatively resistant to common type LPS stimulation [26]. Pretreated LPS protects the epithelial cells against the action of poly(I:C) and human parechovirus by attenuating TANK-binding kinase 1, IRF3, and NF- κB activation [17]. The disparity may be due to the difference of experimental animals, the timing of delivering LPS, the dose of LPS and the pathway (inhaled by aerosol in a chamber, intranasally or intravenously) [17, 21].
Previously, we have demonstrated that Th2 cytokines were triggered and accounted for the persistent airway inflammation induced by RSV [9]. Caballero MT. et al. [27] further revealed that interactions between TLR4 genotype and environmental LPS exposure modulated GATA3/T-bet ratios during RSV infection, and a Th2 bias with high levels of IL-4 increased disease severity in infants and murine models. Thus, in the present study, we first detected levels of IL-4, IL-5 and IL-13. Unexpectedly, as shown in Supplementary data 2, these cytokines were dramatically inhibited but not increased by LPS. The epithelia-derived cytokines TSLP, IL-25 and IL-33, which could promote asthmatic inflammation by driving Th2 responses [28, 29] were also suppressed by LPS. In addition, mRNA level of GATA-3 and ID2, the transcriptional factors related to Th2 cytokines were consistently decreased by LPS. Thus, other divergent pro-inflammatory mechanisms were indicated. LPS can promote a shift from Th2-derived airway eosinophilic inflammation to Th1 or Th17-derived neutrophilic inflammation in murine asthma models [30, 31]. However, the local switching of the airway inflammation from eosinophilia to neutrophilia did not quench the airway inflammation; instead, airway hyperreactivity was increased [27]. Accordingly, levels of IFN-γ, IL-10, IL-6, IL-17, IL-21 and IL-1β were subsequently assessed (Supplementary data 3). Again, none of these cytokines was increased following LPS treatment during the later stage of RSV infection.
The initial episodes of acute inflammation promote airway remodeling by altering the homeostasis of extracellular matrix in lungs. Matrix metalloproteinases (MMPs), characterized by mediating tissue remodeling and inflammatory processes, can help in maintaining homeostasis in basement membrane degeneration, epithelial repair and angiogenesis at the early phase of asthma exacerbation [32]. Several previous studies have shown that RSV triggers dramatic up-regulation of lung MMPs which can delay viral clearance and facilitate airway inflammation and AHR [33, 34]. We further identified MMP-12 as an important culprit in our RSV-infected nude mice and BALB/c mice models [10, 11]. Mebratu et al. [35] revealed that mice injured with elastase and LPS showed an enhanced and prolonged neutrophilic response to RSV that was associated with increased levels of IL-17and MMP-9. Therefore, levels of MMP-9 and MMP-12 were evaluated. MMP-9 was similarly markedly increased in both the RSV + LPS group and the LPS group (Supplementary data 4). MMP-12 was dramatically increased in the RSV + LPS group as compared to the RSV group and LPS group. Moreover, MMP-12 block substantially alleviated the airway disorders in the RSV + LPS mice. Hence, MMP-12 contributes to the exacerbated RSV-associated persistent lung inflammation and AHR caused by LPS treatment. The detrimental role of MMP-12 in the pathogenesis of COPD, emphysema, and asthma is also well established [36, 37].
LPS can activate multiple signaling cascades such as MAP kinases and recruitment of interleukin (IL)-1 receptor signaling complex, which involve Myd88 and IRAK [38]. In primary culture chondrocytes and cartilage explants, IL-1β could induce MMP-12 via MAP kinase signaling pathways [39]. We found that among the three MAPK proteins, p-ERK was significantly increased in the RSV + LPS group. When ERK was inhibited, MMP-12, lung inflammation and AHR were all correspondingly decreased. Xiao X et al. [40] reported that Oridonin, a diterpenoid compound extracted from traditional medicinal herbs, can inhibit the proliferation, invasion, and migration of gefitinib-resistant non-small cell lung cancer cells by suppressing EGFR/ERK/MMP-12 and CIP2A/PP2A/Akt signaling pathways. These observations are in support of our findings, suggesting that ERK signaling pathway is involved in LPS induced MMP-12 over-production during the later stage of RSV infection.
Conclusions
In summary, the LPS-induced exacerbation of RSV-induced lung injury shares key features with respiratory virus-induced exacerbation of asthma in humans. Exposure to LPS in RSV-infected mice is associated with enhanced increases in ERK-MMP-12 expression that translates into increased lung inflammation and AHR. These findings contribute novel information to the field investigating the onset of post-RSV bronchiolitis recurrent wheezing as a result of LPS exposure.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AHR:
-
Airway hyperresponsiveness
- BALF:
-
Bronchoalveolar lavage fluid
- LRTI :
-
Lower respiratory infection
- LPS :
-
Lipopolysaccharide
- MMP-12:
-
Matrix metalloproteinases-12
- RSV:
-
Respiratory syncytial virus
References
Li Y, Reeves RM, Wang X, Bassat Q, Brooks WA, Cohen C, Moore DP, Nunes M, Rath B, Campbell H, Nair H. RSV global epidemiology network; RESCEU investigators. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health. 2019;7(8):e1031–45..
Ferolla FM, Hijano DR, Acosta PL, Rodríguez A, Dueñas K, Sancilio A, Barboza E, Caría A, Gago GF, Almeida RE, Castro L, Pozzolo C, Martínez MV, Grimaldi LA, Rebec B, Calvo M, Henrichsen J, Nocito C, González M, Barbero G, Losada JV, Caballero MT, Zurankovas V, Raggio M, Schavlovsky G, Kobylarz A, Wimmenauer V, Bugna J, Williams JV, Sastre G, Flamenco E, Pérez AR, Ferrero F, Libster R, Grijalva CG, Polack FP. Macronutrients during pregnancy and life-threatening respiratory syncytial virus infections in children. Am J Respir Crit Care Med. 2013;187(9):983–90.
Stein RT. Long-term airway morbidity following viral LRTI in early infancy: recurrent wheezing or asthma? Paediatr Respir Rev. 2009;10(Suppl 1):29–31.
Berry CE, Billheimer D, Jenkins IC, et al. A distinct low lung function trajectory from childhood to the fourth decade of life. Am J Respir Crit Care Med. 2016;194(5):607–12.
Weinberger DM, Klugman KP, Steiner CA, Simonsen L, Viboud C. Association between respiratory syncytial virus activity and pneumococcal disease in infants: a time series analysis of US hospitalization data. PLoS Med. 2015;12(1):e1001776.
Thorburn K, Harigopal S, Reddy V, Taylor N, van Saene HK. High incidence of pulmonary bacterial co-infection in children with severe respiratory syncytial virus (RSV) bronchiolitis. Thorax. 2006;61(7):611–5.
Suárez-Arrabal MC, Mella C, Lopez SM, Brown NV, Hall MW, Hammond S, Shiels W, Groner J, Marcon M, Ramilo O, Mejias A. Nasopharyngeal bacterial burden and antibiotics: influence on inflammatory markers and disease severity in infants with respiratory syncytial virus bronchiolitis. J Inf Secur. 2015;71(4):458–69.
Lau N, Norman A, Smith MJ, Sarkar A, Gao Z. Association between traffic related air pollution and the development of asthma phenotypes in children: a systematic review. Int J Chronic Dis. 2018;2018:4047386.
Long X, Xie J, Zhao K, Li W, Tang W, Chen S, Zang N, Ren L, Deng Y, Xie X, Wang L, Fu Z, Liu E. NK cells contribute to persistent airway inflammation and AHR during the later stage of RSV infection in mice. Med Microbiol Immunol. 2016;205(5):459–70.
Zang N, Zhuang J, Deng Y, Yang Z, Ye Z, Xie X, Ren L, Fu Z, Luo Z, Xu F, Liu E. Pulmonary C fibers modulate MMP-12 production via PAR2 and are involved in the Long-term airway inflammation and airway Hyperresponsiveness induced by respiratory syncytial virus infection. J Virol. 2015;90(5):2536–43.
Long X, Li S, Xie J, Li W, Zang N, Ren L, Deng Y, Xie X, Wang L, Fu Z, Liu E. MMP-12-mediated by SARM-TRIF signaling pathway contributes to IFN-γ-independent airway inflammation and AHR post RSV infection in nude mice. Respir Res. 2015;16:11.
Fonceca AM, Zosky GR, Bozanich EM, Sutanto EN, Kicic A, McNamara PS, Knight DA, Sly PD, Turner DJ, Stick SM. Accumulation mode particles and LPS exposure induce TLR-4 dependent and independent inflammatory responses in the lung. Respir Res. 2018;19(1):15.
Lowe AP, Thomas RS, Nials AT, Kidd EJ, Broadley KJ, Ford WR. LPS exacerbates functional and inflammatory responses to ovalbumin and decreases sensitivity to inhaled fluticasone propionate in a Guinea pig model of asthma. Br J Pharmacol. 2015;172(10):2588–603.
Kováts N, Horváth E, Jancsek-Turóczi B, Hoffer A, Gelencsér A, Urbán P, Kiss ÍE, Bihari Z, Fekete C. Microbiological characterization of stable resuspended dust. Int J Occup Med Environ Health. 2016;29(3):375–80.
Michel O. Role of lipopolysaccharide (LPS) in asthma and other pulmonary conditions. J Endotoxin Res. 2003;9(5):293–300.
Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–51.
Lin TH, Su HH, Kang HY, Chang TH. The Interactive Roles of Lipopolysaccharides and dsRNA/Viruses on Respiratory Epithelial Cells and Dendritic Cells in Allergic Respiratory Disorders: The Hygiene Hypothesis. Int J Mol Sci. 2017;18(10):2219. https://doi.org/10.3390/ijms18102219. PMID: 29065558; PMCID: PMC5666898.
Rego SL, Zakhem E, Orlando G, Bitar KN, et al. Bioengineered human pyloric sphincters using autologous smooth muscle and neural progenitor cells. Tissue Eng A. 2016;22(1–2):151–60.
Pope CA 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–42.
Hauk PJ, Krawiec M, Murphy J, Boguniewicz J, Schiltz A, Goleva E, Liu AH, Leung DY. Neutrophilic airway inflammation and association with bacterial lipopolysaccharide in children with asthma and wheezing. Pediatr Pulmonol. 2008;43:916–23.
Rodriguez D, Keller AC, Faquim-Mauro EL, de Macedo MS, Cunha FQ, Lefort J, Vargaftig BB, Russo M. Bacterial lipopolysaccharide signaling through toll-like receptor 4 suppresses asthma-like responses via nitric oxide synthase 2 activity. J Immunol. 2003;171:1001–8.
Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, Maisch S, Carr D, Gerlach F, Bufe A, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–77.
Riedler J, Braun-Fahrlander C, Eder W, Schreuer M, Waser M, Maisch S, Carr D, Schierl R, Nowak D, von Mutius E. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–33.
Gereda JE, Leung DY, Liu AH. Levels of environmental endotoxin and prevalence of atopic disease. JAMA. 2000;284:1652–3.
Tulic MK, Wale JL, Holt PG, Sly PD. Modification of the inflammatory response to allergen challenge after exposure to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol. 2000;22:604–12.
Schulz C, Farkas L, Wolf K, Kratzel K, Eissner G, Pfeifer M. Differences in LPS-induced activation of bronchial epithelial cells (BEAS-2B) and type II-like pneumocytes (A-549). Scand J Immunol. 2002;56:294–302.
Caballero MT, Serra ME, Acosta PL, Marzec J, Gibbons L, Salim M, Rodriguez A, Reynaldi A, Garcia A, Bado D, Buchholz UJ, Hijano DR, Coviello S, Newcomb D, Bellabarba M, Ferolla FM, Libster R, Berenstein A, Siniawaski S, Blumetti V, Echavarria M, Pinto L, Lawrence A, Ossorio MF, Grosman A, Mateu CG, Bayle C, Dericco A, Pellegrini M, Igarza I, Repetto HA, Grimaldi LA, Gudapati P, Polack NR, Althabe F, Shi M, Ferrero F, Bergel E, Stein RT, Peebles RS, Boothby M, Kleeberger SR, Polack FP. TLR4 genotype and environmental LPS mediate RSV bronchiolitis through Th2 polarization. J Clin Invest. 2015;125(2):571–82.
Patel NN, Kohanski MA, Maina IW, Workman AD, Herbert DR, Cohen NA. Sentinels at the wall: epithelial-derived cytokines serve as triggers of upper airway type 2 inflammation. Int Forum Allergy Rhinol. 2019;9(1):93–9.
Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–90.
De Boer JD, Yang J, van den Boogaard FE, Hoogendijk AJ, de Beer R, van der Zee JS, Roelofs JJ, van’t Veer C, de Vos AF, van der Poll T. Mast cell-deficient kit mice develop house dust mite-induced lung inflammation despite impaired eosinophil recruitment. J Innate Immun. 2014;6:219–26.
Zhao S, Jiang Y, Yang X, Guo D, Wang Y, Wang J, Wang R, Wang C. Lipopolysaccharides promote a shift from Th2-derived airway eosinophilic inflammation to Th17-derived neutrophilic inflammation in an ovalbumin-sensitized murine asthma model. J Asthma. 2017;54(5):447–55.
Hendrix AY, Kheradmand F. The role of matrix Metalloproteinases in development, repair, and destruction of the lungs. Prog Mol Biol Transl Sci. 2017;148:1–29.
Foronjy RF, Taggart CC, Dabo AJ, Weldon S, Cummins N, Geraghty P. Type-I interferons induce lung protease responses following respiratory syncytial virus infection via RIG-I-like receptors. Mucosal Immunol. 2015;8(1):161–75.
Marchant DJ, Bellac CL, Moraes TJ, Wadsworth SJ, Dufour A, Butler GS, et al. A new transcriptional role for matrix metalloproteinase-12 in antiviral immunity. Nat Med. 2014;20:493–502.
Mebratu YA, Tesfaigzi Y. IL-17 plays a role in respiratory syncytial virus-induced lung inflammation and emphysema in Elastase and LPS-injured mice. Am J Respir Cell Mol Biol. 2018;58(6):717–26. https://doi.org/10.1165/rcmb.2017-0265OC.
Chaudhuri R, McSharry C, Brady J, Donnelly I, Grierson C, McGuinness S, Jolly L, Weir CJ, Messow CM, Spears M, Miele G, Nocka K, Crowther D, Thompson J, Brannigan M, Lafferty J, Sproule M, Macnee W, Connell M, Murchison JT, Shepherd MC, Feuerstein G, Miller DK, Thomson NC. Sputum matrix metalloproteinase-12 in patients with chronic obstructive pulmonary disease andasthma: relationship to disease severity. J Allergy Clin Immunol. 2012;129(3):655–63 e8.
Chelluboina B, Nalamolu KR, Klopfenstein JD, Pinson DM, Wang DZ, Vemuganti R, Veeravalli KK. MMP-12, a promising therapeutic target for neurological diseases. Mol Neurobiol. 2018;55(2):1405–9.
Perros F, Lambrecht BN, Hammad H. TLR4 signalling in pulmonary stromal cells is critical for inflammation and immunity in the airways. Respir Res. 2011;12:125.
Oh H, Yang S, Park M, Chun JS. Matrix metalloproteinase (MMP)-12 regulates MMP-9 expression in interleukin-1beta-treated articular chondrocytes. J Cell Biochem. 2008;105(6):1443–50.
Xiao X, He Z, Cao W, Cai F, Zhang L, Huang Q, Fan C, Duan C, Wang X, Wang J, Liu Y. Oridonin inhibits gefitinib-resistant lung cancer cells by suppressing EGFR/ERK/MMP-12 and CIP2A/Akt signaling pathways. Int J Oncol. 2016;48(6):2608–18.
Acknowledgments
No potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article. The sponsor had no role in the design of the study, collection and analysis of the data, or in the preparation of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China for Young Scholars (81600005, 81800009) and the National Natural Science Foundation of China (81670011).
Author information
Authors and Affiliations
Contributions
XR L, SL C contributed to acquisition of data, data analysis and interpretation, and preparation of the manuscript. XR L was a major contributor in writing the manuscript. J X, SY C, KT Z: contributed to design of study and acquisition of data. XH X, L R: contributed to data analysis and interpretation. Y D, HM X, EM L: contributed to conception, design of study, and revision of the manuscript. All authors read and approved the final manuscript and agreed to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All experiments involving animals were in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Committee (IACUC), which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, China and Experimental Animal Committee of the Chongqing Medical University (license numbers: SCXK (Yu) 2012–0001 and SYXK (Yu) 2012–0001).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Supplementary data 1
. JNK and p38 signaling pathway didn’t affect MMP-12 production induced by LPS during the later stage of RSV infection. Mice were treated intraperitoneally with the specific inhibitor of JNK or p38. JNK, p-JNK, p38 and p-p38 expressions were detected by western blot and were semi-quantitatively assessed (A-D). MMP-12 levels in BALF were assessed with ELISA (E). Data are representative of two independent experiments performed on 6 animals per group. ***, p < 0.001, shown comparing the control group with the RSV + LPS group; ##, p < 0.01, shown comparing the RSV + LPS group with the LPS group; ^, p < 0.05, shown comparing the RSV + LPS group with the RSV group; ϕ, p < 0.05, shown comparing the RSV + LPS group with the inhibitor-treated mice groups.
Additional file 2: Supplementary data 2.
The Th2 responses were deduced by LPS during the later stage of RSV infection. Levels of IL-4 (A), IL-5 (B), IL-13 (C), TSLP (D), IL-25 (E), IL-33 (F) in BALF were detected with ELISA-based assays. mRNA levels of GATA-3 (G) and ID2 (H) were assessed with Q-PCR. Values are expressed as mean ± sem. Data are representative of two independent experiments performed on 6 animals per group. *, p < 0.05, **, p < 0.01, ***, p < 0.001, shown comparing the control group with the other groups; ^, p < 0.05, ^^, p < 0.01, ^^^, p < 0.001, shown comparing the RSV + LPS group with the RSV group.
Additional file 3: Supplementary data 3.
The Th1 and Th17 cytokines in BALF were not provoked by LPS during the later stage of RSV infection. Levels of IFN-γ (A), IL-6 (B), IL-10 (C), IL-17A (D), IL-21 (E), IL-1β(F) in BALF were detected with ELISA-based assays. Values are expressed as mean ± sem. Data are representative of two independent experiments performed on 6 animals per group. *, p < 0.05, **, p < 0.01, shown comparing the control group with the other groups; ^, p < 0.05, shown comparing the RSV + LPS group with the RSV group.
Additional file 4: Supplementary data 4
. The levels of MMP-9 in BALF. Values are expressed as mean ± sem. Data are representative of two independent experiments performed on 6 animals per group. *, p < 0.05, shown comparing the control group with the other groups; ^, p < 0.05, shown comparing the RSV + LPS group with the RSV group.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, S., Xie, J., Zhao, K. et al. LPS aggravates lung inflammation induced by RSV by promoting the ERK-MMP-12 signaling pathway in mice. Respir Res 21, 193 (2020). https://doi.org/10.1186/s12931-020-01453-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-020-01453-6