Abstract
Osteosarcoma (OS) is the most prevalent and fatal type of bone tumor. It is characterized by great heterogeneity of genomic aberrations, mutated genes, and cell types contribution, making therapy and patients management particularly challenging. A unifying picture of molecular mechanisms underlying the disease could help to transform those challenges into opportunities.
This review deeply explores the occurrence in OS of large-scale RNA regulatory networks, denominated “competing endogenous RNA network” (ceRNET), wherein different RNA biotypes, such as long non-coding RNAs, circular RNAs and mRNAs can functionally interact each other by competitively binding to shared microRNAs. Here, we discuss how the unbalancing of any network component can derail the entire circuit, driving OS onset and progression by impacting on cell proliferation, migration, invasion, tumor growth and metastasis, and even chemotherapeutic resistance, as distilled from many studies. Intriguingly, the aberrant expression of the networks components in OS cells can be triggered also by the surroundings, through cytokines and vesicles, with their bioactive cargo of proteins and non-coding RNAs, highlighting the relevance of tumor microenvironment. A comprehensive picture of RNA regulatory networks underlying OS could pave the way for the development of innovative RNA-targeted and RNA-based therapies and new diagnostic tools, also in the perspective of precision oncology.
Similar content being viewed by others
Introduction
Osteosarcoma (OS) is the most severe and common primary malignant tumor of the bone, which shows a bimodal incidence with a first peak in children, as primary bone cancer, and another peak in adults, as secondary cancer related to radiative therapies or other pathologies [1,2,3]. It is mainly observed in lower long bones and has a high risk of distant metastasis and invasion to the other bones and particularly to lung tissue; in fact, at the time of diagnosis, 20% of OS patients have already developed metastases, out of which 90% are lung metastases [1, 4]. Although OS represents only 5% of tumors in pediatric patients, its severity and ability to metastasize early are responsible for a high cancer-related mortality rate [1, 5]. The five-year survival rate for patients with localized OS is about 60–70%, whereas it is less than 20% for patients with metastatic OS. Chemotherapy is responsible for the impairment of bone metabolism and for the onset of osteoporosis leading to a decrease of bone mineral density in OS patients and predisposing OS long-term survivors to a high risk of bone fractures [6].
OS arises from primitive mesenchymal bone-forming cells within the osteoblastic lineage, undergoing aberrant alterations at any stage of differentiation; the bone niches and their microenvironment are strictly linked, and tumor microenvironment (TME) greatly contribute to OS progression and metastasis [1, 7,8,9]. Vast genomic instability and multiple genomic aberrations characterize the majority of OS cases (58%), such as gain or loss of some portions or entire chromosomes [10]. Apart from these characteristic structural alterations, large-scale sequencing analyses have also identified recurrently mutated genes, such as TP53 (lost in > 90% of OS), deletion of RB (up to 30% of OS) and other drivers lesions such as MYC amplification, PTEN loss and deletion of ATRX [10]. Related to genetic mutations, alterations in many signaling pathways, such as Notch and Wnt, contribute to osteosarcoma genesis. Most of OS cases are sporadic, however a considerable subset of cases occurs in the setting of established cancer predisposition syndromes [3, 10].
In recent years, an increasing number of studies have been published on the possible role of non-coding RNAs contributing to pathophysiology of OS, firstly regarding microRNA (2065 results retrieved by PubMed searching by keywords “osteosarcoma AND miRNA” by March 2024), then lncRNA (1024 results retrieved by PubMed searching by keywords “osteosarcoma AND lncRNA” by March 2024), and more recently circular RNA (326 results retrieved by PubMed searching by keywords “osteosarcoma AND circRNA” by March 2024).
microRNAs (miRNA) are short non-coding RNAs (approximately 20 nt long) that work by driving multiprotein complexes on complementary sequences of target transcripts, thus affecting their translation and/or stability [11]. One miRNA can bind various transcripts, and vice versa one transcript can be targeted by different miRNAs, giving rise to complex regulatory networks controlling more than 30% of protein-coding genes, thus playing key roles in almost all physiological pathways and in the pathogenesis of several diseases [12, 13]. Much evidence has shown that microRNAs can function as either oncogene by downregulating oncosuppressive proteins, or tumor suppressor by negatively regulating oncogenic targets, thus contributing to the onset and progression of osteosarcoma [14, 15]. As an example of oncomiR, miR-21 is able to down-regulate PTEN, TPM1, PDCD4, thus inducing OS cell growth, migration, invasion and metastasis [16, 17]. miR-34a is an example of oncosuppressive miRNAs, whose expression restoration could rescue the abnormal cellular processes in preclinical OS models [18]; several miR-34a oncotargets have been validated and a special link with TP53 has been also highlighted, since miR-34 is a direct transcriptional target of p53, with different feedback regulatory loops contributing to OS [19].
Long ncRNAs (lncRNAs), with a size longer than 200nt and up to several kilobases (up to 100 kb), represent the largest class of ncRNAs in the mammalian genome, and further classified into subclasses, depending on their genomic locations, origins, and transcription directions [20]. LncRNAs are structurally and functionally very versatile, so that they can interact with DNA, other RNA molecules, and proteins, thus regulating gene expression at epigenetic, transcriptional, post-transcriptional, and translational level [21]. The number of lncRNAs involved in cancer initiation and progression is continuously growing and can also be found in curated databases such as Lnc2Cancer or the Cancer LncRNA Census [22, 23]. Some lncRNAs have long been known, such as MALAT1, acting as oncogene in different tumors, including OS; many others have been more recently annotated due to the increasing advances of high-throughput RNA sequencing technologies [24].
Circular RNAs (circRNAs) are covalently closed continuous RNA loops, originated from the primary form of transcripts, mainly mRNAs; through their interaction with DNA, other coding or non-coding RNAs, and proteins, circRNAs can control gene expression at different levels, from transcriptional to post-transcriptional level [25, 26]. The expression of many circRNAs is abnormal in different cancer types, and they have been demonstrated to play relevant roles in carcinogenesis [27].
It is becoming increasingly clear that beyond “conventional” unidirectional regulation of a specific gene expression exerted by the molecules above described (e.g., one miRNA versus one target), the different RNA biotypes can engage in intricate regulatory networks, underlying physiological homeostasis and whose derailing has pathological consequences [28,29,30]. In fact, even unrelated and unexpected transcripts, coding or non-coding, can be functionally linked through miRNAs if they share their binding sites; in this scenario, different RNA species can regulate each other’s by competitively binding to shared miRNAs, thus titrating their availability and preventing their inhibitory binding to the other RNA targets. The binding miRNAs sites become “the letters” of an “RNA code” by which different RNA biotypes, independently from their coding potentiality, form large-scale regulatory networks across the transcriptome, reciprocally fine-tuning their expression levels and thus governing different biological pathways. The described functional relationships are denominated “competing endogenous RNA (ceRNA) networks”, abbreviated as “ceRNET”. In a physiological state, an optimal crosstalk among the RNA molecules occurs, so that the shared pool of miRNAs is sufficient to target repression and govern different biological pathways; however, the unbalancing of any network component, such as an aberrant expression, can affect the entire regulatory circuit acting as a driving force for human diseases, including carcinogenesis.
In recent years, literature concerning osteosarcoma is becoming dominated by association with non-coding RNA biology. Many studies profile microRNAs expression in different patient cohorts, finding differentially expressed miRNAs with a diagnostic/prognostic potential; some others go deeply into the mechanisms trying to piece together the molecular events and identify the governed biological pathways contributing to osteosarcoma genesis. This review aims to give a comprehensive view of RNA regulatory networks involving lncRNA-miRNA-mRNA axes and circRNA-miRNA-mRNA axes in the ceRNET perspective. Literature on PubMed published before 31 March 2024 was screened by the following keywords: “(osteosarcoma) AND (ceRNA),” retrieving 173 results; “((osteosarcoma) AND (lncRNA)) AND (axis),” retrieving 233 results; “((osteosarcoma) AND (circRNA)) AND (axis),” retrieving 123 results. Then, we selected for this study only those articles reporting experimental validation of interaction between lncRNA or circRNA versus miRNA, and miRNA versus mRNA target by RNA immunoprecipitation (RIP) and/or luciferase and/or RNA pull-down assays; duplicate results from our screening were excluded. The ceRNETs were listed grouping together those involving lncRNAs, those involving circRNAs, in alphabetic order referred to lncRNAs or circRNAs, with the aim to put together the networks governed by the same lncRNA or circRNA; the lists were further processed by subgrouping those endowed with oncogenic power, or tumor suppressor action, or involvement in chemoresistance. The described literature processing criteria led to the results presented in Tables 1, 2 and 3, discussed in the next sections.
CeRNETs involving lncRNAs
An increasing number of studies demonstrated that lncRNAs are key players in osteosarcomagenesis, triggering different molecular pathways involved in biological processes such as cell proliferation, migration, invasion, apoptosis, tumor growth and metastasis.
In this section, we discuss the role of lncRNAs as oncogenes and then as tumor suppressors through their ceRNA activity, as distilled from many studies (Fig. 1). The extensive list of lncRNAs, mechanisms and phenotypic effects is reported in Table 1.
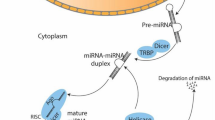
Competing endogenous RNA networks (ceRNET) relying on lncRNAs, circRNAs, miRNAs and mRNAs in osteosarcoma. Coding and non-coding RNAs can compete for binding to a shared pool of miRNAs. Optimal ceRNA crosstalk occurs in a physiological homeostasis condition; however, an aberrant expression of any circuit component can derail the network, thus contributing to the OS onset and progression by repressing tumor suppressive activities (left side) or prompting oncogenic activities (right side). The up and down arrows indicate increased or decreased expression, respectively. Some representative examples discussed in the text are reported in the figure. Figure created with BioRender.com
Oncogenic ceRNA activity
Different biological processes and molecular pathways have been demonstrated to play a key role in OS onset and progression. Among them, the early metastatic potential is a feature of OS; ceRNA activity of many lncRNAs greatly contribute to cell invasiveness and metastasis, a key to a poor prognosis (Table 1). They are also able to contribute to the “Warburg effect”, i.e. a metabolic switch from oxidative phosphorylation to aerobic glycolysis that leads to the enhancement of cell proliferation, and the rapid growth of tumor. In fact, although aerobic glycolysis is less efficient in the generation of ATP, it increases proliferation, inhibits apoptosis, and generates signaling metabolites to enhance cancer cell survival under stressful conditions, such as hypoxia. Regarding molecular pathways, different ceRNETs involving lncRNAs are consistently indicated to be able to trigger PI3K/AKT/mTOR and Wnt/beta-catenin signaling pathways, indeed promoting cell proliferation, invasion and metastasis and inhibiting apoptosis. Then, ceRNA activity can have an impact on the entire transcriptome of OS cells, when lncRNAs share miRNA binding sites with transcripts encoding transcription factors or chromatin remodeling enzymes, that can result upregulated by an overexpression of the sponging lncRNA, with pathological consequences. Among the 118 oncogenic lncRNAs reported in Table 1, below we discuss some of them consistently and more frequently reported (from 16 to 3 papers per lncRNA) to be involved in ceRNETs prompting osteosarcomagenesis, also in relation to biological processes and molecular pathways cited above.
Different members of lncRNA SNHGs family (small nucleolar RNA host genes) have received increasing attention regarding their roles in multiple bone diseases, since their unique expression profile during osteoblast differentiation and involvement in relevant pathways for osteogenesis of mesenchymal stem cells [346]. Furthermore, various lncRNAs SNHGs have been already demonstrated to be involved in different human cancers, including glioma, esophageal cancer, HCC, lung adenocarcinoma and gastric cancer [347,348,349,350,351,352]. Nine members of the family have been also reported to drive osteosarcomagenesis through different ceRNETs (Table 1). Among them, SNHG1 is consistently reported to be upregulated in OS tissues and cells, correlated with tumor size, TNM stage and lymph node metastasis, predicting poor overall survival [162,163,164,165]. In particular, the lncRNA is able to promote proliferation, migration, tumor growth and metastasis, as demonstrated in vitro and in vivo experiments through the ceRNA activity involving the miR-326/NOB1, miR-493/S100A6 and miR-577/Wnt2B axes, with the last one also activating the Wnt/beta-catenin signal pathway, one of the most critical pathway relevant for both cell proliferation and metastasis [162,163,164]. SNH10 is also able to activate the Wnt/beta-catenin pathway by promoting the beta-catenin transfer into nucleus to maintain the activation of the Wnt signaling by miR-182-5p/FZD3 axis, as demonstrated by in vitro and in vivo experiments [168]. The ability to promote tumor growth and metastasis in animal models by ceRNA activity has also been demonstrated for other members of the SNHGs family, i.e. SNHG12, SNHG15 and SNHG16 (Table 1). In addition, different members of the family are also involved in chemoresistance (Table 3).
MALAT1 (Metastasis associated lung adenocarcinoma transcript 1) is a well-known oncogenic lncRNA that is upregulated in several types of tumors, including lung, breast, cervical, and nasopharyngeal cancers [353]. Other studies also support its upregulation in OS tissues and cells, correlation with a poor prognosis and an oncogenic role in the initiation and progression of osteosarcoma, mainly performed by sponging specific miRNAs, as detailed below. In 2016, Luo W. et al. showed that knockdown of MALAT1 in osteosarcoma cells inhibited cell proliferation. This effect was attributed to the ability of MALAT1 to sponge miR-376a thus upregulating TGFA (Transforming Growth Factor Alpha). Since then, several reports confirmed the pro-proliferative activity of MALAT1 toward osteosarcoma cells and showed concomitant inhibition of apoptosis and induction of migration (Table 1). In particular, MALAT1 shares miR-144-3p binding sites with ROCK1 and ROCK2, two small G proteins, belonging to the Rho family, regulating cytoskeletal activities and pericellular matrix degradation involved in cell movement, proliferation and migration/invasion; indeed, the ceRNET MALAT1/miR-144-3p/ROCK1/2 is a molecular mechanism contributing to the ability of the lncRNA to promote tumor growth and lung metastasis, as demonstrated in vivo [125]. The oncogenic power of MALAT1 was also demonstrated in vivo by other ceRNA mechanisms involving the upregulation of histone deacetylase HDAC4 and the cyclin D1 via miR-140-5p and miR-34a, respectively [126, 130]. Worth of note, in 2021 Li F. et al. pointed out that MALAT1 may be released to osteosarcoma cells by surrounding cells. In particular, Bone Marrow Mesenchymal Stem Cells-Derived Extracellular Vesicles (BMSC-EVs) were found able to promote proliferation, invasion and migration of osteosarcoma cells via the MALAT1/miR-143/NRSN2/Wnt/beta-catenin axis both in vitro and in vivo, as detailed in a next section.
The lncRNA TUG1 (Taurine upregulated 1) is abnormally expressed in many cancer types and reported as an oncogene promoting cell proliferation, glycolysis, metastasis, angiogenesis and chemoradioresistance [354]. Consistently, TUG1 has been found upregulated in OS tissues and cells, and highly correlated with clinical stage, metastasis, and poor prognosis; through its ceRNA activity, it is able to increase the expression of different targets, thus promoting cell proliferation, migration, invasion, tumor growth and metastasis, as consistently demonstrated in vitro and in vivo [183,184,185]. TUG1 is also a relevant mediator of crosstalk between cancer-associated fibroblasts and OS cells in TME to promote invasion and distant metastasis, as detailed in a next section [188]. Different molecular pathways are triggered by TUG1 via miRNAs-sponging, such as the Wnt/beta-catenin activation and chromatin remodeling by increasing the expression of miR-144-3p target, EZH2, an H3K27me3 methyltransferase able to epigenetically silence different tumor suppressor genes [184]. In addition, TUG1 increased expression is able to turn the transcriptome of OS cells, up-regulating the transcription factors POU2F1, FOXA1, and HIF-1alfa, by sponging their targeting miRNAs, i.e. miR-9-5p, miR-212-3p, miR-143-5p, respectively, thus deeply contributing to OS progression [183, 186, 188].
KCNQ1OT1 (KCNQ1 Opposite Strand/Antisense Transcript 1) is a lncRNA transcribed in the antisense direction to the KCNQ1 gene, in the chromosomal region 11p15.5 containing two clusters of imprinted genes; KCNQ1OT1 is exclusively expressed from the paternal allele, however it is abnormally expressed from both chromosomes in most patients with the imprinting disorder of Beckwith-Wiedemann syndrome, and in multiple types of cancers (https://www.genecards.org) [355]. In particular, KCNQ1OT1 has been widely reported to be a cancer promoter in various types of tumors, such as non-small cell lung carcinoma, colorectal cancer, tongue cancer, and breast cancer [356,357,358,359]. Recently, it has been reported as a powerful oncogene also in OS, contributing to cell proliferation, migration, invasion, and tumor growth and correlating with a worse prognosis [80,81,82,83]. Its overexpression has a deep impact on the transcriptome of OS cells, since it is able to sponge miR-3666 and miR-154-3p, thus upregulating two members of Kruppel-like family of transcription factors, KLF7 and KLF12, respectively, and indeed activating the Wnt/beta‑catenin signaling [82, 83]. In addition, KCNQ1OT1 contributed to the Warburg effect by sponging miR-34c-5p and thus acting as a ceRNA for the mRNA encoding the key glycolytic enzyme aldolase A (ALDOA), thereby increasing its expression and contributing to glucose metabolism reprogramming [81].
Another lncRNA indicated as an oncogene in OS is LINC00662; it was found upregulated in OS tissues and cells, and correlated with poor prognosis; through its ceRNA activity, LINC00662 is able to promote cell proliferation, migration, invasion and tumor growth [100,101,102,103]. In particular, by miRNAs-sponging LINC00662 can enhance the expression of the mammalian Notch receptor NOTCH2 and IP receptor type 1 (ITPR1), and of a member of ETS family ELK1, thus eliciting signaling pathways reported to be involved in OS progression [100, 102, 103].
The lncRNA NEAT1 (Nuclear enriched abundant transcript 1) has been recognized as an important regulator of the expression of different genes, including some involved in cancer progression and as an activator of Wnt/beta-catenin pathway in OS, similarly to that reported for non-small-cell lung carcinoma (NSCLC) [141, 360]. It is upregulated in OS tissues and cell lines and high NEAT1 expression was associated with advanced clinical stage, distant metastasis, and poor overall survival of patients, consistent with data reported for breast cancer [141,142,143, 361]. In OS, its ceRNA activity greatly contribute to the promotion of cell proliferation, migration, invasion, tumor growth and metastasis; in particular, it is able to competitively bind to miR‐186‐5p, miR-339‐5p, miR-34a-5p, and miR-483, thus upregulating HIF‐1alfa, the cytokine TGF‐β1, HOXA13, and STAT3, as indicated in Table 1.
Similarly to NEAT1, the lncRNA DLX6-AS1 works as an oncogene in OS by triggering the Wnt signaling and augmenting stemness of OS cells through miR-129-5p/DLK1 axis, as demonstrated by in vitro and in vivo experiments [52]. Other studies consistently confirmed that the lncRNA is upregulated in OS tissues and cells, correlates with poor patient survival, and mechanistically contributes to OS hallmarks by the other ceRNETs reported in Table 1 [53, 54].
Tumor suppressive ceRNA activity
A minority of lncRNAs involved in OS are reported as tumor suppressors through their ceRNA activity. One example is represented by GAS5, reported to play a tumor suppressive role in several cancers, associated with clinic-pathological traits and patient survival, and functionally involved in in cell proliferation, apoptosis, invasion, epithelial–mesenchymal transition (EMT), metastasis, and drug resistance, via multiple molecular mechanisms [362]. In OS tissues and cells GAS5 expression level was found significantly decreased in comparison to normal tissues and cells, as expected for a tumor suppressor gene. Furthermore, its overexpression suppresses OS cell proliferation, migration, and invasion in vitro; vice versa, miR-663a is highly expressed in osteosarcoma and promotes cell proliferation and migration by down-regulating its targets, MYL9 and RHOB [201, 202]. Gas5 and miR-663 are functionally linked, since Gas5 is able to sponge the miRNA: when Gas5 is down-regulated, the entire ceRNET is derailed due to the increased level of the miRNA [201, 202, 362].
Another lncRNA downregulated in OS tissues and cell lines compared with the normal ones is TUSC7. Consistently, its experimental overexpression was able to inhibit OS cell proliferation, migration and invasion, and inhibit tumor growth in vivo. Mechanistically, TUSC7 exert its role by sponging miR‑181a, resulting in an increased level of the miRNA target RASSF6 [210]. Similar results have been reported for another lncRNA, TUSC8, acting through miR-197-3p/EHD2 axis, overall pointing to possible new therapeutic approaches based on the enhancement of the tumor suppressive lncRNAs [211].
In this regard, the tumor suppressive lncRNA LINC00261 has been found to potentiate the aptanib drug effectiveness [203]. Apatinib has been recently identified as a potential treatment option for OS; its mechanism of action is well characterized, since it is a high-affinity selective inhibitor of VEGFR2; however, it is also known that different drugs can even engage ncRNAs contributing to their effectiveness [363, 364]. This is the case of apatinib, since it was demonstrated that the drug is able to increase the expression of LINC00261, that in turn can sponge miR-620, thus up-regulating the miRNA target PTEN, a well-known oncosuppressor; importantly, the activation of LINC00261/miR-620/PTEN ceRNET by Apatinib has been demonstrated also in vivo, suggesting LINC00261 as a promising target to improve the efficacy of Apatinib.
CeRNETs involving circRNAs
CircRNAs are aberrantly expressed in almost all types of cancer [365, 366], including OS [367]. In this section, we discuss how different oncogenic circRNAs contribute to the OS onset and progression through the ceRNA mechanism, focusing the attention on those involved in cell invasiveness, Warburg effect, PI3K/AKT/mTOR and Wnt/beta-catenin signaling pathways, and chromatin remodeling, the same biological process and molecular pathways highlighted in the previous section. Then, we examine ceRNETs involving circRNAs functioning as tumor suppressors to hinder malignant growth (Fig. 1). The extensive list, their molecular mechanisms and phenotypic effect is reported in Table 2.
Oncogenic ceRNA activity
As described above, cell invasiveness plays a pivotal role in OS progression. Interestingly, several circRNAs have been found to be involved in the modulation of OS invasiveness. For instance, Yan and colleagues found that circPVT1 is upregulated in OS tissues and is more commonly overexpressed in samples with lung metastasis. Moreover, they demonstrated that downregulation of circPVT1 can reduce cell migration and invasion via regulating of miR-526b/FOXC2 axis. Likewise, another study demonstrated that circPVT1 facilitates OS invasion and metastasis via enhancing cell epithelial–mesenchymal transition (EMT). At the molecular level, circPVT1 may enhance the invasion and metastasis by releasing c-FLIP through the interaction with miR-205-5p, highlighting a new ceRNA network [280]. Furthermore, knockdown of circPVT1 can notably inhibit the severity of tumor metastasis in lung tissues of mice modulating the 26b-5p/CCNB1 axis [281]. Consistently, circPVT1 was also found upregulated in several cancers, such as bladder cancer, oral squamous cell carcinoma, and small cell lung cancer, highlighting its relevance in cancer progression. [368,369,370]. Another circRNA involved in the regulation of metastasis in different tumor types, including OS, is circUBAP2 [371,372,373,374]. Upregulation of circUBAP2 is found in OS tissues and is associated with short survival of patients, TNM stage and distant metastasis. In addition, circUBAP2 knockdown can significantly inhibit OS cell proliferation, migration and invasion in vitro by sponging miR-204 -3p to upregulate HMGA2. Additionally, circUBAP2 can regulate cell invasion and tumor growth in vivo by regulating the miR-637/HMGB2 axis [291]. Also, CircUBAP2 knockdown increased the expression of E-cadherin while it downregulated Vimentin, two markers of EMT, thus inhibiting cell invasion. Wu and colleagues demonstrated that circUBAP2 can exert that effect through upregulating the expression of YAP1 by targeting miR‑641 in OS cells [292]. Yap1 is a key element in the Hippo signaling pathway that plays an important role in the control of cell proliferation, EMT and metastasis [375]. In this regard, the circPIP5K1A can contribute to cancer cell stemness by targeting miR‑515‑5p/YAP axis. Shi and colleagues demonstrated that circPIP5K1A knockdown, or miR-515-5p mimic, repressed the protein levels of ALDH1 and Nanog, while miR-515-5p inhibitor or YAP overexpression can reverse this effect [279]. A similar role in promoting cancer progression and metastasis has been found for other tumor types. For instance, circPIP5K1A may function as a miR-600 sponge to facilitate non-small cell lung cancer proliferation and metastasis by promoting HIF-1α [376]. Moreover, circPIP5K1A can regulate glioma progression by modulating the miR-515-5p/TCF12/PI3K/AKT axis [377]. In gastric cancer, circPIP5K1A can regulate PI3K/AKT pathway through miR-671-5p/KRT80 axis [378]. Those cross-cancer insights highlight the strong and general involvement of ncRNA function in cancer.
As discussed in the previous section, cancer cells can enhance their metabolism for rapid growth, and one of the most common metabolic changes is enhanced glycolysis, the “Warburg effect”. Several studies indicated that hyperactive glycolysis is the main metabolic alteration in OS and it is involved in cell growth, invasion, and treatment effectiveness [379]. Interestingly, circRNAs have been also described to be involved in the regulation of glucose metabolism in OS through the ceRNA mechanism, as well as lncRNAs. For instance, it has been demonstrated that circATRNL1 overexpression promoted glucose uptake and lactate production thus accelerating the Warburg effect. Mechanistically, circATRNL1 can sponge mir-409-3p to upregulate the expression level of LDHA, a key enzyme in the glycolytic pathway [258]. Likewise, Hu and colleagues demonstrated that circCNST knockdown can decrease glucose consumption, lactate production, and ATP/ADP ratio downregulating LDHA through the circCNST-miR578-LDHA/PDK1 ceRNA regulatory network [263]. Another circRNA involved in glucose metabolism is circ_0056285 which can regulate the expression of TRIM44 by sponging miR-1244, which in turn can regulate the expression of key enzymes such as HK2 and LDHA [249]. Moreover, the circCYP51A1, that was upregulated under hypoxia conditions, can markedly induce the lactate production and glucose consumption by sponging miR-490-3p and regulating KLF12. Interestingly, the knockdown of circCYP51A1 in xenograft mice models can reduce tumor growth by downregulating KLF12 and consequently reducing glycolysis associated markers, such as GLUT1, HK2 and LDHA [264].
Alteration of different molecular pathways is known to be involved in OS onset and progression. Among them, the PI3K/AKT/mTOR signaling pathway has been demonstrated to have the ability to enhance the cell cycle, inhibit apoptosis, and promote cellular proliferation, invasion, and metastasis in OS [380]. Interestingly, several studies indicate that circRNAs may play an important role in the regulation of the PI3K/AKT/mTOR pathway. In this regard, circ_001422, that is found to be upregulated in OS tissues and correlated with clinical features, can promote proliferation and metastasis, in vitro and in vivo, via the miR-195-5p/FGF2/PI3K/Akt axis [240]. Shi and colleagues demonstrated that circNRIP1 derived from BMSC-EVs can upregulate AKT3 expression by competitively binding to miR-532-3p, thus promoting proliferation and tumor growth activating the PI3K/AKT/mTOR pathway. This pathway is also triggered by circNRIP1 to promote gastric cancer progression via miR-149-5p sponging [381]. Moreover, circ_0005909 may increase viability and invasion of OS upregulating expression of HGMA1 through sponging miR-338-3p, which activated PI3K-Akt signaling pathway [232].
Another pathway playing a crucial role in OS development is the Wnt/β-catenin signaling pathway. The Wnt/β-catenin pathway is a well-known oncogenic pathway responsible for cell fate determination, stem cell replication, survival, differentiation, cell polarity, and osteogenic differentiation [380, 382]. In this regard, circ_001350, that is upregulated in OS tissues, is able to activate the Wnt pathway by inducing the β-catenin protein expression and its downstream effector cyclin D1, and c-myc. Xu and colleagues demonstrated that circ_001350 can regulate the Wnt pathway and the malignant progression by regulating the miR-578/CNOT7 axis [239]. Moreover, circMYO10 was found to regulate the Wnt signaling to induce proliferation and EMT in OS cells. At the molecular level, circMYO10 can sponge miR-370-3p and upregulate RUVBL1 expression to promote the interaction between RUVBL1 and β-catenin/LEF1 complex and thus promoting Wnt/β-catenin signaling. Interestingly, the authors demonstrated that circMYO10/miR-370-3p/RUVBL1 axis enhanced the transcription activity of β-catenin/LEF1 via histone H4K16 acetylation [277].
Histone modification is a dynamic process that alters the structure of chromatin, leading to the expression or repression of local genes. In cancer, the normal balance between active and repressive histone modification modifies the expression of oncogenes and tumor suppressor genes, leading to tumorigenesis. Recently, some evidence highlighted that deregulation of genes involved in these processes has been associated with OS tumorigenesis, progression and chemoresistance [383]. circLRP6, that is upregulated in OS and is associated with poor prognosis, was found to enhance the expression of histone deacetylase 4 (HDAC4) in OS cells via sponging miR-141-3p promoting cell proliferation, invasion [274]. The relevance of histone modification in OS was also demonstrated by Wang and colleagues showing that circABCC1 knockdown can stop the malignant progression of OS by attenuating HDAC4 expression through sponging miR-591, highlighting a new ceRNA network [256].
Tumor suppressive ceRNA activity
Although most of the annotated circRNAs are reported to be oncogenic, different circRNAs have been found to act as tumor suppressors in OS.
For instance, circ_0046264 may exert a tumor-suppressive role in OS. Low expression of circ_0046264 was found in OS tissues and correlated with larger tumor. At cellular level, circ_0046264 can inhibit the proliferation, migration and invasion of OS cells. Du and colleagues, demonstrated that SFRP1, that is known to inhibit the proliferation, migration and invasion of OS cells by inhibiting Wnt/β-catenin signaling [384], is upregulated in OS cells overexpressing circ_0046264. Mechanistically, circ_0046264 can upregulate SFRP1 expression by sponging miR-940 [300]. The tumor-suppressive role of circ_0046264 was also demonstrated in lung cancer where it can inhibit viability, invasion, and induce apoptosis by upregulating BRCA2 expression through down-regulating miR-1245 [385].
It is well recognized that abnormal activation of the AKT/mTOR signaling pathway is one pivotal cause of OS development and progression [380]. PF4V1 is a negative regulator of the AKT signaling pathway and negatively regulates OS progression [386]. Interestingly, circ_0069117 might promote the expression of PF4V1 by sponging miR-875-3p, thus regulating the progress of OS [301].
Also, circ_0000658 was found to inhibit cell proliferation and invasion in vitro and impede tumor growth in vivo. At the molecular level, circ_0000658 can exert a tumor suppressive effect by targeting the miR-1227/IRF2 axis in OS cells [296].
Moreover, circ_0088212, which is poorly expressed in osteosarcoma tissues and cells, may function as a tumor suppressor by inhibiting cell proliferation and invasion and limiting tumorigenesis in vivo through miR-520 h/APOA1 axis [302]. Likewise, circ_0102049 could suppress the progression of OS by activating PLK2 by targeting miR-520 g-3e [303].
CircROCK1-E3/E4, a circular RNA derived from exons 3 and 4 of the ROCK1 gene, was found downregulated in OS patients with lymph node metastasis and distant metastasis. Liu and colleagues demonstrated that expression of circROCK1-E3/E4 was partially regulated by QKI, a well-known RNA Binding Protein (RBP) belonging to the STAR family of KH domain-containing RBPs. Moreover, they demonstrated that overexpression of circROCK1-E3/E4 may inhibit cell proliferation and lung metastasis in vivo by regulating miR-532-5p/PTEN axis in osteosarcoma [305].
Mir-21 is a well-known oncomiR also for OS [16, 17]. Interestingly, circ_0008259, which is downregulated in OS, can increase PDCD4 expression via adsorbing miR-21 and repressing the OS progression, thus depicting a new ceRNET involved in tumor suppressive activity [298].
CeRNETs in chemoresistance
Osteosarcoma treatment typically involves surgery and chemotherapy; radiation therapy might be an option in certain situations. In the 1970s, amputation or limb-sparing surgery represented the standard OS treatment, yielding a 5-year survival rate of only 20%; then, chemotherapy agents elevated the post-treatment 5-year OS survival rate. The current treatment strategy usually consists of several weeks of neoadjuvant preoperative chemotherapy followed by the surgical removal of primary tumor, and also several weeks of postoperative adjuvant chemotherapy [387]. Indeed, the 5-year survival rate has increased to 70%-80% by the wide resection surgery combined with adjuvant chemotherapy. However, long-term chemotherapy poses the risk that the patient’s cells develop resistance to the chemotherapeutic drug, even to combinations of different ones, culminating in OS recurrence, distant metastasis, and treatment failure. In fact, the 5-year survival rate of patients who experience chemoresistance decreased to less than 20%. The present standard treatment chemotherapy mainly consists in the combined administration of high dose methotrexate, doxorubicin and cisplatin (MAP) [1, 387, 388]. Methotrexate is a folate analogue designed to inhibit dihydrofolate reductase; reduced folate (tetrahydrofolate) is the proximal single carbon donor in several reactions involved in the de novo synthetic pathway for purines and pyrimidines, formation of polyamines, and transmethylation of phospholipids and proteins; as consequence of methotrexate treatment, the malignant cells become starved for the purine and pyrimidine precursors of DNA and RNA and unable to synthesize DNA and RNA and proliferate [389]. Doxorubicin acts in the cancer cell according to two proposed mechanisms: by intercalating into DNA and disrupting topoisomerase-II-mediated DNA repair and by generating free radicals with consequent damage to cellular membranes, DNA and proteins [390]. Cisplatin mode of action has been linked to its ability to crosslink with the purine bases on the DNA, thus interfering with DNA repair mechanisms, causing DNA damage, and subsequently inducing apoptosis in cancer cells [391].
The effectiveness of chemotherapy in OS is markedly impacted by chemoresistance. Presently, there exist no conventional methods to overcome chemotherapy resistance in malignancies without inducing adverse side effects. The knowledge of molecular mechanisms underlying drug and multidrugs resistance is essential to investigate potential strategies for reversing this process and avoid the high doses with severe side effects [392]. Numerous studies have linked OS chemotherapy resistance to abnormal expression of different ncRNA biotypes (lncRNA, circRNA and miRNA) and it is now increasingly clear that they can mechanistically contribute to OS chemoresistance. Also considering that single mechanisms don’t fully explain chemotherapeutic resistance, but many factors can be responsible for drug resistance, the study of large-scale RNA regulatory networks can be useful to explore innovative RNA-based and RNA-targeted therapy that surmount and/or prevent chemotherapy resistance, even in a perspective of personalized treatment. Table 3 reports validated networks contributing to chemoresistance.
Most of ceRNETs involving lncRNAs contribute to cisplatin resistance, whereas most of ceRNETs involving circRNAs contribute to doxorubicin resistance; indeed, there are also some examples of ceRNETs responsible for multidrug resistance.
An example of lncRNAs enhancing cisplatin resistance is HOTAIR: in different OS cell lines it promoted the cisplatin resistance by regulating cell proliferation, invasion, and apoptosis via miR-106a-5p/STAT3 Axis [310]. HOTAIR seems to be particularly linked to cisplatin resistance, since it can induce that drug resistance also in other tumors, such as non-small cell lung cancer and nasopharyngeal carcinoma [393, 394]. OIP5-AS1 contributes to cisplatin resistance via miR-377-3p/FOSL2 axis [319], but also to doxorubicin resistance by different molecular axis, i.e. miR-137-3p/PTN [320] and miR-200b-3p/fibronect-1 axis [321]; the lncRNA is significantly upregulated in OS chemo-resistant tissues and cell lines and its knock-down reduced doxorubicin resistance in vitro and in vivo [320]. In particular, fibronectin-1, a glycoprotein related to cellular adhesion and migration processes, was demonstrated to be functionally related to the oncogenic OIP5-AS1, because the lncRNA is able to sponge the shared miR-200b-3p; this mechanism could explain fibronectin-1 upregulation in the chemo-resistant OS cell lines and tissues and its relation to unfavorable prognosis [321]. Different members of lncRNA SNHGs family can drive osteosarcomagenesis through various ceRNETs, as shown in Table 1 and discussed in a previous section; they can also contribute to chemoresistance (Table 3). As an example, SNHG15 can contribute to both cisplatin and doxorubicin resistance through miR-335-3p/ZNF32 and miR-381-3p/GFRA1 axes, respectively [327, 328]. Intriguingly, p53, very frequently lost in OS, is able to transcriptionally repress SNHG15, thus depicting regulatory pathways wherein p53 dysfunction substantially increased SNHG15 expression, that in turn sponges specific miRNAs, thus downregulating their oncogenic targets [328]. Aberrant expression of SNHG15 can also contribute to the resistance of lung adenocarcinoma and breast cancer cells to gefitinib and cisplatin, respectively, highlighting its general relevance in drug resistance [395, 396].
Whole-transcriptome sequencing of three paired multi-drug chemoresistant and chemosensitive OS cell lines and exploitation of different interaction predictive tools have highlighted how extensive and relevant such regulatory networks are, placing in functional relation unexpected lncRNAs or circRNAs with mRNAs via miRNAs [316]. Then, luciferase, RIP and RNA-pull down assays were used to validate different ceRNETs, such as that involving circ_0001258 through miR-744-3p/GSTM2 axis or another one involving circ_0004674 through miR-142-5p/MCL1 axis [316, 335]. Circ_0004674 promoted the DXR resistance also through Wnt/β-catenin pathway via regulating the miR-342-3p/FBN1 axis [334]. Among circRNAs, circPVT1 also contributed to doxorubicin resistance, as demonstrated in vitro and in vivo; it contributes to tumor growth; its silencing increased the drug sensitivity of osteosarcoma in vivo; it has an increased expression in DXR-resistant osteosarcoma tissues and cells. TP53-regulated inhibitor of apoptosis 1 (TRIAP1), an apoptosis inhibitor, paralleled circPVT1 increased expression in OS and in fact, they are functionally linked through miR-137, since circPVT1 is able to sponge miR-137, thus de-repressing TRIAP1 [344]. The role of PVT1 in chemoresistance was also extended to cisplatin and methotrexate other than doxorubicin by demonstrating its ability to sponge miR-24-3p and thus up-regulating KLF8 [343].
Overall, those studies turn the spotlight on intricate RNA regulatory networks underlying chemotherapeutic drug resistance mechanisms, inspiring new strategies based on management of such networks, e.g. by down-regulating or overexpressing any network component functionally linked in the ceRNET, and possibly overcome, revert and even prevent chemoresistance, prospectively also in terms of precision medicine.
ceRNETs contributing to TME relevance
The tumor microenvironment (TME) plays a key role in OS onset and progression. TME is a mixture of cancer and non-cancer cells and their stroma, that can be categorized in two major categories of components: cellular components, including different cell types, such as osteoblasts, osteoclasts, mesenchymal stem cells, cancer-associated fibroblasts (CAFs), endothelial cells, adipocytes and immune cells, especially tumor-associated macrophages (TAMs); acellular components, such as the extracellular matrix (ECM), cytokines, growth factors and extracellular vesicles (EVs), with their bioactive cargo of proteins and different RNA biotypes [397].
Cells in the TME are in constant autocrine and paracrine communication, which contributes to tumor development, progression, drug resistance and metastasis. The local microenvironment provides a fertile niche for tumor growth, wherein interaction between cancer and bone cells leads to an increase in OS cell proliferation and altered bone remodeling. In particular, a crucial role is played by TAMs which represent the most abundant cells of TME and are involved in tumor growth and progression [398]. Macrophages exist in two different phenotypes: the classically activated macrophages M1 and the alternatively activated macrophages M2 [7]. M1 macrophages exhibit pro-inflammatory and anti-cancer effects by releasing pro-inflammatory cytokines and inducible factors against pathogens; instead, M2 ones have anti-inflammatory, pro-tumoral and pro-angiogenic properties [7]. It has been reported that the prevalence of M2 phenotype in TME is generally associated with a poorer 5-year event free survival in patients. Surprisingly, the presence of M2 macrophages in OS counteracts metastasis formation and increases the survival rate of high-grade OS patients [7, 399].
In addition, OS cells can produce EVs containing TGF-beta that activate local mesenchymal stem cells, which in turn release EVs containing IL-6, facilitating tumor progression. Furthermore, cytokine-containing EVs prepare the lung metastatic niche to receive OS circulating tumor cells and, acting as the main messenger between OS cells and the pulmonary parenchyma, contributing to the local tumor development [3]. Exosomes, nano-sized extracellular vesicles up to 100 nm in diameter, have a relevant cargo of miRNAs involved in cells crosstalk for physiological homeostasis maintenance, but also contributing to progression of different cancer types [400]. By releasing exosomes, tumor cells can reprogram their surroundings and shaping the TME into a tumor-permissive or tumor-promoting environment [401]. As an example specifically for OS, OS-derived exosomal miR-21 regulates the tumor microenvironment by targeting specific molecules in tumor cells, endothelial cells, cancer-associated fibroblasts and immune cells [17]. Indeed, exosomal miRNAs, secreted in different body fluids, on one hand represent a gold mine for identifying new diagnostic biomarkers, on the other hand represent a therapeutic opportunity by engineering them to deliver beneficial molecules [402]. In fact, cell–cell communication mediated by extracellular RNA is becoming increasingly appreciated, so much so that a data repository, the exRNA Atlas, has been created by the NIH Extracellular RNA Communication Consortium (https://exrna-atlas.org/), representing a resource for translational studies for diagnostics and therapeutics [403].
In particular, normalizing the TME may have therapeutic relevance, however, the high genetic heterogeneity of OS makes the TME much more complex than that of other tumors, and thus, potential TME normalizing drugs should have multiple targets. In this regard, exploring the RNA networks may pave the way for innovative therapeutic strategies. In fact, different studies highlighted the role of miRNAs in the crosstalk between OS cells and the TME; taking into consideration that the miRNA binding sites can be envisioned as the letters of an “RNA code”, the knowledge of ceRNETs involved in the communication between OS cells and the surrounding TME may offer the opportunity to manipulate them for normalizing TME. The combination of TME-normalizing drugs, including those RNA-based, and chemotherapy may offer promise for innovative therapeutic approaches.
Recent evidence revealed that also lncRNAs can be abundant and stable in EVs [404]. The lncRNA CASC15 was significantly upregulated in OS plasma exosomes as well as in OS tissues and cell lines (Table 1). Interestingly, CASC15 knockdown can restrain the proliferation, migration, and invasion of OS cells, and inhibit the growth of OS in xenograft models. Mechanistically, CASC15 is able to sponge miR-338-3p, thus up-regulating its oncogenic target RAB14; rescue experiments verified that CASC15 can promote OS cell growth and metastasis by upregulating RAB14 expression [43].
The role of macrophages-derived exosomal lncRNAs in osteosarcoma development has been studied in vitro by differentiating the human mononuclear cells THP-1 in tumor associated macrophages (TAMs) and then performing a high-throughput microarray assay to analyze the dysregulated lncRNAs and miRNAs in osteosarcoma cells co-cultured with macrophages-derived exosomes. Then, functional analyses revealed that macrophages-derived exosomal lncRNA LIFR-AS1 can be delivered to OS cells, and there its increased expression unbalance the ceRNET LIFR-AS1/miR-29a/NFIA, with the consequent promotion of cell proliferation, migration, invasion, and apoptosis inhibition [88] (Table 1). TAMs were also obtained by inducing CD14 + peripheral blood mononuclear cells (PBMCs); then, it was demonstrated that TAMs increased the lncRNA PURPL expression in OS cells, promoting cell proliferation, migration, invasion by miR-363/PDZD2 axis; this same axis can also modulate TAM migration, highlighting a possible feedback crosstalk between TAMs and OS cells [155] (Table 1).
As a critical component of TME, bone marrow-derived mesenchymal stem cells (BMSCs) have been demonstrated to modulate the cancer hallmarks. Li et al. demonstrated that BMSC-EVs facilitated proliferation, invasion and migration of osteosarcoma cells and promoted tumor growth in nude mice. In particular, BMSC can load MALAT-1 in EVs and deliver it to OS cells, potentially unbalancing all the ceRNETs mediated by the lncRNA. In particular, it has been demonstrated that BMSC-EVs-treated osteosarcoma cells showed increased MALAT1 and NRSN2 expressions, and activated Wnt/β-catenin pathway due to MALAT-1 sponging activity versus miR-143 [131]. BMSCs are also able to deliver the lncRNA NORAD through EVs to OS cells; there, NORAD promoted OS cell proliferation and invasion by sponging miR-30c-5p and thus increasing KLF10. Very importantly, these results were confirmed in vivo, where BMSC-EV-NORAD was also able to promote lung metastasis of osteosarcoma [145]. Intriguingly, KLF10 was also involved in another ceRNET, Circ-0003998/miR-197-3p/KLF10, promoting OS cell proliferative and invasiveness [230].
Finally, cytokines produced by different cell types in TME can increase the expression of oncogenic ncRNAs, derailing their governed network. One example is represented by cancer-associated fibroblasts (CAFs)-derived TGF-beta, that is able to upregulate the expression of TUG1 in OS cells [188]. There, up-regulated TUG1 is able to sponge different miRNAs, thus increasing the expression of their oncogenic targets prompting cell proliferation, migration, angiogenesis, tumor growth, and metastasis (Table 1).
Overall, molecular mechanisms underlying the crosstalk among OS cells each other and the other components of TME are much more complex than expected and relying also on RNA regulatory networks that can be unbalanced in each cell type component by the surroundings (Fig. 2). These features are challenging for the comprehension of OS progression and patients’ management, but can offer innovative therapeutic opportunities, also in the direction of precision oncology.
ceRNETs contributing to tumor microenvironment. A continuous crosstalk occurs among osteosarcoma cells (OS cells), bone mesenchymal cells (BMCs), immune cells, especially tumor-associated macrophages (TAFs), cancer-associated fibroblasts (CAFs) through cytokines and vesicles, with their cargo of proteins and RNAs, that can unbalance the RNA regulatory networks in the recipient cells, thus contributing to OS progression. Examples discussed in the text are reported. Figure created with BioRender.com
Conclusion and next challenges
OS is a very complex cancer: mesenchymal bone-forming cells can undergo aberrant alterations at any stage of differentiation; vast genomic instability and multiple genomic aberrations characterize the majority of OS cases; different mutated genes have been identified. The heterogeneity of genetic drivers and of cell types contribution to OS onset and progression, especially in TME, makes therapy and patients management particularly challenging. The deep understanding of OS biology and a unifying picture of molecular mechanisms could help to transform those challenges into opportunities. The ceRNET perspective may be a key to understand how different transcripts, coding and non-coding, are functionally linked and talk each other using the microRNA binding sites as the letters of an “RNA language”; indeed, the unbalancing of the networks can drive OS onset, progression and even chemotherapeutic resistance.
The knowledge of those mechanisms could inspire innovative therapeutic approaches based on restoring the optimal ceRNA crosstalk for the homeostasis equilibrium, with a view to achieving drugs for multiple targets, required by OS heterogeneity. It is becoming increasingly clear that RNA molecules as therapeutic agents are more cost-effective and easier to develop than traditional therapeutics based on small molecule chemicals or proteins, due to their structural/functional versatility allowing them to interact with DNA, other RNA biotypes and proteins and thus broadening the range of druggable targets. Different FDA and EMA drugs approved in clinical care or in clinical development cover the five different categories of RNA therapeutics, i.e.: mRNAs, RNA encoding for proteins; antisense oligonucleotides (ASOs), small single-stranded nucleic acids binding target RNA with perfect complementarity and thus inducing post-transcriptional gene silencing; small interfering RNAs (siRNAs), double-stranded RNA causing degradation or translational block of target RNAs; miRNA mimic or inhibitor, respectively small double-stranded RNA molecules boosting the miRNA level or small single-stranded RNA binding and suppressing the miRNA silencing activity; aptamers, RNA, DNA, or RNA/DNA hybrids that form secondary or tertiary structures binding to a target molecule, either suppressing or enhancing the pathway relying on that target [405, 406]. Even more RNA-targeted and RNA-based strategies have been found to have possible therapeutic potential, such as circRNA molecules carrying multiple binding sites for sponging oncomiRs and thus preventing their activity [407]. The basic concept is to restore the expression of beneficial molecules (such as a tumor suppressive miRNA) or silence the oncogenic molecules. Intriguingly, among the RNA therapeutics developed for other diseases, some strategies useful for OS can be found. One example is represented by MRX34, a miRNA mimic for miR-34a, that gained the Phase I of clinical trial for melanoma and other cancer types (NCT01829971), but that could be useful also for OS, due to its oncosuppressive activity [408]. Although the FDA halted the clinical trial for immune-related adverse events, it could be worth to develop other miR-34a mimic-based strategies, since its effectiveness has been also demonstrated by another miR-34a prodrug (chimeric recombinant tRNA fusion pre-miR-34a) that has anti-tumor activity just for OS, in a canine model [409]. Vice versa, RG-012 is a miR-21 inhibitor developed for Alport Syndrome (NCT03373786, Phase II), but that could be useful also for OS due to its oncogenic activity [410]. Other interesting approaches targeting lncRNAs, even for unrelated diseases and that could be inspiring and beneficial also for OS, have been found for the oncogenic lncRNA MALAT1 and the tumor suppressor lncRNA GAS5 (Table 1); in the first case, multiple structural element lockers are being developed for disrupting a stabilizing triple helix structure at its 3’ end, resulting in MALAT1 destabilization and downregulation; in the second case, the interaction element blocker, NP-C86 molecule, blocks the interaction with UPF1, which normally results in nonsense-mediated decay, thus increasing the stability and half-life of GAS5 [406, 411]. The next overcoming challenges for successful RNA therapy are probably represented by stable and possibly specific delivery of the molecule through the extracellular and intracellular barriers; for those obstacles, various chemical modifications and the engineering of delivery formulations have been explored to improve pharmacodynamics and pharmacokinetics. In particular, five nanocarriers delivery strategies have been developed, i.e. lipid nanoparticles, cationic polymers, engineered exosomes, spherical nucleic acid nanoparticles, self-assembled DNA cage tetrahedron nanostructures, and they can keep the promise to deliver RNA molecules through binding to the cell membrane, endocytosis, endosome escape and release RNAs in the cytoplasm for translation or incorporation into appropriate ribonucleoproteins complexes [407].
Indeed, the combination of RNA-targeted and RNA-based therapies with lower doses of current treatments could be exploited, also in an attempt to normalize TME, inhibit metastasis, prevent or overcome the chemoresistance, in the perspective of personalized therapeutic plans.
Finally, many RNAs discussed here and listed in the Tables are consistently reported as up-regulated or down-regulated in OS tissues and cells, and related to tumor stage, progression, prognosis and survival. It would be worth setting-up PCR arrays for simultaneously and systematically measuring, in large cohorts, the different candidates to find those ones useful as biomarkers for an early diagnosis, prognosis and monitoring therapy response. Different diagnostic panels are now commercially available for various diseases, including cancer, and more than 150 clinical studies are registered at clinicaltrials.gov, wherein the value of a miRNA or miRNA signature is being investigated for a variety of clinical applications from early disease detection to treatment response [412,413,414]. A clear trend in the recent literature and on-going clinical trials can be envisaged, that is the development of miRNA-based noninvasive detection assays using liquid biopsies (mainly blood or serum samples) as starting material to inform clinical decisions, whereas initial diagnostic/prognostic studies used tissues from diagnostic biopsies or surgical procedures. Some challenges with circulating RNA-based diagnostic applications are related to their specificity, since certain miRNAs can be altered in other physiological (e.g., pregnancy) and pathological conditions and their diagnostic performance could be lower compared to other investigational and clinically established biomarkers; however, combining the detection of different RNA molecules, for example involved in various ceRNETs consistently reported to be related to tumor stage, progression, prognosis, survival and therapy response, may be the linchpin to overcome the problems. Those approaches will be useful in the near future for defining new diagnostic tools and supporting precision oncology.
Overall, a multidisciplinary approach, based on deep knowledge crossing the field of both RNA and cancer biology, is increasingly required, especially when the subject of study is so complex and heterogenous such as OS and patients care.
Availability of data and materials
Not applicable.
References
Argenziano M, Tortora C, Pota E, Di Paola A, Di Martino M, Di Leva C, et al. Osteosarcoma in children: not only chemotherapy. Pharmaceuticals (Basel). 2021;14:923.
Punzo F, Tortora C, Argenziano M, Pinto DD, Pota E, Martino MD, et al. Can Denosumab be used in combination with Doxorubicin in Osteosarcoma? Oncotarget. 2020;11:2763–73.
Beird HC, Bielack SS, Flanagan AM, Gill J, Heymann D, Janeway KA, et al. Osteosarcoma. Nat Rev Dis Primers. 2022;8:77.
Li M, Jin X, Li H, Wu G, Wang S, Yang C, et al. Key genes with prognostic values in suppression of osteosarcoma metastasis using comprehensive analysis. BMC Cancer. 2020;20:65.
Kleinerman ES, Mary V, John A. Osteosarcoma: the state of affairs dictates a change. What do we know? Adv Exp Med Biol. 2014;804:vii–viii.
Ahn JH, Cho WH, Lee JA, Kim DH, Seo J-H, Lim JS. Bone mineral density change during adjuvant chemotherapy in pediatric osteosarcoma. Ann Pediatr Endocrinol Metab. 2015;20:150–4.
Punzo F, Bellini G, Tortora C, Pinto DD, Argenziano M, Pota E, et al. Mifamurtide and TAM-like macrophages: effect on proliferation, migration and differentiation of osteosarcoma cells. Oncotarget. 2020;11:687–98.
Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33:3029–35.
Belayneh R, Fourman MS, Bhogal S, Weiss KR. Update on Osteosarcoma. Curr Oncol Rep. 2021;23:71.
Czarnecka AM, Synoradzki K, Firlej W, Bartnik E, Sobczuk P, Fiedorowicz M, et al. Molecular biology of osteosarcoma. Cancers (Basel). 2020;12:2130.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
Chang T-C, Mendell JT. microRNAs in vertebrate physiology and human disease. Annu Rev Genomics Hum Genet. 2007;8:215–39.
Di Palo A, Siniscalchi C, Salerno M, Russo A, Gravholt CH, Potenza N. What microRNAs could tell us about the human X chromosome. Cell Mol Life Sci. 2020;77:4069–80.
Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314.
Scuderi SA, Calabrese G, Paterniti I, Campolo M, Lanza M, Capra AP, et al. The biological function of MicroRNAs in bone tumors. Int J Mol Sci. 2022;23:2348.
Sekar D, Mani P, Biruntha M, Sivagurunathan P, Karthigeyan M. Dissecting the functional role of microRNA 21 in osteosarcoma. Cancer Gene Ther. 2019;26:179–82.
Wang S, Ma F, Feng Y, Liu T, He S. Role of exosomal miR-21 in the tumor microenvironment and osteosarcoma tumorigenesis and progression (Review). Int J Oncol. 2020;56:1055–63.
Zhang L, Liao Y, Tang L. MicroRNA-34 family: a potential tumor suppressor and therapeutic candidate in cancer. J Exp Clin Cancer Res. 2019;38:53.
Yang Z, Liu T, Ren X, Yang M, Tu C, Li Z. Mir-34a: a regulatory hub with versatile functions that controls osteosarcoma networks. Cell Cycle. 2022;21:2121–31.
Mosca N, Russo A, Potenza N. Making Sense of Antisense lncRNAs in Hepatocellular Carcinoma. Int J Mol Sci. 2023;24:8886.
Beermann J, Piccoli M-T, Viereck J, Thum T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol Rev. 2016;96:1297–325.
Gao Y, Wang P, Wang Y, Ma X, Zhi H, Zhou D, et al. Lnc2Cancer v2.0: updated database of experimentally supported long non-coding RNAs in human cancers. Nucleic Acids Res. 2019;47:D1028-33.
Carlevaro-Fita J, Lanzós A, Feuerbach L, Hong C, Mas-Ponte D, Pedersen JS, et al. Cancer LncRNA Census reveals evidence for deep functional conservation of long noncoding RNAs in tumorigenesis. Commun Biol. 2020;3:56.
Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–89.
Braicu C, Zimta A-A, Gulei D, Olariu A, Berindan-Neagoe I. Comprehensive analysis of circular RNAs in pathological states: biogenesis, cellular regulation, and therapeutic relevance. Cell Mol Life Sci. 2019;76:1559–77.
Yeh S-C, Cheong FJF, Tay Y. Circular RNAs and Untranslated Regions in Acute Myeloid Leukemia. Int J Mol Sci. 2023;24:3215.
Zhao X, Cai Y, Xu J. Circular RNAs: Biogenesis, Mechanism, and Function in Human Cancers. Int J Mol Sci. 2019;20:3926.
Chan JJ, Tay Y. Noncoding RNA:RNA Regulatory Networks in Cancer. Int J Mol Sci. 2018;19:1310.
Siniscalchi C, Di Palo A, Russo A, Potenza N. The lncRNAs at X Chromosome Inactivation Center: Not Just a Matter of Sex Dosage Compensation. Int J Mol Sci. 2022;23:611.
Di Palo A, Siniscalchi C, Mosca N, Russo A, Potenza N. A Novel ceRNA Regulatory Network Involving the Long Non-Coding Antisense RNA SPACA6P-AS, miR-125a and its mRNA Targets in Hepatocarcinoma Cells. Int J Mol Sci. 2020;21:5068.
Dang Y, Zhou Y, Ou X, Wang Q, Wei D, Xie F. lncRNA AC007207.2 promotes malignant properties of osteosarcoma via the miR-1306–5p/SIRT7 Axis. Cancer Manag Res. 2021;13:7277–88.
Xu L, Tan Y, Xu F, Zhang Y. Long noncoding RNA ADIRF antisense RNA 1 upregulates insulin receptor substrate 1 to decrease the aggressiveness of osteosarcoma by sponging microRNA-761. Bioengineered. 2022;13:2028–43.
Li R, Liu S, Li Y, Tang Q, Xie Y, Zhai R. Long noncoding RNA AFAP1-AS1 enhances cell proliferation and invasion in osteosarcoma through regulating miR-4695-5p/TCF4-β-catenin signaling. Mol Med Rep. 2018;18:1616–22.
Fei D, Zhang X, Lu Y, Tan L, Xu M, Zhang Y. Long noncoding RNA AFAP1-AS1 promotes osteosarcoma progression by regulating miR-497/IGF1R axis. Am J Transl Res. 2020;12:2155–68.
Yu H, Zhang B, Qi L, Han J, Guan M, Li J, et al. AP003352.1/miR-141–3p axis enhances the proliferation of osteosarcoma by LPAR3. PeerJ. 2023;11:e15937.
Guan H, Shang G, Cui Y, Liu J, Sun X, Cao W, et al. Long noncoding RNA APTR contributes to osteosarcoma progression through repression of miR-132-3p and upregulation of yes-associated protein 1. J Cell Physiol. 2019;234:8998–9007.
Hou C, Sun F, Sun M. Long non-coding RNA ASMTL-AS1 deteriorates the oncogenicity of osteosarcoma by decoying microRNA-342-3p and consequently raising ADAM9 expression. Biochem Biophys Res Commun. 2021;579:89–96.
Li C, Wang F, Wei B, Wang L, Kong D. LncRNA AWPPH promotes osteosarcoma progression via activation of Wnt/β-catenin pathway through modulating miR-93-3p/FZD7 axis. Biochem Biophys Res Commun. 2019;514:1017–22.
Han G, Guo Q, Ma N, Bi W, Xu M, Jia J, et al. LncRNA BCRT1 facilitates osteosarcoma progression via regulating miR-1303/FGF7 axis. Aging (Albany NY). 2021;13:15501–10.
Chen X, Cui Y, Ma Y. Long non-coding RNA BLACAT1 expedites osteosarcoma cell proliferation, migration and invasion via up-regulating SOX12 through miR-608. J Bone Oncol. 2020;25:100314.
Zhou X, Li J, Teng J, Liu Y, Zhang D, Liu L, et al. Long noncoding RNA BSN-AS2 induced by E2F1 promotes spinal osteosarcoma progression by targeting miR-654-3p/SYTL2 axis. Cancer Cell Int. 2020;20:133.
Qiu H, Yang D, Li X, Feng F. LncRNA CASC9 promotes cell proliferation and invasion in osteosarcoma through targeting miR-874-3p/SOX12 axis. J Orthop Surg Res. 2022;17:460.
Zhang H, Wang J, Ren T, Huang Y, Yu Y, Chen C, et al. LncRNA CASC15 is Upregulated in Osteosarcoma Plasma Exosomes and CASC15 Knockdown Inhibits osteosarcoma progression by regulating miR-338-3p/RAB14 Axis. Onco Targets Ther. 2020;13:12055–66.
Chen S, Yang M, Chang S. LncRNA CCAL promotes angiogenesis through regulating the MiR-29b/ANGPTL4 Axis in osteosarcoma. Cancer Manag Res. 2020;12:10521–30.
Abula A, Saimaiti G, Maimaiti X, Wuqikun W, Abulaiti A, Ren P, et al. The stimulative function of long noncoding RNA CDKN2B-AS1 in osteosarcoma by targeting the microRNA-122/CCNG1 axis. J Recept Signal Transduct Res. 2022;42:71–9.
Ding X, Zhang Y, Liang J, Yin J, Akbar N, Miguel V, et al. The long non-coding RNA CRNDE promotes osteosarcoma proliferation and migration by sponging miR-136-5p/MRP9 axis. Ann Transl Med. 2022;10:835.
Wang Y, Zeng X, Wang N, Zhao W, Zhang X, Teng S, et al. Long noncoding RNA DANCR, working as a competitive endogenous RNA, promotes ROCK1-mediated proliferation and metastasis via decoying of miR-335-5p and miR-1972 in osteosarcoma. Mol Cancer. 2018;17:89.
Zhang W, Li J-Z, Tai Q-Y, Tang J-J, Huang Y-H, Gao S-B. LncRNA DANCR regulates osteosarcoma migration and invasion by targeting miR-149/MSI2 axis. Eur Rev Med Pharmacol Sci. 2020;24:6551–60.
Gu Z, Hou Z, Zheng L, Wang X, Wu L, Zhang C. LncRNA DICER1-AS1 promotes the proliferation, invasion and autophagy of osteosarcoma cells via miR-30b/ATG5. Biomed Pharmacother. 2018;104:110–8.
Chen X, Zhang C, Wang X. Long noncoding RNA DLEU1 aggravates osteosarcoma carcinogenesis via regulating the miR-671-5p/DDX5 axis. Artif Cells Nanomed Biotechnol. 2019;47:3322–8.
Zheng C, Li R, Zheng S, Fang H, Xu M, Zhong L. The knockdown of lncRNA DLGAP1-AS2 suppresses osteosarcoma progression by inhibiting aerobic glycolysis via the miR-451a/HK2 axis. Cancer Sci. 2023;114:4747–62.
Zhang R-M, Tang T, Yu H-M, Yao X-D. LncRNA DLX6-AS1/miR-129-5p/DLK1 axis aggravates stemness of osteosarcoma through Wnt signaling. Biochem Biophys Res Commun. 2018;507:260–6.
Zhang N, Meng X, Mei L, Zhao C, Chen W. LncRNA DLX6-AS1 promotes tumor proliferation and metastasis in osteosarcoma through modulating miR-641/HOXA9 signaling pathway. J Cell Biochem. 2019;120:11478–89.
Guo Q, Sun H, Zheng K, Yin S, Niu J. Long non-coding RNA DLX6-AS1/miR-141-3p axis regulates osteosarcoma proliferation, migration and invasion through regulating Rab10. RSC Adv. 2019;9:33823–33.
Zhang S, Ding L, Gao F, Fan H. Long non-coding RNA DSCAM-AS1 upregulates USP47 expression through sponging miR-101-3p to accelerate osteosarcoma progression. Biochem Cell Biol. 2020;98:600–11.
Yang T, Guo JP, Li F, Xiu C, Wang H, Duan XL. Long non-coding RNA-DUXAP8 regulates TOP2A in the growth and metastasis of osteosarcoma via microRNA-635. Mol Med Rep. 2021;24:511.
Dai S, Li N, Zhou M, Yuan Y, Yue D, Li T, et al. LncRNA EBLN3P promotes the progression of osteosarcoma through modifying the miR-224-5p/Rab10 signaling axis. Sci Rep. 2021;11:1992.
Xie H, Dai L, Ye B, Chen R, Wang B, Zhang N, et al. Long non-coding RNA ERVK13-1 aggravates osteosarcoma through the involvement of microRNA-873-5p/KLF5 axis. Acta Biochim Pol. 2022;69:703–10.
He P, Ding J. EWS promotes cell proliferation and inhibits cell apoptosis by regulating miR-199a-5p/Sox2 axis in osteosarcoma. Biotechnol Lett. 2020;42:1263–74.
Zhou C, Xu J, Lin J, Lin R, Chen K, Kong J, et al. Long noncoding RNA FEZF1-AS1 promotes osteosarcoma progression by regulating the miR-4443/NUPR1 Axis. Oncol Res. 2018;26:1335–43.
Liu J, Feng G, Li Z, Li R, Xia P. Long non-coding RNA FEZF1-AS1 modulates CXCR4 to promote cell proliferation, warburg effect and suppress cell apoptosis in osteosarcoma by sponging miR-144. Onco Targets Ther. 2020;13:2899–910.
Li C, Lin X, Zhang C, Wan L, Yin J, Wang B. Long non-coding RNA FGD5-AS1 enhances osteosarcoma cell proliferation and migration by targeting miR-506-3p/RAB3D axis. Hum Cell. 2021;34:1255–65.
Shuang O, Zhou J, Cai Z, Liao L, Wang Y, Wang W, et al. EBF1-mediated up-regulation of lncRNA FGD5-AS1 facilitates osteosarcoma progression by regulating miR-124-3p/G3BP2 axis as a ceRNA. J Orthop Surg Res. 2022;17:332.
Wei G, Zhang T, Li Z, Yu N, Xue X, Zhou D, et al. USF1-mediated upregulation of lncRNA GAS6-AS2 facilitates osteosarcoma progression through miR-934/BCAT1 axis. Aging (Albany NY). 2020;12:6172–90.
Liu R, Ju C, Zhang F, Tang X, Yan J, Sun J, et al. LncRNA GSEC promotes the proliferation, migration and invasion by sponging miR-588/ EIF5A2 axis in osteosarcoma. Biochem Biophys Res Commun. 2020;532:300–7.
Wang L, Zhou J, Zhang Y, Hu T, Sun Y. Long non-coding RNA HCG11 aggravates osteosarcoma carcinogenesis via regulating the microRNA-579/MMP13 Axis. Int J Gen Med. 2020;13:1685–95.
Yan H, Zhou Y, Chen Z, Yan X, Zhu L. Long non-coding RNA HCG11 enhances osteosarcoma phenotypes by sponging miR-1245b-5p that directly inhibits plakophilin 2. Bioengineered. 2022;13:140–54.
Zhao Z, Chen J, Xia D. Knockdown of HCG18 inhibits cell viability, migration and invasion in pediatric osteosarcoma by targeting miR-188-5p/FOXC1 Axis. Mol Biotechnol. 2021;63:807–17.
Pan X, Guo J, Liu C, Pan Z, Yang Z, Yao X, et al. LncRNA HCG18 promotes osteosarcoma growth by enhanced aerobic glycolysis via the miR-365a-3p/PGK1 axis. Cell Mol Biol Lett. 2022;27:5.
Tan J-D, Zhou M-F, Yang S, Lin J-P. Long noncoding RNA HCP5 promotes osteosarcoma cell proliferation, invasion, and migration via the miR-29b-3p-LOXL2 axis. Kaohsiung J Med Sci. 2022;38:960–70.
Lou P, Ding T, Zhan X. Long noncoding RNA HNF1A-AS1 regulates osteosarcoma advancement through modulating the miR-32-5p/HMGB1 Axis. Cancer Biother Radiopharm. 2021;36:371–81.
Lin H, Zhao Z, Hao Y, He J, He J. Long noncoding RNA HIF1A-AS2 facilitates cell survival and migration by sponging miR-33b-5p to modulate SIRT6 expression in osteosarcoma. Biochem Cell Biol. 2020;98:284–92.
Wang B, Qu X-L, Liu J, Lu J, Zhou Z-Y. HOTAIR promotes osteosarcoma development by sponging miR-217 and targeting ZEB1. J Cell Physiol. 2019;234:6173–81.
Wu W, Wang L, Li S. Hox transcript antisense RNA knockdown inhibits osteosarcoma progression by regulating the phosphoinositide 3-kinase/AKT pathway through the microRNA miR-6888-3p/spleen tyrosine kinase axis. Bioengineered. 2022;13:9397–410.
Yu X, Duan W, Wu F, Yang D, Wang X, Wu J, et al. LncRNA-HOTAIRM1 promotes aerobic glycolysis and proliferation in osteosarcoma via the miR-664b-3p/Rheb/mTOR pathway. Cancer Sci. 2023;114:3537–52.
Xiao X, Liu M, Xie S, Liu C, Huang X, Huang X. Long non-coding HOXA-AS3 contributes to osteosarcoma progression through the miR-1286/TEAD1 axis. J Orthop Surg Res. 2023;18:730.
Cui M, Wang J, Li Q, Zhang J, Jia J, Zhan X. Long non-coding RNA HOXA11-AS functions as a competing endogenous RNA to regulate ROCK1 expression by sponging miR-124-3p in osteosarcoma. Biomed Pharmacother. 2017;92:437–44.
Li Y, Liu J-J, Zhou J-H, Chen R, Cen C-Q. LncRNA HULC induces the progression of osteosarcoma by regulating the miR-372-3p/HMGB1 signalling axis. Mol Med. 2020;26:26.
Hu X-H, Dai J, Shang H-L, Zhao Z-X, Hao Y-D. SP1-mediated upregulation of lncRNA ILF3-AS1 functions a ceRNA for miR-212 to contribute to osteosarcoma progression via modulation of SOX5. Biochem Biophys Res Commun. 2019;511:510–7.
Wang M, Wang Z, Zhu X, Guan S, Liu Z. LncRNA KCNQ1OT1 acting as a ceRNA for miR-4458 enhances osteosarcoma progression by regulating CCND2 expression. In Vitro Cell Dev Biol Anim. 2019;55:694–702.
Shen Y, Xu J, Pan X, Zhang Y, Weng Y, Zhou D, et al. LncRNA KCNQ1OT1 sponges miR-34c-5p to promote osteosarcoma growth via ALDOA enhanced aerobic glycolysis. Cell Death Dis. 2020;11:278.
Zhang Q, Jiang H, Jin Y, Zhang N, Mu Z, Guo Y, et al. Long noncoding RNA KCNQ1 opposite strand/antisense transcript 1 promotes osteosarcoma progression through miR-154-3p/KLF12. Am J Transl Res. 2021;13:12285–301.
Huang A, Jin S, Han W, Wang Y, Ma S, Wang Z, et al. Long noncoding RNA KCNQ1OT1 contributes to tumor growth and activates Wnt/β-catenin signaling in osteosarcoma by targeting the miR-3666/KLF7 axis. Int J Mol Med. 2021;47:387–96.
Chen C, Liu L. Silencing of lncRNA KLF3-AS1 represses cell growth in osteosarcoma via miR-338-3p/MEF2C axis. J Clin Lab Anal. 2022;36: e24698.
Han J, Hu Y, Ding S, Liu S, Wang H. The analysis of the pyroptosis-related genes and hub gene TP63 ceRNA axis in osteosarcoma. Front Immunol. 2022;13:974916.
Li J, Yuan X, Ma C, Li J, Qu G, Yu B, et al. LncRNA LBX2-AS1 impacts osteosarcoma sensitivity to JQ-1 by sequestering miR-597-3p away from BRD4. Front Oncol. 2023;13:1139588.
Wang G, Cui T, Sun L, Peng N, Yang C. Long noncoding RNA LeXis promotes osteosarcoma growth through upregulation of CTNNB1 expression. Am J Cancer Res. 2017;7:1577–87.
Zhang H, Yu Y, Wang J, Han Y, Ren T, Huang Y, et al. Macrophages-derived exosomal lncRNA LIFR-AS1 promotes osteosarcoma cell progression via miR-29a/NFIA axis. Cancer Cell Int. 2021;21:192.
Zheng L, Hu N, Zhou X. TCF3-activated LINC00152 exerts oncogenic role in osteosarcoma through regulating miR-1182/CDK14 axis. Pathol Res Pract. 2019;215:373–80.
Xiao Y, Li C, Wang H, Liu Y. LINC00265 targets miR-382-5p to regulate SAT1, VAV3 and angiogenesis in osteosarcoma. Aging (Albany NY). 2020;12:20212–25.
Chen T, Liu J, Zhang H, Li J, Shang G. Long Intergenic Noncoding RNA00265 Enhances Cell Viability and Metastasis via Targeting miR-485-5p/USP22 Axis in Osteosarcoma. Front Oncol. 2022;12:907472.
Chen H, Wahafu P, Wang L, Chen X. LncRNA LINC00313 Knockdown Inhibits Tumorigenesis and Metastasis in Human Osteosarcoma by Upregulating FOSL2 through Sponging miR-342-3p. Yonsei Med J. 2020;61:359–70.
Sun F, Yu Z, Wu B, Zhang H, Ruan J. LINC00319 promotes osteosarcoma progression by regulating the miR-455-3p/NFIB axis. J Gene Med. 2020;22:e3248.
Lian H, Xie P, Yin N, Zhang J, Zhang X, Li J, et al. Linc00460 promotes osteosarcoma progression via miR-1224-5p/FADS1 axis. Life Sci. 2019;233:116757.
Yan J, Fang T, Zhang M, Zhou Q. LINC00467 facilitates osteosarcoma progression by sponging miR-217 to regulate KPNA4 expression. Int J Mol Med. 2021;47:26.
Guo W, Yu Q, Zhang M, Li F, Liu Y, Jiang W, et al. Long intergenic non-protein coding RNA 511 promotes the progression of osteosarcoma cells through sponging microRNA 618 to upregulate the expression of maelstrom. Aging (Albany NY). 2019;11:5351–67.
Xu J, Chen G, Zhang Y, Huang Z, Cheng X, Gu H, et al. LINC00511 Promotes Osteosarcoma Tumorigenesis and Invasiveness through the miR-185-3p/E2F1 Axis. Biomed Res Int. 2020;2020:1974506.
Zhou F-C, Zhang Y-H, Liu H-T, Song J, Shao J. LncRNA LINC00588 Suppresses the Progression of Osteosarcoma by Acting as a ceRNA for miRNA-1972. Front Pharmacol. 2020;11:255.
Zhou Y, Li X, Yang H. LINC00612 functions as a ceRNA for miR-214-5p to promote the proliferation and invasion of osteosarcoma in vitro and in vivo. Exp Cell Res. 2020;392:112012.
Liu S, Meng X. LINC00662 long non-coding rna knockdown attenuates the proliferation, migration, and invasion of osteosarcoma cells by regulating the microRNA-15a-5p/Notch2 Axis. Onco Targets Ther. 2020;13:7517–30.
Huang J, Lin F, Xu C, Xu Y. LINC00662 facilitates osteosarcoma progression via sponging miR-103a-3p and regulating SIK2 expression. J Tissue Eng Regen Med. 2021;15:1082–91.
Yu M, Lu W, Cao Z, Xuan T. LncRNA LINC00662 exerts an oncogenic effect on osteosarcoma by the miR-16-5p/ITPR1 Axis. J Oncol. 2021;2021:8493431.
Wang B, Xu Z, Wang X, Xia S, Cai P, Wang M, et al. Knockdown of lncRNA LINC00662 suppresses malignant behaviour of osteosarcoma cells via competition with miR-30b-3p to regulate ELK1 expression. J Orthop Surg Res. 2022;17:74.
A feedback loop of LINC00665 and the Wnt signaling pathway expedites osteosarcoma cell proliferation, invasion, and epithelial-mesenchymal transition - PubMed [Internet]. [cited 2023 Nov 28]. Available from: https://pubmed.ncbi.nlm.nih.gov/36387061/
Xing W, Xu W-Y, Chang L, Zhang K, Wang S-R. SP1-induced lncRNA LINC00689 overexpression contributes to osteosarcoma progression via the miR-655/SOX18 axis. Eur Rev Med Pharmacol Sci. 2020;24:2205–17.
Yao P, Ni Y, Liu C. Long non-coding RNA 691 regulated PTEN/PI3K/AKT signaling pathway in osteosarcoma through miRNA-9-5p. Onco Targets Ther. 2020;13:4597–606.
Zhang X-R, Shao J-L, Li H, Wang L. Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis. Open Life Sci. 2021;16:728–36.
Chang X, Tan Q, Xu J, Wu X, Wang Y, Zhang Y, et al. Tumor-derived exosomal linc00881 induces lung fibroblast activation and promotes osteosarcoma lung migration. Cancer Cell Int. 2023;23:287.
Liu W, Zhang Q, Shen K, Li K, Chang J, Li H, et al. Long noncoding RNA LINC00909 induces epithelial-mesenchymal transition and contributes to osteosarcoma tumorigenesis and metastasis. J Oncol. 2022;2022:8660965.
Zhou Y, Mu T. LncRNA LINC00958 promotes tumor progression through miR-4306/CEMIP axis in osteosarcoma. Eur Rev Med Pharmacol Sci. 2021;25:3182–99.
Zhou Y, Yin L, Li H, Liu L-H, Xiao T. The LncRNA LINC00963 facilitates osteosarcoma proliferation and invasion by suppressing miR-204-3p/FN1 axis. Cancer Biol Ther. 2019;20:1141–8.
Pan X, Tan J, Tao T, Zhang X, Weng Y, Weng X, et al. LINC01123 enhances osteosarcoma cell growth by activating the Hedgehog pathway via the miR-516b-5p/Gli1 axis. Cancer Sci. 2021;112:2260–71.
Yao Q, Chen T. LINC01128 regulates the development of osteosarcoma by sponging miR-299-3p to mediate MMP2 expression and activating Wnt/β-catenin signalling pathway. J Cell Mol Med. 2020;24:14293–305.
Zhang S, Chen R. LINC01140 regulates osteosarcoma proliferation and invasion by targeting the miR-139-5p/HOXA9 axis. Biochem Biophys Rep. 2022;31:101301.
Qu Z, Li S. Long noncoding RNA LINC01278 favors the progression of osteosarcoma via modulating miR-133a-3p/PTHR1 signaling. J Cell Physiol. 2020;1–13. https://doi.org/10.1002/jcp.29582.
Zhang G-F, Zhou B-S, An X-C, An F-M, Li S-H. LINC01278 is highly expressed in osteosarcoma and participates in the development of tumors by mediating the miR-134-5p/KRAS axis. Onco Targets Ther. 2021;14:683–95.
Ma W, Zhao X, Xue N, Gao Y, Xu Q. The LINC01410/miR-122-5p/NDRG3 axis is involved in the proliferation and migration of osteosarcoma cells. IUBMB Life. 2021;73:705–17.
Ma W, Gao Y, Zhang J, Yao X, Jia L, Xu Q. Long noncoding RNA LINC01410 promotes tumorigenesis of osteosarcoma cells via miR-497-5p/HMGA2 axis. J Biochem Mol Toxicol. 2021;35:e22921.
Gu Z, Wu S, Wang J, Zhao S. Long non-coding RNA LINC01419 mediates miR-519a-3p/PDRG1 axis to promote cell progression in osteosarcoma. Cancer Cell Int. 2020;20:147.
Yao X, Wu L, Gu Z, Li J. LINC01535 promotes the development of osteosarcoma through modulating miR-214-3p/KCNC4 Axis. Cancer Manag Res. 2020;12:5575–85.
Cai Q, Zhao X, Wang Y, Li S, Wang J, Xin Z, et al. LINC01614 promotes osteosarcoma progression via miR-520a-3p/SNX3 axis. Cell Signal. 2021;83:109985.
Bian X, Sun Y-M, Wang L-M, Shang Y-L. ELK1-induced upregulation lncRNA LINC02381 accelerates the osteosarcoma tumorigenesis through targeting CDCA4 via sponging miR-503-5p. Biochem Biophys Res Commun. 2021;548:112–9.
Luo W, He H, Xiao W, Liu Q, Deng Z, Lu Y, et al. MALAT1 promotes osteosarcoma development by targeting TGFA via MIR376A. Oncotarget. 2016;7:54733–43.
Liu K, Huang J, Ni J, Song D, Ding M, Wang J, et al. MALAT1 promotes osteosarcoma development by regulation of HMGB1 via miR-142-3p and miR-129-5p. Cell Cycle. 2017;16:578–87.
Wang Y, Zhang Y, Yang T, Zhao W, Wang N, Li P, et al. Long non-coding RNA MALAT1 for promoting metastasis and proliferation by acting as a ceRNA of miR-144-3p in osteosarcoma cells. Oncotarget. 2017;8:59417–34.
Sun Y, Qin B. Long noncoding RNA MALAT1 regulates HDAC4-mediated proliferation and apoptosis via decoying of miR-140-5p in osteosarcoma cells. Cancer Med. 2018;7:4584–97.
Ren D, Zheng H, Fei S, Zhao J-L. MALAT1 induces osteosarcoma progression by targeting miR-206/CDK9 axis. J Cell Physiol. 2018;234:950–7.
Zhang Y, Dai Q, Zeng F, Liu H. MALAT1 promotes the proliferation and metastasis of osteosarcoma cells by activating the Rac1/JNK pathway via targeting MiR-509. Oncol Res. 2018.
Sun Z, Zhang T, Chen B. Long non-coding RNA Metastasis-Associated Lung Adenocarcinoma Transcript 1 (MALAT1) promotes proliferation and metastasis of osteosarcoma cells by targeting c-Met and SOX4 via miR-34a/c-5p and miR-449a/b. Med Sci Monit. 2019;25:1410–22.
Duan G, Zhang C, Xu C, Xu C, Zhang L, Zhang Y. Knockdown of MALAT1 inhibits osteosarcoma progression via regulating the miR-34a/cyclin D1 axis. Int J Oncol. 2019;54:17–28.
Li F, Chen X, Shang C, Ying Q, Zhou X, Zhu R, et al. Bone marrow mesenchymal stem cells-derived extracellular vesicles promote proliferation, invasion and migration of osteosarcoma cells via the lncRNA MALAT1/miR-143/NRSN2/Wnt/β-Catenin axis. Onco Targets Ther. 2021;14:737–49.
Yang F, Wang M, Shi J, Xu G. IncRNA MALAT1 regulates the proliferation, apoptosis, migration, and invasion of osteosarcoma cells by targeting miR-873-5p/ROCK1. Crit Rev Eukaryot Gene Expr. 2023;33:67–79.
Zhang C, Xie L, Liang H, Cui Y. LncRNA MIAT facilitates osteosarcoma progression by regulating mir-128-3p/VEGFC axis. IUBMB Life. 2019;71:845–53.
Ji Q, Zhu J, Fang C-L, Jin H, Zhan D-P, Huang J. Down-regulation of MIAT suppresses osteosarcoma progression by acting as a ceRNA for miR-141-3p to regulate SIX1-mediated PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 2020;24:2218–28.
Wang X, Yu X, Long X, Pu Q. MIR205 host gene (MIR205HG) drives osteosarcoma metastasis via regulating the microRNA 2114–3p (miR-2114-3p)/twist family bHLH transcription factor 2 (TWIST2) axis. Bioengineered. 2021;12:1576–86.
Zhang H, Liu S, Tang L, Ge J, Lu X. Long non-coding RNA (LncRNA) MRPL23-AS1 promotes tumor progression and carcinogenesis in osteosarcoma by activating Wnt/β-catenin signaling via inhibiting microRNA miR-30b and upregulating myosin heavy chain 9 (MYH9). Bioengineered. 2021;12:162–71.
Tian C, Sun X, Han K, Zhu H, Min D, Lin S. Long non-coding RNA MRUL contributes to osteosarcoma progression through the miR-125a-5p/FUT4 Axis. Front Genet. 2020;11:672.
Zhang L, Zhao G, Ji S, Yuan Q, Zhou H. Downregulated long non-coding RNA MSC-AS1 inhibits osteosarcoma progression and increases sensitivity to cisplatin by binding to MicroRNA-142. Med Sci Monit. 2020;26:e921594.
Yang S, Chen M, Lin C. A Novel lncRNA MYOSLID/miR-1286/RAB13 axis plays a critical role in osteosarcoma progression. Cancer Manag Res. 2019;11:10345–51.
Liao Y, Liu Q, Xiao C, Zhou J. Machine learning and experimental validation to construct a metastasis-related gene signature and ceRNA network for predicting osteosarcoma prognosis. J Orthop Surg Res. 2022;17:516.
Tan H, Zhao L. lncRNA nuclear-enriched abundant transcript 1 promotes cell proliferation and invasion by targeting miR-186-5p/HIF-1α in osteosarcoma. J Cell Biochem. 2019;120:6502–14.
Zhang L, Lu X-Q, Zhou X-Q, Liu Q-B, Chen L, Cai F. NEAT1 induces osteosarcoma development by modulating the miR-339-5p/TGF-β1 pathway. J Cell Physiol. 2019;234:5097–105.
Ji S, Wang S, Zhao X, Lv L. Long noncoding RNA NEAT1 regulates the development of osteosarcoma through sponging miR-34a-5p to mediate HOXA13 expression as a competitive endogenous RNA. Mol Genet Genomic Med. 2019;7:e673.
Chen Y, Li J, Xiao J-K, Xiao L, Xu B-W, Li C. The lncRNA NEAT1 promotes the epithelial-mesenchymal transition and metastasis of osteosarcoma cells by sponging miR-483 to upregulate STAT3 expression. Cancer Cell Int. 2021;21:90.
He H, Ding M, Li T, Zhao W, Zhang L, Yin P, et al. Bone mesenchymal stem cell-derived extracellular vesicles containing NORAD promote osteosarcoma by miR-30c-5p. Lab Invest. 2022;102:826–37.
Jia G, Wang Y, Yu Y, Li Z, Wang X. Long non-coding RNA NR2F1-AS1 facilitates the osteosarcoma cell malignant phenotype via the miR-485-5p/miR-218-5p/BIRC5 axis. Oncol Rep. 2020;44:1583–95.
Ye J, He H, Chen S, Ren Y, Guo W, Jin Z. Long non-coding RNA NR2F2-AS1 regulates human osteosarcoma growth and metastasis through miR-425-5p-mediated HMGB2. Int J Clin Oncol. 2022;27:1891–903.
Zhu K-P, Ma X-L, Zhang C-L. LncRNA ODRUL Contributes to Osteosarcoma Progression through the miR-3182/MMP2 Axis. Mol Ther. 2017;25:2383–93.
Dai J, Xu L, Hu X, Han G, Jiang H, Sun H, et al. Long noncoding RNA OIP5-AS1 accelerates CDK14 expression to promote osteosarcoma tumorigenesis via targeting miR-223. Biomed Pharmacother. 2018;106:1441–7.
Wang X, Chen K, Zhao Z. LncRNA OR3A4 regulated the growth of osteosarcoma cells by modulating the miR-1207-5p/G6PD signaling. Onco Targets Ther. 2020;13:3117–28.
Pan Z, Mo F, Liu H, Zeng J, Huang K, Huang S, et al. LncRNA prostate androgen-regulated transcript 1 (PART 1) functions as an oncogene in osteosarcoma via sponging miR-20b-5p to upregulate BAMBI. Ann Transl Med. 2021;9:488.
Chang L, Jia D-L, Cao C-S, Wei H, Li Z-Q. LncRNA PCAT-1 promotes the progression of osteosarcoma via miR-508-3p/ZEB1 axis. Eur Rev Med Pharmacol Sci. 2021;25:2517–27.
Zhu C, Huang L, Xu F, Li P, Li P, Hu F. LncRNA PCAT6 promotes tumor progression in osteosarcoma via activation of TGF-β pathway by sponging miR-185-5p. Biochem Biophys Res Commun. 2020;521:463–70.
Wu K, Feng Q, Li L, Xiong Y, Liu S, Liu J, et al. Long-noncoding RNA PCAT6 aggravates osteosarcoma tumourigenesis via the MiR-143-3p/ZEB1 Axis. Onco Targets Ther. 2020;13:8705–14.
He F, Ding G, Jiang W, Fan X, Zhu L. Effect of tumor-associated macrophages on lncRNA PURPL/miR-363/PDZD2 axis in osteosarcoma cells. Cell Death Discov. 2021;7:307.
Song J, Wu X, Liu F, Li M, Sun Y, Wang Y, et al. Long non-coding RNA PVT1 promotes glycolysis and tumor progression by regulating miR-497/HK2 axis in osteosarcoma. Biochem Biophys Res Commun. 2017;490:217–24.
Yin P, Tong C. LncRNA RGMB-AS1 up-regulates ANKRD1 through competitively sponging miR-3614-5p to promote OSA cell proliferation and invasion. Arch Med Res. 2022;53:131–7.
Wang W, Li Y, Zhi S, Li J, Miao J, Ding Z, et al. LncRNA-ROR/microRNA-185-3p/YAP1 axis exerts function in biological characteristics of osteosarcoma cells. Genomics. 2021;113:450–61.
Yang D, Liu K, Fan L, Liang W, Xu T, Jiang W, et al. LncRNA RP11–361F15.2 promotes osteosarcoma tumorigenesis by inhibiting M2-Like polarization of tumor-associated macrophages of CPEB4. Cancer Lett. 2020;473:33–49.
Jiang R, Zhang Z, Zhong Z, Zhang C. Long-non-coding RNA RUSC1-AS1 accelerates osteosarcoma development by miR-101-3p-mediated Notch1 signalling pathway. J Bone Oncol. 2021;30:100382.
Li R, Chen Z, Zhou Y, Maimaitirexiati G, Yan Q, Li Y, et al. LncRNA SCAMP1 disrupts the balance between miR-26a-5p and ZEB2 to promote osteosarcoma cell viability and invasion. Front Oncol. 2022;12:967000.
Jiang Z, Jiang C, Fang J. Up-regulated lnc-SNHG1 contributes to osteosarcoma progression through sequestration of miR-577 and activation of WNT2B/Wnt/β-catenin pathway. Biochem Biophys Res Commun. 2018;495:238–45.
Wang J, Cao L, Wu J, Wang Q. Long non-coding RNA SNHG1 regulates NOB1 expression by sponging miR-326 and promotes tumorigenesis in osteosarcoma. Int J Oncol. 2018;52:77–88.
Liu Q, Luo J, Wang H, Zhang L, Jin G. SNHG1 functions as an oncogenic lncRNA and promotes osteosarcoma progression by up-regulating S100A6 via miR-493-5p. Acta Biochim Biophys Sin (Shanghai). 2022;54:137–47.
Li Z, Wang X, Liang S. Long non-coding RNA small nucleolar RNA host gene 1 knockdown suppresses the proliferation, migration and invasion of osteosarcoma cells by regulating microRNA-424-5p/FGF2 in vitro. Exp Ther Med. 2021;21:325.
Zheng S, Jiang F, Ge D, Tang J, Chen H, Yang J, et al. LncRNA SNHG3/miRNA-151a-3p/RAB22A axis regulates invasion and migration of osteosarcoma. Biomed Pharmacother. 2019;112:108695.
Wang Z, Wang Z, Liu J, Yang H. Long non-coding RNA SNHG5 sponges miR-26a to promote the tumorigenesis of osteosarcoma by targeting ROCK1. Biomed Pharmacother. 2018;107:598–605.
Zhu S, Liu Y, Wang X, Wang J, Xi G. lncRNA SNHG10 Promotes the Proliferation and Invasion of Osteosarcoma via Wnt/β-Catenin Signaling. Mol Ther Nucleic Acids. 2020;22:957–70.
Ge J, Liu M, Zhang Y, Xie L, Shi Z, Wang G. SNHG10/miR-141-3p/WTAP axis promotes osteosarcoma proliferation and migration. J Biochem Mol Toxicol. 2022;36:e23031.
Zhou S, Yu L, Xiong M, Dai G. LncRNA SNHG12 promotes tumorigenesis and metastasis in osteosarcoma by upregulating Notch2 by sponging miR-195-5p. Biochem Biophys Res Commun. 2018;495:1822–32.
Xu N, Xu J, Zuo Z, Liu Y, Yan F, Han C. Downregulation of lncRNA SNHG12 reversed IGF1R-induced osteosarcoma metastasis and proliferation by targeting miR-195-5p. Gene. 2020;726:144145.
Hou X-K, Mao J-S. Long noncoding RNA SNHG14 promotes osteosarcoma progression via miR-433-3p/FBXO22 axis. Biochem Biophys Res Commun. 2020;523:766–72.
Chen X, Xu H. LncRNA SNHG15 regulates osteosarcoma progression in vitro and in vivo via sponging miR-346 and regulating TRAF4 expression. Open Life Sci. 2020;15:423–36.
Wang X, Hu K, Chao Y, Wang L. LncRNA SNHG16 promotes proliferation, migration and invasion of osteosarcoma cells by targeting miR-1301/BCL9 axis. Biomed Pharmacother. 2019;114:108798.
Bu J, Guo R, Xu X-Z, Luo Y, Liu J-F. LncRNA SNHG16 promotes epithelial-mesenchymal transition by upregulating ITGA6 through miR-488 inhibition in osteosarcoma. J Bone Oncol. 2021;27:100348.
Wang W, Luo P, Guo W, Shi Y, Xu D, Zheng H, et al. LncRNA SNHG20 knockdown suppresses the osteosarcoma tumorigenesis through the mitochondrial apoptosis pathway by miR-139/RUNX2 axis. Biochem Biophys Res Commun. 2018;503:1927–33.
Wan N, Liu Q, Shi J, Wang S. LncRNA SNHG25 Predicts Poor Prognosis and Promotes Progression in Osteosarcoma via the miR-497-5p/SOX4 Axis. Comb Chem High Throughput Screen. 2024;27(5):725–44. https://doi.org/10.2174/1386207326666230602122618.
Chen H, Chen J. LncRNA SOX21-AS1 Promotes the Growth and Invasiveness of Osteosarcoma Cells Through miR-7-5p/IRS2 Regulatory Network. Arch Med Res. 2021;52:294–303.
Liu X, Wang H, Tao G-L, Chu T-B, Wang Y-X, Liu L. LncRNA-TMPO-AS1 promotes apoptosis of osteosarcoma cells by targeting miR-329 and regulating E2F1. Eur Rev Med Pharmacol Sci. 2020;24:11006–15.
Cui H, Zhao J. LncRNA TMPO-AS1 serves as a ceRNA to promote osteosarcoma tumorigenesis by regulating miR-199a-5p/WNT7B axis. J Cell Biochem. 2020;121:2284–93.
Cai Y, Yang Y, Zhang X, Ma Q, Li M. TRPM2-AS promotes the malignancy of osteosarcoma cells by targeting miR-15b-5p/PPM1D axis. Cell Cycle. 2022;21:835–50.
Meng X, Zhang Z, Chen L, Wang X, Zhang Q, Liu S. Silencing of the Long Non-Coding RNA TTN-AS1 Attenuates the Malignant Progression of Osteosarcoma Cells by Regulating the miR-16-1-3p/TFAP4 Axis. Front Oncol. 2021;11:652835.
Xie C-H, Cao Y-M, Huang Y, Shi Q-W, Guo J-H, Fan Z-W, et al. Long non-coding RNA TUG1 contributes to tumorigenesis of human osteosarcoma by sponging miR-9-5p and regulating POU2F1 expression. Tumour Biol. 2016;37:15031–41.
Cao J, Han X, Qi X, Jin X, Li X. TUG1 promotes osteosarcoma tumorigenesis by upregulating EZH2 expression via miR-144-3p. Int J Oncol. 2017;51:1115–23.
Wang Y, Yang T, Zhang Z, Lu M, Zhao W, Zeng X, et al. Long non-coding RNA TUG1 promotes migration and invasion by acting as a ceRNA of miR-335-5p in osteosarcoma cells. Cancer Sci. 2017;108:859–67.
Xie C, Chen B, Wu B, Guo J, Cao Y. LncRNA TUG1 promotes cell proliferation and suppresses apoptosis in osteosarcoma by regulating miR-212-3p/FOXA1 axis. Biomed Pharmacother. 2018;97:1645–53.
Li G, Liu K, Du X. Long Non-Coding RNA TUG1 Promotes Proliferation and Inhibits Apoptosis of Osteosarcoma Cells by Sponging miR-132-3p and Upregulating SOX4 Expression. Yonsei Med J. 2018;59:226–35.
Yu X, Hu L, Li S, Shen J, Wang D, Xu R, et al. Long non-coding RNA Taurine upregulated gene 1 promotes osteosarcoma cell metastasis by mediating HIF-1α via miR-143-5p. Cell Death Dis. 2019;10:280.
Zhao Z-Y, Zhao Y-C, Liu W. Long non-coding RNA TUG1 regulates the progression and metastasis of osteosarcoma cells via miR-140-5p/PFN2 axis. Eur Rev Med Pharmacol Sci. 2019;23:9781–92.
Zhang Z, Wang J, Zhang X, Ran B, Wen J, Zhang H. TYMSOS-miR-101-3p-NETO2 axis promotes osteosarcoma progression. Mol Cell Probes. 2023;67:101887.
Ma H, Su R, Feng H, Guo Y, Su G. Long noncoding RNA UCA1 promotes osteosarcoma metastasis through CREB1-mediated epithelial-mesenchymal transition and activating PI3K/AKT/mTOR pathway. J Bone Oncol. 2019;16:100228.
Zhang Z, Wu X, Han Q, Huang Z. Downregulation of long non-coding RNA UCA1 represses tumorigenesis and metastasis of osteosarcoma via miR-513b-5p/E2F5 axis. Anticancer Drugs. 2021;32:602–13.
Yang Z, Liu Z, Lu W, Guo H, Chen J, Zhang Y. LncRNA WAC-AS1 promotes osteosarcoma Metastasis and stemness by sponging miR-5047 to upregulate SOX2. Biol Direct. 2023;18:74.
Wu D, Nie X, Ma C, Liu X, Liang X, An Y, et al. RSF1 functions as an oncogene in osteosarcoma and is regulated by XIST/miR-193a-3p axis. Biomed Pharmacother. 2017;95:207–14.
Lv G-Y, Miao J, Zhang X-L. Long noncoding RNA XIST promotes osteosarcoma progression by targeting ras-related protein RAP2B via miR-320b. Oncol Res. 2018;26:837–46.
Wen J-F, Jiang Y-Q, Li C, Dai X-K, Wu T, Yin W-Z. LncRNA-XIST promotes the oxidative stress-induced migration, invasion, and epithelial-to-mesenchymal transition of osteosarcoma cancer cells through miR-153-SNAI1 axis. Cell Biol Int. 2020;44:1991–2001.
Liu W, Long Q, Zhang L, Zeng D, Hu B, Zhang W, et al. Long non-coding RNA X-inactive specific transcript promotes osteosarcoma metastasis via modulating microRNA-758/Rab16. Ann Transl Med. 2021;9:841.
Jia Z, Wang Y, Sun X, Zhao X, Zhang Y, Xu S, et al. Effect of lncRNA XLOC_005950 knockout by CRISPR/Cas9 gene editing on energy metabolism and proliferation in osteosarcoma MG63 cells mediated by hsa-miR-542-3p. Oncol Lett. 2021;22:669.
Zhao Z, Lin X, Tong Y, Li W. Silencing lncRNA ZFAS1 or elevated microRNA-135a represses proliferation, migration, invasion and resistance to apoptosis of osteosarcoma cells. Cancer Cell Int. 2019;19:326.
Liu C-W, Liu D, Peng D. Long non-coding RNA ZFAS1 regulates NOB1 expression through interacting with miR-646 and promotes tumorigenesis in osteosarcoma. Eur Rev Med Pharmacol Sci. 2019;23:3206–16.
Zhao S, Xiong W, Xu K. MiR-663a, regulated by lncRNA GAS5, contributes to osteosarcoma development through targeting MYL9. Hum Exp Toxicol. 2020;39:1607–18.
Yao X, Li X, Luo Y, Xu X, Liu J, Bu J. LncRNA GAS5 Regulates Osteosarcoma Cell Proliferation, Migration, and Invasion by Regulating RHOB via Sponging miR-663a. Cancer Manag Res. 2020;12:8253–61.
Han G, Guo Q, Ma N, Bi W, Xu M, Jia J. Apatinib inhibits cell proliferation and migration of osteosarcoma via activating LINC00261/miR-620/PTEN axis. Cell Cycle. 2021;20:1785–98.
Wan D, Qu Y, Zhang L, Ai S, Cheng L. The lncRNA LINC00691Functions as a ceRNA for miRNA-1256 to Suppress Osteosarcoma by Regulating the Expression of ST5. Onco Targets Ther. 2020;13:13171–81.
Shen B, Zhou N, Hu T, Zhao W, Wu D, Wang S. LncRNA MEG3 negatively modified osteosarcoma development through regulation of miR-361-5p and FoxM1. J Cell Physiol. 2019;234:13464–80.
Zhao H, Zhang M, Yang X, Song D. Overexpression of long non-coding RNA MIR22HG represses proliferation and enhances apoptosis via miR-629-5p/TET3 axis in osteosarcoma cells. J Microbiol Biotechnol. 2021;31:1331–42.
Chen J, Miao W, Yang S, Yin M, Zhao J, Song D. LncRNA NR_027471 Functions as a ceRNA for miRNA-8055 leading to suppression of osteosarcoma by regulating the expression of TP53INP1. Front Oncol. 2020;10:563255.
Liu L, Zheng M, Wang X, Gao Y, Gu Q. LncRNA NR_136400 suppresses cell proliferation and invasion by acting as a ceRNA of TUSC5 that is modulated by miR-8081 in osteosarcoma. Front Pharmacol. 2020;11:641.
Zheng H-L, Yang R-Z, Xu W-N, Liu T, Chen P-B, Zheng X-F, et al. Characterization of LncRNA SNHG22 as a protector of NKIRAS2 through miR-4492 binding in osteosarcoma. Aging (Albany NY). 2020;12:18571–87.
Zhao A, Liu W, Cui X, Wang N, Wang Y, Sun L, et al. lncRNA TUSC7 inhibits osteosarcoma progression through the miR-181a/RASSF6 axis. Int J Mol Med. 2021;47:583–94.
Fan H, Liu T, Tian H, Zhang S. TUSC8 inhibits the development of osteosarcoma by sponging miR-197-3p and targeting EHD2. Int J Mol Med. 2020;46:1311–20.
Zhang R, Xia T. Long non-coding RNA XIST regulates PDCD4 expression by interacting with miR-21-5p and inhibits osteosarcoma cell growth and metastasis. Int J Oncol. 2017;51:1460–70.
Trang NTN, Lai C-Y, Tsai H-C, Huang Y-L, Liu S-C, Tsai C-H, et al. Apelin promotes osteosarcoma metastasis by upregulating PLOD2 expression via the Hippo signaling pathway and hsa_circ_0000004/miR-1303 axis. Int J Biol Sci. 2023;19:412–25.
Ren Z, Wang S, Li B, Huang H, Zhang H, Yang Z, et al. Hsa_circ_0000073 promotes lipid synthesis of osteosarcoma through hsa-miR-1184/ FADS2 pathway. Cell Signal. 2023;110:110829.
Xu L, Duan J, Li M, Zhou C, Wang Q. Circ_0000253 promotes the progression of osteosarcoma via the miR-1236-3p/SP1 axis. J Pharm Pharmacol. 2023;75:227–35.
Li H, He L, Tuo Y, Huang Y, Qian B. Circular RNA hsa_circ_0000282 contributes to osteosarcoma cell proliferation by regulating miR-192/XIAP axis. BMC Cancer. 2020;20:1026.
Long Z, Gong F, Li Y, Fan Z, Li J. Circ_0000285 regulates proliferation, migration, invasion and apoptosis of osteosarcoma by miR-409-3p/IGFBP3 axis. Cancer Cell Int. 2020;20:481.
Zhang Z, Pu F, Wang B, Wu Q, Liu J, Shao Z. Hsa_circ_0000285 functions as a competitive endogenous RNA to promote osteosarcoma progression by sponging hsa-miRNA-599. Gene Ther. 2020;27:186–95.
Dai H, Yi G, Jiang D, Min Y, Li Z. Circ_0000376 regulates miR-577/HK2/LDHA signaling pathway to promote the growth, invasion and glycolysis of osteosarcoma. J Orthop Surg Res. 2024;19:67.
Ye B, Qiao K, Zhao Q, Jiang Z, Hu N, Wang F. Tanshinone I restrains osteosarcoma progression by regulating circ_0000376/miR-432-5p/BCL2 axis. Mol Cell Biochem. 2022;477:1–13.
Wu X, Yan L, Liu Y, Shang L. Circ_0000527 promotes osteosarcoma cell progression through modulating miR-646/ARL2 axis. Aging (Albany NY). 2021;13:6091–102.
Gao Y, Ma H, Gao Y, Tao K, Fu L, Ren R, et al. CircRNA Circ_0001721 promotes the progression of osteosarcoma through miR-372-3p/MAPK7 axis. Cancer Manag Res. 2020;12:8287–302.
Gong S, Zhang Y, Pang L, Wang L, He W. A novel CircRNA Circ_0001722 regulates proliferation and invasion of osteosarcoma cells through targeting miR-204-5p/RUNX2 axis. J Cancer Res Clin Oncol. 2023;149:12779–90.
Li S, Pei Y, Wang W, Liu F, Zheng K, Zhang X. Circular RNA 0001785 regulates the pathogenesis of osteosarcoma as a ceRNA by sponging miR-1200 to upregulate HOXB2. Cell Cycle. 2019;18:1281–91.
Zhang P, Ren J, Wan J, Sun R, Li Y. Circular RNA hsa_circ_0002052 promotes osteosarcoma via modulating miR-382/STX6 axis. Hum Cell. 2020;33:810–8.
Zhang M, Yu G-Y, Liu G, Liu W-D. Circular RNA circ_0002137 regulated the progression of osteosarcoma through regulating miR-433-3p/ IGF1R axis. J Cell Mol Med. 2022;26:1806–16.
Wang T, Zhang C, Wang S. Ginsenoside Rg3 inhibits osteosarcoma progression by reducing circ_0003074 expression in a miR-516b-5p/KPNA4-dependent manner. J Orthop Surg Res. 2021;16:724.
Li L, Kong X-A, Zang M, Dong J, Feng Y, Gui B, et al. Hsa_circ_0003732 promotes osteosarcoma cells proliferation via miR-545/CCNA2 axis. Biosci Rep. 2020;40:BSR20191552.
Zhou Z, Liu T, Li Z, Wang L. Circ_0003732 promotes osteosarcoma progression through regulating miR-377-3p/CPEB1 axis and Wnt/β-catenin signaling pathway. Anticancer Drugs. 2022;33:e299.
Wang L, Du Z-G, Huang H, Li F-S, Li G-S, Xu S-N. Circ-0003998 promotes cell proliferative ability and invasiveness by binding to miR-197-3p in osteosarcoma. Eur Rev Med Pharmacol Sci. 2019;23:10638–46.
Xu M, Sun X, Liu Y, Chang L, Wang HT, Wang S. hsa_circ_0005721 triggers proliferation, migration and invasion of osteosarcoma by upregulating the linear transcript TEP1. J BUON. 2021;26:1588–94.
Zhang C, Na N, Liu L, Qiu Y. CircRNA hsa_circ_0005909 Promotes Cell Proliferation of Osteosarcoma Cells by Targeting miR-338-3p/HMGA1 Axis. Cancer Manag Res. 2021;13:795–803.
Ding S, Zhang G, Gao Y, Chen S, Cao C. Circular RNA hsa_circ_0005909 modulates osteosarcoma progression via the miR-936/HMGB1 axis. Cancer Cell Int. 2020;20:305.
Huang H, Lu D, Li K, Zheng M, Qin X, Cui X, et al. Hsa_circ_0007031 promotes the proliferation and migration of osteosarcoma cells by sponging miR-196a-5p to regulate the HOXB6. Biochem Pharmacol. 2023;214:115667.
Zhang P, Li J. Down-regulation of circular RNA hsa_circ_0007534 suppresses cell growth by regulating miR-219a-5p/SOX5 axis in osteosarcoma. J Bone Oncol. 2021;27:100349.
Deng N, Li L, Gao J, Zhou J, Wang Y, Wang C, et al. Hsa_circ_0009910 promotes carcinogenesis by promoting the expression of miR-449a target IL6R in osteosarcoma. Biochem Biophys Res Commun. 2018;495:189–96.
Lu Z, Wang C, Lv X, Dai W. Hsa_circ_0010220 regulates miR-198/Syntaxin 6 axis to promote osteosarcoma progression. J Bone Oncol. 2021;28:100360.
Li J, Zhang F, Li H, Peng F, Wang Z, Peng H, et al. Circ_0010220-mediated miR-503-5p/CDCA4 axis contributes to osteosarcoma progression tumorigenesis. Gene. 2020;763:145068.
Xu R, Ci X, He F, Chen Y. Circular RNA hsa_circ_001350 contributes to osteosarcoma progression by regulating microRNA-578/CCR4-NOT transcription complex and subunit 7/Wnt signaling. Am J Cancer Res. 2023;13:2360–75.
Yang B, Li L, Tong G, Zeng Z, Tan J, Su Z, et al. Circular RNA circ_001422 promotes the progression and metastasis of osteosarcoma via the miR-195-5p/FGF2/PI3K/Akt axis. J Exp Clin Cancer Res. 2021;40:235.
Li Z, Fu Y, Ouyang W, He M, Wang Y, Wang X, et al. Circ_0016347 Promotes Osteosarcoma Progression by Regulating miR-1225–3p/KCNH1 Axis. Cancer Biother Radiopharmaceut. 2021. [cited 2023 Sep 15]; Available from: https://www.liebertpub.com/doi/10.1089/cbr.2019.3349
Jin H, Jin X, Zhang H, Wang W. Circular RNA hsa-circ-0016347 promotes proliferation, invasion and metastasis of osteosarcoma cells. Oncotarget. 2017;8:25571–81.
Li Z, Zheng L, Yang L, Chen D, Ren G, Yan X, et al. Hsa_circ_0020378 targets miR-556-5p/MAPK1 to regulate osteosarcoma cell proliferation and migration. Gene. 2023;856:147135.
Pan F, Zhang J, Tang B, Jing L, Qiu B, Zha Z. The novel circ_0028171/miR-218-5p/IKBKB axis promotes osteosarcoma cancer progression. Cancer Cell Int. 2020;20:484.
Qin G, Wu X. Circular RNA hsa_circ_0032463 Acts as the tumor promoter in osteosarcoma by regulating the MicroRNA 498/LEF1 Axis. Mol Cell Biol. 2021;41:e00100-e121.
Qin G, Wu X. Hsa_circ_0032463 acts as the tumor promoter in osteosarcoma by regulating the miR-330-3p/PNN axis. Int J Mol Med. 2021;47:92.
Huang Z, Chen P, Jia R, Liu Y. Circ_0051079 functions as an oncogenic regulator in osteosarcoma by leading to MAFB expression upregulation by competitively interacting with miR-1286. J Orthop Surg Res. 2022;17:428.
Wang W, Wang J, Li Y, Zhao Y. Circ_0051079 silencing inhibits the malignant phenotypes of osteosarcoma cells by the TRIM66/Wnt/β-catenin pathway in a miR-625-5p-dependent manner. J Bone Oncol. 2022;35:100436.
Huo S, Dou D. Circ_0056285 regulates proliferation, apoptosis and glycolysis of osteosarcoma cells via miR-1244/TRIM44 Axis. Cancer Manag Res. 2021;13:1257–70.
Zhang G, Zhu Y, Jin C, Shi Q, An X, Song L, et al. CircRNA_0078767 promotes osteosarcoma progression by increasing CDK14 expression through sponging microRNA-330-3p. Chem Biol Interact. 2022;360:109903.
Gao P, Zhao X, Yu K, Zhu Z. Circ_0084582 facilitates cell growth, migration, invasion, and angiopoiesis in osteosarcoma via mediating the miR-485-3p/JAG1 Axis. Front Genet. 2021;12:690956.
Zhou F, Wu H, Yin Z, Zhou W, Chen W. Circular RNA circ_0096041 promotes osteosarcoma cell proliferation and migration via sponging miR-556–5p and regulating LIN28A expression. Cell Mol Biol (Noisy-le-grand). 2024;70:113–9.
Jin Y, Li L, Zhu T, Liu G. Circular RNA circ_0102049 promotes cell progression as ceRNA to target MDM2 via sponging miR-1304-5p in osteosarcoma. Pathol Res Pract. 2019;215:152688.
Gao X, Xu N, Miao K, Huang G, Huang Y. Circ_0136666 aggravates osteosarcoma development through mediating miR-1244/CEP55 axis. J Orthop Surg Res. 2022;17:421.
Wang Z, Deng M, Chen L, Wang W, Liu G, Liu D, et al. Circular RNA Circ-03955 promotes epithelial-mesenchymal transition in osteosarcoma by regulating miR-3662/Metadherin pathway. Front Oncol. 2020;10:545460.
Wang K, Wang N, Liu J, Zhou J, Lei S, Yue H, et al. Silencing circular RNA hsa_circABCC1 inhibits osteosarcoma progression through down-regulating HDAC4 via sponging miR-591. Environ Toxicol. 2023;38:1565–76.
Zhu X, Liu C, Shi J, Zhou Z, Chen S, Jami SA. Circular RNA circANKIB1 promotes the progression of osteosarcoma by regulating miR-217/PAX3 axis. J Bone Oncol. 2021;27:100347.
Zhang Q, Wang L, Cao L, Wei T. Novel circular RNA circATRNL1 accelerates the osteosarcoma aerobic glycolysis through targeting miR-409-3p/LDHA. Bioengineered. 2021;12:9965–75.
Li Z, Luo Y, Wang C, Han D, Sun W. Circular RNA circBLNK promotes osteosarcoma progression and inhibits ferroptosis in osteosarcoma cells by sponging miR-188-3p and regulating GPX4 expression. Oncol Rep. 2023;50:1–11.
Xiang D, Li Y, Lin Y. Circular RNA circCCDC66 Contributes to Malignant Phenotype of Osteosarcoma by Sponging miR-338-3p to Upregulate the Expression of PTP1B. Biomed Res Int. 2020;2020:4637109.
Wu P-F, Tang X-M, Sun H-L, Jiang H-T, Ma J, Kong Y-H. Depletion of circRNA circ_CDK14 inhibits osteosarcoma progression by regulating the miR-520a-3p/GAB1 axis. Neo. 2021;68:798–809.
Liu J, Zhao J, Feng G, Li R, Jiao J. Silencing of circ-CDK14 suppresses osteosarcoma progression through the miR-198/E2F2 axis. Exp Cell Res. 2022;414:113082.
Hu R, Chen S, Yan J. Blocking circ-CNST suppresses malignant behaviors of osteosarcoma cells and inhibits glycolysis through circ-CNST-miR-578-LDHA/PDK1 ceRNA networks. J Orthop Surg Res. 2021;16:300.
Yang J, Liu Z, Liu B, Sun L. Silencing of circCYP51A1 represses cell progression and glycolysis by regulating miR-490-3p/KLF12 axis in osteosarcoma under hypoxia. J Bone Oncol. 2022;37:100455.
Xu G, Zhang H, Shi Y, Yang F. Circular RNA circDOCK1 contributes to osteosarcoma progression by acting as a ceRNA for miR-936 to regulate LEF1. Journal of Bone Oncology. 2022;36:100453.
Tan J, Yang B, Zhong H, Luo M, Su Z, Xie C, et al. Circular RNA circEMB promotes osteosarcoma progression and metastasis by sponging miR-3184-5p and regulating EGFR expression. Biomark Res. 2023;11:3.
Liang Z, Shi Y, Guan Z. CircECE1 promotes osteosarcoma progression through regulating RAB3D by sponging miR-588. J Orthop Surg Res. 2023;18:587.
Tan X, Tan D, Li H, Lin Y, Wen Z, Zeng C. circEPSTI1 Acts as a ceRNA to Regulate the Progression of Osteosarcoma. Curr Cancer Drug Targets. 2020;20:288–94.
Li J-J, Xiong M-Y, Sun T-Y, Ji C-B, Guo R-T, Li Y-W, et al. CircFAM120B knockdown inhibits osteosarcoma tumorigenesis via the miR-1205/PTBP1 axis. Aging. 2021;13:23831–41.
Gu H, Cheng X, Xu J, Zhou K, Bian C, Chen G, et al. Circular RNA circFAT1(e2) Promotes Osteosarcoma Progression and Metastasis by Sponging miR-181b and Regulating HK2 Expression. Biomed Res Int. 2020;2020:3589871.
Wen Y, Li B, He M, Teng S, Sun Y, Wang G. circHIPK3 promotes proliferation and migration and invasion via regulation of miR-637/HDAC4 signaling in osteosarcoma cells. Oncol Rep. 2021;45:169–79.
Huang Z, Yuan C, Gu H, Cheng X, Zhou K, Xu J, et al. Circular RNA circHIPK3 promotes cell metastasis through miR-637/STAT3 Axis in osteosarcoma. Biomed Res Int. 2020;2020:2727060.
He P, Liu F, Wang Z, Gong H, Zhang M, Jia Z, et al. CircKIF4A enhances osteosarcoma proliferation and metastasis by sponging MiR-515-5p and upregulating SLC7A11. Mol Biol Rep. 2022;49:4525–35.
Yu Y, Dong G, Li Z, Zheng Y, Shi Z, Wang G. circ-LRP6 contributes to osteosarcoma progression by regulating the miR-141-3p/HDAC4/HMGB1 axis. Int J Oncol. 2022;60:38.
Liu W, Long Q, Zhang W, Zeng D, Hu B, Liu S, et al. Circular RNA expression profile identifies circMGEA5 as a novel metastasis-promoting factor and potential biomarker in osteosarcoma. J Biochem Mol Toxicol. 2023;37:e23286.
Pan G, Hu T, Chen X, Zhang C. Upregulation of circMMP9 promotes osteosarcoma progression via targeting miR-1265/CHI3L1 Axis. Cancer Manag Res. 2019;11:9225–31.
Chen J, Liu G, Wu Y, Ma J, Wu H, Xie Z, et al. CircMYO10 promotes osteosarcoma progression by regulating miR-370-3p/RUVBL1 axis to enhance the transcriptional activity of β-catenin/LEF1 complex via effects on chromatin remodeling. Mol Cancer. 2019;18:150.
Shi Z, Wang K, Xing Y, Yang X. CircNRIP1 encapsulated by bone marrow mesenchymal stem cell-derived extracellular vesicles aggravates osteosarcoma by modulating the miR-532-3p/AKT3/PI3K/AKT Axis. Front Oncol. 2021;11:658139.
Shi P, Li Y, Guo Q. Circular RNA circPIP5K1A contributes to cancer stemness of osteosarcoma by miR-515-5p/YAP axis. J Transl Med. 2021;19:464.
Liu Y-P, Wan J, Long F, Tian J, Zhang C. circPVT1 facilitates invasion and metastasis by regulating miR-205-5p/c-FLIP axis in osteosarcoma. Cancer Manag Res. 2020;12:1229–40.
Huang S-X, Mei H-B, Liu K, Tang J, Wu J-Y, Zhu G-H, et al. CircPVT1 promotes the tumorigenesis and metastasis of osteosarcoma via mediation of miR-26b-5p/CCNB1 axis. J Bone Miner Metab. 2022;40:581–93.
Yan M, Gao H, Lv Z, Liu Y, Zhao S, Gong W, et al. Circular RNA PVT1 promotes metastasis via regulating of miR-526b/FOXC2 signals in OS cells. J Cell Mol Med. 2020;24:5593–604.
Zhou C, Balmer L, Song M, Wu K, Wang W, Wang H. CircPVT1 promotes migration and invasion by regulating miR-490-5p/HAVCR2 axis in osteosarcoma cells. J Cell Mol Med. 2024;28:e18269.
Tang G, Liu L, Xiao Z, Wen S, Chen L, Yang P. CircRAB3IP upregulates twist family BHLH transcription factor (TWIST1) to promote osteosarcoma progression by sponging miR-580-3p. Bioengineered. 2021;12:3385–97.
Gong Z, Shen P, Wang H, Zhu J, Liang K, Wang K, et al. A novel circular RNA circRBMS3 regulates proliferation and metastasis of osteosarcoma by targeting miR-424-eIF4B/YRDC axis. Aging. 2023;15:1564–90.
Liu F, Li W, Jin Z, Ye J. METTL3-mediated m6A modification of circRNF220 modulates miR-330-5p/survivin axis to promote osteosarcoma progression. J Cancer Res Clin Oncol. 2023;149:17347–60.
Jun L, Xuhong L, Hui L. Circ_SIPA1L1 promotes osteosarcoma progression via miR-379–5p/MAP3K9 axis. Cancer Biother Radiopharmaceut. 2020 [cited 2023 Sep 19]; Available from: https://www.liebertpub.com/doi/10.1089/cbr.2020.3891
Sun X, Zhao X, Xu S, Zhou Y, Jia Z, Li Y. CircSRSF4 enhances proliferation, invasion, and migration to promote the progression of osteosarcoma via Rac1. Int J Mol Sci. 2022;23:6200.
Wu Y, Xie Z, Chen J, Chen J, Ni W, Ma Y, et al. Circular RNA circTADA2A promotes osteosarcoma progression and metastasis by sponging miR-203a-3p and regulating CREB3 expression. Mol Cancer. 2019;18:73.
Shi Y, Tian Y, Wu Y, Zhao Y. CircTNPO1 promotes the tumorigenesis of osteosarcoma by sequestering miR-578 to upregulate WNT5A expression. Cell Signal. 2023;111:110858.
Ma W, Zhao X, Gao Y, Yao X, Zhang J, Xu Q. Circular RNA circ_UBAP2 facilitates the progression of osteosarcoma by regulating microRNA miR-637/high-mobility group box (HMGB) 2 axis. Bioengineered. 2022;13:4411–27.
Wu H, Li W, Zhu S, Zhang D, Zhang M. Circular RNA circUBAP2 regulates proliferation and invasion of osteosarcoma cells through miR-641/YAP1 axis. Cancer Cell Int. 2020;20:223.
Ma W, Xue N, Zhang J, Wang D, Yao X, Lin L, et al. circUBAP2 regulates osteosarcoma progression via the miR-204-3p/HMGA2 axis. Int J Oncol. 2021;58:298–311.
Jiang Y, Hou J, Zhang X, Xu G, Wang Y, Shen L, et al. Circ-XPO1 upregulates XPO1 expression by sponging multiple miRNAs to facilitate osteosarcoma cell progression. Exp Mol Pathol. 2020;117:104553.
Mao X, Guo S, Gao L, Li G. Circ-XPR1 promotes osteosarcoma proliferation through regulating the miR-214-5p/DDX5 axis. Hum Cell. 2021;34:122–31.
Jiang X, Chen D. Circular RNA hsa_circ_0000658 inhibits osteosarcoma cell proliferation and migration via the miR-1227/IRF2 axis. J Cell Mol Med. 2021;25:510–20.
Wu Z, Shi W, Jiang C. Overexpressing circular RNA hsa_circ_0002052 impairs osteosarcoma progression via inhibiting Wnt/β-catenin pathway by regulating miR-1205/APC2 axis. Biochem Biophys Res Commun. 2018;502:465–71.
Guan K, Liu S, Duan K, Zhang X, Liu H, Xu B, et al. Hsa_circ_0008259 modulates miR-21-5p and PDCD4 expression to restrain osteosarcoma progression. Aging. 2021;13:25484–95.
Chen L, Shan Y, Zhang H, Wang H, Chen Y. Up-regulation of Hsa_circ_0008792 inhibits osteosarcoma cell invasion and migration and promotes apoptosis by regulating Hsa-miR-711/ZFP1</p>. OTT. 2020;13:2173–81.
Du R, Fu B, Sun G, Ma B, Deng M, Zhu X, et al. Circular RNA circ_0046264 suppresses osteosarcoma progression via microRNA-940/Secreted frizzled related protein 1 axis. Tohoku J Exp Med. 2021;254:189–97.
Zhang Z, Zhou L, Zhou S, Li X. Circular RNA hsa_circ_0069117 suppresses proliferation and migration of osteosarcoma cells lines via miR-875-3p/PF4V1 axis. J Orthop Surg Res. 2022;17:37.
Liu F, Zhang X, Wu F, Peng H. Hsa_circ_0088212-mediated miR-520 h/APOA1 axis inhibits osteosarcoma progression. Transl Oncol. 2021;14:101219.
Zhang X, Hu Z, Li W, Liu Z, Li J, Wang Z, et al. Circular RNA 0102049 suppresses the progression of osteosarcoma through modulating miR-520g-3p/PLK2 axis. Bioengineered. 2021;12:2022–32.
Liu D-Y, Li Z, Zhang K, Jiao N, Lu D-G, Zhou D-W, et al. Circular RNA CircMTO1 suppressed proliferation and metastasis of osteosarcoma through miR-630/KLF6 axis. Eur Rev Med Pharmacol Sci. 2021;25:86–93.
Liu Y, Qiu G, Luo Y, Li S, Xu Y, Zhang Y, et al. Circular RNA ROCK1, a novel circRNA, suppresses osteosarcoma proliferation and migration via altering the miR-532-5p/PTEN axis. Exp Mol Med. 2022;54:1024–37.
Cheng C, Zhang H, Dai Z, Zheng J. Circular RNA circVRK1 suppresses the proliferation, migration and invasion of osteosarcoma cells by regulating zinc finger protein ZNF652 expression via microRNA miR-337-3p. Bioengineered. 2021;12:5411–27.
Liu S, Zhang J, Zheng T, Mou X, Xin W. Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis. Open Life Sci. 2021;16:229–41.
Fei D, Yuan H, Zhao M, Zhao D. LncRNA FGD5-AS1 potentiates autophagy-associated doxorubicin resistance by regulating the miR-154-5p/WNT5A axis in osteosarcoma. Cell Biol Int. 2022;46:1937–46.
Li G, Yan X. Long non-coding RNA GAS5 promotes cisplatin-chemosensitivity of osteosarcoma cells via microRNA-26b-5p/TP53INP1 axis. J Orthop Surg Res. 2023;18:890.
Guo J, Dou D, Zhang T, Wang B. HOTAIR promotes cisplatin resistance of osteosarcoma cells by regulating cell proliferation, invasion, and apoptosis via miR-106a-5p/STAT3 Axis. Cell Transplant. 2020;29:963689720948447.
Zhan H, Xiao J, Wang P, Mo F, Li K, Guo F, et al. Exosomal CTCF Confers Cisplatin Resistance in Osteosarcoma by Promoting Autophagy via the IGF2-AS/miR-579-3p/MSH6 Axis. J Oncol. 2022;2022:9390611.
Wang Y, Zhang L, Zheng X, Zhong W, Tian X, Yin B, et al. Long non-coding RNA LINC00161 sensitises osteosarcoma cells to cisplatin-induced apoptosis by regulating the miR-645-IFIT2 axis. Cancer Lett. 2016;382:137–46.
He P, Xu Y-Q, Wang Z-J, Sheng B. LncRNA LINC00210 regulated radiosensitivity of osteosarcoma cells via miR-342-3p/GFRA1 axis. J Clin Lab Anal. 2020;34:e23540.
Tang J, Zhu Z, Dong S, Wang Y, Wang J, Chen H, et al. Long non-coding RNA long intergenic non-coding 00641 mediates cell progression with stimulating cisplatin-resistance in osteosarcoma cells via microRNA-320d/myeloid cell leukemia-1 axis. Bioengineered. 2022;13:7238–52.
Li R, Ruan Q, Zheng J, Zhang B, Yang H. LINC01116 promotes doxorubicin resistance in osteosarcoma by epigenetically silencing miR-424-5p and inducing epithelial-mesenchymal transition. Front Pharmacol. 2021;12:632206.
Zhu K-P, Zhang C-L, Ma X-L, Hu J-P, Cai T, Zhang L. Analyzing the Interactions of mRNAs and ncRNAs to Predict Competing Endogenous RNA Networks in Osteosarcoma Chemo-Resistance. Mol Ther. 2019;27:518–30.
Meng Y, Hao D, Huang Y, Jia S, Zhang J, He X, et al. Positive feedback loop SP1/MIR17HG/miR-130a-3p promotes osteosarcoma proliferation and cisplatin resistance. Biochem Biophys Res Commun. 2020;521:739–45.
Han Z, Shi L. Long non-coding RNA LUCAT1 modulates methotrexate resistance in osteosarcoma via miR-200c/ABCB1 axis. Biochem Biophys Res Commun. 2018;495:947–53.
Liu L, Wang S. Long Non-Coding RNA OIP5-AS1 Knockdown Enhances CDDP Sensitivity in Osteosarcoma via miR-377-3p/FOSL2 Axis. Onco Targets Ther. 2020;13:3853–66.
Sun X, Tian C, Zhang H, Han K, Zhou M, Gan Z, et al. Long noncoding RNA OIP5-AS1 mediates resistance to doxorubicin by regulating miR-137-3p/PTN axis in osteosarcoma. Biomed Pharmacother. 2020;128:110201.
Kun-Peng Z, Chun-Lin Z, Xiao-Long M, Lei Z. Fibronectin-1 modulated by the long noncoding RNA OIP5-AS1/miR-200b-3p axis contributes to doxorubicin resistance of osteosarcoma cells. J Cell Physiol. 2019;234:6927–39.
Li Z, Tian J-M, Chu Y, Zhu H-Y, Wang J-J, Huang J. Long non-coding RNA PVT1 (PVT1) affects the expression of CCND1 and promotes doxorubicin resistance in osteosarcoma cells. J Bone Oncol. 2023;43:100512.
Cheng F-H, Zhao Z-S, Liu W-D. Long non-coding RNA ROR regulated ABCB1 to induce cisplatin resistance in osteosarcoma by sponging miR-153-3p. Eur Rev Med Pharmacol Sci. 2019;23:7256–65.
Wen J-F, Jiang Y-Q, Li C, Dai X-K, Wu T, Yin W-Z. LncRNA-SARCC sensitizes osteosarcoma to cisplatin through the miR-143-mediated glycolysis inhibition by targeting Hexokinase 2. Cancer Biomark. 2020;28:231–46.
Zhou B, Li L, Li Y, Sun H, Zeng C. Long noncoding RNA SNHG12 mediates doxorubicin resistance of osteosarcoma via miR-320a/MCL1 axis. Biomed Pharmacother. 2018;106:850–7.
Li L, Zhang Y, Gao Y, Hu Y, Wang R, Wang S, et al. LncSNHG14 promotes nutlin3a resistance by inhibiting ferroptosis via the miR-206 /SLC7A11 axis in osteosarcoma cells. Cancer Gene Ther. 2023;30:704–15.
Zhang J, Rao D, Ma H, Kong D, Xu X, Lu H. LncRNA SNHG15 contributes to doxorubicin resistance of osteosarcoma cells through targeting the miR-381-3p/GFRA1 axis. Open Life Sci. 2020;15:871–83.
Sun Y-F, Wang Y, Li X-D, Wang H. SNHG15, a p53-regulated lncRNA, suppresses cisplatin-induced apoptosis and ROS accumulation through the miR-335-3p/ZNF32 axis. Am J Cancer Res. 2022;12:816–28.
Liu Y, Gu S, Li H, Wang J, Wei C, Liu Q. SNHG16 promotes osteosarcoma progression and enhances cisplatin resistance by sponging miR-16 to upregulate ATG4B expression. Biochem Biophys Res Commun. 2019;518:127–33.
Zhu K, Yuan Y, Wen J, Chen D, Zhu W, Ouyang Z, et al. LncRNA Sox2OT-V7 promotes doxorubicin-induced autophagy and chemoresistance in osteosarcoma via tumor-suppressive miR-142/miR-22. Aging (Albany NY). 2020;12:6644–66.
Fu D, Lu C, Qu X, Li P, Chen K, Shan L, et al. LncRNA TTN-AS1 regulates osteosarcoma cell apoptosis and drug resistance via the miR-134-5p/MBTD1 axis. Aging (Albany NY). 2019;11:8374–85.
Amuti A, Liu D, Maimaiti A, Yu Y, Yasen Y, Ma H, et al. Doxorubicin inhibits osteosarcoma progression by regulating circ_0000006/miR-646/ BDNF axis. J Orthop Surg Res. 2021;16:645.
Xie C, Liang G, Xu Y, Lin E. Circular RNA hsa_circ_0003496 contributes to tumorigenesis and chemoresistance in osteosarcoma through targeting (microRNA) miR-370/Krüppel-Like Factor 12 Axis. Cancer Manag Res. 2020;12:8229–40.
Bai Y, Li Y, Bai J, Zhang Y. Hsa_circ_0004674 promotes osteosarcoma doxorubicin resistance by regulating the miR-342-3p/FBN1 axis. J Orthop Surg Res. 2021;16:510.
Ma X-L, Zhan T-C, Hu J-P, Zhang C-L, Zhu K-P. Doxorubicin-induced novel circRNA_0004674 facilitates osteosarcoma progression and chemoresistance by upregulating MCL1 through miR-142-5p. Cell Death Discov. 2021;7:309.
Gu Y, Wang G, Ran B. Has_circ_0010220 regulates the miR-574-3p/IL-6 axis to increase doxorubicin resistance in osteosarcoma. Hum Exp Toxicol. 2022;41:09603271221131307.
Wei W, Ji L, Duan W, Zhu J. Circular RNA circ_0081001 knockdown enhances methotrexate sensitivity in osteosarcoma cells by regulating miR-494-3p/TGM2 axis. J Orthop Surg Res. 2021;16:50.
Zhang Z, Zhou Q, Luo F, Zhou R, Xu J, Xiao J, et al. Circular RNA circ-CHI3L1.2 modulates cisplatin resistance of osteosarcoma cells via the miR-340–5p/LPAATβ axis. Human Cell. 2021;34:1558–68.
Li S, Liu F, Zheng K, Wang W, Qiu E, Pei Y, et al. CircDOCK1 promotes the tumorigenesis and cisplatin resistance of osteogenic sarcoma via the miR-339-3p/IGF1R axis. Mol Cancer. 2021;20:161.
Zhou W, Liu Y, Wu X. Down-regulation of circITCH promotes osteosarcoma development and resistance to doxorubicin via the miR-524/RASSF6 axis. J Gene Med. 2021;23:e3373.
Feng Z-H, Zheng L, Yao T, Tao S-Y, Wei X-A, Zheng Z-Y, et al. EIF4A3-induced circular RNA PRKAR1B promotes osteosarcoma progression by miR-361-3p-mediated induction of FZD4 expression. Cell Death Dis. 2021;12:1025.
Yuan J, Liu Y, Zhang Q, Ren Z, Li G, Tian R. CircPRDM2 contributes to doxorubicin resistance of osteosarcoma by elevating EZH2 via sponging miR-760. CMAR. 2021;13:4433–45.
Wang B, Yan L, Shi W, Xie H, Chen R, Shao Y, et al. CircRNA PVT1 promotes proliferation and chemoresistance of osteosarcoma cells via the miR-24-3p/KLF8 axis. Int J Clin Oncol. 2022;27:811–22.
Li D, Huang Y, Wang G. Circular RNA circPVT1 Contributes to Doxorubicin (DXR) Resistance of Osteosarcoma Cells by Regulating TRIAP1 via miR-137. Biomed Res Int. 2021;2021:7463867.
Wei W, Ji L, Duan W, Zhu J. CircSAMD4A contributes to cell doxorubicin resistance in osteosarcoma by regulating the miR-218-5p/KLF8 axis. Open Life Sci. 2020;15:848–59.
Tan A-Q, Zheng Y-F. The Roles of SNHG Family in Osteoblast Differentiation. Genes (Basel). 2022;13:2268.
Wang Q, Li Q, Zhou P, Deng D, Xue L, Shao N, et al. Upregulation of the long non-coding RNA SNHG1 predicts poor prognosis, promotes cell proliferation and invasion, and reduces apoptosis in glioma. Biomed Pharmacother. 2017;91:906–11.
Yan Y, Fan Q, Wang L, Zhou Y, Li J, Zhou K. LncRNA Snhg1, a non-degradable sponge for miR-338, promotes expression of proto-oncogene CST3 in primary esophageal cancer cells. Oncotarget. 2017;8:35750–60.
Zhang M, Wang W, Li T, Yu X, Zhu Y, Ding F, et al. Long noncoding RNA SNHG1 predicts a poor prognosis and promotes hepatocellular carcinoma tumorigenesis. Biomed Pharmacother. 2016;80:73–9.
Lan T, Yuan K, Yan X, Xu L, Liao H, Hao X, et al. LncRNA SNHG10 Facilitates Hepatocarcinogenesis and Metastasis by Modulating Its Homolog SCARNA13 via a Positive Feedback Loop. Can Res. 2019;79:3220–34.
Li D-S, Ainiwaer J-L, Sheyhiding I, Zhang Z, Zhang L-W. Identification of key long non-coding RNAs as competing endogenous RNAs for miRNA-mRNA in lung adenocarcinoma. Eur Rev Med Pharmacol Sci. 2016;20:2285–95.
Zhang Y, Guo H, Zhang H. SNHG10/DDX54/PBX3 Feedback Loop Contributes to Gastric Cancer Cell Growth. Dig Dis Sci. 2021;66:1875–84.
Hao L, Wu W, Xu Y, Chen Y, Meng C, Yun J, et al. LncRNA-MALAT1: a key participant in the occurrence and development of cancer. Molecules. 2023;28:2126.
Da M, Zhuang J, Zhou Y, Qi Q, Han S. Role of long noncoding RNA taurine-upregulated gene 1 in cancers. Mol Med. 2021;27:51.
Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, et al. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. 2016;54:1.30.1-1.30.33.
Dong Z, Yang P, Qiu X, Liang S, Guan B, Yang H, et al. KCNQ1OT1 facilitates progression of non-small-cell lung carcinoma via modulating miRNA-27b-3p/HSP90AA1 axis. J Cell Physiol. 2019;234:11304–14.
Bian Y, Gao G, Zhang Q, Qian H, Yu L, Yao N, et al. KCNQ1OT1/miR-217/ZEB1 feedback loop facilitates cell migration and epithelial-mesenchymal transition in colorectal cancer. Cancer Biol Ther. 2019;20:886–96.
Zhang S, Ma H, Zhang D, Xie S, Wang W, Li Q, et al. LncRNA KCNQ1OT1 regulates proliferation and cisplatin resistance in tongue cancer via miR-211-5p mediated Ezrin/Fak/Src signaling. Cell Death Dis. 2018;9:742.
Feng W, Wang C, Liang C, Yang H, Chen D, Yu X, et al. The dysregulated expression of KCNQ1OT1 and its interaction with downstream factors miR-145/CCNE2 in breast cancer cells. Cell Physiol Biochem. 2018;49:432–46.
Sun S-J, Lin Q, Ma J-X, Shi W-W, Yang B, Li F. Long non-coding RNA NEAT1 acts as oncogene in NSCLC by regulating the Wnt signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21:504–10.
Zhang M, Wu W-B, Wang Z-W, Wang X-H. lncRNA NEAT1 is closely related with progression of breast cancer via promoting proliferation and EMT. Eur Rev Med Pharmacol Sci. 2017;21:1020–6.
Yu Y, Hann SS. Novel tumor suppressor lncRNA growth arrest-specific 5 (GAS5) in human cancer. Onco Targets Ther. 2019;12:8421–36.
Liu K, Ren T, Huang Y, Sun K, Bao X, Wang S, et al. Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death Dis. 2017;8:e3015.
Potenza N, Mosca N, Zappavigna S, Castiello F, Panella M, Ferri C, et al. MicroRNA-125a-5p is a downstream effector of sorafenib in its antiproliferative activity toward human hepatocellular carcinoma cells. J Cell Physiol. 2017;232:1907–13.
Greene J, Baird A-M, Brady L, Lim M, Gray SG, McDermott R, et al. Circular RNAs: biogenesis, function and role in human diseases. Front Mol Biosci. 2017;4:38.
He Z, Zhu Q. Circular RNAs: emerging roles and new insights in human cancers. Biomed Pharmacother. 2023;165:115217.
Wang J, Zhang Y, Li Z. Advancements in understanding the role of circular RNA in Osteosarcoma. Mol Biotechnol. 2023 [cited 2023 Oct 2]https://doi.org/10.1007/s12033-023-00838-4
Zhou H, Cui X, Zhu L, Xu Z, Wang Z, Shao J. circPVT1 inhibits the proliferation and aids in prediction of the prognosis of bladder cancer. Pharmgenomics Pers Med. 2024;17:1–11.
Wang S, Li W, Yang L, Yuan J, Wang L, Li N, et al. CircPVT1 facilitates the progression of oral squamous cell carcinoma by regulating miR-143-3p/SLC7A11 axis through MAPK signaling pathway. Funct Integr Genomics. 2022;22:891–903.
Tolomeo D, Traversa D, Venuto S, Ebbesen KK, García Rodríguez JL, Tamma G, et al. circPVT1 and PVT1/AKT3 show a role in cell proliferation, apoptosis, and tumor subtype-definition in small cell lung cancer. Genes Chromosomes Cancer. 2023;62:377–91.
Yu M-C, Ding G-Y, Ma P, Chen Y-D, Zhu X-D, Cai J-B, et al. CircRNA UBAP2 serves as a sponge of miR-1294 to increase tumorigenesis in hepatocellular carcinoma through regulating c-Myc expression. Carcinogenesis. 2021;42:1293–303.
Li X, Azhati B, Wang W, Rexiati M, Xing C, Wang Y. Circular RNA UBAP2 promotes the proliferation of prostate cancer cells via the miR-1244/MAP3K2 axis. Oncol Lett. 2021;21:486.
Chen F, Guo L, Di J, Li M, Dong D, Pei D. Circular RNA ubiquitin-associated protein 2 enhances autophagy and promotes colorectal cancer progression and metastasis via miR-582-5p/FOXO1 signaling. J Genet Genomics. 2021;48:1091–103.
Lv Z, Shi Y, Wu H, Cao K, Liu X, Wang C. Novel circular RNA CircUBAP2 drives tumor progression by regulating the miR-143/TFAP2B Axis in prostate cancer. Protein Pept Lett. 2024;31:61–73.
Thompson BJ. YAP/TAZ: drivers of tumor growth, metastasis, and resistance to therapy. BioEssays. 2020;42:1900162.
Chi Y, Luo Q, Song Y, Yang F, Wang Y, Jin M, et al. Circular RNA circPIP5K1A promotes non-small cell lung cancer proliferation and metastasis through miR-600/HIF-1α regulation. J Cell Biochem. 2019;120:19019–30.
Zheng K, Xie H, Wu W, Wen X, Zeng Z, Shi Y. CircRNA PIP5K1A promotes the progression of glioma through upregulation of the TCF12/PI3K/AKT pathway by sponging miR-515-5p. Cancer Cell Int. 2021;21:27.
Song H, Xu Y, Xu T, Fan R, Jiang T, Cao M, et al. CircPIP5K1A activates KRT80 and PI3K/AKT pathway to promote gastric cancer development through sponging miR-671-5p. Biomed Pharmacother. 2020;126:109941.
Feng Z, Ou Y, Hao L. The roles of glycolysis in osteosarcoma. Front Pharmacol. 2022;13:950886.
Ji Z, Shen J, Lan Y, Yi Q, Liu H. Targeting signaling pathways in osteosarcoma: Mechanisms and clinical studies. MedComm. 2020;2023(4):e308.
Zhang X, Wang S, Wang H, Cao J, Huang X, Chen Z, et al. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol Cancer. 2019;18:20.
Panez-Toro I, Muñoz-García J, Vargas-Franco JW, Renodon-Cornière A, Heymann M-F, Lézot F, et al. Advances in osteosarcoma. Curr Osteoporos Rep. 2023;21:330–43.
Twenhafel L, Moreno D, Punt T, Kinney M, Ryznar R. Epigenetic changes associated with osteosarcoma: a comprehensive review. Cells. 2023;12:1595.
Yang H-W, Liu G-H, Liu Y-Q, Zhao H-C, Yang Z, Zhao C-L, et al. Over-expression of microRNA-940 promotes cell proliferation by targeting GSK3β and sFRP1 in human pancreatic carcinoma. Biomed Pharmacother. 2016;83:593–601.
Yang L, Wang J, Fan Y, Yu K, Jiao B, Su X. Hsa_circ_0046264 up-regulated BRCA2 to suppress lung cancer through targeting hsa-miR-1245. Respir Res. 2018;19:115.
Li D, Hao X, Dong Y, Zhang M, Song Y. PF4V1, an miRNA-875-3p target, suppresses cell proliferation, migration, and invasion in prostate cancer and serves as a potential prognostic biomarker. Cancer Manag Res. 2019;11:2299–312.
Zhang B, Zhang Y, Li R, Li J, Lu X, Zhang Y. The efficacy and safety comparison of first-line chemotherapeutic agents (high-dose methotrexate, doxorubicin, cisplatin, and ifosfamide) for osteosarcoma: a network meta-analysis. J Orthop Surg Res. 2020;15:51.
Benner B, Scarberry L, Suarez-Kelly LP, Duggan MC, Campbell AR, Smith E, et al. Generation of monocyte-derived tumor-associated macrophages using tumor-conditioned media provides a novel method to study tumor-associated macrophages in vitro. J Immunother Cancer. 2019;7:140.
Cronstein BN. The mechanism of action of methotrexate. Rheum Dis Clin North Am. 1997;23:739–55.
Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, et al. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21:440–6.
Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78.
Dumars C, Ngyuen J-M, Gaultier A, Lanel R, Corradini N, Gouin F, et al. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget. 2016;7:78343–54.
Guo F, Cao Z, Guo H, Li S. The action mechanism of lncRNA-HOTAIR on the drug resistance of non-small cell lung cancer by regulating Wnt signaling pathway. Exp Ther Med. 2018;15:4885–9.
Cao W, Sun Y, Liu L, Yu J, Ji J, Wang Y, et al. HOTAIR mediates cisplatin resistance in nasopharyngeal carcinoma by regulating miR-106a-5p/SOX4 axis. Bioengineered. 2022;13:6567–78.
Huang J, Pan B, Xia G, Zhu J, Li C, Feng J. LncRNA SNHG15 regulates EGFR-TKI acquired resistance in lung adenocarcinoma through sponging miR-451 to upregulate MDR-1. Cell Death Dis. 2020;11:525.
Mi H, Wang X, Wang F, Li L, Zhu M, Wang N, et al. SNHG15 Contributes To Cisplatin Resistance In Breast Cancer Through Sponging miR-381. Onco Targets Ther. 2020;13:657–66.
Lei Y, Junxin C, Yongcan H, Xiaoguang L, Binsheng Y. Role of microRNAs in the crosstalk between osteosarcoma cells and the tumour microenvironment. J Bone Oncol. 2020;25:100322.
Corre I, Verrecchia F, Crenn V, Redini F, Trichet V. The osteosarcoma microenvironment: a complex but targetable ecosystem. Cells. 2020;9:976.
Yang C, Tian Y, Zhao F, Chen Z, Su P, Li Y, et al. Bone microenvironment and osteosarcoma metastasis. Int J Mol Sci. 2020;21:6985.
Neviani P, Fabbri M. Exosomic microRNAs in the tumor microenvironment. Front Med (Lausanne). 2015;2:47.
Fanini F, Fabbri M. Cancer-derived exosomic microRNAs shape the immune system within the tumor microenvironment: State of the art. Semin Cell Dev Biol. 2017;67:23–8.
Yao P, Lu Y, Cai Z, Yu T, Kang Y, Zhang Y, et al. Research progress of exosome-loaded mirna in osteosarcoma. Cancer Control. 2022;29:10732748221076684.
Murillo OD, Thistlethwaite W, Rozowsky J, Subramanian SL, Lucero R, Shah N, et al. exRNA atlas analysis reveals distinct extracellular RNA cargo types and their carriers present across human biofluids. Cell. 2019;177:463-477.e15.
Sun Z, Yang S, Zhou Q, Wang G, Song J, Li Z, et al. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol Cancer. 2018;17:82.
Zogg H, Singh R, Ro S. Current advances in RNA therapeutics for human diseases. Int J Mol Sci. 2022;23:2736.
Winkle M, El-Daly SM, Fabbri M, Calin GA. Noncoding RNA therapeutics - challenges and potential solutions. Nat Rev Drug Discov. 2021;20:629–51.
Zhu Y, Zhu L, Wang X, Jin H. RNA-based therapeutics: an overview and prospectus. Cell Death Dis. 2022;13:644.
Beg MS, Brenner AJ, Sachdev J, Borad M, Kang Y-K, Stoudemire J, et al. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs. 2017;35:180–8.
Alegre F, Ormonde AR, Snider KM, Woolard K, Yu A-M, Wittenburg LA. A genetically engineered microRNA-34a prodrug demonstrates anti-tumor activity in a canine model of osteosarcoma. PLoS ONE. 2018;13:e0209941.
Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S, et al. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J Clin Invest. 2015;125:141–56.
Shi Y, Parag S, Patel R, Lui A, Murr M, Cai J, et al. Stabilization of lncRNA GAS5 by a small molecule and its implications in diabetic adipocytes. Cell Chem Biol. 2019;26:319-330.e6.
Sempere LF, Azmi AS, Moore A. microRNA-based diagnostic and therapeutic applications in cancer medicine. Wiley Interdiscip Rev RNA. 2021;12:e1662.
Bonneau E, Neveu B, Kostantin E, Tsongalis GJ, De Guire V. How close are miRNAs from clinical practice? A perspective on the diagnostic and therapeutic market. EJIFCC. 2019;30:114–27.
Ho PTB, Clark IM, Le LTT. MicroRNA-Based Diagnosis and Therapy. Int J Mol Sci. 2022;23:7167.
Acknowledgements
Not applicable.
Funding
The authors are supported for osteosarcoma studies by a PNRR grant “National Centre for Gene Therapy and Drugs based on RNA Technology”, CUP B63C22000600001, MUR CN00000041.
Author information
Authors and Affiliations
Contributions
NP and NM conceptualized the manuscript, performed the literature search and data analysis. All authors drafted and critically revised the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mosca, N., Alessio, N., Di Paola, A. et al. Osteosarcoma in a ceRNET perspective. J Biomed Sci 31, 59 (2024). https://doi.org/10.1186/s12929-024-01049-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12929-024-01049-y