Abstract
Background
Escherichia coli is known to cause about 2 million deaths annually of which diarrhea infection is leading and typically occurs in children under 5 years old. Although Africa is the most affected region there is little information on their pathotypes diversity and their antimicrobial resistance.
Objective
To determine the pathotype diversity and antimicrobial resistance among E. coli from patients attending regional referral hospitals in Tanzania.
Materials and methods
A retrospective cross-section laboratory-based study where a total of 138 archived E. coli isolates collected from 2020 to 2021 from selected regional referral hospitals in Tanzania were sequenced using the Illumina Nextseq550 sequencer platform. Analysis of the sequences was done in the CGE tool for the identification of resistance genes and virulence genes. SPSS version 20 was used to summarize data using frequency and proportion.
Results
Among all 138 sequenced E. coli isolates, the most prevalent observed pathotype virulence genes were of extraintestinal E. coli UPEC fyuA gene 82.6% (114/138) and NMEC irp gene 81.9% (113/138). Most of the E. coli pathotypes observed exist as a hybrid due to gene overlapping, the most prevalent pathotypes observed were NMEC/UPEC hybrid 29.7% (41/138), NMEC/UPEC/EAEC hybrid 26.1% (36/138), NMEC/UPEC/DAEC hybrid 18.1% (25/138) and EAEC 15.2% (21/138). Overall most E. coli carried resistance gene to ampicillin 90.6% (125/138), trimethoprim 85.5% (118/138), tetracycline 79.9% (110/138), ciprofloxacin 76.1% (105/138) and 72.5% (100/138) Nalidixic acid. Hybrid pathotypes were more resistant than non-hybrid pathotypes.
Conclusion
Whole genome sequencing reveals the presence of hybrid pathotypes with increased drug resistance among E. coli isolated from regional referral hospitals in Tanzania.
Similar content being viewed by others
Introduction
Escherichia coli is a gram-negative rod-shaped bacterium from the family Enterobacteriaceae known to cause a variety of diseases ranging from diarrheagenic to extraintestinal infections due to their pathogenic effects and productions of various toxins and other virulence factor [1]. It is known to cause about 2 million deaths annually as a result of diarrhea infection typically occurring in children under 5 years old and mostly affecting tropical and sub-tropical regions [2]. In Dar es Salaam Tanzania, a previous study reported that about 51.1% of UTI cases are caused by E. coli [3]. Similarly, in the northern part of Tanzania E. coli account for 46.2% of all UTI cases among children [4]. Another study conducted in HIV-infected people in the northern part of Tanzania reported the prevalence of bacteriuria in HIV patients 12.3% whereby 16.2% of the causative agent is E. coli [5]. It has also been demonstrated that E. coli causes about 11.7% of the human bacterial infection [6].
World Health Organization reorganizes antimicrobial resistance as among the top 10 global public health threats facing humanity today. Resistance bacterial infections are associated with 4.95 million deaths per year [7]. Antimicrobial resistance increases healthcare costs due to more expensive therapy, prolonged hospitalization, and high morbidity rates [8]. It is estimated that by 2050, drug resistance will cause more deaths than all cancers combined [9].
Ability of the pathogenic E. coli to cause a variety of diseases from intestinal to extra-intestinal infection is a result of a unique acquired virulence factor that differentiates E. coli into pathotypes based on the specific disease and the site of infections [10,11,12]. Intestinal E. coli pathotypes include; Enterotoxigenic E. coli (ETEC), Enteropathogenic E. coli (EPEC), Shiga toxin-producing E. coli (STEC), Enteroinvasive E. coli (EIEC), Enteroaggregative E. coli (EAEC) and diffusely adherent E. coli (DAEC) [11] while Extraintestinal E. coli pathotypes are; Neonatal meningitis E. coli (NMEC), Uropathogenic E. coli (UPEC) and Septicemic E. coli [1, 13].
Horizontal gene transfer of the resistance and virulence genes among different E. coli increases antimicrobial resistance and creates diversity. In addition, hybrid pathotypes may result from gene overlapping processes [14,15,16]. Hybrid pathotypes cause more severe disease and complications. Evidently, a single E. coli has been reported to cause both diarrhea and hemolytic uremic syndrome [17]. Another report suggested that 93.5% (101/108) of the E. coli isolates that were originally known as intestinal E. coli carry genes also for extraintestinal E. coli [18].
Despite of the significance of next-generation sequencing in analyzing pathogenic bacteria, their utilization is rare in low- and middle-income countries like Tanzania, leading to a scarcity of information regarding diversity among E. coli pathotypes. Whole genome sequencing (WGS) of the particular pathogen provides precise information on the species identity, resistance gene, plasmid, virulence gene, multi-locus sequence typing (MLST), or serotyping compared to other methods [19, 20]. Understanding diversity among the pathotypes is important for determining the genetic relatedness and variability among the strains while assessing drug resistance patterns is essential for guiding appropriate treatment strategies. This study aims to determine the level of antimicrobial resistance and pathotype diversity among E. coli isolated from regional referral hospitals in Tanzania.
Materials and methods
Study design, study participants, and study sites
This was a retrospective cross-section laboratory-based study using achieved E. coli culture sample isolated from urine, wound, pus, blood, sputum, and stool collected from January 2020 to December 2021 at Regional Referral Hospital of Tanzania. This study was nested from SeqAfrica project with the main objective of developing, expanding, and supporting whole genome sequencing and bioinformatics capacity for antimicrobial resistance surveillance across Africa. The project is functioning in four countries across Africa; Ghana, Nigeria, South Africa, and Tanzania. In Tanzania six study areas were selected; Tabora, Dodoma, Songea, Kigoma, Morogoro, and Zanzibar.
DNA extraction and whole genomic sequencing
DNA from E. coli strains was extracted using Quick-DNA™ Fungal/Bacteria Miniprep Kit as per manufacturer instructions. The purity and the quantity of the extracted DNA were checked by using a Qubit® version 4.0 fluorometer. Library preparation was performed based on the NEBNext® Ultra™ II FS DNA Library Prep Kit manual 2020. Briefly, library preparation involves fragmentation, adaptor ligation, size selection, and indexing or barcoding of each extracted DNA from different E. coli. The prepared library was normalized and combined with Phix control before loading in the Illumina Nextseq550 sequencer platform for sequencing.
Bioinformatics analysis
Quality control of the sequenced raw data was performed using FastQC 0.12.0 [21]. De novo assembly was performed using SPAdes 3.15.5 [22] and the final output files were in Fasta format. Bacterial Analysis Pipeline (BAP 3.3.2) which is based on the services available at the Center for Genomic Epidemiology (CGE) (https://www.genomicepidemiology.org/services/) was used. Species identification was determined using Kmerfinder 3.2 [23,24,25], the Resistance gene was determined using Resfinder 4.1 [26,27,28] and the virulence gene was determined using VirulenceFinder 2.0 [26, 29, 30]. The virulence genes that were used to define E. coli pathotypes are presented in supplementary Table 1. The assembled E. coli genome from this study has been submitted to the European Nucleotide Archive with project accession number PRJEB71714. SPSS version 20 was used to summarize data using frequency and proportion.
Results
Distribution of E. Coli across the hospitals
A total of 138 non-duplicate E. coli isolates were retrieved of which 60 (43.5%) were from urine, 45 (32.6%) from pus wound swabs, 15 (10.9%) from high vaginal swabs, and 13 (9.4%) from blood. 48 (34.8%) of the E. coli isolates were collected from Dodoma regional referral hospitals, 36 (26.1%) from Morogoro regional referral hospitals, and only 5 (3.6%) from Kigoma regional referral hospitals. The majority 136 (98.6%) of the isolates were cultured in 2021 and half of the participants (50.0%) were aged 18–34 years old (Table 1).
Antimicrobial resistance genes and predicted phenotypic resistance profiles
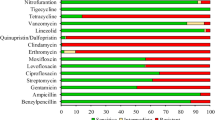
Among all 138 sequenced E. coli, 125 (90.6%) were harboring at least one of blaOXA-1, blaTEM-1B, and blaCTX-M-15 gene which can confer resistance to resistant to ampicillin, 118 (85.5%) were harboring dfrA1, dfrA5, dfrA7, dfrA8, dfrA12, dfrA14, and dfrA1 gene which can confer resistance to resistant to trimethoprim, 110 (79.7%) were harboring at least one of tet(A), tet(B) and tet(D) gene which can confer resistance to tetracycline, 105 (76.1%) were harboring at least one of aac(6’)-Ib-cr gene, qnrS1 gene, gyrA mutation,, parC mutation and parE mutation which can confer resistance to ciprofloxacin and 100 (71.9%) were harboring at least one of qnrS1 gene, gyrA mutation, parC mutation and parE mutation which can confer resistance to Nalidixic acid. (Table 2).
E. Coli pathotypes virulence genes
Among all sequenced E. coli isolate, 114 (82.6%) were harboring the fyuA virulence gene for UPEC pathotype, 113 (81.9%) were harboring irp virulence gene for NMEC pathotype, 95 (68.8%) were harboring chuA virulence gene for UPEC, 80 (58.0%) were harboring yfcV virulence gene for UPEC, and 71 (51.4) were harboring hlyE virulence gene for EAEC (Table 3).
Predicted E. Coli pathotypes using virulence gene
Most of the E. coli pathotypes observed from sequenced isolates exist as a hybrid due to gene overlapping, the most prevalent pathotypes observed were 41 (29.7%) NMEC/UPEC hybrid, 36 (26.1%) NMEC/UPEC/EAEC hybrid, 25 (18.1%) NMEC/UPEC/DAEC hybrid, and 21 (15.2%) EAEC. The least observed pathotypes were 1 (0.7%) UPEC, 1 (0.7%) EPEC/EAEC hybrid, 1 (0.7%) UPEC/EAEC/DAEC hybrid, and 1 (0.7%) NMEC/UPEC/EAEC/EIEC hybrid (Table 4).
Association between E. Coli pathotypes and their antimicrobial resistance profiles
Among all pathotypes, high resistance was observed for the antibiotic ampicillin and trimethoprim in which the hybrid pathotypes were more resistant than non-hybrid pathotypes. All pathotypes were sensitive to the antibiotic meropenem with a slight resistance of 2.4% and 2.8% among the NMEC/UPEC hybrid and NMEC/UPEC/EAEC hybrid respectively (Table 5).
Discussion
The present study aimed to determine the diversity among the E. coli pathotypes and their associated drug resistance in regional referral hospitals in Tanzania by the use of whole genome sequencing technology. High diversity in the E. coli pathotypes was observed in which extra-intestinal E. coli virulence genes for UPEC and NMEC pathotypes were most prevalent while the least virulence genes were of diarrheagenic E. coli pathotypes (Table 3.). These specific virulence genes together with other factors such as tissue tropisms, pathogenesis, and clinical features as explained by [1, 31, 32] are what divide E. coli into either intestinal or extraintestinal pathotypes. But also, the highly prevalent UPEC reported in this study might be due to more urine samples that were used in this study. Horizontal gene transfer of the specific pathotyped virulence factor can cause diversity and formation of hybrid pathotypes [15, 16].
As a result of virulence gene overlapping reported in this study, seven different types of hybrid pathotypes were observed (Table 4). The only pathotypes that were not hybrid were EAEC and UPEC. The combination of virulent factors to form hybrid or hetero-hybrid pathotypes results in more severity of the disease by creating more complications [33]. A similar case was also reported by [17] where a single E. coli strain causes both diarrhea and hemolytic uremic syndrome which creates more complications and questions on the best therapy to use. Another study from Norway reported that 93.5% (101/108) of the E. coli isolates that were originally known as intestinal E. coli carry genes also for extra-intestinal E. coli [18].
Following analysis for resistance and prediction of phenotypic resistance among all pathotypes, hybrid pathotypes were more resistant than non-hybrid in which high resistance was observed for the antibiotic ampicillin, trimethoprim ciprofloxacin, tetracycline, and nalidixic acid. All pathotypes were sensitive to the antibiotic meropenem with a slight resistance of 2.4% and 2.8% among the NMEC/UPEC hybrid and NMEC/UPEC/EAEC hybrid respectively (Table 5). Overall, most E. coli isolates carried resistance genes for ampicillin (90.6%) (blaOXA-1, blaTEM-1B and blaCTX-M-15), trimethoprim (85.5%) (dfrA1, dfrA5, dfrA7, dfrA8, dfrA12, dfrA14 and dfrA17), tetracycline (79.7%) (tet(A), tet(B) and tet(D)) and ciprofloxacin (76.1%) (aac(6’)-Ib-cr, gyrA mutation, qnrS1, parC mutation and parE mutation) and (71.9%) Nalidixic acid (qnrS1, gyrA mutation, parC mutation and parE mutation). A longitudinal assessment of antibiotic resistance in fecal E. coli in Tanzanian children also reports high resistance to ampicillin but low resistance to ciprofloxacin [34]. Ciprofloxacin was reported as among the most used antibiotics by 9.27 (6.0%) Daily Doses per 1000 inhabitants per day from 2010 to 2016 [35] which could be the factor for the observed increase in the resistance.
Resistance gene mcr-1 and blaCTX-M-15-like were also reported in Zanzibar among the sequenced E. coli with a prevalence of 55% and 51.0% respectively. These resistance genes code for colistin and extended-spectrum cephalosporines resistance genes respectively [36, 37]. Another study conducted in Tanzania also reported that 63% (77/123) of gram-negative bacteria carry resistance gene dfrA of which dfrA1 was frequently in E. coli isolates [38].
Despite the observed high resistance gene among the sequenced E. coli, Meropenem, Amoxicillin-clavunated and Gentamicin antibiotics were less occupied with the resistance gene with the resistance prevalence of 1.4%, 29.7%, and 37.7% respectively. This brings hope for an alternative drug of choice for treatment of the E. coli infection despite the reduced treatment options for E. coli infections.
Furthermore, the resistance gene observed in the present study was observed to cause resistance in more than one antibiotic of either one or different class, this was also reported in other studies such that; the aac(6’)-Ib-c gene was reported to cause antibiotic resistance to aminoglycoside antibiotic amikacin and quinolone antibiotic ciprofloxacin [39, 40]; gyrA gene mutation can also confer resistance to nalidixic acid and ciprofloxacin antibiotics of which they both belong to quinolone antibiotic group [41, 42]. The clinical implications for the observed results are that; treatment options and management for the E. coli infection should be reviewed mostly in low- and middle-income countries. Additionally, observed findings suggest the proper use of antibiotics to reduce the development of drug resistance and disease severity.
Conclusion
There is a reduced treatment option for E. coli infections due to increased drug resistance, the only drug with less observed resistance in our setting was meropenem 1.4% a third generation β-lactam antibiotic of the carbapenem class. There is an increase in E. coli pathotype diversity, severity, and complications of the disease due to the large number of observed pathotype hybrid.
Data availability
Assembled E. coli genome from this study have been submitted to European Nucleotide Archive with project accession number PRJEB71714.
References
Robbens J, Devriese L, Verstraete K, Heyndrickx M. Escherichia coli. Encycl Toxicol. Third Edit. Elsevier; 2014. pp. 459–61.
Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet [Internet]. 2013;382:209–22. https://doi.org/10.1016/S0140-6736(13)60844-2.
Moyo SJ, Aboud S, Kasubi M, Lyamuya EF, Maselle SY. Antimicrobial resistance among producers and non-producers of extended spectrum beta-lactamases in urinary isolates at a tertiary Hospital in Tanzania. BMC Res Notes [Internet]. 2010;3:348. https://bmcresnotes.biomedcentral.com/articles/https://doi.org/10.1186/1756-0500-3-348.
Gidabayda J, Philemon R, Abdallah M, Saajan A, Temu T, Kunjumu I et al. Prevalence, Aetiology, and Antimicrobial Susceptibility Patterns of Urinary Tract Infection Amongst Children Admitted at Kilimanjaro Christian Medical Centre, Moshi, Tanzania. East African Heal Res J. 2017;1:53–61. http://www.eac.int/sites/default/files/docs/eahrj_i1v1_0.pdf.
Ngowi BN, Sunguya B, Herman A, Chacha A, Maro E, Rugarabamu LF et al. Prevalence of Multidrug Resistant UTI Among People Living with HIV in Northern Tanzania. Infect Drug Resist [Internet]. 2021;Volume 14:1623–33. https://www.dovepress.com/prevalence-of-multidrug-resistant-uti-among-people-living-with-hiv-in--peer-reviewed-fulltext-article-IDR.
Kumburu HH, Sonda T, Mmbaga BT, Alifrangis M, Lund O, Kibiki G et al. Patterns of infections, aetiological agents and antimicrobial resistance at a tertiary care hospital in northern Tanzania. Trop Med Int Heal [Internet]. 2017;22:454–64. https://onlinelibrary.wiley.com/doi/https://doi.org/10.1111/tmi.12836.
WHO. Antimicrobial resistance [Internet]. World Heal. Organ. 2023. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
Dadgostar P. Antimicrobial Resistance: Implications and Costs. Infect Drug Resist [Internet]. 2019;Volume 12:3903–10. https://www.dovepress.com/antimicrobial-resistance-implications-and-costs-peer-reviewed-article-IDR.
Avershina E, Shapovalova V, Shipulin G. Fighting Antibiotic Resistance in Hospital-Acquired Infections: Current State and Emerging Technologies in Disease Prevention, Diagnostics and Therapy. Front Microbiol [Internet]. 2021;12. https://www.frontiersin.org/articles/https://doi.org/10.3389/fmicb.2021.707330/full.
Leimbach A, Hacker J, Dobrindt U. E. coli as an All-Rounder: The Thin Line Between Commensalism and Pathogenicity. Assess Eval High Educ [Internet]. 2013. pp. 3–32. http://link.springer.com/10.1007/82_2012_303.
Jesser KJ, Levy K. Updates on defining and detecting diarrheagenic Escherichia coli pathotypes. Curr Opin Infect Dis [Internet]. 2020;33:372–80. https://journals.lww.com/https://doi.org/10.1097/QCO.0000000000000665.
da Silva GJ, Mendonça N. Association between antimicrobial resistance and virulence in Escherichia coli. Virulence [Internet]. 2012;3:18–28. http://www.tandfonline.com/doi/abs/10.4161/viru.3.1.18382.
Vila J, Sáez-López E, Johnson JR, Römling U, Dobrindt U, Cantón R, et al. Escherichia coli: an old friend with new tidings. FEMS Microbiol Rev. 2016;40:437–63.
Lerminiaux NA, Cameron ADS. Horizontal transfer of antibiotic resistance genes in clinical environments. Can J Microbiol [Internet]. 2019;65:34–44. http://www.nrcresearchpress.com/doi/https://doi.org/10.1139/cjm-2018-0275.
Chib S, Ali F, Seshasayee ASN. Genomewide Mutational Diversity in Escherichia coli Population Evolving in Prolonged Stationary Phase. Bowman GR, editor. mSphere [Internet]. 2017;2:1–15. https://doi.org/10.1128/mSphere.00059-17.
Javadi M, Bouzari S, Oloomi M. Horizontal Gene Transfer and the Diversity of Escherichia coli. Escherichia coli - Recent Adv Physiol Pathog Biotechnol Appl [Internet]. InTech; 2017. http://www.intechopen.com/books/-i-escherichia-coli-i-recent-advances-on-physiology-pathogenesis-and-biotechnological-applications/horizontal-gene-transfer-and-the-diversity-of-i-escherichia-coli-i.
Mariani-Kurkdjian P, Lemaître C, Bidet P, Perez D, Boggini L, Kwon T et al. Haemolytic-uraemic syndrome with bacteraemia caused by a new hybrid Escherichia coli pathotype. New Microbes New Infect [Internet]. 2014;2:127–31. https://linkinghub.elsevier.com/retrieve/pii/S2052297514500212.
Lindstedt BA, Finton MD, Porcellato D, Brandal LT. High frequency of hybrid Escherichia coli strains with combined intestinal pathogenic Escherichia coli (IPEC) and Extraintestinal Pathogenic Escherichia coli (ExPEC) virulence factors isolated from human faecal samples. BMC Infect Dis. 2018;18:1–12.
Shelenkov A. Whole-Genome Sequencing of Pathogenic Bacteria—New Insights into Antibiotic Resistance Spreading. Microorganisms [Internet]. 2021;9:2624. https://www.mdpi.com/2076-2607/9/12/2624.
Iskandar K, Molinier L, Hallit S, Sartelli M, Hardcastle TC, Haque M et al. Surveillance of antimicrobial resistance in low- and middle-income countries: a scattered picture. Antimicrob Resist Infect Control [Internet]. 2021;10:63. https://aricjournal.biomedcentral.com/articles/https://doi.org/10.1186/s13756-021-00931-w.
Simon Andrews. Babraham Bioinformatics - FastQC A Quality Control tool for High Throughput Sequence Data [Internet]. Soil. 2020. pp. 47–81. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J Comput Biol. 2012;19:455–77. http://www.liebertpub.com/doi/https://doi.org/10.1089/cmb.2012.0021.
Hasman H, Saputra D, Sicheritz-Ponten T, Lund O, Svendsen CA, Frimodt-Moller N, et al. Rapid whole-genome sequencing for detection and characterization of microorganisms directly from clinical samples. J Clin Microbiol. 2014;52:139–46.
Larsen MV, Cosentino S, Lukjancenko O, Saputra D, Rasmussen S, Hasman H, et al. Benchmarking of methods for genomic taxonomy. J Clin Microbiol. 2014;52:1529–39.
Clausen PTLC, Aarestrup FM, Lund O. Rapid and precise alignment of raw reads against redundant databases with KMA. BMC Bioinformatics. 2018;19:1–8.
Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, et al. BLAST+: Architecture and applications. BMC Bioinformatics. 2009;10:1–9.
Zankari E, Allesøe R, Joensen KG, Cavaco LM, Lund O, Aarestrup FM. PointFinder: a novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J Antimicrob Chemother. 2017;72:2764–8.
Bortolaia V, Kaas RS, Ruppe E, Roberts MC, Schwarz S, Cattoir V, et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob Chemother. 2020;75:3491–500.
Malberg Tetzschner AM, Johnson JR, Johnston BD, Lund O, Scheutz F. In Silico Genotyping of Escherichia coli Isolates for Extraintestinal Virulence Genes by Use of Whole-Genome Sequencing Data. Dekker JP, editor. J Clin Microbiol [Internet]. 2020;58:1–13. https://doi.org/10.1128/JCM.01269-20.
Joensen KG, Scheutz F, Lund O, Hasman H, Kaas RS, Nielsen EM, et al. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J Clin Microbiol. 2014;52:1501–10.
Pokharel P, Dhakal S, Dozois CM. The Diversity of Escherichia coli Pathotypes and Vaccination Strategies against This Versatile Bacterial Pathogen. Microorganisms [Internet]. 2023;11:344. https://www.mdpi.com/2076-2607/11/2/344.
Cabal A, García-Castillo M, Cantón R, Gortázar C, Domínguez L, Álvarez J. Prevalence of Escherichia coli virulence genes in patients with diarrhea and a subpopulation of healthy volunteers in Madrid, Spain. Front Microbiol. 2016;7:1–6.
Santos AC, de Santos M, Silva FF, Gomes RM. TAT. Diversity of Hybrid- and Hetero-Pathogenic Escherichia coli and Their Potential Implication in More Severe Diseases. Front Cell Infect Microbiol [Internet]. 2020;10:1–11. https://www.frontiersin.org/article/https://doi.org/10.3389/fcimb.2020.00339/full.
Fleece ME, Nshama R, Walongo T, Kimathi C, Gratz J, Rogawski McQuade ET et al. Longitudinal Assessment of Antibiotic Resistance in Fecal Escherichia coli in Tanzanian Children. Am J Trop Med Hyg [Internet]. 2019;100:1110–4. https://doi.org/10.4269/ajtmh.18-0789.
Sangeda RZ, Saburi HA, Masatu FC, Aiko BG, Mboya EA, Mkumbwa S et al. National Antibiotics Utilization Trends for Human Use in Tanzania from 2010 to 2016 Inferred from Tanzania Medicines and Medical Devices Authority Importation Data. Antibiotics [Internet]. 2021;10:1249. https://www.mdpi.com/2079-6382/10/10/1249.
Büdel T, Kuenzli E, Clément M, Bernasconi OJ, Fehr J, Mohammed AH et al. Polyclonal gut colonization with extended-spectrum cephalosporin- and/or colistin-resistant Enterobacteriaceae: a normal status for hotel employees on the island of Zanzibar, Tanzania. J Antimicrob Chemother. 2019;74:2880–90. https://academic.oup.com/jac/article/74/10/2880/5540741.
Moser AI, Kuenzli E, Büdel T, Campos-Madueno EI, Bernasconi OJ, DeCrom-Beer S et al. Travellers returning from the island of Zanzibar colonized with MDR Escherichia coli strains: assessing the impact of local people and other sources. J Antimicrob Chemother [Internet]. 2021;76:330–7. https://academic.oup.com/jac/article/76/2/330/6013159.
Manyahi J, Tellevik MG, Ndugulile F, Moyo SJ, Langeland N, Blomberg B. Molecular Characterization of Cotrimoxazole Resistance Genes and Their Associated Integrons in Clinical Isolates of Gram-Negative Bacteria from Tanzania. Microb Drug Resist [Internet]. 2017;23:37–43. http://www.liebertpub.com/doi/https://doi.org/10.1089/mdr.2016.0074.
Machuca J, Ortiz M, Recacha E, Díaz-De-Alba P, Docobo-Perez F, Rodríguez-Martínez JM, et al. Impact of AAC(6’)-Ib-cr in combination with chromosomal-mediated mechanisms on clinical quinolone resistance in Escherichia coli. J Antimicrob Chemother. 2016;71:3066–71.
Reeves CM, Magallon J, Rocha K, Tran T, Phan K, Vu P et al. Aminoglycoside 6’-N-acetyltransferase type Ib [AAC(6’)-Ib]-mediated aminoglycoside resistance: Phenotypic conversion to susceptibility by silver ions. Antibiotics [Internet]. 2021;10:1–9. https://www.mdpi.com/2079-6382/10/1/29.
Bhatnagar K, Wong A. The mutational landscape of quinolone resistance in Escherichia coli. Karunasagar I, editor. PLoS One [Internet]. 2019;14:e0224650. https://doi.org/10.1371/journal.pone.0224650.
Vinué L, Corcoran MA, Hooper DC, Jacoby GA. Mutations That Enhance the Ciprofloxacin Resistance of Escherichia coli with qnrA1. Antimicrob Agents Chemother [Internet]. 2016;60:1537–45. https://doi.org/10.1128/AAC.02167-15.
Acknowledgements
We thank the management of all regional referral hospitals of Tanzania who participate in this study.
Funding
Whole-genome sequencing of the bacterial isolates was funded by the SeqAfrica, project number FF25-286. The SeqAfrica project is funded by the Department of Health and Social Care’s Fleming Fund using UK aid. The views expressed in this publication are those of the authors and not necessarily those of the UK Department of Health and Social Care or its Management Agent, Mott MacDonald.
Author information
Authors and Affiliations
Contributions
LK, SM, TS and HK develop an idea and present to all others. All authors prepare and read the final proposal. LK MJ and BW conduct DNA sequencing together with DK, MS, PK, MB, MZ and BM for data analysis and interpretation. LK was the major contributor in writing the manuscript together with discussion from all authors. All authors read and approve the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was granted ethical approval by the National Institute for Medical Research (NIMR) and Kilimanjaro Christian Medical University College (KCMUCo) Research Ethics Committee with approval number NIMR/HQ/R.8a/Vol.IX/3273 and PG-180/2023 respectively. Informed consent was obtained from all subjects or their legal guardian before using the sample for research purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kanje, L.E., Kumburu, H., Kuchaka, D. et al. Short reads-based characterization of pathotype diversity and drug resistance among Escherichia coli isolated from patients attending regional referral hospitals in Tanzania. BMC Med Genomics 17, 110 (2024). https://doi.org/10.1186/s12920-024-01882-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-024-01882-y