Abstract
Background
Mutations in MPZL2, the characteristic genetic etiology of autosomal recessive deafness loci 111 (DFNB111), cause non-syndromic and moderate sensorineural hearing loss.
Methods
In this study, we analyzed the phenotype and genotype of eight pedigrees consisting of 10 hearing loss patients with bi-allelic pathogenic or likely pathogenic variants in MPZL2. These patients were identified from a 3272 Chinese patient cohort who underwent genetic testing.
Results
Apart from symmetrical and moderate sensorineural hearing loss, the MPZL2-related phenotype was characterized by progressive hearing loss with variation in the onset age (congenital defect to onset at the young adult stage). We determined that in the Chinese population, the genetic load of MPZL2 defects was 0.24% (8/3272) in patients diagnosed with hearing loss and 7.02% (8/114) in patients diagnosed with hereditary moderate sensorineural hearing loss caused by STRC, OTOA, OTOG, OTOGL, TECTA, MPZL2 and others. Three known MPZL2 variants (c.220C > T (p.Gln74*), c.68delC (p.Pro23Leufs*2), c.463delG (p.Ala155Leufs*10)) and a novel start loss variant (c.3G > T (p.Met1?)) were identified. MPZL2 c.220C > T was identified as the hotspot variant in the Chinese population and even in East Asia compared with c.72delA (p.Ile24Metfs*22) in European and West Asia through allele frequency.
Conclusions
We concluded that apart from moderate HL, progressive HL is another character of MPZL2-related HL. No specified variant was verified for the progression of HL, the penetrance and expressivity cannot be determined yet. A novel MPZL2 variant at the start codon was identified, enriching the variant spectrum of MPZL2. The hotspot variants of MPZL2 vary in different ethnicities. This study provides valuable data for the diagnosis, prognosis evaluation and genetic counseling of patients with moderate sensorineural hearing loss related to MPZL2.
Similar content being viewed by others
Introduction
An estimated 1.57 billion (95% uncertainty interval 1.51 ~ 1.64) people globally had hearing loss (HL) in 2019, accounting for one in five people. And By 2050, a projected 2.45 billion (2.35 ~ 2.56) people will have hearing loss, a 56.1% (47.3 ~ 65.2) increase from 2019 [1]. Genetic etiology can be found in about 60% of congenital hearing loss [2]. In terms of authoritative statistics, as of August 4, 2021 (last update), a total of 124 genes were reported to related to hereditary non-syndromic hearing loss on the website (https://hereditaryhearingloss.org/). However, according to the most recent literature report, more than 150 genes have been identified to link to hereditary non-syndromic hearing loss [3]. Only several genes have been clearly identified to be associated with mild-to-moderate HL [4]. It is crucial to determine the phenotype-genotype correlation of patients’ specific genetic mutations, which can not only predict and help in arresting the development of HL but also provide specific guidance for health management and family genetic counseling throughout the life cycle of patient [5]. In a word, an in-depth analysis of genetic and phenotypic data is necessary for determining the clinical characteristics of different types of genetic variations and help in patient-specific genetic counseling.
Some genes closely related to mild-to-moderate HL have been reported, such as Stereocilin (STRC, MIM: 606440), Otogelin (OTOG, MIM: 604487), Otogelin-like protein (OTOGL, MIM: 614925), Myelin protein zero-like 2 (MPZL2, MIM: 604873), Otoancorin (OTOA, MIM: 607038), and Alpha-tectorin (TECTA, MIM:602574) [6,7,8,9,10,11]. Common variant in GJB2 can also cause mild-to-moderate HL, such as the p.V37I [12]. There exist similarities in the protein expression in the cochlea of STRC, OTOG, OTOGL, OTOA and TECTA [7, 11, 13]. Different from the most of the genes mentioned above, MPZL2 expresses in the organs of Corti and the stria vascularis, especially in the basal regions of Deiter cells [8] (Fig. 1). However, there are relatively few reports on MPZL2.
Genetic defects in MPZL2 were determined to be the cause of autosomal recessive deafness-111 (DFNB111, MIM: 618145) with non-syndromic, early-onset, symmetrical and moderate sensorineural HL, which was first identified by Wesdorp in 2018 [8]. The recent studies on DFNB111 have mainly focused on the Dutch, Moroccan, and some Middle Eastern countries [8, 14, 15]. In East Asia countries such as Korea [4, 16] and China [17], only a few families were identified with these defects and to date, no systematic phenotype-genotype correlation analysis for MPZL2 has been performed.
In this study, by analyzing the genetic data of 3272 Chinese patients with HL, we determined the correlation between MPZL2 defects and deafness, especially in those with mild-to-moderate HL. By analyzing the phenotypic and genotypic characteristic of these patients and reviewing the reported studies on MPZL2 defects in other races, we determined the characteristics of deafness caused by MPZL2 defects and attempted to provide precise guidance for the prognosis, treatment and family genetic counselling of the related hearing loss patients.
Material and methods
Ethical considerations
This study was approved by the Ethics Committee of Chinese PLA General Hospital (approval number S2018-088–01). Informed written consent was obtained from all subjects or their guardians for genetic analysis and the publication of the clinical data.
Clinical data
Eight MPZL2-related HL pedigrees were enrolled in this study. The pedigrees were from a hearing loss patient cohort of 3272 different families, who underwent genetic testing from December 2015 to November 2022 in the Genetic Testing Center of Chinese PLA General Hospital. The medical history of each family member was obtained by using a questionnaire that included questions on the degree of HL, the age of onset of HL, the progression of HL, symmetry of HL, the use of hearing aids or cochlea implants, the presence of tinnitus and vertigo, infection, use of ototoxic drugs, noise exposure, and other relevant clinical manifestations to understand the otological manifestation and exclude any history of other diseases and environmental factors. Pure-tone audiometry with air and bone conduction was performed in accordance with the standard protocols in a sound-controlled room at frequencies ranging from of 125 ~ 8000 Hz. The patients underwent otoscopic examinations to evaluate the integrity of the tympanic membrane. High-resolute CT scans of the temporal bone were performed in some patients to exclude infection, space occupying lesions, and middle or inner ear malformations. If syndromic HL was suspicious, the probands underwent further general physical examinations, ultrasound of the heart, thyroid as well as other visceral organs, and craniocerebral MRI.
Gene capture and next-generation sequencing (NGS)
Genomic DNA was extracted from the peripheral blood using a blood DNA extraction kit (TianGen, Beijing, China) in accordance with the manufacturer’s instructions.
Family 1 and family 3 were tested with deafness gene panels containing 415 known deafness genes, six mitochondrial regions associated with deafness, and three deafness-related microRNAs (Table S1), and other six families were tested with whole exome sequencing (WES). All coding exons, along with 100-bp flanking regions, were captured for each gene and then sequenced. The details of the deafness gene capture, sequencing, and bioinformatics analysis methods have been described previously [18]. Illumina NovaSeq6000 sequencing platform was used to conduct the WES. DNA samples from three MPZL2-related cases and their parents (family 2, family 6 and family 7) were subjected to trio WES, Sanger sequencing is used to verify the sequencing results and whether other family members carry the mutation. The nomenclature of the mutation described in Table 1 is based on MPZL2 cDNA and protein accession numbers NM_005797.4 and NP_005788.1, respectively. We used the genomic coordinates from GRCH37/hg19 constructed from the human genome.
Bioinformatic analyses
All detected variants were filtered against the 1000 Genomes Project, Exome Aggregation Consortium (ExAC), Human Gene Mutation Database (HGMD), and Exome Sequencing Project (ESP) databases. Changes with an unknown frequency or minor allele frequency < 1% were retained. Variants with no annotated frequency information in the databases were included. Variants predicted as low impact, missense changes predicted as not damaging by both SIFT and Polyphen-2, and synonymous variants were excluded. CNV Analysis along with both WES and targeted panel was conducted by use of Cnvkit software. The variants were comprehensively interpreted with the VarSome [19]. The manual variant classification was performed using the American College of Medical Genetics and Genomics (ACMG)/Association for Molecular Pathology (AMP) guidelines for genetic HL [20]. The final screened potential pathogenic variants were verified by Sanger sequencing and validated by parental testing on condition that the DNA samples of the parents were available. Novel variants were defined when it was absent form the HGMD professional database, ClinVar database, gnomAD database and published literature previouely.
The data, including phenotypes and observed variants, have been submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) under the accession numbers SCV002762660—SCV002762663.
Sanger sequencing
Primers were designed for the sequence variations detected in MPZL2. Polymerase Chain Reaction (PCR) was used to amplify the corresponding DNA fragments of the probands and their parents, and Sanger sequencing was used to sequence the amplified products. The sequencing results were analyzed by DNASTAR (Madison) software. Primers sequences (reference genome: GRCh37/hg19) are as follows: MPZL2 c.3G > T: upstream 5' AACCTGTTTGTCCGAGAGCAG 3'; downstream 5' AGTCACAGGCACAGGTGAGG 3'; MPZL2 c.68delC: upstream 5' GCTCTGTCACTTGCTTTCCC 3', downstream 5' GAGGCGAGGCTTTAATGCTG 3'; MPZL2 c.220C > T: upstream 5' TGCAGCTCTGTCACTTGCTT 3', downstream 5' GGGACAGATGCTCGGTTAAA 3'; MPZL2 c.463delG: upstream 5' CTGCCCAGCCTTACGATTTT 3', downstream 5' AAGACACAGATTGCTCAGCC 3'.
Results
Clinical findings
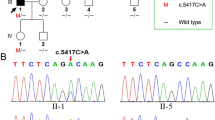
Eight pedigrees with homozygous or compound heterozygous variants of MPZL2 from a hearing loss patient cohort of 3272 who underwent genetic testing from December 2015 to November 2022 in the Genetic Testing Center of Chinese PLA General Hospital, were included in this study. There were totally 11 patients diagnosed as moderate sensorineural HL in the eight pedigrees (Fig. 2), among which the phenotype of 10 patients except for III-1 from Family 1 was confirmed to be MPZL2-related by next-generation and subsequent Sanger sequencing. III-1 in Family 1 carried heterozygous MPZL2 c.220C > T (p.Gln74*) and compound heterozygous GJB2 c.109G > A(p.Val37Ile)/c.235delC(p.Leu79Cysfs*3) inherited from her parents (Fig. 2). The HL level was classified according to 2021 WHO classification of HL (https://www.who.int/publications/i/item/world-report-on-hearing).
A Family tree of eight inherited HL pedigrees caused by bi-allelic variants of MPZL2. A-H The pedigree trees of Family 1 to Family 8, respectively. Six pathogenic or likely pathogenic variants including four in MPZL2 and two in GJB2 were identified in these families. All patients, except III-1 in Family 1, carried bi-allelic MPZL2 variants. The variant of III-1 in Family 1 was identified as a carrier of MPZL2:c.220C > T(p.Gln74*) and compound heterozygotes of GJB2 c.109G > A/c.235delC, suggesting that her HL is caused by GJB2 mutations
For the 10 patients carrying homozygous or compound heterozygous variants in MPZL2, progressive HL was reported by four patients (for more details please see Table 1). Moreover, we observed a 17.75 dBHL and 18 dBHL decrease in PTA of the right and left ears, respectively, during 17 years in patient no.30, and a 17.5 dBHL and 22.5 dBHL decrease in PTA of the right and left ears, respectively, during three years in patient no.37. The hearing level changes could not be determined because of the incomplete data for patients no.34 and 35.
The onset age of HL ranged from infant to 21 years, and the median age was 13.5 years. All but patient no.32 visited the hospital with HL as the first symptom. The main complaint of patient no.32 were the feeling of ear fullness and tinnitus but not HL. Interestingly, patient no.39 reported that he developed HL successively in either ear with a time interval of 5 years, and a subsequent hearing test conformed symmetrical moderate sensorineural HL in both ears. None of the patients complained of vertigo and hence the vestibular examinations were not performed.
Detected variants of MPZL2
Four types of variants of MPZL2 were detected in our study, including three truncation variants (MPZL2:c.220C > T (p.Gln74*), MPZL2:c.463delG (p.Ala155Leufs*10), and MPZL2:c.68delC (p.Pro23Leufs*2)), which result in the early termination of protein synthesis, and one start loss variant (MPZL2:c.3G > T (p.Met1?)). The start loss variant has not been reported previously and nor was it detected in the 686 individuals with normal hearing included in this study, but the three truncation variants are known pathogenic ones. The start loss variant was classified as likely pathogenic (PVS1_Moderate, PM2, PM3) according to the expert specification of the ACMG/AMP variant interpretation guidelines for genetic HL [20]. The allele frequency distribution of each mutation in different nationalities has been summarized in Table 2.
Genetic load of MPZL2 defects in the Chinese population with hearing loss
In a cohort of 3272 patients, 114 probands clinically diagnosed with moderate sensorineural HL were confirmed as hereditary by performing a genetic diagnosis. The related genes involved in the molecular pathogenesis of the 114 patients mainly included STRC, OTOA, OTOG, OTOGL, TECTA, GJB2, KCNQ4, USH2A, PDZD7, MPZL2 and et al. MPZL2 accounted for 7.02% (n = 8, 8/114) among the above genes resulting in a characteristic moderate HL. HL caused by MPZL2 defects accounted for 0.24% (n = 8, 8/3272) of the Chinese population with HL.
Discussion
At present, several studies on hereditary HL have been published, most of which have focused on congenital and severe-to-profound sensorineural HL. There are a few pathogenic genes associated with mild-to-moderate HL, and most patients with mild-to-moderate HL rarely opt for genetic counseling. Therefore, studies on the genetic factors causing mild-to-moderate HL are relatively less.
We retrospectively analyzed 3272 patients with HL who had undergone genetic testing from December 2015 to November 2022, and the results showed that MPZL2 accounted for 0.24% of all 3272 patients diagnosed with HL. Furthermore, the molecular etiologies of 114 patients (3.48%) with mild-to-moderate HL were identified. The pathogenic genes consisted of STRC, OTOA, TECTA, MPZL2, OTOG, OTOGL and so on. Among them, MPZL2 mutation was observed in 7.02% of patients with mild-to-moderate hereditary deafness, after STRC, GJB2, OTOA and TECTA. This finding is similar with the data (7.02%) reported by Kim [4], in whose study the related genes mainly included STRC, OTOA, OTOG, OTOGL, TECTA, GJB2, KCNQ4, USH2A, PDZD7 and et al.. However, both our and the Korean studies [4] suggest the contribution of variations in MPZL2 should not be ignored as the molecular genetic etiology in patients with mild-to-moderate sensorineural HL in East Asia.
In this study, 10 individuals from eight families were diagnosed with moderate HL caused by bi-allelic MPZL2 variants. The following four pathogenic and likely pathogenic variations were involved: c.220C > T, c.463delG, c.68delC, and c.3G > T. The start loss variant c.3G > T, which may affect the initiation codon and translation of RNA, has been reported for the first time in this study. Another reported variant, c.72delA, was not found in our cases. So far, only five pathogenic variants were identified in MPZL2. Among them, 4 variants are truncation variants that could result in the early termination of protein synthesis, but it did not rule out the possibility that these variants would undergo nonsense-mediated decay (NMD). NMD is a quality control pathway that removes transcripts bearing premature termination codons (PTCs) unless PTCs are in the last exon and last ~ 50 nt of the penultimate exon [21]. Since MPZL2 has six exons and the three truncation variants (c.68delC, c.72delA, and c.220C > T) are located in exons 2 or 4, these variants of MPZL2 would likely undergo NMD, consistent with the evaluation of automatic PVS1 interpretation (AutoPVS1), an automatic classification tool (http://autopvs1.genetics.bgi.com/).
We also analyzed the allele frequency of all the reported variants. From the distribution of allele frequency, we concluded that c.220C > T and c.72delA are the most common variants of MPZL2. However, these two variants are distributed in different nationalities. MPZL2 c.220C > T mainly occurs in East Asian populations, such as Chinese and South Korean, whereas c.72delA mainly exists in people with European ethnicities such as Dutch, Turkish and Italian. In the case of the Moroccan population, since only two cases were reported, its main variation cannot be accurately known yet. The other three variants (c.463delG, c.68delC and c.3G > T) were found only in Chinese and Korean patients. Since the number of reported cases is not large, we cannot regard them as unique variations only in the Chinese and Korean populations. For the common variations c.220C > T and c.72delA of MPZL2, previous studies also analyzed the founder effect. Haplotype analysis showed that the MPZL2 c.72delA allele shared a common haplotype delimited by markers D11S1341 and D11S4104 in Dutch and Turkmen ethnicities from north-eastern Iran, and Turkish and Moroccan ethnicities [8, 14, 15]. Kim's study [4] showed that the MPZL2 c.220C > T allele with an exclusively high minor allele frequency in the East Asian group of 1000 Genomes (0.0069) was carried by every patient of Han Chinese descent. Therefore, we believe that c.220C > T is a very ancient founding allele in East Asians and not just in Koreans. The results of the founder effect are consistent with our conclusion from the distribution of allele frequency.
At present, MPZL2 gene is mainly associated with mild-to-moderate HL and progressive HL. These phenotypes are probably the result of the Mplz2 variant that affects the adhesion of the inner ear epithelium, which leads to the loss of the structural integrity of the organ of Corti and the progressive degeneration of hair cells, supporting cells, and spiral ganglion neurons [8]. Moreover, the Mpzl2 mutant mice showed early-onset progressive HL and a significantly increased ABR threshold [8], which was similar to the findings in human. However, the rate of HL development in the Mpzl2 mutant mice was much faster than that in humans, and the HL in Mpzl2 mutant mice rapidly developed to moderate and severe at 8 and 12 weeks, respectively (ABR threshold higher than 70 dB SPL) [8, 17]. To better demonstrated the extent of HL caused by MPZL2 variants, we combined our results with those from previous studies (a total of 39 cases in Table 1) to comprehensively analyze the genotype and phenotype correlation of the MPZL2 variant. The onset age of HL is wide, ranging from infant to 21 years, but is mainly early-onset. During the first consultation, all patients had moderate HL and could benefit from wearing hearing aids. In total, progressive HL was reported in four patients in this study and six patients in previous studies (Table 1). By comparing the hearing level at the first consultation and follow-up, we observed that 35.71% (10/28) patients had reduced hearing; however, the progression of HL could not be determined in 2 patients (patients no.34 and 35) in this study. The rate of HL was 2.10 and 13.33 dBHL per year in patient nos.30 and 37, respectively, which is higher than that reported in the literature (0.59 dBHL/year) [17]. A total of 64.29% (18/28) of patients did not report reduced hearing. Interestingly, all the audiograms of the subjects in our study showed higher thresholds in the high-frequencies (~ 4 kHz to 8 kHz) than in other frequencies (~ 0.125 kHz to 2 kHz), which could be partly explained by the histological abnormalities in the cochleae of the Mpzl2-mutant mice. The Mpzl2-mutant mice exhibited an altered organization of the outer hair cells and supporting cells, and degeneration of the organ of Corti, in addition to a mild degeneration of spiral ganglion neurons that was most pronounced at the cochlear base [8]. This conclusion needs to be confirmed by early diagnosis and long follow-ups.
In conclusion, in this study, a novel MPZL2 variant at the start codon was identified, which enriched the variant spectrum of MPZL2. By analyzing the variant spectrum of MPZL2 in the Chinese population, we further confirmed that c.220C > T has the founder effect on the East Asian population. The genotypic and phenotypic characteristics of MPZL2 were analyzed more systematically by summarizing the findings of all the patients carrying MPZL2 variants from this study and those reported in the previous studies, and we found apart from moderate HL, progressive HL is another character of MPZL2-related HL, which proposes the importance of pre-warning risk factors in order to arrest or put off the development of HL in patients. Thus, we believe that our findings have contributed to the early diagnosis of HL, prognosis evaluation, and could facilitate the genetic counselling of related patients.
Availability of data and materials
The datasets presented in this study are available in the clinVAR (https://submit.ncbi.nlm.nih.gov/clinvar/).
References
Hearing loss prevalence and years lived with disability. 1990–2019: findings from the Global Burden of Disease Study 2019. Lancet. 2021;397(10278):996–1009.
Korver AM, Smith RJ, Van Camp G, et al. Congenital hearing loss. Nat Rev Dis Primers. 2017;3:16094.
Li Y, Ning G, Kang B, et al. A novel recessive mutation in OXR1 is identified in patient with hearing loss recapitulated by the knockdown zebrafish. Hum Mol Genet. 2023;32(5):764–72.
Kim BJ, Oh DY, Han JH, et al. Significant Mendelian genetic contribution to pediatric mild-to-moderate hearing loss and its comprehensive diagnostic approach. Genet Med. 2020;22(6):1119–28.
Writing Group For Practice Guidelines For D, Treatment Of Genetic Diseases Medical Genetics Branch Of Chinese Medical A, Yuan H, Dai P, Liu Y, Yang T. Clinical practice guidelines for hereditary non-syndromic deafness. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2020;37(3):269–76.
Čada Z, Šafka Brožková D, Balatková Z, et al. Moderate sensorineural hearing loss is typical for DFNB16 caused by various types of mutations affecting the STRC gene. Eur Arch Otorhinolaryngol. 2019;276(12):3353–8.
Oonk AM, Leijendeckers JM, Huygen PL, et al. Similar phenotypes caused by mutations in OTOG and OTOGL. Ear Hear. 2014;35(3):e84–91.
Wesdorp M, Murillo-Cuesta S, Peters T, et al. MPZL2, Encoding the Epithelial Junctional Protein Myelin Protein Zero-like 2, Is Essential for Hearing in Man and Mouse. Am J Hum Genet. 2018;103(1):74–88.
Laurent S, Gehrig C, Nouspikel T, et al. Molecular characterization of pathogenic OTOA gene conversions in hearing loss patients. Hum Mutat. 2021;42(4):373–7.
Iwasaki S, Harada D, Usami S, Nagura M, Takeshita T, Hoshino T. Association of clinical features with mutation of TECTA in a family with autosomal dominant hearing loss. Arch Otolaryngol Head Neck Surg. 2002;128(8):913–7.
Verpy E, Masmoudi S, Zwaenepoel I, et al. Mutations in a new gene encoding a protein of the hair bundle cause non-syndromic deafness at the DFNB16 locus. Nat Genet. 2001;29(3):345–9.
Huang S, Huang B, Wang G, Yuan Y, Dai P. The Relationship between the p.V37I Mutation in GJB2 and Hearing Phenotypes in Chinese Individuals. PLoS One. 2015;10(6):e0129662.
Zwaenepoel I, Mustapha M, Leibovici M, et al. Otoancorin, an inner ear protein restricted to the interface between the apical surface of sensory epithelia and their overlying acellular gels, is defective in autosomal recessive deafness DFNB22. Proc Natl Acad Sci U S A. 2002;99(9):6240–5.
Bademci G, Abad C, Incesulu A, et al. MPZL2 is a novel gene associated with autosomal recessive nonsyndromic moderate hearing loss. Hum Genet. 2018;137(6–7):479–86.
Amalou G, Bonnet C, Riahi Z, et al. A homozygous MPZL2 deletion is associated with nonsyndromic hearing loss in a moroccan family. Int J Pediatr Otorhinolaryngol. 2021;140:110481.
Lee SY, Oh DY, Han JH, et al. Flexible Real-Time Polymerase Chain Reaction-Based Platforms for Detecting Deafness Mutations in Koreans: A Proposed Guideline for the Etiologic Diagnosis of Auditory Neuropathy Spectrum Disorder. Diagnostics (Basel). 2020;10(9):672.
Wang Z, Jiang M, Wu H, Li Y, Chen Y. A novel MPZL2 c.68delC variant is associated with progressive hearing loss in Chinese population and literature review. Laryngoscope Investig Otolaryngol. 2022;7(3):870–6.
Li X, Huang S, Yuan Y, et al. Detecting novel mutations and combined Klinefelter syndrome in Usher syndrome cases. Acta Otolaryngol. 2019;139(6):479–86.
Kopanos C, Tsiolkas V, Kouris A, et al. VarSome: the human genomic variant search engine. Bioinformatics. 2019;35(11):1978–80.
Oza AM, DiStefano MT, Hemphill SE, et al. Expert specification of the ACMG/AMP variant interpretation guidelines for genetic hearing loss. Hum Mutat. 2018;39(11):1593–613.
Supek F, Lehner B, Lindeboom RGH. To NMD or Not To NMD: Nonsense-Mediated mRNA Decay in Cancer and Other Genetic Diseases. Trends Genet. 2021;37(7):657–68.
Acknowledgements
The authors would like to thank all the patients who participated in the study.
Funding
This study was funded by grants from National key Research and development project of China (grant number2022YFC2703602) and National Natural Science Foundation of China (grant number 82271177, 82271185, 81873704, 82171155, 82171158, and 81900953). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.Y.Y., S.S.H, and D.Y.H.; Methodology, J.Y.Y., L.Z., and D.Y.K.; Software, J.Y.Y, G.J.W., and M.Y.H.; Data Analysis and Curation, Q.Q.W. and X.G.; Writing-Original Draft Preparation, J.Y.Y. and L.Z.; Writing-Review & Editing, Y.Y.Y. and S.S.H.; Project Administration, D.Y.H. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Chinese PLA General Hospital (approval number S2018-088–01). Informed written consent was obtained from all subjects or their guardians for genetic analysis and the publication of the clinical data. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Informed written consent was obtained from all subjects or their guardians for genetic analysis and the publication of the clinical data.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
List of genes included in the deafness gene panels.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, L., Yang, JY., Wang, QQ. et al. MPZL2—a common autosomal recessive deafness gene related to moderate sensorineural hearing loss in the Chinese population. BMC Med Genomics 17, 32 (2024). https://doi.org/10.1186/s12920-023-01786-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-023-01786-3