Abstract
Background
Polycystic ovary syndrome (PCOS) is a common endocrine disorder in premenopausal women, whose etiology remains uncertain, although it is known to be highly heterogeneous and genetically complex. PCOS often presents with hyperandrogenism symptoms. The present study aimed to determine whether polymorphisms in the FK-506 binding protein 5 (FKBP5) gene (androgen target gene) are associated with an association for PCOS and hyperandrogenism.
Methods
This is a case–control study, and association analyses were conducted. A total of 13 single-nucleotide polymorphisms (SNPs) in the FKBP5 gene were evaluated in 775 PCOS patients who were diagnosed based on the Rotterdam Standard and 783 healthy Chinese Han women. Associations between FKBP5 SNPs and hormone levels were investigated. These 13 SNPs were genotyped using the Sequenom MassARRAY system, and an association analysis between the phenotype and alleles and genotypes were conducted.
Results
The genotype frequencies for the rs1360780 and rs3800373 SNPs differed significantly between the PCOS cases and healthy controls (p = 0.025, OR is 1.63 (1.05–2.53) and p = 0.029, OR is 1.59 (1.03–2.45) respectively under co-dominant model). Moreover, the genotype frequencies and genetic model analysis for the SNPs rs1360780, rs9470080, rs9296158, rs1043805 and rs7757037 differed significantly between the hyperandrogenism and non-hyperandrogenism groups of PCOS patients. The TT genotype of rs1360780, the TT genotype of rs9470080, the TT genotype of rs1043805 or the GG genotype of rs7705037 (ORs are 2.13 (1.03–4.39), 1.81 (1.03–3.17), 2.94 (1.32–6.53) and 1.72 (1.04–2.84) respectively) were correlated with androgen level of PCOS patients.
Conclusion
Our study showed that FKBP5 gene polymorphisms are associated with PCOS generally (rs1360780 and rs3800373) and with the hyperandrogenism subtype specifically (rs1360780, rs9470080, rs9296158, rs1043805 and rs7757037).
Similar content being viewed by others
Background
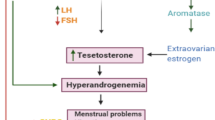
Polycystic ovary syndrome (PCOS) is an endocrine disorder which afflicts females of reproductive age [1]. While the etiology of PCOS remains unclear, PCOS patients differ from healthy women in many aspects, often presenting with chronic anovulation (ovulation dysfunction or loss), insulin resistance, polycystic ovarian morphology (PCOM) under B-ultrasound (multiple cysts are formed in the ovarian follicles), and hyperandrogenism (high levels of androgens in females) [2]. Notably, interplay between hyperandrogenism and insulin resistance has been reported to influence the occurrence and development of PCOS [3]. Disrupted secretion of the pulsatile gonadotropin-releasing hormone (GnRH) from the hypothalamus has also been associated with PCOS, as have imbalances in regulation of both the hypothalamic–pituitary–adrenal (HPA) axis and the hypothalamic–pituitary–ovary (HPO) axis [3, 4]. High androgen exposure during the embryonic period, endocrine disorders, and immune dysregulation have, among other factors, also been linked to PCOS [5, 6].
PCOS is considered to be a chronic inflammatory disease [1], and multiple inflammation-related genes (such as interleukin-1 beta (IL1B), prostaglandin-endoperoxide synthase 2 (PTGS2)), as well as granule cells(GCs) in the inflammatory environment of PCOS patients, have been linked to hyperandrogenism [7,8,9]. Hyperandrogenism is understood to damage the health of PCOS patients, and hyperandrogenism is now an important criterion for PCOS diagnosis. The most common clinical manifestation of hyperandrogenism in women is hirsutism and excessive terminal hair growth in androgen-dependent areas of the body. Other clinical manifestations of hyperandrogenism include acne vulgaris, weight gain, menstrual irregularities, and acanthosis nigricans [10]. The incidence rate of hyperandrogenism is as high as 60–80% [11, 12]. Androgens can be biosynthesized from cholesterol by the theca cells in the ovary [13]; these hormones function in regulating metabolic homeostasis and reproductive health in both men and women. In PCOS, excess androgens lead to follicular dysplasia and anovulatory infertility, with studies suggesting that theca cell androgen receptors likely mediate many of these negative effects [14]. Moreover, disrupted androgen levels have been linked to other clinical manifestations common to PCOS patients, including obesity, type 2 diabetes, hypertension and atherosclerosis, cardiac hypertrophy, and coronary heart disease, as well as kidney diseases [11].
PCOS has a strong genetic component [15]. Some SNPs of PCOS-related genes are partially associated with the morbidity of PCOS [16,17,18]. For example, the GG genotype of LHCGR (rs2293275) in PCOS showed strong correlations with body-mass index (BMI), waist to hip ratio, insulin resistance, luteinizing hormone, and LH/FSH ratio [19]. Moreover, transmission disequilibrium testing revealed a strong genetic association between the D19S884 allelic marker located near the INSR gene and the development of PCOS. Additionally, a T/C polymorphism was also reported among the Chinese population in the CYP17A gene: affected females having the CC genotype had increased testosterone levels compared to individuals harboring the TC and TT genotypes. Additional genes with PCOS-associations include the androgen receptor (AR) gene, the sex hormone-binding globulin (SHBG) gene, and the insulin-like factor 3 (INSL3) gene [20].
FKBP5 encodes the FK-506 binding protein 5 (a co-chaperone of a heat shock protein (Hsp90)) [21], which is known to be directly regulated by androgens and has been implicated in metabolism-related disorders. Specifically, there is a distal enhancer located 65 kb downstream of the transcription start site in the fifth intron of the FKBP5 gene, and a study showed that FKBP5 expression is regulated by an interaction between the AR and this enhancer; specifically, AR selectively recruits cAMP response element-binding protein to this enhancer [22]. Moreover, it is notable that previous studies have shown that FKBP5 is highly expressed in muscle and adipose tissue, and human FKBP5 is associated with type 2 diabetes(T2D) and with markers for both insulin resistance and obesity [23, 24]. Given that PCOS patients show symptoms like endocrine disorders, metabolic disorders, and obesity, we were interested in exploring potential relationships between FKBP5 and androgens in the context of PCOS etiology. The objective of the present case–control study was to investigate potential associations between single nucleotide polymorphisms of FKBP5 and PCOS pathogenesis and symptoms.
Methods
Ethics statement
All patients in this study gave their informed written consent; the protocol for this study was reviewed and approved by the Institutional Review Board of Reproductive Medicine of Shandong University ([2020] Ethical Review #44).
Study subjects
A total of 1558 women from the Reproductive Hospital affiliated with Shandong University were included in the present case–control study. Among them, 775 women had PCOS, while the other 783 were healthy control subjects, whose blood and other samples have been kept in the sample bank of the Affiliated Reproductive Hospital of Shandong University for research. The patients with PCOS and the healthy control subjects were diagnosed based on the presence of two out of three criteria of proposed by the Rotterdam European Society for Human Reproduction and Embryology (ESHRE)/American Society for Reproductive Medicine (ASRM)-sponsored PCOS Consensus Workshop Group; patients with PCOS were included if they met at least two of the following three criteria: (1) chronic oligoovulation and/or anovulation; (2) clinical or biochemical hyperandrogenism; and (3) polycystic ovaries on ultrasound, ≥ 12 ovary follicles measuring 2–9 mm in diameter in one or both ovaries, or ovarian volume > 10 mL [25]. Other etiologies (congenital adrenal hyperplasias, androgen-secreting tumors, and Cushing's syndrome) were excluded. According to the level of androgen, there were 588 cases are with hyperandrogenism subtype; and 187 cases were of the non-hyperandrogenism subtype. Clinical or biochemical hyperandrogenism was defined on the basis of hirsutism (modified Ferriman-Gallwey score ≥ 6) or elevated circulating total testosterone ≥ 60 ng/dl [26, 27].
Controls were gathered primarily from healthy women who presented with regular menstrual cycles, excluding hyperandrogenism and PCOS. Clinical parameters of the patients with PCOS and the healthy control subjects are shown in Table 1.
Measurement of clinical data
The patients with PCOS and the healthy control subjects were diagnosed and examined at the Center for Reproductive Medicine, Shandong Provincial Hospital, Shandong University, China. Weight and height were measured using standard protocols with calibrated instruments. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Peripheral blood samples were collected on days 2–5 of a spontaneous cycle or after withdrawal of bleeding from the subjects in a fasting state. Measurement with a chemiluminescent analyzer (Beckman Access Health Company, Chaska, Minnesota, USA) was done for the following hormones: follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone (T). Blood samples, which were collected with EDTA as an anticoagulant and stored at − 80 °C, were prepared for genomic DNA extraction.
Clinical data of the PCOS Group and the control group
We analyzed subjects from the two groups (PCOS group and control group); the clinical characteristics of groups are shown in Table 1. The mean age did not different between the two groups. BMI and WHR were significantly higher in the PCOS group than in the control group (p < 0.001). The levels of LH, LH/FSH, and T in the PCOS group were higher in the PCOS group than in the controls. The FSH level was significantly lower in the PCOS group compared to the controls.
SNP genotyping
To investigate potential associations between single nucleotide polymorphisms of FKBP5 and PCOS pathogenesis and symptoms, for the total 54,836 variants in the FKBP5 gene, we chose 13 SNPs. Selected criteria: functional region SNP were selected, including promoter, exon and 3′ UTR of gene, by SNP Function Prediction (https://snpinfo.niehs.nih.gov/) [28] to evaluate potential functional mechanism, this step includes 3 functional region SNPs: rs3800373 and rs1043805 belonging to the 3′ UTR region, and rs2817035 belonging to the promoter region. Furthermore, we searched literature to filter known important risk SNP of genes, for our sample validation, this step incorporates the remaining 10 SNPs. MAF of above all SNP were verified through 1000 Genomes CHB (https://www.internationalgenome.org/), setting up cutoff MAF > 0.05. Among them, the SNPs rs2817035 and rs4713902 of the FKBP5 gene were reported as linked to risk for coronary artery disease [24]. A study of interindividual response differences to inhaled corticosteroids in patients with chronic obstructive pulmonary disease showed that rs4713916 is associated with sensitivity/resistance to corticosteroids [29]. Considering that rs1360780, rs3800373, rs9296158, and rs9470080 are commonly detected SNPs of the FKBP5 gene [30], we focused on these SNPs in this study. In total, we selected 13 SNPs in FKBP5 (Table 2).
The genomic DNA of every subject was extracted from peripheral blood using DNA Blood Mini Kits (QIAGEN, 51,106, Germany). Thirteen SNPs in the FKBP5 gene were genotyped using the MassARRAY RS1000 platform (Sequenom, San Diego, CA, USA) according to the standard protocol. The genotyping primers were designed using MassARRAY Assay Design 3.1 Software (Sequenom) and were synthesized by Biomiao Biological Technology Company (Beijing, China). Sequencing was performed on a MassARRAY Compact System (Sequenom), and the genotype data were analyzed using MassARRAY TYPER Analyzer Software version 4.0 (Sequenom).
Statistical analysis
Tests of Hardy–Weinberg equilibrium and linkage disequilibrium analysis were performed using HaploView software. The analysis of the association between clinical characteristics and genotypes was performed with independent sample t tests. The allele frequencies and genotype frequencies of the 13 SNPs were tested with chi-square tests, and were adjusted using logistic regression analysis [31]. The genotype frequency of the 13 SNPs were analyzed in terms of five genetic models using SNPStats software (http://bioinfo.iconcologia.net/snpstats/start.htm): the log-additive model (+/+ vs. −/−), the dominant model (+/+ plus +/− vs. −/−), the recessive model (+/+ vs. +/− plus −/−), the co-dominant model (+/+ vs. +/− vs. −/−), and the overdominant model (+/+ plus −/− vs. +/−). Considering that the outcome variables are dichotomous (PCOS and control, hyperandrogenism and non-hyperandrogenism), the log-additive model was performed. The genetic model was assessed using the Akaike information criteria (AIC) and the Bayesian information criteria (BIC); the model with the lowest values was considered to have the best fit. Genotype differences and other statistical analyses, including chi-square tests, independent t tests, and logistic regression analysis, were performed using SPSS software version 20.0 (SPSS, Chicago, IL, USA). Data are expressed as means ± SD. Categorical data are expressed as frequencies or percentages. Associations with a two-tailed p value < 0.05 were considered statistically significant.
Results
The genotype frequency of two SNPs differed significantly between the PCOS group and the control group
Prior to the case-control study, we confirmed that all of the genotyped SNPs agreed with Hardy-Weinberg equilibrium, thus ensuring that our sampling is representative of a truly large population (MAF: minor allele frequency > 0.01, Hardy–Weinberg equilibrium p > 0.001 (the independent sample t test), call rate > 95%,). The distribution of the genotypes and allelic frequencies of the 13 SNPs were analyzed with chi-square tests, and we investigated the 13 SNPs of the samples from PCOS and control groups in five genetic models: the log-additive model, the dominant model, the recessive model, the co-dominant model, and the overdominant model.
Significant differences were detected for two SNPs: rs1360780 and rs3800373 (Table 3). The rs1360780 genotype frequencies for CC, CT, and TT were 54.2% (425/775), 41.3% (324/775), and 4.5% (35/775) in the PCOS patients, versus 55.2% (432/783), 37.4% (293/783), and 7.4% (58/783) in the healthy controls. Under the co-dominant genetic model, the frequencies of the three genotypes differed significantly between the PCOS patients and healthy controls (p = 0.025, AIC = 2717, BIC = 2187). Under the recessive genetic model, there were more significant carriers of the C allele in the PCOS group (95.5% (749/775) than in the control group (92.6% (725/783) (p = 0.013, AIC = 2170.2, BIC = 2180.9).
For rs3800373, the frequencies of AA, CA, and CC were 55% (430/775), 40.3% (315/775), and 4.7% (37/775) in the PCOS patients, versus 56% (432/783), 36.3% (280/783), and 4.7% (59/783) in the healthy controls, with significant differences under the co-dominant genetic model (p = 0.029, AIC = 2151.8, BIC = 2167.8). In the recessive genetic model, the frequencies of genotype AA and CA (A allele carrier) in PCOS patients and healthy control also showed a significant difference (p = 0.016, AIC = 2151.1, BIC = 2161.8). Regarding allele frequencies, no significant differences were found for any of the 13SNPs between the PCOS group and the control group (p > 0.05) (Additional file1: Table S1). Notably, none of the heterozygous conditions for the two SNPs (rs1360780 and rs3800373) showed any association between PCOS and control group.
The genotype frequency of five snps differed significantly between the HA subtype and the NHA subtype
Recall that the PCOS patients can be divided into two groups: the hyperandrogenism (HA) and non-hyperandrogenism (NHA) subtypes. Clinical or biochemical hyperandrogenism was defined on the basis of hirsutism (modified Ferriman-Gallwey score ≥ 6) or elevated circulating total testosterone ≥ 60 ng/dl. Our study analyzed 588 HA and 187 NHA PCOS patients. The allele frequencies and genotype frequencies of the 13 SNPs were tested, and the genotype frequency of the 13 SNPs was analyzed with five genetic models (Table 4).
Using the log-additive model, significant differences were found for all five SNPs: rs1360780 (OR is 1.35(1.02–1.77), p = 0.036), rs9470080(OR is 1.33(1.03–1.71), p = 0.028), rs9296158(OR is 1.33(1.03–1.72), p = 0.031), rs1043805(OR is 1.37(1.03–1.83), p = 0.034) and rs7757037(OR is 1.32(1.04–1.69), p = 0.023). Under the codominant genetic model, the proportions of each genotype are shown on the Table 4, the PCOS cases with following homozygous genotypes displayed a significantly lower level of testosterone: the TT genotype of rs1360780, the TT genotype of rs9470080, the TT genotype of rs1043805 and the GG genotype of rs7705037 (ORs are 2.13 (1.03–4.39), 1.81 (1.03–3.17), 2.94 (1.32–6.53) and 1.72 (1.04–2.84), and p values are 0.09, 0.09, 0.03 and 0.07 respectively).
Under a dominant genetic model, analysis of rs9296158 showed that the frequencies of GG and GA + AA were 47% (276/587) and 53% (311/587), and 38.5% (72/187) and 61.5% (115/187) in the HA PCOS patients and in the NHA PCOS patients, respectively. Notably, the genotypes carrying A alleles (G/A-A/A) were more strongly associated with NHA (OR value of 1.42 (1.01–1.98) and p-value of 0.041 under the dominant model). For rs7757037, the frequencies of AA and GA + GG were 38.3% (225/587) and 61.7% (362/587) in the HA PCOS patients, and 29.9% (56/187), and 70% (131/187) in the NHA PCOS patients. The genotypes carrying G alleles (G/A-G/G) were more strongly associated with NHA under the dominant genetic model (OR is 1.45 (1.02–2.07), p = 0.036, AIC = 855.5, BIC = 864.8). Under a recessive genetic model, analysis of rs1043805 showed that the frequencies of AA + TA and TT were 97.6% (572/586) and 14%(14/586), and 93.6%(175/187) and 6.4% (12/187) in the HA PCOS patients and in the NHA PCOS patients, respectively, OR is 2.80 (1.27–6.17) and p value is 0.01. In addition, the genotype frequencies and genetic model analysis of each SNP between the insulin resistance(IR) group and the control group were conducted, while no significant differences were found for any of the 13SNPs between the two group (P > 0.05) (Additional file 2: Table S2).
Discussion
In this study, we evaluated potential genetic influences of 13 SNPs of androgen-responsive gene FKBP5 in Han Chinese women with PCOS, with a particular focus on the hyperandrogenism PCOS subtype. The 13 SNPs were selected based on previous reports and database information. The frequencies of the TT genotype of rs1360780 and the CC genotype of rs3800373 were significantly lower in the PCOS patients than in the healthy controls. And we detected five FKBP5 SNPs that showed significant differences in genotype frequency analysis: the PCOS cases showing an elevated frequency for the TT genotype of rs1360780, TT genotype of rs9470080, TT genotype of rs1043805, GG genotype of rs7757037 and GA and AA of rs9296158 genotypes displayed lower level of testosterone. Our study showed that the FKBP5 variations in combination with PCOS, with two SNP genotypes associated with PCOS generally and five for the hyperandrogenism subtype specifically, while this kind of marginal association of the FKBP5 rs1360780 and rs3800373 with PCOS patients and FKBP5 rs1360780, rs9470080, rs9296158, rs1043805, rs7757037 with hyperandrogenism subtype need to be viewed with caution before further validation and subsequent experimentation are conducted.
In recent years, a large number of studies have shown that genetic factors play a role in the etiology of PCOS [25, 32, 33], the contribution of heritability of this disorder has been explained based on the approaches used in the twin and linkage studies [20]. In a discovery cohort, FKBP4 (a kind of androgen receptor gene) SNPs rs2968909 and rs4409904 were associated with lower odds of PCOS [34]. Among the multiple SNPs associated with PCOS [35, 36], an example is a SNP at exon 17 of insulin receptor gene (INSR), for which the CC genotype showed a higher frequency in PCOS women than in controls [37]. Additionally, PCOS patients show an elevated frequency for the G allele of a SNP in sorbin and SH3-domain-containing-1 (SORBS1), which encodes a protein known to function in both insulin resistance and glucose uptake [38]. Additionally, a strong association between follistatin and the Cytochrome P450 Family 11 Subfamily A Member 1 (CYP11A1) gene in affected siblings with hyperandrogenism and PCOS related traits was predicted by studying 37 candidate genes [20].
Whereas these two previous studies linked PCOS to SNPs in genes related to glucose metabolism and insulin resistance, we focused on FKBP5, which is known to function as an androgen receptor. While hyperandrogenism is understood as a common clinical feature in PCOS patients, the specific contributions of androgens and related events in PCOS and PCOS-related complications are not yet clear [11]. Unlike most studies—which have compared the PCOS group with a suitable healthy control group, our present study additionally compared the hyperandrogenism and non-hyperandrogenism subtypes of PCOS patients. We detected differences between the hyperandrogenism and non-hyperandrogenism PCOS subtype groups for five SNP alleles of the FKBP5 gene. The frequencies of TT genotype of rs1360780, the TT genotype of rs9470080, the TT genotype of rs1043805 or the GG genotype of rs7705037 were significantly higher in the NHA PCOS group than that in the HA PCOS group. Regarding genetic model analysis, the AA genotype frequency of the HA group is higher than that in the HA group in the dominant model for rs7757037, and for rs9296158, the GG genotype showed the same trend in the dominant model. While for rs9470080, the frequency of the TT genotype in NHA is higher than that in the HA group. Previous reports have proposed that the effects of androgens on cell activities in PCOS may relate to apoptosis, autophagy, mitochondrial dysfunction, and/or endoplasmic reticulum stress in granule cells and oocytes [11]. Excessive androgens could aggravate the development of hypertension and atherosclerosis in patients with PCOS [39]. Particularly, previous studies examined the association of PCOS with genes involved in androgen biosynthesis and action, which supports the hypothesis that inherited abnormalities in genes involved in androgen signaling may contribute to PCOS [34].
FKBP5 is associated with endocrine disorders, which are highly consistent with the symptoms of PCOS. FKBP5 is involved in the regulation of glucose homeostasis through its regulation of AKT2 signaling [23]. Reports have suggested that SNPs within the FKBP5 gene may be linked to the susceptibility to develop insulin resistance and dyslipidemia [40]. FKBP5 functions in responses to steroids, and FKBP5 is strongly transcriptionally activated by androgens, glucocorticoids, and progestins [41]; Several polymorphisms in the FKBP5 gene have been associated with differences in glucocorticoid sensitivity; notably many of the clinically linked SNPs localize close to (or overlap with) regions that encompass steroid-regulated enhancers [42]. The polymorphisms may contribute to the steroid up-regulation of FKBP5 and could thereby influence the complex regulatory loops of steroid signaling [42]. Also, a study by Ortiz and colleagues reported an association between FKBP5 intronic methylation and the risk of cardiovascular disease in T2D patients [43].
Possible functional significance of FKBP5 polymorphisms includes association of rs1360780 with increased protein levels [44] and differences in chromatin conformation [45] due to its location in the region adjacent to the hormone response element (HRE) binding sequence. And a study examining immune cells reported that higher FKBP5 levels promotes inflammation by activating the master immune regulator NF-κB [46]. We paid attention to FKBP5 because of its function as an androgen receptor gene, our study showed FKBP5 gene polymorphisms are associated with PCOS generally (rs1360780, rs3800373) and with the hyperandrogenism subtype specifically (rs1360780, rs9470080, rs9296158, rs1043805, rs7757037), among which rs3800373 and rs1043805 belong to 3’ UTR variant of FKBP5 gene. There may be FKBP5 variants exhibiting yet to be discovered interaction effects with PCOS. Since this is the first study investigating the role of FKBP5 single nuclear polymorphism in PCOS it is not possible to compare our results with the data in literature. While in a discovery cohort, FKBP4 (a kind of androgen receptor gene) SNPs rs2968909 and rs4409904 were associated with lower odds of PCOS [34].
Another study which focused on the relationship between FKBP5 SNPs and PTSD reported that the rs9470080 TT genotype carriers had a higher risk of developing high co-occurring PTSD and depression symptoms than the C allele carriers [30]. A previous study showed that participants exposed to childhood abuse and carrying the TT genotype of the FKBP5 SNP rs1360780 had an increased susceptibility to stress-related disorders [47]. In our study, inversely, the frequency of the TT genotype of FKBP5 SNP rs9470080 and the TT genotype of rs1360780 was significantly higher in PCOS NHA group than PCOS HA group, and the TT of rs1043805, the GG of rs7757037 showed the same tendency. We mentioned above the SNPs of FKBP4 rs2968909 and rs4409904 were associated with lower odds of PCOS [34]. As noted above, there are two homozygous genotypes between PCOS and control group (the TT of rs1360780 and the CC of rs3800373) and four homozygous genotypes between NHA and HA group (the TT of rs1360780, the TT of rs9470080, the TT of rs1043805, the GG of rs7757037) showing associations. For these SNPs, only the homozygous mutant might be associated with PCOS and hyperandrogenism risk.
In another study [17], more comprehensive clinical data were incorporated into an association analysis, including prolactin, blood lipid (CHOL total cholesterol, low-density lipoprotein cholesterol LDL-C, HDL-C high-density lipoprotein cholesterol, triglycerides TG, etc.), OGTT (oral glucose tolerance test), FPG (fasting plasma glucose), HOMA-IR (homeostasis model assessment-insulin resistance) and other clinical indices. Note that we were particularly focused on the hyperandrogenism symptom in our case–control study.
There are some limitations to this case–control study that bear mention. First, the participants were all Han Chinese women, so there was very little variation in genetic background. Genetic factors that influence the pathogenesis of PCOS are known to differ by ethnic group [33, 48], so the associations we detected between FKBP5 and PCOS need to be confirmed in a larger sample size that includes multiple ethnic groups. Second, the functional significance of the SNPs remains unknown and molecular mechanisms regarding FKBP5 in the pathophysiology of PCOS should be examined in future studies.
Our study covered a relatively large cohort of Chinese women with PCOS and represents the first study providing data about the FKBP5 gene and PCOS. On the basis of the available evidence, we conclude that the SNPs, rs1360780, rs3800373, rs9470080, rs9296158, rs1043805, and rs7757037 in the FKBP5 gene are strongly associated with PCOS among Han Chinese women. These SNPs we detected should be useful in future investigations of HA in PCOS, and especially for supporting clinical diagnosis of PCOS as informed by genetic testing. The major limitation of our study was the small number of the studied sample. Further studies in humans and potentially some functional studies will be required to validate the predictive value of these SNPs as potential biomarkers and to elucidate the effects of FKBP5 on PCOS development and clinical outcomes.
Conclusions
Our study showed that the FKBP5 gene is a potential association gene for PCOS, with some SNP genotypes associated with PCOS generally and with the hyperandrogenism subtype specifically. On the basis of the available evidence, we conclude that the SNPs, rs1360780, rs3800373, rs9470080, rs9296158, rs1043805, and rs7757037 in the FKBP5 gene are strongly associated with PCOS among Han Chinese women. Assuming the further validation including functional significance and molecular mechanisms of the SNPs and other evidence appears, we indicate that the SNPs we detected will be useful in future investigations of HA in PCOS, especially for supporting clinical diagnosis of PCOS as informed by genetic testing.
Availability of data and materials
All data supporting the findings of this study are available within the manuscript except for the raw sequence data. Any data providing genotype information is considered to be personal property by Chinese law, hence the submission to public achieves is prohibited. The raw sequence data can be acquired upon reasonable request from the authors (yrzhao@sdu.edu.cn), if approval could be granted from the Ethics Committee of Reproductive Medicine of Shandong University.
Abbreviations
- PCOS:
-
Polycystic ovary syndrome
- FKBP5 :
-
FK-506 binding protein 5
- SNP:
-
Single-nucleotide polymorphisms
- HA:
-
Hyperandrogenism
- NHA:
-
Non-hyperandrogenism
- BMI:
-
Body mass index
- AR:
-
Androgen receptor
- T2D:
-
Type 2 diabetes
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- T:
-
Testosterone
- MAF:
-
Minor allele frequency
- AIC:
-
Akaike information criteria
- BIC:
-
Bayesian information criteria
References
Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018;182:27–36. https://doi.org/10.1016/j.jsbmb.2018.04.008.
Szydlarska D. History of discovery of polycystic ovary syndrome. Adv Clin Exp Med. 2017;26(3):555–8. https://doi.org/10.17219/acem/61987.
Wang J, et al. Hyperandrogenemia and insulin resistance: The chief culprit of polycystic ovary syndrome. Life Sci. 2019;236:116940. https://doi.org/10.1016/j.lfs.2019.116940.
Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J. 2009;56(6):729–37. https://doi.org/10.1507/endocrj.k09e-185.
Dumesic DA, et al. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487–525. https://doi.org/10.1210/er.2015-1018.
Karjula S, et al. Psychological distress is more prevalent in fertile age and premenopausal women with PCOS symptoms: 15-year follow-up. J Clin Endocrinol Metab. 2017;102(6):1861–9. https://doi.org/10.1210/jc.2016-3863.
Lagana AS, et al. Metabolism and ovarian function in pcos women: a therapeutic approach with Inositols. Int J Endocrinol. 2016;2016:6306410. https://doi.org/10.1155/2016/6306410.
Schmidt J, et al. Differential expression of inflammation-related genes in the ovarian stroma and granulosa cells of PCOS women. Mol Hum Reprod. 2014;20(1):49–58. https://doi.org/10.1093/molehr/gat051.
Hewlett M, et al. Prenatal exposure to endocrine disruptors: a developmental etiology for polycystic ovary syndrome. Reprod Sci. 2017;24(1):19–27. https://doi.org/10.1177/1933719116654992.
Dadachanji R, Shaikh N, Mukherjee S. Genetic variants associated with hyperandrogenemia in PCOS pathophysiology. Genet Res Int. 2018;2018:7624932. https://doi.org/10.1155/2018/7624932.
Ye W, et al. The role of androgen and its related signals in PCOS. J Cell Mol Med. 2021;25(4):1825–37. https://doi.org/10.1111/jcmm.16205.
Qu JW, et al. Insulin resistance directly contributes to androgenic potential within ovarian theca cells. Fertil Steril. 2009;91(5):1990–7. https://doi.org/10.1016/j.fertnstert.2008.02.167.
Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of pcos as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467–520. https://doi.org/10.1210/er.2015-1104.
Astapova O, Minor BMN, Hammes SR. Physiological and pathological androgen actions in the ovary. Endocrinology. 2019;160(5):1166–74. https://doi.org/10.1210/en.2019-00101.
Fratantonio E, et al. Genetics of polycystic ovarian syndrome. Reprod Biomed Online. 2005;10(6):713–20. https://doi.org/10.1016/s1472-6483(10)61114-5.
Spinedi E, Cardinali DP. The polycystic ovary syndrome and the metabolic syndrome: a possible chronobiotic-cytoprotective adjuvant therapy. Int J Endocrinol. 2018;2018:1349868. https://doi.org/10.1155/2018/1349868.
Li C, et al. Association of rs10830963 and rs10830962 SNPs in the melatonin receptor (MTNR1B) gene among Han Chinese women with polycystic ovary syndrome. Mol Hum Reprod. 2011;17(3):193–8. https://doi.org/10.1093/molehr/gaq087.
Sagvekar P, et al. LINE1 CpG-DNA hypomethylation in granulosa cells and blood leukocytes is associated with pcos and related traits. J Clin Endocrinol Metab. 2017;102(4):1396–405. https://doi.org/10.1210/jc.2016-2645.
Thathapudi S, et al. Association of luteinizing hormone chorionic gonadotropin receptor gene polymorphism (rs2293275) with polycystic ovarian syndrome. Genet Test Mol Biomarkers. 2015;19(3):128–32. https://doi.org/10.1089/gtmb.2014.0249.
Saddick SY. Identifying genes associated with the development of human polycystic ovary syndrome. Saudi J Biol Sci. 2020;27(5):1271–9. https://doi.org/10.1016/j.sjbs.2020.01.012.
Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–95. https://doi.org/10.1016/j.psyneuen.2009.05.021.
Magee JA, et al. Direct, androgen receptor-mediated regulation of the FKBP5 gene via a distal enhancer element. Endocrinology. 2006;147(1):590–8. https://doi.org/10.1210/en.2005-1001.
Hausl AS, et al. Focus on FKBP51: a molecular link between stress and metabolic disorders. Mol Metab. 2019;29:170–81. https://doi.org/10.1016/j.molmet.2019.09.003.
Wang H, et al. Association of FKBP5 polymorphisms with patient susceptibility to coronary artery disease comorbid with depression. PeerJ. 2020;8:e9286. https://doi.org/10.7717/peerj.9286.
Nam-Menke M, Strauss JF. Genetics of polycystic ovarian syndrome. Clin Obstet Gynecol. 2007;50(1):188–204. https://doi.org/10.1097/GRF.0b013e3180305f7c.
Chen ZJ, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43(1):55–9. https://doi.org/10.1038/ng.732.
Chazenbalk G, et al. Regulation of adiponectin secretion by adipocytes in the polycystic ovary syndrome: role of tumor necrosis factor-α. J Clin Endocrinol Metabol. 2010;95(2):935–42. https://doi.org/10.1210/jc.2009-1158.
Xu Z, Taylor JA. SNPinfo: integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res. 2009;37(Web Server issue):W600–5. https://doi.org/10.1093/nar/gkp290.
Russo P, et al. FKBP5 rs4713916: a potential genetic predictor of interindividual different response to inhaled corticosteroids in patients with chronic obstructive pulmonary disease in a real-life setting. Int J Mol Sci. 2019;20(8):2024. https://doi.org/10.3390/ijms20082024.
Li G, et al. FKBP5 genotype linked to combined PTSD-depression symptom in Chinese earthquake survivors. Can J Psychiatry. 2019;64(12):863–71. https://doi.org/10.1177/0706743719870505.
Chen CC, et al. Methods for identifying SNP interactions: a review on variations of logic regression, random forest and bayesian logistic regression. IEEE/ACM Trans Comput Biol Bioinform. 2011;8(6):1580–91. https://doi.org/10.1109/TCBB.2011.46.
Gilling-Smith C, et al. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J Clin Endocrinol Metab. 1994;79(4):1158–65. https://doi.org/10.1210/jcem.79.4.7962289.
Ha L, et al. Association study between polycystic ovarian syndrome and the susceptibility genes polymorphisms in Hui Chinese women. PLoS ONE. 2015;10(5):e0126505. https://doi.org/10.1371/journal.pone.0126505.
Ketefian A, et al. Association study of androgen signaling pathway genes in polycystic ovary syndrome. Fertil Steril. 2016;105(2):467-73 e4. https://doi.org/10.1016/j.fertnstert.2015.09.043.
Flores-Martinez SE, et al. Analysis of the association of SNP-63 and indel-19 variant in the calpain-10 gene with polycystic ovary syndrome in women of reproductive age. Cir Cir. 2015;83(1):35–42.
Dadachanji R, et al. PON1 polymorphisms are associated with polycystic ovary syndrome susceptibility, related traits, and PON1 activity in Indian women with the syndrome. Fertil Steril. 2015;104(1):207–16. https://doi.org/10.1016/j.fertnstert.2015.03.037.
Gangopadhyay S, et al. Single-nucleotide polymorphism on exon 17 of insulin receptor gene influences insulin resistance in PCOS: a pilot study on North Indian women. Biochem Genet. 2016;54(2):158–68. https://doi.org/10.1007/s10528-015-9708-7.
Park JM, et al. A single nucleotide polymorphism in exon 7 of sorbin and SH3-domain-containing-1 (SORBS1) in Korean PCOS patients. Mol Med Rep. 2008;1(1):93–7.
Escobar-Morreale HF, et al. The striking similarities in the metabolic associations of female androgen excess and male androgen deficiency. Hum Reprod. 2014;29(10):2083–91. https://doi.org/10.1093/humrep/deu198.
Sidibeh CO, et al. FKBP5 expression in human adipose tissue: potential role in glucose and lipid metabolism, adipogenesis and type 2 diabetes. Endocrine. 2018;62(1):116–28. https://doi.org/10.1007/s12020-018-1674-5.
Hubler TR, Scammell JG. Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids. Cell Stress Chaperones. 2004;9(3):243–52. https://doi.org/10.1379/csc-32r.1.
Jaaskelainen T, Makkonen H, Palvimo JJ. Steroid up-regulation of FKBP51 and its role in hormone signaling. Curr Opin Pharmacol. 2011;11(4):326–31. https://doi.org/10.1016/j.coph.2011.04.006.
Ortiz R, et al. Type 2 diabetes and cardiometabolic risk may be associated with increase in DNA methylation of FKBP5. Clin Epigenetics. 2018. https://doi.org/10.1186/s13148-018-0513-0.
Binder EB, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36(12):1319–25. https://doi.org/10.1038/ng1479.
Klengel T, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16(1):33–41. https://doi.org/10.1038/nn.3275.
Zannas AS, et al. Epigenetic upregulation of FKBP5 by aging and stress contributes to NF-kappaB-driven inflammation and cardiovascular risk. Proc Natl Acad Sci USA. 2019;116(23):11370–9. https://doi.org/10.1073/pnas.1816847116.
Grabe HJ, et al. Effect of the interaction between childhood abuse and rs1360780 of the FKBP5 gene on gray matter volume in a general population sample. Hum Brain Mapp. 2016;37(4):1602–13. https://doi.org/10.1002/hbm.23123.
Wan JP, et al. The common variant rs11646213 is associated with preeclampsia in Han Chinese women. PLoS ONE. 2013;8(8):e71202. https://doi.org/10.1371/journal.pone.0071202.
Acknowledgements
We are grateful to all participants for donating their blood for this study.
Funding
The study was supported by The National Key Research and Development Program of China (2017YFC1001002).
Author information
Authors and Affiliations
Contributions
YZ and YC conceived idea, and XM performed most of the tests, analyzed the date, and wrote the manuscript. XZ and XL did the work of preparing samples, ZW and CZ did the work of extracting DNA and sequencing, YB assisted in data analysis and data interpretation, and YC revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All patients in this study gave their informed written consent; the protocol for this study was reviewed and approved by the Institutional Review Board of Reproductive Medicine of Shandong University ([2020] Ethical Review #44). All methods of this study were carried out in accordance with relevant guidelines and regulations addressed in the Declaration of Helsinki, adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1
. Table S1: Allele frequencies of FKBP5 SNPs in the PCOS patients and in healthy controls.
Additional file2.
Table S2: Genotype frequencies and genetic model analysis of FKBP5 SNPs in the IR group and the control group.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ma, X., Wang, Z., Zhang, C. et al. Association of SNPs in the FK-506 binding protein (FKBP5) gene among Han Chinese women with polycystic ovary syndrome. BMC Med Genomics 15, 149 (2022). https://doi.org/10.1186/s12920-022-01301-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-022-01301-0