Abstract
Background
Small cell lung cancer (SCLC) is an aggressive disease with poor survival. Although molecular and clinical characteristics have been established for SCLC in western patients, limited investigation has been performed for Chinese SCLC patients.
Objective
In this study, we investigated the genomic features of Chinese SCLC patients.
Methods
A total of 75 SCLC patients were enrolled. Genomic alterations in 618 selected genes were analyzed by targeted next-generation sequencing.
Results
Here, we showed that TP53 (77.30%) and RB1 (30.70%) were the most prevalent genes alterations, followed by KMT2D, ALK, LRP1B, EGFR, NOTCH3, AR, CREBBP, ROS1, and BRCA2. And the most common genetic alterations were enriched in the cell cycle signaling pathway (84.00%) of Chinese SCLC patients. DNA damage repair (DDR) pathway analysis showed that the most frequently enriched DDR pathways were fanconi anaemia (FA, 29.41%) and homology recombination (HR, 21.57%). Notably, 9.33% SCLC patients in our cohort had pathogenic or likely pathogenic germline gene variants. Compared with the U Cologne cohort, a higher prevalence in EGFR, AR, BRCA2, TSC1, ATXN3, MET, MSH2, ERBB3 and FOXA1 were found in our cohort; while compared to the data from the Johns Hopkins cohort, a higher mutated frequency in TP53, KMT2D, ALK, and EGFR were found in our cohort. Moreover, a significant association was found between high tumor mutation burden (TMB) and mutations involved in TP53, CREBBP, EPHA3, KMT2D, ALK and RB1. Approximately 33.33% of patients with SCLC harbored at least one actionable alteration annotated by OncoKB, of which one patient had alterations of level 1; seventeen patients had level 3; fifteen patients possessed level 4.
Conclusion
Our data might provide an insightful meaning in targeted therapy for Chinese SCLC patients.
Similar content being viewed by others
Introduction
Small cell lung cancer (SCLC) is a highly malignant form of lung cancer that kills ~ 250,000 people worldwide annually and accounts for approximately 15% of lung cancer cases [1]. Biologically, rapid doubling time and early widespread metastases are characteristic of SCLC. Around 70% of cases present with the extensive-stage disease at diagnosis (ES-SCLC); the remaining 30% of patients have the limited-stage disease (LS-SCLC), in which tumor involvement is confined to one hemithorax and can be treated in a tolerable radiation field. The overall prognosis of SCLC patients is poor, with a median overall survival (OS) of 15–20 months for LS-SCLC and 8–13 months for ES-SCLC [2, 3].
Chemotherapy has been the bedrock of the treatment of SCLC for over two decades, now is replaced by immuno-chemotherapy strategy. Compared with chemotherapy alone, combination therapy with atezolizumab, an anti-program death ligand 1 (PD-L1) antibody and chemotherapy as the first-line treatment of ES-SCLC significantly prolonged overall and progression-free survival [4]. In a subsequent study, durvalumab, another PD-L1 antibody, in combination with platinum and etoposide also significantly improved overall survival in ES-SCLC patients [5]. Although the advent of immunotherapy has benefited SCLC patients, with only a modest efficacy, compared to other solid tumors. In addition, there is still a lack of targeted therapy for SCLC. Therefore, therapeutic strategy for SCLC treatment still has a lot of room for improve, and there are many problems and limitations that need to be solved urgently.
Some studies based on Caucasian population identified alterations in TP53 and RB1 were the most prevalent in SCLC [6,7,8]. In addition, PIK3CA, EGFR and KRAS also have high mutation frequency in SCLC [6]. Specifically, biallelic inactivation of TP53 and RB1 can be detected in almost all the SCLC tumors, suggesting that loss of the tumor suppressors TP53 and RB1 is obligatory in SCLC [6]. However, mutations in other genes varied from study to study. The majority of mutations have little significance for the SCLC pathogenesis and are described as passenger mutations. Finding the driving mutations of heterogenous diseases among SCLC patients and developing them into actionable targets for treatment are the primary issues to be faced [9]. There are very few genomic data of SCLC in China. In order to fill the gap of comprehensive genomic variation of SCLC, it is necessary to track more genomic variation of SCLC from different populations. In addition, the prognostic value of mutated genes in SCLC has not been well investigated.
With the in-depth research on the mechanism of DNA damage repair (DDR), people have a further understanding of improving sensitivity and overcoming resistance to traditional DNA damage treatment [10]. Although DDR data are scarce in SCLC, Byers et al. identified the DNA repair protein poly ADP-ribose polymerase 1 (PARP 1) as a therapeutic target [11]. Preclinical SCLC models were sensitive to PARP inhibition alone and the efficacy of chemotherapy was also enhanced by the addition of a PARP inhibitor [12, 13]. Despite of this, definite recurrent and targetable genomic alterations have not been identified in SCLC at present, especially in the Chinese population. Moreover, the DDR profile of Chinese SCLC patients was still not very clear yet.
Here, we carried out this study to clarify the genomic alterations and molecular characteristics of Chinese SCLC patients, especially DDR alterations and TMB levels. We attempted to better understand the association of genomic alterations with TMB levels in SCLC, and identify candidate prognostic biomarkers. Additionally, we tried to figure out whether there were significant differences in the mutational data between our cohort and the other two cohorts from cBioportal database. We further investigated the germline mutations and defined the frequency of actionable alterations to catch sight of the genetic features as well as corresponding target therapies in Chinese SCLC patients.
Materials and methods
Biospecimen collection and clinical data
Biospecimens of 75 SCLC patients were collected. All patients provided written informed consent for publication of their clinical details. Formalin-fixed, paraffin-embedded (FFPE) tumor tissues were pathologically assessed to have at least 20% tumor cells. Blood samples were drawn into Cell-free DNA BCT tubes (Streck, Inc.). Blood Cell-free DNA (cfDNA) testing were performed in 50 patients who could not provide sufficient or valid tumor tissue samples.
DNA isolation
The FFPE samples and peripheral blood mononuclear cells were collected using DNeasy Blood &Tissue Kit (Qiagen, Inc.) to isolate gDNA following the manufacturer’s instruction [14]. cfDNA was extracted from blood was using the QIAamp Circulating Nucleic Acid Kit (Qiagen, Inc.) according to the protocol of the manufacturer. The purified gDNA and cfDNA were quantified using the Qubit 3.0 Fluorometer (Life Technologies, Inc.) and StepOnePlus System (Life Technologies, Inc.) [14].
Target next-generation sequencing
For the tumor and blood samples, 100 ng gDNA was sheared to target 200 bp fragment sizes with the Covaris E210 system (Covaris, Inc.). Next-generation sequencing of gDNA and cfDNA was performed, in which Accel-NGS 2S DNA Library Kit (Swift Biosciences, Inc.) was used for library preparation and xGen Lockdown Probes kit (IDT, Inc.) for target enrichment [14]. The custom xGen Lockdown probe was synthesized by IDT, Inc. for the exons and selected intronic regions of 618 genes (Additional file 1: Table S1). The prepared library was quantified using the Qubit 3.0 Fluorometer (Life Technologies, Inc.), and quality and fragment size were measured with an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.). Samples underwent paired-end sequencing on an Illumina Nextseq CN500 platform (Illumina Inc) with a 150-bp read length [15]. The mean coverage of tumor gDNA, blood cfDNA and peripheral blood mononuclear cells was more than 1000 × , 3500 × and 200 × , respectively.
Tumor mutation burden analysis
Tumor mutation burden (TMB) was defined as the total somatic nonsynonymous mutation counts in coding regions [16]. TMB was classified into high and low categories, with the top quartile as the cutoff value.
Interpretation of pathogenicity of germline variants
Variants were detected in the white blood cells with at least 8 supporting reads and allele frequency beyond 20% were considered as germline variants. Then those variants with population allele frequency over 1% (from 1000 genomes and ExAC database), labeled as benign or likely benign in the latest Clinvar database and/or synonymous were excluded. The interpretation of germline variants followed the standards and guidelines of American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG/AMP) and independently reviewed by two genetic consultants [17].
Data and statistical analysis
Raw sequencing data were aligned to the reference human genome (UCSC hg19) through Burrows-Wheeler Aligner and producing a BAM (binary alignment/map) file [18]. After removing duplicate and local realignment, single nucleotide variation (SNV)/indel calls were performed using the Genome Analysis Toolkit (GATK) [19]. Somatic variants were generated for the patient by subtracting the germline variants from the tumor to keep only variants unique to a tumor. Variants were annotated using the ANNOVAR software tool. Somatic mutations were annotated with information from the Catalog of Somatic Mutations in Cancer (COSMIC) database [20]. The Genomic alterations data of Johns Hopkins, Nat Genet 2012 (80 patients) and U Cologne Nature 2015 (120 patients) was downloaded from OncoKB (https://www.oncokb.org/) [21]. The survival data was downloaded from National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/pmc/). Differential mutations analysis was performed under a dominant model using Chi Square test or Fisher exact test. P values less than 0.05 on two-sides were considered statistically significant. All analyses were performed by SPSS 25.0 software.
Results
Clinicopathological characteristics of SCLC patients
This study enrolled a total of 75 Chinese SCLC patients, among whom 52 were males and 23 patients were female. The clinical characteristic obtained are summarized in Table 1. The ages of the patients ranged from 39 to 89 with a median age of 66. Eight (10.7%) SCLC patients had been diagnosed with II-III stage, and 67 (89.3%) patients with IV stage. Moreover, 18 (24.0%) cases presented a family cancer history, and 55 (73.3%) individuals without it. All tumor samples were pathologically assessed to have a purity of at least 20%.
The landscape of mutation profiles in SCLC
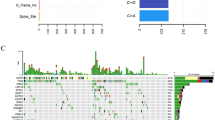
Through targeted deep sequencing of all exons and selected introns of 618 cancer-related genes (Additional file 1: Table S1) in 75 SCLC tissue and blood samples, a total of 97.3% (73/75) of the samples were identified as valid somatic mutations. As shown in Fig. 1A, a total of 978 mutations were identified in 75 cases, with a median mutation number of 13 per patient (range 1–60). Missense mutation was the main mutation type, and the frequency of single nucleotide polymorphism was higher than that of insertion and deletion, C > T was the most common single nucleotide variation in SCLC (Fig. 1B–D). In addition, we counted the number of altered bases in each sample and color-coded the types of mutations (Fig. 1E, F). The top 10 mutated genes with highest prevalence were TP53 (77.30%), RB1 (30.70%), KMT2D (17.30%), ALK (16.00%), LRP1B (14.70%), EGFR (14.70%), NOTCH3 (14.70%), AR (14.70%), CREBBP (12.00%), ROS1 (12.00%), and BRCA2 (12.00%), respectively (Fig. 1G). Pathway analysis indicated that the most frequently enriched pathways were Cell Cycle (84.00%), RTK-RAS-MAPK (72.00%), DNA damage repair (DDR, 49.33%), Epigenetic_modifiers/Chromatin_remodelers (40.00%), and NOTCH (36.00%), respectively (Fig. 1H).
The landscape of mutated genes in a series of 75 SCLC patients. A Oncoprint of the 30 most frequently mutated genes in our cohort. B Summary of the mutation information with statistical calculations. C, D Classification of mutation types according to different categories, in which missense mutation accounts for the most fraction, SNP showed more frequency than insertion or deletion, and C > T was the most common of SNV; E, F) Tumor mutation burden in specific samples. G The top 10 mutated genes in SCLC. H The prevalence of total and oncogenic alterations in specified signaling pathways in SCLC. SNV: single nucleotide variation
Germline mutations in Chinese SCLC patients
In our cohort, 60.00% (45/75) patients harbored at least one germline mutation, and the total number of germline mutations was 105. The patients with germline mutation were further divided into pathogenic/likely pathogenic and non-pathogenic types, and the patients with pathogenic/likely pathogenic germline mutation accounted for 9.33% of the 75 SCLC patients. These germline mutation genes included BRCA2, BRCA1, ATM, UCP3, GCDH, MPL, SMO, FGFR4 and TP53 genes. A deleterious mutation in the germline may indicate family heredity, so we investigated the familial history of cancer in 75 SCLC patients. After excluding 2 patients who were unwilling to provide a family history of cancer, we found that 24.00% (18/75) had a family history of cancer, with most family members being diagnosed with respiratory and digestive tract tumors. We further screened 5 patients with germline mutations and found that only 1 carrier had susceptible genetic hereditary phenomena in his family (Table 2). Notably, this patient has been identified with dual deleterious variants, including an ATM-c.2376 + 1G > A and a TP53-p.Arg273His.
Genetic alterations in DNA damage repair pathway
A total of 28 patients (37.33%) harbored at least one alteration in DNA repair genes. The distribution of specific genes was exhibited in Fig. 2A, and the most frequently mutated DDR genes with known or likely deleterious variants were BRCA2 (n = 8, 13.33%) and MSH2 (n = 6, 10.00%), followed by ATM (n = 4, 6.67%), ATR (n = 4, 6.67%) and BRCA1 (n = 4, 6.67%). DDR pathway analysis showed that the most frequently enriched DDR pathways were homology recombination (HR, 35.00%), fanconi anaemia (FA, 20.00%), mismatch repair (MMR, 16.67%), DNA sensor (DS, 13.33%), base excision repair (BER, 8.33%) and nucleotide excision repair (NER, 6.67%) respectively (Fig. 2B). We also analyzed the clinical significance of DDR-related genes and these genes are listed below (Table 3). Particularly, HR pathway accounts for the most among these genes, followed by MMR and DS pathway.
Differences of somatic gene mutations in SCLC patients between our cohort and Western cohorts
Comparing the significantly mutated genes with U Cologne cohort showed that there were several significantly lower mutated genes in RB1 (30.67 vs 79.09%), LRP1B (14.67 vs 46.36%) and TP53 (77.33 vs 93.64%), but a higher prevalence in EGFR (14.67% vs 3.64%), AR (14.67 vs 4.55%), BRCA2 (12 vs 1.82%), TSC1 (10.67 vs 0.91%), ATXN3 (9.33 vs 1.82%), MET (9.33 vs 1.82%), MSH2 (8 vs 0.91%), ERBB3 (8 vs 0.91%) and FOXA1 (8% vs none) were presented in our cohort (Fig. 3A). While compared to the data from Johns Hopkins cohort, a higher mutated frequency in TP53 (77.33 vs 45.0%), KMT2D (17.33 vs 3.75%), ALK (16 vs 2.5%), and EGFR (14.67% vs none) were found in our cohort (Fig. 3B).
A Comparisons of the gene prevalence identified in our cohort (red bars) and U Cologne cohort (green bars). B Comparisons of the gene prevalence identified in our cohort and in Johns Hopkins cohort. Two-sided Fisher’s tests were conducted to compare the different frequency between two cohorts. ***p \(\le\) 0.001, **p \(\le\) 0.01, *p \(\le\) 0.05
TMB analysis in the Chinese cohort
The TMB values in our cohort ranged from 2.00/Mb to 64.29/Mb with a median value of 14.53/Mb. And the TMB was significantly higher in blood samples than in the tissue sample group (p = 0.028) as more extensive stage cases involved. However, there were no significant differences in TMB were observed between each of these compared groups with age, gender and DDR mutation (Fig. 4A–D). Moreover, the median TMB of patients with alterations in TP53 (p = 0.018), CREBBP (p = 0.013), EPHA3 (p = 0.013), KMT2D (p = 0.03), ALK (p = 0.046) and RB1 (p = 0.05) genes were higher than those without the alterations, on the contrary the median TMB of patients with PIK3CA alteration (p = 0.019) was lower (Fig. 4E).
Clinically actionable alterations
To evaluate the clinical utility of prospective molecular profiling to guide treatment decisions, we used OncoKB (http://oncokb.org/) to group all mutations into various levels according to evidence of clinical actionability. Overall, 33.33% (25/75) of patients harbored at least one actionable alteration (Table 4). We found a group of gene mutations as standard care biomarkers for an FDA-approved drug in another indication. 5.56% of tumors harbored level_1 gene alteration (Fig. 5A) including NTRK (Fig. 5B). Level_3 accounted for 61.11% (Fig. 5A), including ALK, BRAF, CDK12, ERBB2, TSC1, ATM, BRCA1/2, EGFR, PIK3CA (Fig. 5B). Level_4 accounted for 33.33% (Fig. 5A), including AKT, U2AF1, SF3B1, FGFR1, HRAS (Fig. 5B). Additionally, two germline alterations including one BRCA1 germline alterations and one ATM germline alteration may confer sensitivity to corresponding target therapy (Table 4) (Fig. 5).
Discussion
SCLC is an aggressive and refractory form of lung cancer originated from neuroendocrine cells. It must be emphasized that although immune checkpoint therapy has paved the new way for the treatment of SCLC, more precise and effective therapy for SCLC still need to be explored. However, due to the lack of treatable oncogene mutations, molecular targeted therapies for SCLC have not yet been developed. Modern technologies such as next-generation sequencing (NGS) can carry out gene profiling of cancer cells, making the successful development of molecular targeted therapy possible, and has remarkable potential to realize the precision medicine for cancers. In this study, we used NGS to elucidate the genomic characteristics of Chinese SCLC patients, especially DDR alterations and TMB levels, to provide a basis for the development of precision targeted therapy.
As expected, we detected the most frequent mutations in TP53 (77.3%) and RB1 (30.7%), which in line with previous publications [6, 22]. In addition to common genomic alterations, alterations in other tumor-related genes displayed a unique feature in Chinese populations. The prevalence of EGFR, BRCA2, TSC1, KMT2D and ALK gene alterations was higher in the Chinese cohort than in the Western population. Among those differences, BRCA2 was the well-known biomarkers for PARP inhibitors [23], and TSC1 naturally suppressed the overactivity of downstream mammalian target of rapamycin (mTOR), which indicated the potential clinical benefits of patients with TSC1 loss of function mutations from mTOR inhibitors [24, 25]. EGFR mutation and ALK rearrangement are meaningful targetable driver alterations in lung adenocarcinoma (LUAD) and non-small-cell lung cancer (NSCLC), respectively [26, 27]. Histological transformation of EGFR-driven or ALK-driven LUAD to SCLC has been reported in some cases [28]. The conversion of LUAD to SCLC has been shown to be associated with acquired resistance to EGFR or other tyrosine kinase receptor inhibitors [29,30,31]. However, the patients enrolled in our study were all patients with primary SCLC who had not been converted to SCLC from other cancer types after multiline therapy. Moreover, the frequency of EGFR and ALK mutations measured in our cohort is higher than previously reported, which is a finding worthy of further exploration. However, compared with the Western SCLC patients (U Cologne cohort), the incidence of LRP1B in Chinese patients with SCLC was lower. Due to its long coding sequence, LRP1B is often omitted from genomic research, but its mutation may still have a functional consequence in tumorigenesis and heterogeneity [32]. This gene encoded lipoprotein receptor-related protein 1B, and was suggested as a novel tumor suppressor gene and associated with better efficacy with immunotherapy in NSCLC and melanoma [33]. In patients with multiple primary lung cancers, LRP1B alterations were also associated with higher TMB value and positive tumor PDL1 expression [34]. There was poor number of studies reporting on the prevalence and function of LRP1B in SCLC, and our study provided a clue of the difference role of it in the carcinogenesis of SCLC between Western and Chinese patients. Whether loss of function or deletion of LRP1B related to the clinical outcome of LRP1B inhibitors was not clear, but the lower incidence of this gene may indicate the differences in the pathogenesis between different ethnic groups. Our genomic analyses further compared the genetic alterations involved in several cancer-related signaling pathways in the Chinese cohort. We found that most of the mutant genes were enriched in the Cell Cycle, RTK-RAS-MAPK and DDR signaling pathways, suggesting that the molecular characterization of these pathways is closely related to the development of SCLC.
Previous studies have similarly examined the prevalence and spectrum of germline variants in SCLC patients, but they are primarily focused on limited genes or in a small subset [35]. Our findings provide a novel insight on the SCLC with germline alterations in the Chinese population tested by an NGS panel with 618 cancer-related genes. Specifically, 9.33% of Chinese SCLC patients had pathogenic or likely pathogenic germline gene variants, including BRCA2, BRCA1, ATM, UCP3, GCDH, MPL, SMO, FGFR4 and TP53. Moreover, 24% of patients had a family history of cancer, highlighting the necessity of risk assessment for those patients and their first-degree family members. Additionally, some publications have investigated the roles of germline alternations, mostly selected mutations, in genetic susceptibility to lung cancer [36, 37], while systematic studies of the germline mutations potentially predisposing to lung cancer. For example, the identification of germline mutations in driver oncogenes like EGFR, has heightened interest in identifying germline mutations carrying a high inherited risk of lung cancer [38]. However, EGFR mutations are not conventional germline mutations associated with hereditary cancers, and are not common in our cohort as well [39]. Liu et al. found that BRCA2 and ATM were germline mutations with the highest mutation frequency in Chinese lung cancer patients, similar to our results [40].
Unlike NSCLC, SCLC harbors few actionable mutations that can be used for therapeutic intervention. Actionability is defined as a molecular alteration that has clinical or strong preclinical evidence of a predictive benefit from a specific therapy (in any cancer type) [41, 42]. Here, we detected that 33.33% of SCLC patients had at least one actionable alteration with any level of evidence from OncoKB. Our results provide a new insight into patients with SCLC tumors who harbor actionable molecular alterations and receive appropriately matched therapy. Pishvaian’s investigation showed that patients with actionable molecular alterations could benefit considerably from receiving matched therapy [43]. It has been reported that patients with advanced pancreatic cancer with actionable alterations who received matched therapy had a one-year increase in median overall survival compared with patients with or without actionable alterations who did not receive matched therapy. However, other therapeutic modality did not offer such a huge advantage for this patient population. Thus, these findings set the stage for prospective clinical trials guided by molecular profiling. Previous findings revealed that the median PFS of patients with actionable alterations undergoing molecularly matched therapies is significantly longer than that of historical controls. To our knowledge, there is no systematic assessment of median overall survival of SCLC patients with molecularly matched therapies [44]. The sensitivity of these analyses to molecular profiling warrants further investigation.
DDR pathway defects may lead to severe DNA damage, resulting in genome instability and trigger malignant transformation [45]. Therefore, targeting the DDR pathway may be a promising therapeutic strategy for SCLC [9, 46]. The high frequency of DDR gene and pathway alterations in our cohort and other studies identifies opportunities to improve cancer therapy. For example, HR defects are relatively common in cancer and may compromise DNA replication and genome stability [47]. Thus, combination therapies that induce or potentiate replication stress or impair replication fork protection may effectively inhibit HR-deficient cancers like SCLC. PARP inhibitors have demonstrated great promise in the treatment of patients with deficiencies in HR DNA repair. Among the DDR proteins, PARP inhibitors are the most attractive agents in clinical research [48]. Farago et al. conducted a phase I/II trial combining olaparib (PARP inhibitor) with temozolomide in previously treated SCLC patients. The results showed that the overall response rate was 41.7%, the median overall survival was 8.5 months, and the median progression-free survival was 4.2 months [49]. Their findings provide a promising new therapeutic strategy for SCLC. PARP inhibitors are active in SCLC models and clinical trials are in progress as well [9, 13], so the clinical benefit of these biomarker-targeted therapies for patients with SCLC will hopefully be realized.
This study also has some limitations. Firstly, serial analyses of tumor biopsies have not been performed in some SCLC patients, limiting molecular studies and biomarker assessments of treatment-induced changes in this cancer type. Secondly, due to the limited sample size, the results may have some deviation.
Conclusions
Our study describes the clinical characteristics of SCLC in China and identifies many novel candidate genes, some of which may have therapeutic implications. Our results further figure out there were significant differences between our cohort and other two cohorts from cBioportal database of the mutational data. Analysis of these altered genes provided information regarding the molecular mechanisms of SCLC and significant biomarkers or targets for the diagnosis and treatment of SCLC. However, further molecular biological experiments are required to confirm the function of the pathways in SCLC.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SCLC:
-
Small cell lung cancer
- DDR:
-
DNA damage repair
- TMB:
-
Tumor mutation burden
- FA:
-
Fanconi anaemia
- HR:
-
Homology recombination
- MMR:
-
Mismatch repair
- DS:
-
DNA sensor
- BER:
-
Base excision repair
- NER:
-
Nucleotide excision repair
- OS:
-
Overall survival
- ICI:
-
Immune checkpoint inhibitors
- PARP:
-
Poly ADP-ribose polymerase
- FFPE:
-
Formalin-fixed, paraffin-embedded
- cfDNA:
-
Cell-free DNA
References
Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17(12):765.
Pietanza MC, Byers LA, Minna JD, Rudin CM. Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res. 2015;21(10):2244–55.
Kalemkerian GP, Akerley W, Bogner P, Borghaei H, Chow LQ, Downey RJ, Gandhi L, Ganti AK, Govindan R, Grecula JC, et al. Small cell lung cancer. J Natl Compr Cancer Netw. 2013;11(1):78–98.
Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. New Engl J Med. 2018;379(23):2220–9.
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–39.
George J, Lim JS, Jang SJ, Cun Y, Ozretić L, Kong G, Leenders F, Lu X, Fernández-Cuesta L, Bosco G, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524(7563):47–53.
Peifer M, Fernández-Cuesta L, Sos ML, George J, Seidel D, Kasper LH, Plenker D, Leenders F, Sun R, Zander T, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet. 2012;44(10):1104–10.
Rudin CM, Durinck S, Stawiski EW, Poirier JT, Modrusan Z, Shames DS, Bergbower EA, Guan Y, Shin J, Guillory J, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet. 2012;44(10):1111–6.
Foy V, Schenk MW, Baker K, Gomes F, Lallo A, Frese KK, Forster M, Dive C, Blackhall F. Targeting DNA damage in SCLC. Lung Cancer. 2017;114:12–22.
Thomas A, Pommier Y. Small cell lung cancer: time to revisit DNA-damaging chemotherapy. Sci Transl Med. 2016. https://doi.org/10.1126/scitranslmed.aaf6282.
Byers LA, Wang J, Nilsson MB, Fujimoto J, Saintigny P, Yordy J, Giri U, Peyton M, Fan YH, Diao L, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012;2(9):798–811.
Cardnell RJ, Feng Y, Diao L, Fan YH, Masrorpour F, Wang J, Shen Y, Mills GB, Minna JD, Heymach JV, et al. Proteomic markers of DNA repair and PI3K pathway activation predict response to the PARP inhibitor BMN 673 in small cell lung cancer. Clin Cancer Res. 2013;19(22):6322–8.
Lok BH, Gardner EE, Schneeberger VE, Ni A, Desmeules P, Rekhtman N, de Stanchina E, Teicher BA, Riaz N, Powell SN, et al. PARP inhibitor activity correlates with SLFN11 expression and demonstrates synergy with temozolomide in small cell lung cancer. Clin Cancer Res. 2017;23(2):523–35.
Yang B, Zhao X, Wan C, Ma X, Niu S, Guo A, Wang J, Wang J, Sun D, Jiao S. Genomic profiling of Chinese patients with urothelial carcinoma. BMC Cancer. 2021;21(1):162.
Wen L, Li X, Shi J, Zhang S, Wang R, Yao M, Guo J. Allele-specific expression mediates primary resistance to poly (ADP-ribose) polymerase inhibitor therapy in a case of BRCA1/2 double-germline mutant gastric cancer. J Int Med Res. 2020;48(3):300060519886226.
Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015;17(5):405–24.
Yang L, Zhang X, Wang T. The Burrows-Wheeler similarity distribution between biological sequences based on Burrows-Wheeler transform. J Theor Biol. 2010;262(4):742–9.
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–303.
Tate JG, Bamford S, Jubb HC, Sondka Z, Beare DM, Bindal N, Boutselakis H, Cole CG, Creatore C, Dawson E, et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019;47(D1):D941-d947.
Chakravarty D, Gao J, Phillips S, Kundra R, Zhang H, Wang J, Rudolph JE, Yaeger R, Soumerai T, Nissan MH, et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol. 2017. https://doi.org/10.1200/PO.17.00011.
Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, Heymach JV, Johnson JE, Lehman JM, MacPherson D, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. 2019;19(5):289–97.
Reisländer T, Lombardi EP, Groelly FJ, Miar A, Porru M, Di Vito S, Wright B, Lockstone H, Biroccio A, Harris A, et al. BRCA2 abrogation triggers innate immune responses potentiated by treatment with PARP inhibitors. Nat Commun. 2019;10(1):3143.
Luo YD, Fang L, Yu HQ, Zhang J, Lin XT, Liu XY, Wu D, Li GX, Huang D, Zhang YJ, et al. p53 haploinsufficiency and increased mTOR signalling define a subset of aggressive hepatocellular carcinoma. J Hepatol. 2021;74(1):96–108.
Mrozek EM, Bajaj V, Guo Y, Malinowska IA, Zhang J, Kwiatkowski DJ. Evaluation of Hsp90 and mTOR inhibitors as potential drugs for the treatment of TSC1/TSC2 deficient cancer. PLoS ONE. 2021;16(4): e0248380.
Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. 2021;398(10299):535–54.
Golding B, Luu A, Jones R, Viloria-Petit AM. The function and therapeutic targeting of anaplastic lymphoma kinase (ALK) in non-small cell lung cancer (NSCLC). Mol Cancer. 2018;17(1):52.
Quintanal-Villalonga Á, Chan JM, Yu HA, Pe’er D, Sawyers CL, Sen T, Rudin CM. Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat Rev Clin Oncol. 2020;17(6):360–71.
Niederst MJ, Sequist LV, Poirier JT, Mermel CH, Lockerman EL, Garcia AR, Katayama R, Costa C, Ross KN, Moran T, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun. 2015;6:6377.
Popat S. Histologically transformed SCLC from EGFR-mutant NSCLC: understanding the wolf in sheep’s clothing. J Thorac Oncol. 2019;14(10):1689–91.
Offin M, Chan JM, Tenet M, Rizvi HA, Shen R, Riely GJ, Rekhtman N, Daneshbod Y, Quintanal-Villalonga A, Penson A, et al. Concurrent RB1 and TP53 alterations define a subset of EGFR-Mutant lung cancers at risk for histologic transformation and inferior clinical outcomes. J Thorac Oncol. 2019;14(10):1784–93.
Chen H, Chong W, Wu Q, Yao Y, Mao M, Wang X. Association of LRP1B Mutation with tumor mutation burden and outcomes in melanoma and non-small cell lung cancer patients treated with immune check-point blockades. Front Immunol. 2019;10:1113.
Chen H, Chong W, Wu Q, Yao Y, Mao M, Wang X. LRP1BAssociation of mutation with tumor mutation burden and outcomes in melanoma and non-small cell lung cancer patients treated with immune check-point blockades. Front Immunol. 2019;10:1113.
Chunhong H, Zhao L, Liu W, Fan S, Liu J, Liu Y, Liu X, Shu L, Liu X, Liu P, et al. Genomic profiles and their associations with TMB, PD-L1 expression, and immune cell infiltration landscapes in synchronous multiple primary lung cancers. J Immunother Cancer. 2021;9(12):e003773. https://doi.org/10.1136/jitc-2021-003773.
Tlemsani C, Takahashi N, Pongor L, Rajapakse VN, Tyagi M, Wen X, Fasaye GA, Schmidt KT, Desai P, Kim C, et al. Whole-exome sequencing reveals germline-mutated small cell lung cancer subtype with favorable response to DNA repair–targeted therapies. Sci Transl Med. 2021. https://doi.org/10.1126/scitranslmed.abc7488.
Slavin TP, Banks KC, Chudova D, Oxnard GR, Odegaard JI, Nagy RJ, Tsang KWK, Neuhausen SL, Gray SW, Cristofanilli M, et al. Identification of incidental germline mutations in patients with advanced solid tumors who underwent cell free circulating tumor DNA sequencing. J Clin Oncol. 2018;36(35):3459.
Yamamoto H, Yatabe Y, Toyooka S. Inherited lung cancer syndromes targeting never smokers. Transl Lung Cancer Res. 2018;7(4):498–504.
Hu Y, Alden RS, Odegaard JI, Fairclough SR, Chen R, Heng J, Feeney N, Nagy RJ, Shah J, Ulrich B, et al. Discrimination of Germline EGFR T790M Mutations in Plasma Cell-Free DNA Allows Study of Prevalence Across 31,414 Cancer Patients. Clin Cancer Res. 2017;23(23):7351–9.
Parry EM, Gable DL, Stanley SE, Khalil SE, Antonescu V, Florea L, Armanios M. Germline mutations in DNA repair genes in lung adenocarcinoma. J Thorac Oncol. 2017;12(11):1673–8.
Liu Y, Lusk CM, Cho MH, Silverman EK, Qiao D, Zhang R, Scheurer ME, Kheradmand F, Wheeler DA, Tsavachidis S, et al. Rare variants in known susceptibility loci and their contribution to risk of lung cancer. J Thorac Oncol. 2018;13(10):1483–95.
Aguirre AJ, Nowak JA, Camarda ND, Moffitt RA, Ghazani AA, Hazar-Rethinam M, Raghavan S, Kim J, Brais LK, Ragon D, et al. Real-time genomic characterization of advanced pancreatic cancer to enable precision medicine. Cancer Discov. 2018;8(9):1096–111.
Khemlina G, Ikeda S, Kurzrock R. The biology of Hepatocellular carcinoma: implications for genomic and immune therapies. Mol Cancer. 2017;16(1):149.
Pishvaian MJ, Blais EM, Brody JR, Lyons E, DeArbeloa P, Hendifar A, Mikhail S, Chung V, Sahai V, Sohal DPS, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the know your tumor registry trial. Lancet Oncol. 2020;21(4):508–18.
Redman MW, Papadimitrakopoulou VA, Minichiello K, Hirsch FR, Mack PC, Schwartz LH, Vokes E, Ramalingam S, Leighl N, Bradley J, et al. Biomarker-driven therapies for previously treated squamous non-small-cell lung cancer (Lung-MAP SWOG S1400): a biomarker-driven master protocol. Lancet Oncol. 2020;21(12):1589–601.
Jeggo PA, Pearl LH, Carr AM. DNA repair, genome stability and cancer: a historical perspective. Nat Rev Cancer. 2016;16(1):35–42.
Sen T, Gay CM, Byers LA. Targeting DNA damage repair in small cell lung cancer and the biomarker landscape. Transl Lung Cancer Res. 2018;7(1):50–68.
Kadouri L, Rottenberg Y, Zick A, Hamburger T, Lipson D, Peretz T, Nechushtan H. Homologous recombination in lung cancer, germline and somatic mutations, clinical and phenotype characterization. Lung Cancer. 2019;137:48–51.
Taniguchi H, Sen T, Rudin CM. Targeted therapies and biomarkers in small cell lung cancer. Front Oncol. 2020;10:741.
Farago AF, Yeap BY, Stanzione M, Hung YP, Heist RS, Marcoux JP, Zhong J, Rangachari D, Barbie DA, Phat S, et al. Combination olaparib and temozolomide in relapsed small-cell lung cancer. Cancer Discov. 2019;9(10):1372–87.
Acknowledgements
We are grateful of all the participants in the Second Affiliated Hospital of South China University of Technology.
Funding
The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by JL, ZZ and BL. SW made data supplement and analysis for the article. The first draft of the manuscript was written by ZZ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Guangzhou First People's Hospital. All samples collection and processing were carried out respecting the Declaration of Helsinki. All patients signed informed consent prior to tumor tissues collection treatment, including allowing their data to be used for further research.
Consent for publication
Not Applicable.
Competing interests
All authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
618 selected cancer-related genes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, J., Zhao, Z., Wei, S. et al. Genomic features of Chinese small cell lung cancer. BMC Med Genomics 15, 117 (2022). https://doi.org/10.1186/s12920-022-01255-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-022-01255-3