Abstract
In low- and middle-income countries, data on antimicrobial use (AMU) and antimicrobial resistance (AMR) in aquaculture are scarce. Therefore, summarizing documented data on AMU, antimicrobial residue (AR), and AMR in aquaculture in Africa is key to understanding the risk to public health. Google Scholar, PubMed, African Journals online, and Medline were searched for articles published in English and French following the PRISMA guidelines. A structured search string was used with strict inclusion and exclusion criteria to retrieve and screen the articles. The pooled prevalence and 95% confidence intervals were calculated for each pathogen–antimicrobial pair using random effects models. Among the 113 full-text articles reviewed, 41 met the eligibility criteria. The majority of the articles reported AMR (35; 85.4%), while a few were on AMU (3; 7.3%) and AR (3; 7.3%) in fish. The articles originated from West Africa (23; 56.1%), North Africa (8; 19.7%), and East Africa (7; 17.1%). Concerning the antimicrobial agents used in fish farming, tetracycline was the most common antimicrobial class used, which justified the high prevalence of residues (up to 56.7%) observed in fish. For AMR, a total of 69 antimicrobial agents were tested against 24 types of bacteria isolated. Bacteria were resistant to all classes of antimicrobial agents and exhibited high levels of multidrug resistance. Escherichia coli, Salmonella spp., and Staphylococcus spp. were reported in 16, 10, and 8 studies, respectively, with multidrug resistance rates of 43.1% [95% CI (32.0–55.0)], 40.3% [95% CI (24.1–58.1)] and 31.3% [95% CI (17.5–49.4)], respectively. This review highlights the high multidrug resistance rate of bacteria from aquaculture to commonly used antimicrobial agents, such as tetracycline, ampicillin, cotrimoxazole, gentamicin, and amoxicillin, in Africa. These findings also highlighted the lack of data on AMU and residue in the aquaculture sector, and additional efforts should be made to fill these gaps and mitigate the burden of AMR on public health in Africa.
Similar content being viewed by others
Background
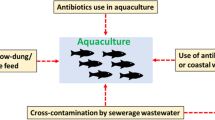
Aquaculture is a rapidly growing livestock production sector with an expected increase of 62% in 2030, and it represents one of the most sustainable and economical sources of protein for humans [1]. It provides approximately 15% of the animal protein needs of more than three billion people worldwide [2]. However, infectious diseases seriously threaten aquaculture production and the livelihoods of many households [3], and fish farmers usually use antimicrobial agents for the prevention and control of diseases and as growth promoters [4]. However, antimicrobials that are not efficiently metabolized by fish are eliminated through urine and feces [3]. Additionally, chemical substances such as disinfectants and biocides used to ensure good water quality [5], together with ARs from integrated production systems, may contribute to the selection, emergence, and spread of drug-resistant pathogens, which pose serious threats to public health [4, 6]. Research has indicated that the use of antimicrobials as growth promoters in agriculture is associated with the emergence of resistant foodborne pathogens, which are relatively risky to human, animal, and environmental health [7]. In most African countries, the choice of antimicrobial agent is not usually based on knowledge of bacterial susceptibility tests [8].
The inappropriate use of antimicrobials has accelerated AMR emergence at animal, human and environmental interfaces [9,10,11]. In various low- and middle-income countries, published data on AMR are more frequently observed in animal and human compartments than in environmental compartments. However, the scarcity of data might hamper efforts to fight AMR from the environment and mainly from aquaculture to human and animal health [2]. Summarized available data are essential for the development of local and regional treatment guidelines, with an emphasis on the need for sustainable efforts by stakeholders for the coordination and harmonization of competencies against the emergence of AMR [8]. Therefore, this study was carried out to systematically analyze validated information on AMU, ARs, and AMR emergence in fish production systems in Africa.
Methods
Search strategy
This systematic review was performed following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines [12]. The PubMed, Google Scholar, and African Journal Online databases were used to search for articles published in English and French on AMU, AR, and AMR in Africa. No limit on publication date was set. The literature search started from November 2020 to August 2021. The reference lists of relevant articles were checked for additional titles for inclusion in the review. The free text was obtained by contacting the authors directly. Additionally, attempts were made to contact the authors to obtain inaccessible abstracts and full texts from the included studies. Boolean operators (AND/OR/NOT) and predefined search terms of relevant studies conducted in African countries in aquaculture and related production sectors were adopted [8, 13]. The following keywords were used: ‘antimicrobial use’, ‘antibiotic use’, ‘chemical use’, ‘antibiotic residue’, ‘antimicrobial residue’, ‘chemical residue’, ‘antimicrobial resistance’, ‘antibiotic resistance’, ‘chemical resistance’, ‘aquaculture’, ‘fish farm’, ‘fish’, ‘shellfish’, ‘shrimp’, ‘Africa’, and ‘specific African countries.
Inclusion and exclusion criteria
The procedure for the inclusion and exclusion of articles in the systematic review and meta-analysis was similar to that described by Mouiche et al. [8]. Briefly, full-text articles published on AMU, AM, and the prevalence of AMR among bacteria isolated in aquaculture or natural aquatic milieu or in association with other food items in African countries were used in the review. After removing duplications and retracted citations in Zotero, the citations were uploaded to Rayyan software for screening. First, the selection process consisted of title and abstract screening. To increase consistency among reviewers, a calibration exercise was carried out on 10 randomly selected articles to enable discussion and resolve disagreements before the full-text selection process. Two authors (MMFN and FM) independently reviewed the publications to determine eligibility. When there was doubt about the decision, this was resolved by consensus or third-party consultation (MMMM and JAN) when consensus could not be reached. Publications that described aquatic subjects or aquatic populations studied or types of aquatic environmental samples, bacteria isolated, specific laboratory methods, antimicrobial sensitivity patterns, and antimicrobial tests were considered and included in the study. Articles obtained through the use of predefined search terms (which were translated to search articles written in French) to identify relevant literature were included. Studies on mycobacteriosis and outbreak disease were not included. Studies reporting aggregated data, such as studies in which resistance rates were aggregated in a large category, were excluded. Additionally, articles identified through a literature search that reported AMR in aquaculture and aquatic environments but that did not report prevalence data were not included in the meta-analysis.
Data extraction
The data were extracted from individual studies using a form and database developed for this review in Microsoft Excel 2013. The data extraction was independently performed by two coauthors (MMFN and FM), while MNT and RNGN conducted the datachecking of the included papers. When there was a confrontation of the data set, third-party (MMMM and JAN) consultation was performed for validation. Articles that met the inclusion criteria and reported AMR data in aquaculture production and aquatic environments were included in the meta-analysis. The extracted information included article information: first author, year of publication, duration of study and country, study design (cross-sectional or longitudinal study), type of aquaculture production or aquatic products (type and species of aquatic species, processed and unprocessed), sampling point and origin of aqua-product, aquatic environment (farm, natural, market for aquatic products), and type of sample (fluids, gastrointestinal content, tissue, organs). Qualitative and quantitative data on AMU (type of antimicrobial agent, frequency and indication of usage), AR (antimicrobial agents investigated, quantity, prevalence), and AMR (number of strains tested for AMR, number of resistant strains, antimicrobial panels tested) as well as laboratory procedures and bacteria investigated were also taken into consideration.
A quality assessment of the articles was performed to evaluate the reliability of the studies using a modified version of a critical appraisal tool developed for use in systematic reviews addressing questions of prevalence [14]. Each publication was assessed using 5 specific questions: (1) If the data included study period, sample type, and study zone? (2) Were the study subjects and setting described in detail? (3) Was the data analysis conducted with sufficient coverage of the identified germ? (4) Were the objectives and standard criteria used to measure the condition? (5) Was the condition measured reliably? Responses to each of the five questions were coded as yes (Y), no (N), or unclear (U) and categorized into three groups. Articles that answered “yes” to ≥ 80% of the items were classified as high quality (H), articles that answered “yes” to 60%—< 80% of the items were considered medium quality (M), and articles that answered “yes” to less than 60% of the items were considered low quality (L). Articles that scored high quality (H) or medium quality (M) were included in the review.
Data analysis
Descriptive statistics were used to summarize the characteristics of the articles included in the review and the AMU and AR data from the fish. For AMR, the point estimate prevalence and 95% confidence interval (CI) of each pathogen–antimicrobial pair were pooled using a random effects model. Resistance rates were pooled if at least four studies reported on a specific bacterium-antimicrobial combination. Random effects meta-analysis was also used to calculate the overall proportion of pathogen–multidrug resistance pairs. If not defined by the study, resistance to three or more antimicrobial classes, frequently used in primary reports, was considered multidrug resistance (MDR) [15].
Subgroup analysis was performed according to the African region
Heterogeneity across the studies was assessed using the Cochrane Q statistic (significant at p < 0.10) and was quantified with the I2 statistic [13, 16]. Sensitivity analysis was performed to evaluate the influence of individual studies on the final effect. The Begg rank correlation [17] and Egger regression asymmetry test [18] were used to examine publication bias. If publication bias was confirmed, a trim-and-fill method developed by Duval and Tweedie [19] was used to adjust for the bias. The funnel plot was replicated with their “missing” counterparts around the adjusted summary estimate. If, after a detailed investigation, there was no obvious cause for the heterogeneity, the data were analyzed with a more conservative statistical method. Random effects analysis attempts to account for the distribution of effects and provides a more conservative estimate of the effect [16, 20]. A p value of 0.05 was considered to indicate statistical significance, except for the test of heterogeneity. The data were analyzed using Comprehensive Meta-Analysis Software (Biostat, Inc., New Jersey) Version 3.0 for Windows.
Results
A total of 113 citations were identified using an online database search strategy in this study. Forty duplicate papers were removed, and 73 records were screened for eligibility based on a review of the title and abstract content. Twelve papers were excluded because they were not relevant to the research objectives. Of the 61 full-text articles assessed for eligibility, 41 met the inclusion criteria and were retained for analysis (Fig. 1). Three (03) studies reported the outcomes of AMU in aquaculture [5, 21, 22], and three (03) studies investigated ARs in fish [21, 23, 24]. A total of 35 studies reported the outcome of AMR in aquaculture [6, 23, 25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. The studied articles were journal papers (100%), published in English (100%), and included cross-sectional perspective (32; 91.4%) and longitudinal studies (3; 8.6%), with the majority originating from West Africa (23; 56.1%) and North Africa (8; 19.5%) (Fig. 2). Most of the articles on AMR originated from Nigeria (13; 37.1%), Egypt (4; 11.4%), Ethiopia (3; 8.6%) and Tanzania (3; 8.6%) and focused on fresh fish (91.4%), with Oreochromis niloticus (13; 59.4%), Clarias grariepinus (11; 34.4%), and Sardina pilchardus (2; 6.3%) being the most represented species. Samples were commonly collected from markets (14; 40.0%), fish farms (13; 37.1%), and natural milieu (8; 22.9%). With regard to the type of sample, the gut (8; 22.9%), muscle (8; 22.9%), gill (7; 20.0%), gut content (5; 14.3%) and skin (3; 8.6%) were the most common pathogens identified.
Overall, 24 types of pathogens were isolated and tested against 62 different antimicrobial agents, 42 of which were critically important antimicrobial agents [β-lactams (17; 27.4%), cephalosporin (9; 14.5%), quinolone (6; 9.7%), macrolide (4; 6.45%), and carbapenem (2; 3.2%)]. Additionally, 20 were classified as important antimicrobial agents [aminoglycoside (8; 12.90%), sulfonamide (5; 8.06%), phenicol (3; 4.84%), and tetracycline (3; 4.84%)]. The bacteria most commonly reported in the articles were Escherichia coli (16; 45.7%), Salmonella spp. (10; 28.6%), Staphylococcus spp. (8; 22.9%), Aeromonas spp. (8; 22.9%), Proteus spp. (8; 22.9%), Klebsiella spp. (8; 22.9%), and Enterobacter spp. (8; 22.9%) (Table 1).
Antimicrobial use in aquaculture
Of the three articles that reported the outcomes of AMU in aquaculture, two were from Nigeria and one was from Ghana (Table 1). Tetracyclines (3/3) and penicillin (2/3) were the most common antimicrobial agents reported in these studies, followed by sulfamethoxazole, virginiomycin, erythromycin, enrofloxacin, and chloramphenicol (1/3). One article reported the frequency of AMU and the indication for usage. Agoba et al.[5] reported that two out of nine hatcheries investigated in Ghana used tetracycline or chloramphenicol in fish feed. Olatoye and Basiru [22] reported that 90% of the 20 fish farmers investigated in their study used oxytetracycline, penicillin, and enrofloxacin for preventive measures, treatment, and growth promotion. Alarape and Adelewo reported that oxytetracycline (69.8%), penicillin (25%), erythromycin (25%), and enrofloxacin (22.4%) were more commonly used in fish farms than were sulfamethazole (12.1%) and virginiomycin (6%) in 116 fish farms in Nigeria [21].
Antimicrobial residues in fish
Of the three articles that reported the outcomes of ARs in fish products, two focused on qualitative analysis of the presence of tetracycline, amphenicols, and beta-lactams, and one focused on the quantitative analysis of tetracycline. Out of a total of 144 samples of Clarias gariepinus and Oreochromis niloticus screened in Benin, a prevalence of 11.1% of tetracycline residue was reported. The residue was more prevalent in Clarias gariepinus (16.7%) than in Oreochromis niloticus (5.6%) [24]. Donkor et al. [23] examined 100 samples of tilapia (Oreochromis niloticus) gills from the Ghana market and reported an overall prevalence of 7% AR in fish. Among the 60 muscle samples of fresh and smoked Clarias gariepinus strains analyzed in Nigeria, 56.7% of the total tetracycline residue was detected. In addition, the reported concentration of 236 ng/g was higher than the recommended maximum residue level (MRL) of 200 ng/g [21].
Antimicrobial resistance in aquaculture products
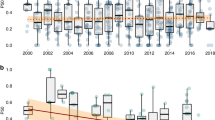
Of the 35 studies that reported outcomes of AMR in aquaculture, 29 were assessed as high quality with AMR prevalence data and were included in the meta-analysis. Higher levels of resistance of Escherichia coli were detected for ampicillin (87.1%) [95% CI (62.8–96.4)], cotrimoxazole (65.1%) [95% CI (38.0–85.1)] and tetracycline (66.4%) [95% CI (46.3–81.8]) than for ceftriaxone (15.0%) [95% CI (3.6–45.2)], ciprofloxacin (15.1%) [95% CI (5.8–33.7)] and gentamicin (18.0%) [95% CI (7.9–36.1)]. Overall, an Escherichia coli multidrug resistance rate of 43.1% [95% CI (32.0–55.0)], I2 = 69.5%, p < 0.001] was observed (Fig. 3).
Concerning Salmonella spp., a higher pooled resistance rate was observed for amoxicillin (74.9%, 95% CI 39.6–93.1) and cotrimoxazole (68.9%, 95% CI 30.3–91.9) than for cefotaxime (7.4%, 95% CI 0.9–42.1), ciprofloxacin (7.8%, 95% CI 1.7–29.6), chloramphenicol (11.3%, 95% CI 3.0–34.2), and gentamicin (17.3%, 95% CI 5.0–45.6). Overall, a Salmonella spp. multidrug resistance rate of 40.3% [95% CI (24.1–58.1)] (I2 = 52.09%, p < 0.03) was observed (Fig. 4).
For Staphylococcus spp., a higher pooled resistance rate was observed for ampicillin (45.6%; 95% CI (11.2–84.8)) and tetracycline (37.5%; 95% CI (18.2–61.8) than for gentamycin (8.9%; 95% CI (1.9–33.3)), nitrofurantoin (11.0%; 95% CI (2.7–35.7]), ciprofloxacin (15.7%; 95% CI (3.9–46.2)) and erythromycin (27.8%; 95% CI (10.4–56.2)) (Table 2). Overall, a Staphylococcus spp. multidrug resistance rate of 31.3% [95% CI (17.5–49.4)], I2 = 69.46%, p < 0.002 was observed (Fig. 5). For the African subregion where the studies were reported, the pooled prevalence of MDR Escherichia coli was significantly (p < 0.05) lower in East Africa than in North and West Africa (Table 3).
Discussion
Despite the decreasing use of antimicrobial agents in recent decades, partly due to the ban on growth promoting treatments in many high-income countries (Sweeden, South Korea, the USA, Canada, Mexico, Japan, and China) [58], information on AMU in fish farming in low- and middle-income countries is scarce, hindering the assessment of human, animal, and environmental risks. This study was performed to summarize published data on AMU, ARs and AMR in aquaculture in Africa as key elements for decision making and policies. At least 27% of fish farmers use antimicrobials for disease prevention and control. Tetracycline was identified as the common class of antimicrobial used in fish farms across the African region [5, 21, 22]. Oxytetracycline is known to be a common antimicrobial agent used in fish farms, especially in hatcheries [59,60,61,62]. The systematic use of tetracycline could be explained by its broad-spectrum activity against furunculsis, Vibrio [63], ulcer disease, Pseudomonas disease, and bacterial hemorrhagic septicaemia [64]. In addition, tetracycline is cheaper and more readily available than other alternative drugs used in aquaculture [65]. Penicillin, erythromycin, enrofloxacin, and sulfamethazole were reported to be used in fish farms in Nigeria. These antimicrobial agents, classified as the highest priority critically important antimicrobial agents or highly important antimicrobial agents by the World Health Organization, highlight the urgent need for antimicrobial regulation, reinforcement, control and reporting in aquaculture [66]. Other consequences of the use of antimicrobial agents in fish farms include the deposition of residues in muscles designated for human consumption irrespective of the route or purpose of administration before they are completely metabolized or excreted from the body [67]. The presence of residues in fish could pose a public health risk to consumers [22]. The prevalence of residue in fish in Africa was higher than the 1% reported in European countries[24, 68]. The main reasons include poor drug regulation in animals, a lack of complete monitoring from prescription to antimicrobial agent use, a lack of updated AMU and treatment guidelines in most African countries[65], the use of noncompliant (substandard drugs with lower concentrations of active ingredients than those stated on labels) veterinary drugs[69], and detection methods that are often inadequate or unavailable at all to comply with limit values and the absence of certification systems regarding food products of animal origin[68].
Tetracyclines, β-lactams (penicillin) and phenicols (chloramphenicol) were mostly detected in fish. Particular attention should be given to antimicrobial agents that are toxic to humans even at low concentrations, such as chloramphenicol and tetracycline. Various studies have shown that ARs from food can negatively impact human health through allergic reactions, mutations in cells, imbalances in the intestinal microbiome, and ultimately, the presence of multiresistant microorganisms [68]. Evidence studies have reported that chloramphenicol residues may be associated with hematological disorders, including aplastic anemia in humans, while sulfametazine, oxytetracycline and furazolidone may induce carcinogenicity[70]. This inability to set the threshold value and the shortcomings of the dossier led to its classification as a substance prohibited for use in food-producing animals[65]. A high concentration of tetracycline residue (236 ng/kg) in fish and products that exceed the allowable residue limits (200 ng/kg) [71] poses a serious threat to public health. Heat treatments that occur during cooking can reduce the risk of ingesting tetracyclines but do not guarantee the breakdown of these antimicrobial residues in animal products, such as broiler meat [72]. The high stability of β-lactams represents a significant risk to human health because the residues of these antimicrobial agents can remain in foodstuff after heat treatment and, therefore, can reach the dairy industry and consumers [73].
In the present study, 35 articles reported the outcome of AMR in various bacteria in Africa. Enterobacterales isolated from aquaculture products, such as Escherichia coli and Klebsiella spp., show a high rate of antimicrobial resistance to ampicillin, cefotaxim, cotrimoxazole and tetracycline, while Salmonella spp. exhibit a high rate of antimicrobial resistance to amoxicillin, ampicillin, and cotrimoxazole. These antimicrobial agents are the most inexpensive broad-spectrum drugs and are therefore frequently used [65]. The use of antimicrobial agents with a broader spectrum affects a greater number of bacterial taxa and may increase the risk of selecting bacteria harboring resistance genes compared with agents with a narrower spectrum. In addition, it may increase the risk of suppressing and eliminating susceptible commensal microbiota, which generally outcompete resistant strains [26]. Approximately 80% of antimicrobials administered through feeds to aquatic farmed animals disseminate to nearby environments (water and sediment), where they remain active for months at concentrations allowing selective pressure on bacterial communities and favoring AMR development [74]. Additionally, manure from treated animals [75], human feces and urine [76] are indirect sources of antimicrobial agents and their residues in aquaculture [75, 76]. Independent of these practices, the aquatic environment is considered the major pool for antimicrobial agents accumulated from effluent discharged after treatment, and surface runoff has the same undesired effect on the sensitivity of aquatic pathogens to antimicrobial agents [77]. In this review, the high rate of resistance to multiple classes of antimicrobial agents in aquatic products raises the urgent question of the therapeutic efficacy of first-line antimicrobial agents and the degradation of last-resort therapeutics during serious infections due to multiresistant bacteria [78].
The high MDR prevalence observed in Enterobacter spp. and Escherichia coli emphasizes the importance of Enterobacteriaceae in aquatic environments as carriers of AMR genes and determinants of virulence. Hence, there is a need for in-depth monitoring of aquatic environments as a source of the emergence and spread of AMR [54]. This review highlights serious concerns relating to the use of ampicillin, tetracycline and cotrimoxazole as antimicrobial agents of choice for optimal therapy of common pathogens and the difficulty of treating Enterobacteriaceae disease in Africa. Although this study is based on the state of knowledge on AMU, ARs, and AMR in aquaculture on the African continent, it suffers from a lack of data concerning AMU and residue in aquaculture. However, the few existing data on AMU are exclusively focused on the percentages of farms using antimicrobial agents rather than on defined daily doses, as recommended by the World Health Organization. Additionally, the majority of studies on AMR have not provided an understanding of the dynamics of resistance transmission because these studies are interested in phenotypic rather than molecular aspects. This review highlights the need for the implementation of AMR surveillance based on one health approach to develop surveillance strategies at the level of each African country. Thus, as suggested by Gazal et al. [78], each state would begin by enforcing the complete restriction of the use of medically important antimicrobial agents for the prevention of pathologies in aquaculture or as growth promoters. The prudent use of antimicrobial agents under veterinary control must be the other line of action to ensure the safety of aquatic products.
Conclusion
The present review highlighted the general lack of information about AMR surveillance in aquaculture, especially concerning AMU and residue. The high prevalence of resistance to the most commonly used antimicrobial agents and the level of MDR bacteria imposed by certain isolated bacteria reveal the real threat posed by AMR to public health. Furthermore, Africa could benefit from developing strategies to increase awareness and understanding of the AMR problem through effective communication, education and training; optimizing the use of antimicrobial agents; reducing the incidence of infection through effective sanitation, hygiene, and implementation of good farm biosecurity practices and prevention measures; and above all, strengthening knowledge through surveillance and research.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Watts JEM, Schreier HJ, Lanska L, Hale MS. The rising tide of antimicrobial resistance in aquaculture: sources. Sinks Solutions Mar Drugs. 2017;15:158. https://doi.org/10.3390/md15060158.
Reverter M, Sarter S, Caruso D, Avarre JC, Combe M, Pepey E, Pouyaud L, Heredía SV, HVRE G. Aquaculture at the crossroads of global warming and antimicrobial resistance. Nat Commun. 2020;11:1870. https://doi.org/10.1038/s41467-020-15735-6.
Pathmalal M. Heavy use of antibiotics in aquaculture: emerging human and animal health problems: a review Sri Lanka. J Aquat Sci. 2018;23:13–27. https://doi.org/10.4038/sljas.v4023i4031.7543.
Muziasari I, Pitkänen KL, Sorum H, Stedtfeld RD, Tiedje JM, Virta M. The resistome of farmed fish feces contributes to the enrichment of antibiotic resistance genes in sediments below baltic sea fish farms. Front Microbiol. 2017;8:1491. https://doi.org/10.3389/fmicb.2017.01491
Agoba EE, Adu F, Agyare C, Boamah VE. Antibiotic use and practices in selected fish farms in the ashanti region of Ghana. J Infect Dis. 2017;3(2):9. https://doi.org/10.21767/2472-1093.100036.
Hamza D, Dorgham S, Ismael E, El-Moez AIS, Elhariri M, Elhelw R, Hamza E. Emergence of β-lactamase- and carbapenemase- producing Enterobacteriaceae at integrated fish farms. Antimicrob Resist Infect Control. 2020;9:67. https://doi.org/10.1186/s13756-020-00736-3.
Manishimwe R, Nishimwe K, Ojok L. Assessment of antibiotic use in farm animals in Rwanda. Trop Anim Health Prod. 2017;49:1101–6.
Mouiche MMM, Moffo F, Akoachere KTJ-F, Okah-Nnane NH, Mapiefou PN, Ndze NV, Wade A, Djuikwo-Teukeng FF, Toghoua TGD, Zambou HR, et al. Antimicrobial resistance from a one health perspective in Cameroon: a systematic review and meta-analysis. BMC Pub Hlth. 2019;19:1135. https://doi.org/10.1186/s12889-019-7450-5.
Ouedraogo AS, Banuls AL, Ouedraogo R, Godreuil S. Emergence and spread of antibiotic resistance in west africa: contributing factors and threat assessment. Med Sant Trop. 2017;27:147–54.
Holman BD, Chenier RM. Impact of subtherapeutic administration of tylosin and chlortetracycline on antimicrobial resistance in farrow-to-finish swine. FEMS Microbiol Ecol. 2013;85(1):1–13.
Clifford K, Desai D, Da Costa PC, Meyer H, Klohe K, Winkler A. Antimicrobial resistance in livestock and poor quality veterinary medicines. Bull World Hlth Organ. 2018;96:662–4.
Page JM, McKenzie EJ, Bossuyt M, Boutron I, Hoffmann CT, Mulrow D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. 2021;372:371. https://doi.org/10.1136/bmjn1171
Naylor RN, Silva S, Kulasabanathan K, Atun R, Zhu N, Knight MG, Robotham J. Methods for estimating the burden ofantimicrobial resistance: a systematic literature review protocol. Sys Rev. 2016;5:187. https://doi.org/10.1186/s13643-13016-10364-13648.
Munn Z, Moola S, Riitano D, Lisy D. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014, 3(3):123–128. 10.15171/ijhpm.12014.15171.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler J, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–328. https://doi.org/10.1111/j.1469-0691.2011.03570.x.
Crowther M, Wendy LW, Crowthe AM. Systematic review and metaanalysis methodology. Blood. 2010, 116(117).
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Brit Med J. 1997;315:629–34.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker JB, Ann Sipe T, Thacke BT. Meta-analysis of observational studies in epidemiology. J Am Med Assoc. 2000;283(15):2008–12.
Alarape SA, Adeyemo OK. Tetracycline residue in fresh and processed Clarias gariepinus from selected fish farms and markets in Ibadan. Nigeria Trop Vet. 2017;35(2):61–71.
Olatoye IO, Basiru A. Antibiotic Usage and Oxytetracycline Residue in African Catfish (Clariasgariepinus in Ibadan, Nigeria). World J Fish Mar Sci. 2013;5(3):302–9. https://doi.org/10.5829/idosi.wjfms.2013.05.03.71214.
Donkor ES, Baidoo IA, Fei E, Amponsah C, Taiwo MO, Adjei DN, Owusu E, Forson AO. Occurrence of antibiotic residues and antibiotic-resistant bacteria in nile tilapia sold in some markets in Accra, Ghana: Public Health Implications. J Food Res. 2018;7:(6). https://doi.org/10.5539/jfr.v7n6p129.
Mensah SEP, Dakpogan H, Aboh AB, Sika KC, Abléto M, Adjahoutonon KYKB, Koudandé OD, Sanders P, Mensah GA. Occurrence of antibiotic residues in raw fish Clarias gariepinus and Oreochromis niloticus from intensive rearing system in Benin. Vet. 2019;68(2):91-94.
Beshiru A, Okareh OT, Okoh AI, Igbinosa IH. Detection of antibiotic resistance and virulence genes of Vibrio strains isolated from ready-to-eat shrimps in Delta and Edo States. Nigeria J Applied Microbiol. 2020;129:17–36. https://doi.org/10.1111/jam.14590.
Tiamiyu AM, Soladoye MO, Adegboyega TT, Adetona MO. Occurrence and antibiotic sensitivity of bacterial strains isolated from nile tilapia, oreochromis niloticus obtained in Ibadan. Southwest Nigeria J Biosci Med. 2015;3:19–26. https://doi.org/10.4236/jbm.2015.35003.
Rabia AR, Wambura PN, Kimera SI, Mdegela RH, Mzula A. Potential public health risks of pathogenic bacteria contaminating marine fish in value chain in Zanzibar. Tanzania Microbiol Res J Int. 2017;20(5):1–11. https://doi.org/10.9734/MRJI/2017/34030.
Rabia AR, Wambura PN, Kimera SI, Mdegela RH, Mzula A, Khamis FA. Phenotypic characterization of escherichia coli isolates from fish, diarrheic and healthy children in Zanzibar, Tanzania. Int J Trop Dis Hlth. 2017;24(3):1-11 https://doi.org/10.9734/IJTDH/2017/34262.
Adekanmbi AO, Adejoba AT, Banjo OA, Saki M. Detection of sul1 and sul2 genes insulfonamide-resistantbacteria (SRB) from sewage, aquaculture sources, animal wastes and hospital wastewater in South-West Nigeria. Gene Rep. 2020. https://doi.org/10.1016/j.genrep.2020.100742.
Hammad MA, Moustafa AH, Mansour MM, Fahmy BM, Hamada MG, Shimamoto T, Shimamoto T. Molecular and phenotypic analysis of hemolytic aeromonas strains isolated from food in Egypt revealed clinically important multidrug resistance and virulence profiles. J Food Prot. 2018;81(6):1015–21. https://doi.org/10.4315/0362-1028X.JFP-1017-1360.
Akande A, Onyedibe KI. First report of enteropathogenic and enteroinvasive Escherichia coli with multiple antibiotic resistance indices from African catfish (Clarias glariepinus) in Nigeria. Afr J Clin Exper Microbiol. 2019;20(2):95–103. https://doi.org/10.4314/ajcem.v4320i4323.4313.
Teklu A, Alemayhu T, Assefa S, Getachew B, Hagos Y, Tkue T, Berhe N. Isolation and antimicrobial sensitivity testing of escherichia coli from fish meat retailing shops of Mekelle City, Ethiopia. Momona Ethio J Sci. 2019;11(2):229–38. https://doi.org/10.4314/mejs.v4311i4312.4314.
Assefa A, Regassa F, Ayana D, Amenu K, Abunna F. Prevalence and antibiotic susceptibility pattern of Escherichia coli 0157:H7isolated from harvested fish at lake Hayq and Tekeze Dam Northen Ethiopia. Heliyon 2019, 5 https://doi.org/10.1016/j.heliyon.2019.e02996
Tilahun A, Isolation Engdawork A. Identification and antimicrobial susceptibility profile of E. coli (O157: H7) from fish in Lake Hawassa. Southern Ethiopia Life Sci J. 2020;17(2):64–72. https://doi.org/10.7537/marslsj170220.170210.
Beshiru A, Igbinosa IH. Prevalence of antimicrobial resistance and virulence gene elements of salmonella serovars from ready-to-eat (RTE) shrimps. Front Microbiol. 2019;10(1613):16. https://doi.org/10.3389/fmicb.2019.01613.
Gufe C, Hodobo CT, Mbonjani B, Majonga O, Marumure J, Musari S, Jongi G, Makaya VP, Machakwa J. Antimicrobial profiling of bacteria isolated from fish sold at informal market in Mufakose. Zimbabwe Int J Microbiol. 2019;2019:7. https://doi.org/10.1155/2019/8759636.
Adinortey AC, Aheto WD, Boateng AA, Agbeko R. Multiple antibiotic resistance-coliform bacteriain some selected fish farms of the central region of Ghana. Scientifica. 2020, 11 https://doi.org/10.1155/2020/6641461.
Mwega E, Chengula A, Colquhoun D, Mutoloki S, Mdegela R, Evensen O, Wasteson Y. Antimicrobial susceptibility of Flavobacteriaceae isolates from Nile Tilapia (Oreochromis niloticus) in Tanzania. Afr J Microbiol Res. 2020;14(1):42–50. https://doi.org/10.5897/AJMR2019.9240.
Falegan CR, Anosike OH, Dairo AM, Akoja SO. Microbiological evaluation and plasmid profile of fresh african mud catfish (Clarius gariepinus) in some Towns in Ekiti State. Nigeria J Adv Botany Zool. 2017;5:1. https://doi.org/10.5281/zenodo.1000075.
Ramadan H, Ibrahim N, Samir M, El-Moaty AA, Gad T. Aeromonas hydrophila from marketed mullet (Mugil cephalus) in Egypt: PCR characterisation of b-lactam resistance and virulence genes. J Appl Microbiol. 2018, 124:1629–1637. 1610.1111/jam.13734.
Raufu AI, Lawan FA, Bello HS, Musa AS, Ameh JA, Ambali AG. Occurence and antimicrobial susceptibility profiles of Salmonella serovars from fish in Maiduguri, Subsahara. Nigeria Egypt J of Aquati Res. 2014;40:59–63.
Oko JO, Adeshina GO, Onaolapo J. Antibiotics Susceptibility Study of Staphylococcus aureus isolates from dry catfish sold in some open markets in Zaria - Nigeria. South Asian J Res Microbiol. 2019;5(2):1–8. https://doi.org/10.9734/SAJRM/2019/v9735i230127.
Osman KM, Al-Maary KS, Mubarak AS, Dawoud TM, Moussa IMI, Ibrahim MDS, Hessain AM, Orabi A, Fawzy NM. Characterisation and susceptibility of streptococci and enterococci isolated from Nile tilapia (Oreochromis niloticus) showing septicaemia in aquaculture and wild sites in Egypt. BMC Vet Res. 2017;13:357. https://doi.org/10.1186/s12917-12017-11289-12918.
Efuntoye MO, Olurin K, Jegede GC. Bacterial flora from healthy clarias gariepinus and their antimicrobial resistance pattern. Adv J Food Sci Technol. 2012;4(3):121–5.
Donkeng NN, Maiwore J, Ngoune LT, Montet D, Mbofung CMF. Characterisation of the bacterial flora of tilapia (Oreochoromis niloticus) harvested from four lakes in the north of Cameroon. Afr J Biotechnol. 2011 10(71):16016–16023. 16010.15897/AJB16010.11491.
Saidi N, Lagha R, Abdallah FB, Rokbani KB, Bakhrouf A. Slime producing, heavy metals and antibiotics resistance in Aeromonas hydrophila isolated in Tunisia. Afr J Microbiol Res. 2013;7(50):5697–708. https://doi.org/10.5897/AJMR5612.2328.
Adenike AOO, Olabode OP. Antimicrobial potentials of indigenous Lactobacillus strains on gram-negative indicator bacterial species from Clarias gariepinus (Burchell.) microbial inhibition of fish-borne pathogens. Afr J Microbiol Res. 2009;3(12):870–6.
Traoré O, Martikainen O, Siitonen A, Traoré AS, Barro N, Haukka K. Occurrence of vibrio cholerae in fish and water from a reservoir and a neighboring channel in Ouagadougou, Burkina Faso J Infect Dev Ctries. 2014, 8(10):1334–1338 1310.3855/jidc.3946.
Traoré O, Nyholm O, Siitonen A, Bonkoungou IJO, Traoré AS, Barro N, Haukka K. Prevalence and diversity of Salmonella enterica in water, fish and lettuce in Ouagadougou, Burkina Faso. BMC Microbiol. 2015;15:151. https://doi.org/10.1186/s12866-015-0484-7.
Wamala SP, Mugimba KK, Mutoloki S, Evensen O, Mdegela R, Byarugaba DK, Sorum H. Occurrence and antibiotic susceptibility of fish bacteria isolated from Oreochromis niloticus (Nile tilapia) and Clarias gariepinus (African catfish) in Uganda Fish Aquatic Sci. 2018, 21(6). https://doi.org/10.1186/s41240-017-0080-x.
Ombede SNN, Dougnon V, Koudokpon H, Deguenon E, Ngomo RPJM, Tchibozo C, Gnimatin JP, Tchoumbougnang F, Yadouleton A, Dougnon J. Antimicrobial resistance and toxigenic profiles of bacteria isolated from tropical shrimps (Farfantepenaeus notialis and Penaeus monodon) in Cameroun. BMC Res Notes. 2020;13:358 https://doi.org/10.1186/s13104-020-05184-1 .
Saka BA, Adeyemo OK, Odeseye AO. Multiple antibiotic resistance indices of aeromonas hydrophila isolates of muscle of catfish (Clarias Gariepinus, Burchell 1822) from selected markets in Ibadan. Nigeria African J Clin Exp Microbiol. 2017;18(2):73–8. https://doi.org/10.4314/ajcem.v4318i4312.4313.
Anyanwu MC, Chah KF, Shoyinka VS. Antibiogram of aerobic bacteria isolated from skin lesions of African catfish cultured in Southeast. Nigeria Int J Fish Aquat Stud. 2014;2(1):134–41.
Brahmi S, Touati A, Remy CD, Sotto A, Pantel A, Lavigne JP. High prevalence of extended-spectrum b-LactamaseProducing enterobacteriaceae in wild fish from the Mediterranean Sea in Algeria. Microb Drug Resist. 2017;00:00. https://doi.org/10.1089/mdr.2017.0149.
Hassen B, Jouini A, Elbour M, Hamrouni S, Maaroufi A. Detection of extended-spectrum β-Lactamases (ESBL) producing enterobacteriaceae from fish trapped in the lagoon area of bizerte. Tunisia BioMed Res Int. 2020;2020:9. https://doi.org/10.1155/2020/7132812.
Aliyu A, Ibrahim YKE, Oyi RA. Bacteriological and elemental quality of clarias gariepinus (cat fish) Samples from river Lavun, Bida Niger state. Nigeria Nig J Pharm Res. 2016;12(2):139–47.
Dib LA, Agabou A, Chahed A, Kurekci C, Moreno E, Espigares ME. Isolation, molecular characterisation and antimicrobial resistance of enterobacteriaceae isolated from fish and seafood. Food Control. 2018;88:54–60. https://doi.org/10.1016/j.foodcont.2018.1001.1005.
Hossan MD, Salim, Khan Shahidul H, Kazi MK, Anwarul HB. Global restriction of using antibiotic growth promoters and alternative strategies in poultry production. Sci Prog. 2018, 101(1): 52 –75. https://doi.org/10.3184/003685018X15173975498947.59 .
Dietze JE, Scribner EA, Meyer MT, Kolpin DW. Occurrence of antibiotics in water from 13 fish hatcheries, 2001–2003. Int J Environ Anal Chem. 2005, 85. doi: 10.1080/03067310500273682.
Miranda CD, Godoy FA, Lee MR. Current status of the use of antibiotics and the antimicrobial resistance in the chilean salmon farms. Front Microbiol. 2018;9:1284. https://doi.org/10.3389/fmicb.2018.01284.
Burridge L, Weis JS, Cabello F, Pizarro J, Bostick K. Chemical use in salmon aquaculture: a review of current practices and possible environmental effects. Aquaculture. 2010;306:7–23.
Guglielmetti E, Korhonen JM, Heikkinen J, Morelli L, Wright AV. Transferofplasmid-mediated resistance to tetracycline in pathogenic bacteria from shand aquaculture environments. FEMS Microbiol Lett. 2009;293:28–34. https://doi.org/10.1111/j.1574-6968.2009.01512.x.
Benbrook MC. Antibiotic Drug Use in U.S. Aquaculture. Idaho: The Northwest Science and Environmental Policy Center Sandpoint; 2002.
Kimera ZI, Mshana SE, Rweyemamu MM, Mboera LEG, Matee MIN. Antimicrobial use and resistance in foodproducing animals and the environment: an African perspective. Antimicrob Resist Infect Control. 2020;9:37. https://doi.org/10.1186/s13756-020-0697-x.
WHO: Critically Important Antimicrobials for Human Medicine: Ranking of medically important antimicrobials for risk management of antimicrobial resistance due to nonhuman use. 6th Revision. World health organisation 2018:52p. https://www.who.int/foodsafety/publications/antimicrobials-sixth/en/. Access 14 Aug 2023.
Okocha CR, Olatoye OI, Adedeji BO. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018;39(21):22. https://doi.org/10.1186/s40985-40018-40099-40982.
Treiber FM, Beranek-Knauer H. Antimicrobial residues in food from animal origin—a review of the literature focusing on products collected in stores and markets worldwide. Antibiotics. 2021;10:534. https://doi.org/10.3390/antibiotics10050534.
Jaime G, Hobeika A, Figuié M. Access to veterinary drugs in sub-saharan africa: roadblocks and current solutions. Front Vet Sci. 2022;8: 558973. https://doi.org/10.3389/fvets.2021.558973.
Balcanli M, Basaran N. Importance of antibiotic residues in animal food. Food Chem Toxicol. 2019;125:462–6. https://doi.org/10.1016/j.fct.2019.01.033.
Codex Alimentarius. Maximum residue limits (MRLS) and risk management recommendations (RMRS) for residues of veterinary drugs in foods. 2021. CX/MRL 2–2021
Gratacós-Cubarsí M, Fernandez-García A, Picouet P, Valero-Pamplona A, García-Regueiro J, Castellari M. Formation of tetracycline degradation products in chicken and pig meat under different thermal processing conditions. J Agr Chem. 2007;55:4610–6.
Roca M, Villegas L, Kortabitarte M, Althaus R, Molina M. Effect of heat treatments on stability of β-lactams in milk. J Dairy Sci. 2011;94:1155–64.
Liu X, Steele JC, Meng XZ. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: a review. Environ Pollut. 2017;223:161–9.
Minich JJ, Zhu Q, Xu ZZ, Amir A, Ngochera M, Simwaka M, Allen EE, Zidana H, Knight R. Microbial effects of livestock manure fertilisation on freshwater aquaculture ponds rearing tilapia (Oreochromis shiranus) and North African catfish (Clarias gariepinus). Microbiol Open. 2018. https://doi.org/10.1002/mbo3.716.
Mdegela RH, Mwakapeje ER, Rubegwa B, Gebeyehu DT, Niyigena S, Msambichaka V, Nonga HE, Moussiaux NA, Fasina FO. Antimicrobial use, residues, resistance and governance in the food and agriculture sectors, Tanzania. Antibiotics. 2021;10:454. https://doi.org/10.3390/antibiotics10040454.
Li Z, Li M, Zhang Z, Li P, Zang Y, Liu X. Antibiotics in aquatic environments of China: A review and meta-analysis. Ecotoxicol Environ Saf. 2020;199:110668. https://doi.org/10.1016/j.ecoenv.2020.110668.
Schar D, Zhao C, Wang Y, Larsson DGJ, Gilbert M, Boeckel TPV. Twenty-year trends in antimicrobial resistance from aquaculture and fisheries in Asia. Nat Commun. 2021;12:5384. https://doi.org/10.1038/s41467-021-25655-8.
Gazal LES, Brito KCT, Kobayashi RKT, Nakazato G, Cavalli LS, Otutumi LK, Brito BG. Antimicrobials and resistant bacteria in global fish farming and the possible risk for public health. Anim Pathol. 2020;87:1–11. https://doi.org/10.1590/1808-1657000362019.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.M.M.M, J.A.N and F.M; investigation and data analysis: M.M.F.N, M.N.T, FM and M.M.M.M; original draft preparation: M.M.F.N, R.N.G.N, M.N.T, F.M and M.M.M.M; review and editing: J.A.N and R.N.G.N. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests in this section.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Moffo, F., Ndebé, M.M.F., Tangu, M.N. et al. Antimicrobial use, residues and resistance in fish production in Africa: systematic review and meta-analysis. BMC Vet Res 20, 307 (2024). https://doi.org/10.1186/s12917-024-04158-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-024-04158-w