Abstract
Background
Coccidiosis is one of the most frequently reported diseases in chickens, causing a significant economic impact on the poultry industry. However, there have been no previous studies evaluating the prevalence of this disease in broiler farms in Guangdong province. Therefore, this study aims to conduct an epidemiological investigation into the occurrence of Eimeria species and associated risk factors in intensive management conditions across four regions in Guangdong province, China. A total of 394 fecal samples were collected from 89 broiler farms in Guangdong province. The prevalence of Eimeria species infection was determined using PCR, and the occurrence of Clostridium perfringens type A was assessed using quantitative real-time PCR.
Results
The results showed an overall prevalence of 98.88% (88/89) at the farm level and 87.06% (343/394) at the flock level. All seven Eimeria species were identified, with E. acervulina (72.53%; 64/89), E. tenella (68.54%; 61/89), and E. mitis (66.29%; 59/89) at the farm level, and E. acervulina (36.55%; 144/394), E. mitis (35.28%; 139/394), and E. tenella (34.01%; 134/394) at the flock level. The predominant species combination observed was a co-infection of all seven Eimeria species (6.74%; 6/89), followed by a combination of E. acervulina, E. tenella, E. mitis, E. necatrix, E. brunetti, and E. maxima (5.62%, 5/89). A combination of E. acervulina, E. tenella, E. mitis, E. necatrix, E. brunetti, and E. praecox (4.49%; 4/89) was also observed at the farm level. Furthermore, the study identified several potential risk factors associated with the prevalence of Eimeria species, including farm location, chicken age, drinking water source, control strategy, and the presence of C. perfringens type A were identified as potential risk factors associated with prevalence of Eimeria species. Univariate and multivariate analyses revealed a significant association between E. necatrix infection and both grower chickens (OR = 10.86; 95% CI: 1.92–61.36; p < 0.05) and adult chickens (OR = 24.97; 95% CI: 4.29–145.15; p < 0.001) compared to starter chickens at the farm level. Additionally, farms that used groundwater (OR = 0.27; 95% CI: 0.08–0.94; p < 0.05) were less likely to have E. maxima compared to those that used running water. At the flock level, the prevalence of E. tenella was significantly higher in the Pearl River Delta (OR = 2.48; 95% CI: 1.0–6.15; p = 0.05) compared to eastern Guangdong. Interestingly, flocks with indigenous birds were less likely to have E. brunetti (OR = 0.48; 95% CI: 0.26–0.89; p < 0.05) compared to flocks with indigenous crossbred birds. Furthermore, flocks that used anticoccidial drugs (OR = 0.09; 95% CI: 0.03–0.31; p < 0.001) or a combination of vaccines and anticoccidial drugs (OR = 0.06; 95% CI: 0.01–0.25; p < 0.001) were less likely to be positive for E. tenella compared to flocks that only used vaccines. Finally, flocks with C. perfringens type A infection were significantly more likely to have E. necatrix (OR = 3.26; 95% CI: 1.96–5.43; p < 0.001), E. tenella (OR = 2.14; 95% CI: 1.36–3.36; p < 0.001), E. brunetti (OR = 2.48; 95% CI: 1.45–4.23; p < 0.001), and E. acervulina (OR = 2.62; 95% CI: 1.69–4.06; p < 0.001) compared to flocks without C. perfringens type A.
Conclusions
This study conducted an investigation on the prevalence, distribution, and risk factors associated with Eimeria species infection in broiler chickens in Guangdong. The farm-level prevalence of Eimeria species was higher than the previous prevalence figures for other areas and countries. E. brunetti was identified at higher prevalence in Guangdong than previously survived prevalence in different regions in China. Farm location, chicken age, drinking water source, control strategy, and the presence of C. perfringens type A were considered as potential risk factors associated with prevalence of Eimeria species. It is imperative to underscore the necessity for further surveys to delve deeper into the occurrence of Eimeria species under intensive management conditions for different flock purposes.
Similar content being viewed by others
Background
Coccidiosis is a highly prevalent disease that affects chickens globally. It is caused by protozoan parasites from the Eimeria genus and can cause significant damage to the intestinal tract. This results in increased mortality rates, reduced weight gain, impaired nutrient absorption, and heightened susceptibility to other enteric pathogens [1]. The far-reaching consequences of this disease have a profound economic impact on the poultry industry [2]. In chickens, there are seven mainly recognized species of Eimeria: E. tenella, E. necatrix, E. brunetti, E. acervulina, E. maxima, E. mitis, and E. praecox. Each of these species has a preference for specific segments of the intestinal tract and exhibits varying levels of pathogenicity, resulting in distinct clinical manifestations [3]. E. necatrix is considered the most pathogenic species, while E. tenella, is relatively prevalent and both can cause bloody lesions and high rates of morbidity and mortality in chickens [4]; E. brunetti is highly pathogenic and is associated with haemorrhagic coccidiosis [5]. On the other hand, E. acervulina and E. maxima are classified as moderately pathogenic, causing inflammation of the intestinal wall characterized by pinpoint haemorrhage and epithelial demolition [5]. Finally, E. mitis and E. praecox are generally considered less pathogenic, causing malabsorption and enteritis [3].
Control strategies for coccidiosis primarily rely on chemotherapy or vaccination. However, the emergence of drug resistance in various regions and the lack of new anticoccidial drugs have led to a decrease in the effectiveness of these agents [6]. In recent decades, live anticoccidial vaccines have been utilized to prevent coccidiosis [7]. Currently, there are three types of live anticoccidial vaccines currently available in China: a trivalent vaccine containing E. tenella, E. acervulina and E. maxima; a tetravalent vaccine containing E. tenella, E. necatrix, E. acervulina, and E. maxima; and an imported vaccine, Coccivac™, containing E. maxima, E. mivati, E. acervulina and E. tenella. In order to accurately assess the effectiveness of these control strategies, including the composition of vaccines, it is crucial to have a thorough understanding of the epidemiology of Eimeria species and the potential risk factors associated with the occurrence of different Eimeria species.
The conventional taxonomy of Eimeria species has traditionally relied on morphological characteristics, the affected segments of the intestinal tract, and the pre-patent period of the Eimeria following in vivo infection in chickens [5]. However, these methods may not always provide precise diagnoses [8]. In recent decades, polymerase chain reaction (PCR) techniques have emerged as a valuable tool for identifying all seven Eimeria species. This molecular method utilizes genetic markers located within the internal transcribed spacer-1 (ITS-1), ITS-2, and the sequence characterized amplified region (SCAR) [9,10,11,12]. Currently, there is a lack of accurate data and previously reported information on the prevalence of Eimeria species in broiler farms in Guangdong province, China. Therefore, the purpose of this study is to investigate the epidemiology of Eimeria species in Guangdong province and analyze the associated risk factors. The findings from this study will not only contribute to our understanding of the occurrence and potential control strategies for coccidiosis in poultry in Guangdong province, China, but also enhance our comprehension of the potential risk factors associated with intensive poultry management practices.
Methods
Study area and farms
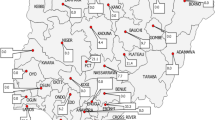
The study was conducted across four distinct regions, spanning geographically between 20°09'–25°31' north latitude and 109°45'–117°20' east longitude located in southern China. These regions covering a total land area of 179,800 km2. The study was carried out over an extensive timeframe, spanning from April 2020 to November 2021. The climate in Guangdong is subtropical, characterized by mild winters and hot, humid summers. The average annual temperature ranged from 23 to 25 °C. Additionally, the relative humidity levels ranged from 57 to 77% on average. The average monthly rainfall was approximately from 118 mm to 150 mm, drawing data from https://www.worldweatheronline.com/ as the source (Table 1). The selection of poultry farms depended on the number of broiler farms across four areas in Guangdong province. This study included 89 broiler farms (21 from eastern Guangdong, 19 from western Guangdong, 24 from northern Guangdong, and 25 from Pearl River Delta) (Fig. 1). Each farm had between 2 and 20 houses, with bird populations ranging from 5,000 to 40,000 individuals and a density of 10 to 16 birds/m2. The most common broiler breeds are the three-yellow chicken and the spotted-brown chicken. The bedding materials in use were wood shavings or rice husk.
Questionnaire design
Based on previous studies collecting data on farm management, performance figures, bird characteristics, chicken health and social factors, a questionnaire was developed for analyzing risk factors in this study to identify risk factors associated with Eimeria species distribution (Supplemental Table S1) [13]. The questionnaire for broiler farmers and/or veterinarians included 21 questions. In detail, the survey gathered information on bird-related factors (e.g., age, breed, flock size, and flock density), along with flock management practices associated with coccidiosis, such as general information on the farm (e.g., farm location, type of production, type of farming, litter composition, source of drinking water, and fecal treatment method), data regarding coccidiosis occurrence (e.g., coccidiosis detection, Eimeria species identification), and strategies for coccidiosis control (e.g., the use of coccidiostats and/or vaccines) (Supplemental Tables S2 and S3).
Fecal sample collection and sample analysis
Broiler flocks were sampled for this study according to the scale of poultry operations on the farm. On small-scale broiler farms, between 1 and 4 flocks were sampled, whereas on large-scale broiler farms, sampling involved 5 to 16 flocks. A total of 394 fecal samples were obtained on 89 farms. For sample collection, fresh fecal samples were obtained from different sites in each poultry house, as previously described by Kumar et al. [14]. This method included tracing a W-shaped pattern along each poultry house. Each sample, weighed approximately 250 g, was made up of 30 fresh fecal droppings collected from a single house. Samples were placed in labelled zipped plastic bags and immediately transported at 4 °C to the laboratory. Each sample was mixed with an equal volume of sterile ddH2O and was homogenized using a blender. 200 µl aliquots of the prepared samples were transferred into a 1.5 ml Eppendorf tubes for DNA extraction. The E.Z.N.A.® Stool DNA Kit (Omega, D4015) was used for genomic DNA extraction, following the manufacturer’s protocol. The extracted DNA was then stored at -20 °C until further use.
PCR was performed separately for each Eimeria species. The primer sequences for each Eimeria species can be found in Table 2, as previously described by Schnitzler et al. [15, 16] and Haug et al. [10]. Each amplification reaction consisted of a total volume of 20 µl, including 10 µl of Premix Taq™ (Takara, RR901A), 500 nm of species-specific for forward and reverse primers, 2 µl of DNA sample, and 6 µl of ddH2O. The amplification was carried out using a T100™ thermal cycler (Bio-Rad, USA) with the following cycling conditions: an initial denaturation step at 95 °C for 2 min, followed by 35 repeat cycles, each consisting of 30 s of denaturation at 95 °C, 30 s at 62 °C for annealing, and 1 min at 72 ° for extension, with a final extension step of 3 min. The resulting amplification products were then analyzed by electrophoresis using a 1.5% agarose gel (Supplemental Figure S1).
The identification of C. perfringens type A in fecal samples was conducted using quantitative real-time PCR (qPCR) targeting the alpha toxin gene, as described by Mohiuddin et al. [17]. The qPCR was carried out in a reaction mixture of 20 ul, containing TB Green Premix Ex Taq II (Takara, RR820B) (10 µL), forward primers (1 µL), reverse primers (1 µL), template DNA 1µL (150–200 ng), and ddH2O (7 µL). The amplification process was performed using CFX Connect™ Real-Time PCR System (Bio-Rad, USA). The amplification program was at 95 °C for 30 s, 35 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s, and a final step for dissociation at 95 °C for 10 s, 65 °C for 5 s, and 95 °C for 5 s.
Statistical analysis
All statistical analyses were performed using software IBM SPSS Statistics 27.0 software (SPSS Inc., http://www.spss.com.hk). Descriptive statistics, including bird age, bird breed, flock size, farming type, type of drinking water, and control strategy were obtained from the questionnaires. The prevalence of Eimeria spp. infections, with a 95% confidence interval (CI), was initially calculated. Univariable and multivariable logistic regression models were then used to assess the predictor variables associated with the presence of Eimeria species. Multivariable models were built using forward stepwise logistic regression procedures, with inclusion if p < 0.05. The prevalence of each species of Eimeria infection in variables such as age, breed, flock size, farming type, drinking water source, control strategy, region, and presence of C. perfringens type A was compared using chi-square test or Fisher’s exact test. The odds ratio (OR) with a 95% CI was calculated to assess the associations between participants’ characteristics and Eimeria species infection. A p value of ≤ 0.05 was considered as statistically significant.
Results
Infection of Eimeria species in broiler chickens in Guangdong
An epidemiological study was conducted in Guangdong province from April 2020 to November 2021 to investigate the prevalence of Eimeria species infection in broiler chickens. A total of 394 flocks from 89 broiler farms were examined for the presence of Eimeria species. The overall farm-level infection rate was 98.88% (88/89; 95% CI: 96.64–100%), while the flock-level prevalence was 87.06% (343/394; 95% CI: 83.73–90.38%) (Table 3). All four regions of Guangdong were found to have seven Eimeria species present. The most common species at the farm-level were E. acervulina (72.53%; 64/89; 95% CI: 63.18–81.88%), E. tenella (68.54%; 61/89; 95% CI: 58.70–78.38%), E. mitis (66.29%; 59/89; 95% CI: 56.28–76.31%), and E. necatrix (61.80%; 55/89; 95% CI: 51.50–72.09%). At the flock-level, the predominant species were E. acervulina (36.55%; 144/394; 95% CI: 31.77–41.32%), E. mitis (35.28%; 139/394; 95% CI: 30.54–40.02%), E. tenella (34.01%; 134/394; 95% CI: 29.31–38.71%), and E. necatrix (30.96%; 122/394; 95% CI: 26.38–35.55%). Geographically, E. necatrix was significantly more prevalent in northern Guangdong (87.50%; 21/24; 95% CI: 73.23–100%) at the farm-level (p < 0.05), as well as at the flock-level with a prevalence of 46.77% in northern Guangdong (58/124; 95% CI: 37.87–55.68%) (p < 0.001). In contrast, E. acervulina was more prevalent in both eastern (47.13%; 41/87; 95% CI: 36.43–57.83%) and western Guangdong (45.71%; 32/70; 95% CI: 33.75–57.68%) at the flock-level (p < 0.05). Additionally, both E. tenella and E. acervulina were more prevalent in eastern Guangdong, with a prevalence of 45.98% (40/87; 95% CI: 35.29–56.66%) (p < 0.05), and 47.13% (41/87; 95% CI: 36.43–57.83%) (p < 0.05), respectively (Table 3).
Mixed infection of Eimeria species
In this study, the prevalence of infection with two or more Eimeria species was found to be 93.25% (83/89) at the farm level and 49.75% (196/394) at the flock level. Co-infection with three and four Eimeria species was more common among the 89 farms included, with a proportion of 20.22% (18/89; 95% CI: 11.72–28.73%) for both, followed by co-infection with five Eimeria species, which was found in 17.95% (16/89; 95% CI: 9.84–26.11%) of the farms. In terms of single-species infections at the flock level, they were prevalent across all four regions of Guangdong, with a proportion of 37.31% (147/394; 95% CI: 32.51–42.11%). This was followed by co-infection with two Eimeria species, which was found in 19.80% (78/394; 95% CI: 15.85–23.75%) of the flocks. Only 49.75% (196/394) of samples contained two or more Eimeria species within a single fecal sample at the flock level (Table 4). At the farm level, the most common combination of Eimeria species was all seven species (6.74%; 6/89), followed by E. acervulina, E. tenella, E. mitis, E. necatrix, E. brunetti, and E. maxima (5.62%, 5/89), and E. acervulina, E. tenella, E. mitis, E. necatrix, E. brunetti, and E. praecox (4.49%; 4/89) (Table 5).
Risk factors associated with Eimeria species infection
Univariate analysis was performed to determine the associations between the prevalence of Eimeria species infection at the farm level and various factors, such as farm location, bird age, drinking water source, control strategy, and presence of C. perfringens type A (Table 6). Multivariate analysis at the farm level revealed significant associations between E. necatrix infection and both grower birds (OR = 10.86; 95% CI: 1.92–61.36; p < 0.05) and adult birds (OR = 24.97; 95% CI: 4.29–145.15; p < 0.001) compared to starter birds. Additionally, a significant positive association was found between E. brunetti infection and adult chickens (OR = 5.02; 95% CI: 1.41–17.83; p < 0.05) compared to starter chickens. Farms that used groundwater (OR = 0.27; 95% CI: 0.08–0.94; p < 0.05) were less likely to have E. maxima compared to farms that used running water. Furthermore, farms with C. perfringens type A infection showed a significant positive association with E. brunetti (OR = 6.53; 95% CI: 1.52–28.09; p < 0.05), E. acervulina (OR = 5.30; 95% CI: 1.41–19.95; p < 0.05), E. mitis (OR = 4.23; 95% CI: 1.17–15.33; p < 0.05), and E. praecox (OR = 7.63; 95% CI: 1.45–40.09; p < 0.05) infections compared to farms without C. perfringens type A detected (Table 7).
In the flock-level analysis, univariate analysis revealed significant associations between Eimeria species infections and several variables, including farm location, bird age, bird breed, farming practices, drinking water source, control strategy, and occurrence of C. perfringens type A (Table 8). Multivariate analysis showed that the prevalence of E. tenella was significantly higher in the Pearl River Delta (OR = 2.48; 95% CI: 1.0–6.15; p = 0.05) compared to eastern Guangdong. Flocks between 4 and 8 weeks of age were significantly associated with E. brunetti (OR = 2.63; 95% CI: 1.15–6.04; p < 0.05), E. maxima (OR = 3.05; 95% CI: 1.23–7.59; p < 0.05), E. mitis (OR = 2.01; 95% CI: 1.08–3.73; p < 0.05), and E. praecox (OR = 3.52; 95% CI: 1.44–8.62; p < 0.05) infections compared to flocks younger than 4 weeks flocks. Additionally, flocks older than 8 weeks were more likely to be positive for E. necatrix (OR = 9.65; 95% CI: 4.45–20.94; p < 0.001), E. brunetti (OR = 2.91; 95% CI: 1.31–6.44; p < 0.05), and E. maxima (OR = 2.88; 95% CI: 1.23–6.77; p < 0.05) infections compared to flocks younger than 4 weeks. Interestingly, flocks with indigenous birds were less likely to be positive for E. brunetti (OR = 0.48; 95% CI: 0.26–0.89; p < 0.05) compared to indigenous crossbred birds. Additionally, ground-floored flocks had a significantly higher prevalence of E. acervulina (OR = 2.63; 95% CI: 1.03–6.74; p < 0.05) compared to multi-layer caged flocks. On the other hand, ground-floored flocks were less likely to be positive for E. necatrix (OR = 0.34; 95% CI: 0.13–0.90; p < 0.05) compared to multi-layer caged flocks. Flocks treated with anticoccidial drugs (OR = 0.09; 95% CI: 0.03–0.31; p < 0.001) or a combination of vaccines and anticoccidial drugs (OR = 0.06; 95% CI: 0.01–0.25; p < 0.001) were less likely to be positive for E. tenella infection compared to flocks immunized with vaccines only. Flocks with C. perfringens type A infection had a significantly higher likelihood of being positive for E. necatrix (OR = 3.26; 95% CI: 1.96–5.43; p < 0.001), E. tenella (OR = 2.14; 95% CI: 1.36–3.36; p < 0.001), E. brunetti (OR = 2.48; 95% CI: 1.45–4.23; p < 0.001), and E. acervulina (OR = 2.62; 95% CI: 1.69–4.06; p < 0.001) infections compared to flocks that C. perfringens type A was not detected (Table 9).
Discussion
Coccidiosis poses a significant economical challenge for the global poultry industry. This study aimed to investigate the prevalence of Eimeria species in Guangdong province, filling a critical research gap [18,19,20,21]. The overall prevalence of coccidiosis in Guangdong (87.06%; 343/394) was found to be higher than that in other regions, such as Zhejiang province in China (30.7%; 95/310) [19], Shandong province in China (65.8%; 50/76) [20], Korean (75%; 291/388) [22], Serbia (59%; 59/100) [23], north India (28.5%; 171/600) [24], and southwestern Nigeria (41.3%; 2292/5544) [25]. The farm-level prevalence of Eimeria species in this study (98.88%; 88/89) was higher than that reported in Romania (92%; 11/12) [6]. The high prevalence of Eimeria species in Guangdong province can be attributed to the climatic conditions, characterized by increased temperature and humidity, which promote the propagation of Eimeria in broiler flocks. Our findings are consistent with previous reports from other tropical and subtropical regions and countries, including Anhui province in China (87.75%; 150/171) [21], two northern Indian states (81.03%; 47/58) [26] and Greece (85.7%; 36/42) [27]. However, higher prevalence rates were documented in Henan province (96.70%; 176/182) and Hubei province in China (97.79%; 133/136) [28], Colombia (96.3%; 236/245) [29], Australia (98%; 255/260) [30], Japan (91.9%; 33/37) [31], and northeastern Algeria (99.5%; 186/187) [32]. This variability can be attributed to differing climate conditions, seasonal variations, different terrains, and management practices in different regions and countries.
Seven distinct Eimeria species were identified within broiler farms in Guangdong province. The most prevalence species at the flock level were E. acervulina (36.55%; 144/394), E. mitis (35.28%; 139/394), E. tenella (34.01%; 134/394), and E. necatrix (30.96%; 122/394). It is well-known that the interactions between Eimeria species and crowing effects play a pivotal role in oocyst production [33]. E. acervulina and E. tenella exhibit higher productive potential, and in cases of mixed infection, E. acervulina tends to suppress the oocyst production of E. necatrix, E. maxima, and E. brunetti [34, 35]. Our study found that single-species infections were predominant at the flock level (37.31%; 147/394), with only 49.75% (196/394) of samples infected with two or more Eimeria species within a single fecal sample. The most common combination found was all seven Eimeria species (6.74%; 6/89), which differs from a previous report that found the most common combination to be E. acervulina, E. maxima, E. necatrix, and E. praecox (23.90%) in Pichincha and Santo Domingo de los Tsáchilas, Ecuador [36].
Univariate and multivariate analyses have identified several potential risk factors associated with the prevalence of Eimeria species. This study found that flocks with adult chickens faced a higher risk of E. necatrix infection (OR = 9.65, 95% CI: 4.45–20.94; p < 0.001) compared to starter chickens. This finding is consistent with previous reports, which have also suggested higher prevalence rates among adult birds compared to birds of other ages [37, 38]. However, it contrasts with studies by Lawal et al. [39] and Khursheed et al. [24], which reported that younger birds were more susceptible to infection than older birds. This discrepancy might be attributed to variations in the prevalence of Eimeria species. E. necatrix is known to have lower reproductive capabilities and is considered a ‘poor competitor’ compared to other species, which may explain its higher prevalence in older birds [40]. Notably, outbreaks due to E. necatrix predominantly occur in older birds aged 9–14 weeks [41]. The increase in epidemic E. necatrix prevalence observed in this study highlights the importance of improving preventative measures.
The association between geographical variation and elevated prevalence of coccidia has been reported in previous studies [42,43,44]. In this study, flocks from the Pearl River Delta had a higher risk of E. tenella occurrence (OR = 2.48, 95% CI: 1.0–6.15; p = 0.05) compared to those from eastern Guangdong. This could be due to the heavier rainfall (approximately 149 mm/year) and relatively lower humidity (approximately 57%) in the Pearl River Delta. These findings are consistent with a previous report by Waldenstedt et al. [45] which found that the sporulation of Eimeria oocysts was poorest under the conditions of high moisture content (62%), suggesting that oocyst sporulation may be more efficient in drier litter [40].
This study observed a lower risk of E. tenella infection in flocks that used anticoccidial drugs (OR = 0.09, 95% CI: 0.03–0.31; p < 0.001) or a combination of vaccines and anticoccidial drugs (OR = 0.06, 95% CI: 0.01–0.25; p < 0.001) compared to flocks that only used vaccines. This result is consistent with previous research, which found that oocyst shedding was significantly lower in medicated flocks compared to vaccinated flocks in chickens younger than 4 weeks (p < 0.05) [46]. Additionally, this study found a high prevalence of E. brunetti (19.80%; 78/394) in Guangdong, compared to a previous study in China (6.6%) [28], where no commercial vaccines containing E. brunetti are available. Given its classification as a highly pathogenic species, it may be necessary to include E. brunetti in vaccines in China. Furthermore, previous studies have shown that chickens raised in free-range systems have a higher occurrence of coccidiosis compared to those raised in cages [22, 28], as the main mode of transmission for sporulated oocysts of coccidia is through the fecal-oral route. In this study, a higher risk of E. acervulina infection (OR = 2.63, 95% CI: 1.03–6.74; p < 0.05) was found in ground-floored flocks compared to multi-layer caged flocks. However, ground-floored flocks were less likely to be positive for E. necatrix (OR = 0.34; 95% CI: 0.13–0.90; p < 0.05) compared to multi-layer caged flocks. The higher prevalence of coccidia in birds raised in multi-layer cages may be attributed to high bird density and suboptimal cage design or maintenance. Further studies with a larger sample size are needed to explore the prevalence of Eimeria in flocks using different farming methods.
In the present study, the occurrence of C. perfringens type A was significantly associated with the flock-level prevalence of E. acervulina (OR = 2.62, 95% CI: 1.69–4.06; p < 0.001), E. necatrix (OR = 3.26, 95% CI: 1.96–5.43; p < 0.001), E. brunetti (OR = 2.48, 95% CI: 1.45–4.23; p < 0.001), and E. maxima (OR = 1.99, 95% CI: 1.16–3.42; p < 0.05) compared to flocks where clostridia were not detected. Similarly, a previous study found that infection rates of Eimeria species were significantly associated with a history of clostridiosis on farms (OR = 2.6, 95% CI: 1.19–2.78; p = 0.006) [47]. The damage to the intestinal epithelium caused by coccidia creates an environment that allows for rapid replication and toxin production of C. perfringens [48]. In addition, experimental use of C. perfringens type A, and E. acervulina or E. necatrix has been shown to produce necrotic enteritis in chickens, with a mortality rate of 53% in chickens infected with E. acervulina before C. perfringens type A [48]. Under field conditions, coccidia can play a significant role in the occurrence of necrotic enteritis when there is a sufficient number of C. perfringens type A present [49].
Conclusion
This study highlights the high prevalence of Eimeria species infections in broiler chickens across Guangdong province, China. The infection is widespread at both the farm and flock levels, with 98.88% (88/89) and 87.06% (343/394) of samples testing positive, respectively. The most common species found was E. acervuline in both farm and flock settings. Univariate and multivariate analysis revealed that geographical location, bird age, drinking water source, control methods, and the presence of C. perfringens type A were all associated with Eimeria species infection in chickens. Based on the identified risk factors, it is crucial to implement effective control strategies and management practices to reduce infections and minimize economic losses in poultry farming.
Availability of data and materials
The data that supporting the findings of this study, and the datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
- E. necatrix :
-
Eimeria necatrix
- E. tenella :
-
Eimeria tenella
- E. brunetti :
-
Eimeria brunetti
- E. acervulina :
-
Eimeria acervulina
- E. maxima :
-
Eimeria maxima
- E. mitis :
-
Eimeria mitis
- E. praecox :
-
Eimeria praecox
- C. perfringens :
-
Clostridium perfringens
- PCR:
-
Polymerase chain reaction
- qPCR:
-
Quantitative real-time polymerase chain reaction
- ITS:
-
Internal transcribed spacer
- SCAR:
-
Sequence characterized amplified region
- OR:
-
Odds ratio
- CI:
-
Confidence interval
References
Chapman HD. Milestones in avian coccidiosis research: a review. Poult Sci. 2014;93(3):501–11.
Blake DP, Knox J, Dehaeck B, Huntington B, Rathinam T, Ravipati V, et al. Re-calculating the cost of coccidiosis in chickens. Vet Res. 2020;51(1):115.
Williams RB, Marshall RN, Pagès M, Dardi M, del Cacho E. Pathogenesis of Eimeria praecox in chickens: virulence of field strains compared with laboratory strains of E. praecox and eimeria acervulina. Avian Pathol. 2009;38(5):359–66.
Blake DP, Clark EL, Macdonald SE, Thenmozhi V, Kundu K, Garg R, et al. Population, genetic, and antigenic diversity of the apicomplexan Eimeria tenella and their relevance to vaccine development. Proc Natl Acad Sci U S A. 2015;112(38):E5343–5350.
Long PL, Millard BJ, Joyner LP, Norton CC. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet Lat. 1976;6(3):201–17.
Györke A, Pop L, Cozma V. Prevalence and distribution of Eimeria species in broiler chicken farms of different capacities. Parasite. 2013;20:50.
Williams RB. Anticoccidial vaccines for broiler chickens: pathways to success. Avian Pathol. 2002;31(4):317–53.
Long PL, Joyner LP. Problems in the identification of species of Eimeria. J Protozool. 1984;31(4):535–41.
Lew AE, Anderson GR, Minchin CM, Jeston PJ, Jorgensen WK. Inter- and intra-strain variation and PCR detection of the internal transcribed spacer 1 (ITS-1) sequences of Australian isolates of Eimeria species from chickens. Vet Parasitol. 2003;112(1–2):33–50.
Haug A, Thebo P, Mattsson JG. A simplified protocol for molecular identification of Eimeria species in field samples. Vet Parasitol. 2007;146(1–2):35–45.
Gasser RB, Woods WG, Wood JM, Ashdown L, Richards G, Whithear KG. Automated, fluorescence-based approach for the specific diagnosis of chicken coccidiosis. Electrophoresis. 2001;22(16):3546–50.
Adeyemi OS, Olatoye IO, Oladele DO, Adejimi JO, Ogundipe GAT. Morphometric and molecular identification of Eimeria species from commercial chickens in Nigeria. J Dairy Vet Anim Res. 2020;9(4):104–8.
Thrusfield M. Veterinary epidemiology. 3rd ed. Oxford: Blackwell Publishing; 2008.
Kumar S, Garg R, Moftah A, Clark EL, Macdonald SE, Chaudhry AS, et al. An optimised protocol for molecular identification of Eimeria from chickens. Vet Parasitol. 2014;199(1–2):24–31.
Schnitzler BE, Thebo PL, Mattsson JG, Tomley FM, Shirley MW. Development of a diagnostic PCR assay for the detection and discrimination of four pathogenic Eimeria species of the chicken. Avian Pathol. 1998;27(5):490–7.
Schnitzler BE, Thebo PL, Tomley FM, Uggla A, Shirley MW. PCR identification of chicken eimeria: a simplified read-out. Avian Pathol. 1999;28(1):89–93.
Mohiuddin M, Song Z, Liao S, Qi N, Li J, Lv M, et al. Animal model studies, antibiotic resistance and toxin gene profile of ne reproducing Clostridium perfringens type A and type G strains isolated from commercial poultry farms in China. Microorganisms. 2023;11(3):622.
Zhang JJ, Wang LX, Ruan WK, An J. Investigation into the prevalence of coccidiosis and maduramycin drug resistance in chickens in China. Vet Parasitol. 2013;191(1–2):29–34.
Lan LH, Sun BB, Zuo BX, Chen XQ, Du AF. Prevalence and drug resistance of avian Eimeria species in broiler chicken farms of Zhejiang Province, China. Poult Sci. 2017;96(7):2104–9.
Sun XM, Pang W, Jia T, Yan WC, He G, Hao LL, et al. Prevalence of Eimeria species in broilers with subclinical signs from fifty farms. Avian Dis. 2009;53(2):301–5.
Huang Y, Ruan X, Li L, Zeng M. Prevalence of Eimeria species in domestic chickens in Anhui province, China. J Parasit Dis. 2017;41(4):1014–9.
Flores RA, Nguyen BT, Cammayo PLT, Vo TC, Naw H, Kim S, et al. Epidemiological investigation and drug resistance of Eimeria species in Korean chicken farms. BMC Vet Res. 2022;18(1):277.
Pajić M, Todorović D, Knežević S, Prunić B, Velhner M, Andrić DO, et al. Molecular investigation of Eimeria species in broiler farms in the province of Vojvodina, Serbia. Life (Basel). 2023;13(4):1039.
Khursheed A, Yadav A, Sofi OM, Kushwaha A, Yadav V, Rafiqi SI, et al. Prevalence and molecular characterization of Eimeria species affecting backyard poultry of Jammu region, North India. Trop Anim Health Prod. 2022;54(5):296.
Ola-Fadunsin SD. Investigations on the occurrence and associated risk factors of avian coccidiosis in Osun State, Southwestern Nigeria. J Parasitol Res. 2017;2017:9264191.
Kumar S, Garg R, Ram H, Maurya PS, Banerjee PS. Gastrointestinal parasitic infections in chickens of upper gangetic plains of India with special reference to poultry coccidiosis. J Parasit Dis. 2015;39(1):22–6.
Andreopoulou M, Chaligiannis I, Sotiraki S, Daugschies A, Bangoura B. Prevalence and molecular detection of Eimeria species in different types of poultry in Greece and associated risk factors. Parasitol Res. 2022;121(7):2051–63.
Geng T, Ye C, Lei Z, Shen B, Fang R, Hu M, et al. Prevalence of Eimeria parasites in the Hubei and Henan provinces of China. Parasitol Res. 2021;120(2):655–63.
Mesa C, Gomez-Osorio LM, Lopez-Osorio S, Williams SM, Chaparro-Gutierrez JJ. Survey of coccidia on commercial broiler farms in Colombia: frequency of Eimeria species, anticoccidial sensitivity, and histopathology. Poult Sci. 2021;100(8):101239.
Godwin RM, Morgan JA. A molecular survey of Eimeria in chickens across Australia. Vet Parasitol. 2015;214(1–2):16–21.
Matsubayashi M, Shibahara T, Matsuo T, Hatabu T, Yamagishi J, Sasai K, et al. Morphological and molecular identification of Eimeria spp. in breeding chicken farms of Japan. J Vet Med Sci. 2020;82(5):516–9.
Djemai S, Ayadi O, Khelifi D, Bellil I, Hide G. Prevalence of Eimeria species, detected by ITS1-PCR, in broiler poultry farms located in seven provinces of northeastern Algeria. Trop Anim Health Prod. 2022;54(5):250.
Williams RB. Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: its importance for experimental designs and the production of oocyst stocks. Int J Parasitol. 2001;31(10):1056–69.
Fatoba AJ, Zishiri OT, Blake DP, Peters SO, Lebepe J, Mukaratirwa S, et al. Study on the prevalence and genetic diversity of Eimeria species from broilers and free-range chickens in KwaZulu-Natal province, South Africa. Onderstepoort J Vet Res. 2020;87(1):e1–10.
da Silva JT, Alvares FBV, de Lima EF, da Silva Filho GM, da Silva ALP, Lima BA, et al. Prevalence and diversity of Eimeria spp. in free-range chickens in northeastern Brazil. Front Vet Sci. 2022;9:1031330.
Cevallos-Gordon A, Molina CA, Radman N, Ron L, Gamboa MI. Prevalence and risk factors of Eimeria spp. in broiler chickens from Pichincha and Santo Domingo De Los Tsáchilas. Ecuador Pathogens. 2024;13(1):48.
Amare A, Worku N, Negussie H. Coccidiosis prevailing in parent stocks a comparative study between growers and adult layers in kombolcha poultry breeding and multiplication center, Ethiopia. Glob Vet. 2012;8(3):285–91.
Dakpogana HB, Salifoua S. Coccidiosis prevalence and intensity in litterbased high stocking density layer rearing system of Benin. J Anim Plant Sci. 2013;17(2):2522–6.
Lawal JR, Jajere SM, Ibrahim UI, Geidam YA, Gulani IA, Musa G, et al. Prevalence of coccidiosis among village and exotic breed of chickens in Maiduguri, Nigeria. Vet World. 2016;9(6):653–9.
Williams RB. Epidemiological aspects of the use of live anticoccidial vaccines for chickens. Int J Parasitol. 1998;28(7):1089–98.
Sawale GK, Rambabu D, Kommu S, Bhandurge MS, Naik R, Lakshman M. Outbreak of intestinal coccidiosis due to Eimeria necatrix in rajasree birds: patho-morphological and electron microscopic study. Int J Livest Res. 2018;8(12):247–51.
Chengat Prakashbabu B, Thenmozhi V, Limon G, Kundu K, Kumar S, Garg R, et al. Eimeria species occurrence varies between geographic regions and poultry production systems and may influence parasite genetic diversity. Vet Parasitol. 2017;233:62–72.
Mohammed BR, Sunday OS. An overview of the prevalence of avian coccidiosis in poultry production and its economic importance in Nigeria. Vet Res Int. 2015;3(3):35–45.
Ekawasti F, Nurcahyo RW, Firdausy LW, Wardhana AH, Sawitri DH, Prastowo J, et al. Prevalence and risk factors associated with Eimeria species infection in cattle of different geographical regions of Indonesia. Vet World. 2021;14(9):2339–45.
Waldenstedt L, Elwinger K, Lundén A, Thebo P, Uggla A. Sporulation of Eimeria maxima oocysts in litter with different moisture contents. Poult Sci. 2001;80(10):1412–5.
Snyder RP, Guerin MT, Hargis BM, Page G, Barta JR. Monitoring coccidia in commercial broiler chicken flocks in Ontario: comparing oocyst cycling patterns in flocks using anticoccidial medications or live vaccination. Poult Sci. 2021;100(1):110–8.
Gharekhani J, Sadeghi-Dehkordi Z, Bahrami M. Prevalence of coccidiosis in broiler chicken farms in Western Iran. J Vet Med. 2014;2014:980604.
Al-Sheikhly F, Al-Saieg A. Role of coccidia in the occurrence of necrotic enteritis of chickens. Avian Dis. 1980;24(2):324–33.
Cooper KK, Songer JG. Necrotic enteritis in chickens: a paradigm of enteric infection by Clostridium perfringens type A. Anaerobe. 2009;15(1–2):55–60.
Acknowledgements
We are so grateful to Dr. Junfen Liang and Dr. Xu Liu from the Institute of Agricultural Economics and Information, Guangdong Academy of Agricultural Sciences, for their technical support in creating and scaling maps to locate the sampled farms. We are thankful for the farm managers/owners, and veterinarians who agreed to participate in this study.
Funding
This work was supported by the National Key Research and Development Program of China (2023YFD1801202), The open competition program of top ten critical priorities of Agricultural Science and Technology Innovation for the 14th Five-Year Plan of Guangdong Province (2023SDZG02), Key Realm R&D Program of Guangdong Province (2023B0202150001), Opening Project of State Key Laboratory of Swine and Poultry Breeding Industry (2023QZ-NK05, 2022GZ07), Science and technology project of Yunfu (2022020202), Science and technology project of Guangzhou (2023B04J0137, 2023A04J0789), Special fund for scientific innovation strategy-construction of high level Academy of Agriculture Science (202110TD, 202122TD, R2020PY-JC001, R2019YJ-YB3010, R2020PY-JG013, R2020QD-048, R2021PY-QY007, R2023PY-JG018), The Project of Collaborative Innovation Center of GDAAS (XTXM202202).
Author information
Authors and Affiliations
Contributions
SL, NQ, and MS designed this study. SL, XL, QZ and ZY collected samples. CW, JL, ML, JH, HC, YS, and XC performed experiments. SL, XL, YZ, LY, JZ, NQ, and MS interpreted the results and drafted the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The questionnaire and fecal sampling protocols were reviewed and approved by the Animal Care and Use Committee of the Institute of Animal Health, Guangdong Academy of Agricultural Sciences. The farm owners were aware of the objectives of this study. An informed consent was obtained from all broiler farm owners. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liao, S., Lin, X., Zhou, Q. et al. Prevalence, geographic distribution and risk factors of Eimeria species on commercial broiler farms in Guangdong, China. BMC Vet Res 20, 171 (2024). https://doi.org/10.1186/s12917-024-03990-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-024-03990-4