Abstract
Background
Worldwide, there is a need to expand the number of drugs available to treat parasitic infections in aquaculture. One of the new materials being tested is metal nanoparticles, which have unique chemical and physical characteristics owing to their extremely small size and high surface area to volume ratio. We examined the effectiveness of gold nanoparticles against the microsporidian parasite Heterosporis saurida, which causes severe economic losses in lizard fish, Saurida undosquamis aquaculture.
Results
We synthesized gold nanoparticles by chemical reduction of tetrachloroauric acid as a metal precursor. We assessed the antimicrosporidial efficacy of the nanoparticles against H. saurida using an in vitro screening approach, which we had developed previously using the eel kidney cell line EK-1. The number of H. saurida spores produced in EK-1 cells was reduced in a proportional manner to the dosage of gold nanoparticles administered. A cell metabolic activity test (MTT) indicated that the gold nanoparticles did not appear to be toxic to the host cells.
Conclusions
Gold nanoparticles can act as an effective antimicrosporidial agent and hold promise to reduce disease in lizardfish aquaculture. Metal nanoparticles should be considered as an alternate choice for development of new antimicrosporidial drugs to combat disease problems in aquaculture.
Similar content being viewed by others
Background
Saurida undosquamis (Richardson, 1848) also known as Brushtooth lizardfish is fish species of the Synodontidae family. S. undosquamis is a Lessepsian migrant species distributed across the Indo-West Pacific including the Red Sea, Persian Gulf, Eastern Africa, Japan and Australia [1]. S. undosquamis invaded the Levant Basin of the Mediterranean Sea, from the Indo-West Pacific through the Suez Canal [2] and is considered one of the most successful colonizers of the Eastern Mediterranean, extending as far as the Aegean Sea [3]. The Mediterranean lizardfish population now has significant commercial value [4] in the eastern Mediterranean, where it is considered one of the most common species caught in the trawl fishery [5].
Diseases are a major obstruction to expansion of fresh water and marine aquaculture. Fish are susceptible to many pathogens, often with severe consequences [6]. Microsporidia are single-celled, obligate intracellular parasites that infect invertebrates and vertebrates. More than one thousand microsporidian species have been recognized as causing diseases in animals and humans [7]. Microsporidia are common in marine, fresh water and estuarine systems, and affect economically important fish species worldwide, including salmonids [8], flatfish [9], greater sand eels (Hyperoplus lanceolatus) [10] and ornamental fish, such as zebrafish (Danio rerio) [11] and killifish (Family Cyprinodontidae) [12]. Infections caused by microsporidia reduce the growth rate of fish and decrease production in aquaculture [13, 14]. Recently, H. saurida was isolated from lizardfish (Fig. 1) where it infects skeletal muscle, body cavity and mesenteric tissues and forms white, cyst-like structures, which contain numerous spores making the fish unsuitable for public consumption [15].
Only a small number of anti-parasitic drugs are permitted in aquaculture, as the cost of drug development is high and the market small. The few available treatments for microsporidiosis differ in their effectiveness, and as with many drug treatments, there is concern for the pathogens developing resistance to one or multiple antibiotics [16]. A promising, alternative approach that has received recent attention is the use of metallic nanoparticles, which have distinct advantages over conventional antimicrobial agents [17, 18]. Nanometer-sized materials have unique, extraordinary and fascinating physical, chemical, and biological properties, particularly a large contact area with microorganisms because of their small size and higher surface-to-volume ratio; a property that expands their biological and chemical activity. Currently, nanoparticles with one dimension of 100 nm or less have received immense interest as an alternative means of combatting infectious agents in medicine. Metal based nanoparticles display broad spectrum antimicrobial activity against bacteria, fungi and viruses [19–24].
The most widespread antimicrobial compounds are silver and benzalkonium chloride [25, 26]. Unfortunately, in a comparable manner where scientists develop new efficient antimicrobial materials, there is no doubt that resistance to silver also is increasing [16]. Therefore, a number of metals, mainly copper, have been used as an antimicrobial agent. Gold has been little explored as an antimicrobial agent, although it has been used as a catalyst [27, 28].
Gold nanoparticles in particular, have a broad range of applications in nano-scale devices and technologies due to their chemical inertness and resistance to surface oxidation [29]. Gold nanoparticles have been investigated as an antimicrobial agent to inhibit the growth of common, waterborne, pathogens Escherichia coli and Salmonella typhi, which are developing resistance to common bactericides [30]. In aquaculture, several studies have been carried out to investigate gold nanoparticles as antimicrobial drugs [31–33].
In vivo efficiency trials for anti-parasitic drugs are expensive, time intensive, and need ethical permission, hence new and efficient systems to screen compounds while reducing animal experiments are required. One alternative, cost-effective approach is to screen compounds using relevant animal cell lines. In the case of H. saurida, in vitro propagation has been demonstrated for both mammal (rabbit kidney; [34]) and fish (eel kidney EK-1; [35]) cell lines and an in vitro screening approach using the EK-1 cells to test antimicrosporidial agents against the parasite was also developed [36], however, our aim in the present study was to investigate the in vitro antimicrosporidial activity of gold nanoparticles as a potential agent against H. saurida infections.
Methods
Ethics statement
No ethical approval was necessary as this study did not involve laboratory animals; it comprised in vitro testing of cultured cell lines and microsporidian spores.
Propagation of H. saurida spores
Heterosporis saurida spores were grown in the eel fish kidney cell line (EK-1) using procedures previously described [35]. Briefly, H. saurida spores were collected from naturally infected lizard fish, Saurida undosquamis [15]. EK-1 cells were sub-cultured, seeded in 24-well plates in triplicate and supplemented with L-15 medium (Leibovitz) containing L-glutamine (Sigma-Aldrich), 10 % fetal bovine serum (FBS) (Sigma-Aldrich), 100 units/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich, Vienna, Austria). Twenty four hours after seeding, 100 μl (~107 spores/ml) of spore suspension was added to each well. Twenty-four hour post-inoculation (p.i.), the medium was removed and the cells rinsed gently 3 times with fresh medium; the medium was replace twice per week.
Preparation and characterization of gold nanoparticles
We followed the protocol of Storhoff et al. [37] to synthesize gold nanoparticles (~13 nm diameter) by reduction of 10 mM tetrachloroauric acid (HAuCl4) using sodium citrate (Sigma-Aldrich, Vienna, Austria). Briefly, an aqueous solution of HAuCl4.3H2O was boiled under reflux while being stirred. The color of the solution changed from yellow to deep red after rapid addition of 10 ml 1 % trisodium citrate. The color change signified formation of monodispersed spherical gold nanoparticles. The solution refluxed for an additional 15 min, then allowed to cool to room temperature. The solution was subsequently filtered through a 0.45 μm acetate filter and stored at 4 °C. A stock concentration of 1 mg/ml gold nanoparticles was used in the assays.
Morphology, size, and shape of synthesized gold nanoparticles were characterized using transmission electron microscopy (TEM). Samples were prepared by drop casting a 2.5 mL aliquot of the Au NPs suspension onto a 300 mesh carbon-coated copper grid. The gold suspension was dried at room temperature for 5 min and overload solution was removed from the grid using blotting paper. Particles were imaged using a Zeiss EM109. The size distribution of particles was estimated by images analysis of 100 nanoparticles located at different regions of the grid (n = 3). Images were taken of several samples (n = 3) to produce statistically meaningful results.
Measurement of anti-microsporidial activity of gold nanoparticles
Twenty-four well culture plates were loaded with EK-1 cells at a concentration of 1.5 × 105 cells/ml in L-15 medium containing 10 % FBS, and penicillin-streptomycin solution (Sigma-Aldrich, Vienna, Austria). The plates were incubated overnight at 26 °C to allow a cell monolayer to form. To reach a final ratio of 3:1 spores/cell, H. saurida spores were added in 1 ml volumes of medium at a concentration of 106 –107 spores/ml. Non-adherent spores were washed off after 24 h, and fresh medium with 0, 0.01, 0.1 and 1.0 μg/ml gold nanoparticles was added to wells, and incubated for 7 days. Media was replaced every three days. Cell monolayers were examined daily with an inverted microscope, and 10 cells in 10 fields were viewed per well using a 40X objective, for each treatment group (6 wells each treatment and control). Each concentration was tested in triplicate and the inhibition of H. saurida spore propagation was calculated as percent inhibition = 100 – [(mean number of H. saurida spores counted in treated cultures/mean number of H. saurida spores counted in non-treated cultures) × 100]. The differences between treated and non-treated H. saurida spores were analyzed using t-tests with Bonferroni α-correction. A p-value < 0.05 was regarded as significant for all statistical tests. Statistical analyses were carried out using SPSS version-20 software.
Visual observations and measurement of drug toxicity
EK-1 cells were plated in 96-well plates at approximately 1.5 × 104 cells per well. After 24 h, the medium was replaced with fresh medium containing different concentrations of gold nanoparticles as described above. Cellular viability of EK-1 cultures after introduction to the gold nanoparticles was examined as above. Any toxic effects were noted, such as cells becoming sub-confluent or altered in morphology compared with non-treated control cells. Plates were incubated at 26 °C for 1 week. The medium was then removed and the viability of cultures was assayed by incubating with MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide, Sigma-Aldrich), following the method described by Mosmann [38]: 20 μl MTT (5 mg/ml in balanced salt solution) was added to each well and incubated for 2 h. After that, medium of each well was discarded, and 200 μl of MTT solubilization solution (Sigma-Aldrich) was added and the plates agitated with an orbital shaker. Triplicate absorbance values of each well were measured in a microplate reader at 570 nm against a reference wavelength of 690 nm. The percentage of cell viability was calculated as the optical density values of treated cells divided by the mean optical density of non-treated cells, multiplied by100.
Re-infection of EK-1 cells with H. saurida recovered after gold nanoparticles treatments
To determine if H. saurida spores were still infectious after treatment with gold nanoparticles, spores were recovered from each culture well on day 7, by addition of 100 μl of 10 % (w/v) sodium dodecyl sulfate. Released spores were centrifuged at 400 g for 15 min and washed 3 times with Tris-buffered saline containing Tween 20 (0.3 %). Spore pellets were re-suspended in medium and used to infect new cultures as previously described. A hemocytometer was used to count spores gold nanoparticles from treated and non-treated wells: 1 ml of spores suspension was adjusted to 1x104 spores/ml and then added to each well. Fresh medium without gold nanoparticles was added on day 3. Each culture well was observed under an inverted microscope and spores were counted 3 times (10 field/well).
Results
Gold nanoparticles
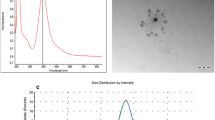
TEM revealed that the mean diameter of gold nanoparticles was 11.06–14.22 nm, and particles were spherical (Fig. 2). TEM also showed that the gold nanoparticles suspension was in a monodispersional state without obvious aggregations.
Measurement of anti-microsporidial activity of gold nanoparticles
Gold nanoparticles significantly reduce (p < 0.001) the number of H. saurida spores produced by infected cells, in a concentration-dependent manner (Fig. 3). The numbers of spores observed in 10 infected EK-1 cells in 10 fields of 6 wells were recorded and are listed in Table 1. Cultures with 0.01 μg/ml gold nanoparticles had fewer H. saurida spores than control cell cultures. Propagation of H. saurida spores was inhibited 68 and 75 % by 0.1 μg/ml and 1 μg/ml gold nanoparticles, respectively. Cellular and nuclear shapes appeared normal at all gold nanoparticle concentrations.
Drug toxicity measurements
Cell viability was monitored using MTT assay after incubation for 7 days with different concentrations of gold nanoparticles. Effects of gold nanoparticles tested concentrations were analyzed using MTT assay and results are shown in Table 1.
Re-infection of EK-1 cells with recovered H. saurida spores
Since it was not appropriate to verify if these spores were mature or infectious by microscopic observation, EK-1 cells were re-infected with recovered H. saurida spores after treatment.
Significantly fewer infectious microsporidia spores were recovered after treatments with gold nanoparticles in a concentration-dependent manner when compared with non-treated cultures (Table 1).
Discussion
Host- pathogen interactions are usually multivalent, and the interplay between microbes and host cells often involves several copies of multiple receptors and ligands that bind in a coordinated way to allow the microbial agent to take the cell under control. Interfering with these recognition events, by effectively crowding out pathogen entry into the cells, is one of the most promising strategies being investigated for drug development [21]. Due to the emergence of diseases and increased mortalities in aquaculture, and the development of drug resistance by aquatic microbes, there is an ongoing effort to investigate alternative methods of prevention and control of diseases [24]. A growing attention in compounds with antimicrobial characteristics is rising due to their wide application potential in various fields including medicine [39, 40].
The present study was conducted to find out if gold nanoparticles demonstrate antimicrosporidial activity against the aquatic microsporidian Heterosporis saurida in vitro.
We synthesized the gold nanoparticles (13 nm diameter) by reduction of 10 mM tetrachloroauric acid (HAuCl4) with sodium citrate.
TEM shows that most of the gold nanoparticles are round and spherical in shape and in a monodispersional state without obvious aggregations that is due to the negatively charged coating layer of citrate ions, which leads to electrostatic repulsion and prevents aggregation of gold nanoparticles.
We screened different concentrations of the nanoparticles for activity against H. saurida in EK-1 cells. Cell cultures treated with even the lowest concentration (0.01 μg/ml) of gold nanoparticles produced fewer H. saurida spores than control cell cultures (Table 1). At a concentration of 0.1 μg/ml, production of spores was inhibited more than 65 %, and at 1.0 μg/ml, inhibition was more than 75 %. The inhibitory rates obtained in this study are close to those observed when other potential antimicrosporidial drugs were used [36].
We conducted MTT assays to determine if health of host cells was adversely affected, after incubation for 7 days with different concentrations of gold nanoparticles. Negligible cytotoxicity was observed up to concentrations of 0.01 μg/ml, and < 35 % toxicity was found at the highest concentrations tested (1 μg/ml). These results are consistent with the low cytotoxicity reported previously for gold nanoparticle conjugates [41]. It has been suggested that gold nanoparticles increase permeability of the cell wall that leads to leakage of cell contents and cell death. Furthermore, gold nanoparticles have been shown to bind to the DNA of microorganisms and inhibit the DNA transcription process [41, 42]. Recently, conjugates of gold nanoparticles were investigated and reported not to be generally cytotoxic and non-specific host cell membrane disruptors but affect the transcription of a number of micro-organisms genes including those involved in cell division [43]. Similarly, in this study gold nanoparticles have been shown not to be generally toxic because the viability of the EK-1 cells was not greatly affected as confirmed with MTT test. Gene expression studies would be required to clarify the molecular mechanistic effects of gold nanoparticles on H. saurida, though it has been suggested that the nanoparticles may alter expression of genes related to parasite cell division [43]. The mechanism of action may also involve an increase in permeability of the cell walls, leading to leakage of cell contents and death [41, 42].
Conclusions
This study shows that gold nanoparticles demonstrate a considerable antimicrosporidial activity against the fish microsporidian H. saurida in vitro. However, further investigations concerning mode of action and functionalization of gold nanoparticles to increase their antimicrosporidial activity, reduce possible in vivo accumulation related toxicity and enhance and promote their use in aquaculture are still needed.
Abbreviations
- S. undosquamis :
-
Saurida undosquamis
- H. saurida :
-
Heterosporis saurida
- EK-1:
-
eel kidney cell line
References
Froese R, Pauly D. Fishbase. World Wide Web electronic publication. 2015. http://www.fishbase.org. (version 10/2015).
Ben-Tuvia A. Red Sea fishes recently found in the Mediterranean. Copeia. 1966;2:254–75.
Bilecenoğlu M, Taşkavak E, Mater S, Kaya M. Checklist of the marine fishes of Turkey. Zootaxa. 2002;113:1–194.
Ben-Yami M, Glazer T. The invasion of Saurida undosquamis (Richardson) into the Levant Basin-an example of biological effect of interoceanic canals. Fish Bull. 1974;72(2):359–73.
Bingel F, Ozsoy E, Unluata U. A review of the state of the fisheries and the environment of the Northeastern Mediterranean (Northern Levantine Basin), Studies and Reviews, General Fisheries Council for the Mediterranenan, vol. 65. Rome: FAO; 1993. p. 74.
Kent ML, Speare DJ. Review of the sequential development of Loma salmonae (Microsporidia) based on experimental infections of rainbow trout (Oncorhynchus mykiss) and Chinook salmon (O. tshawytscha). Folia Parasitol. 2005;52(1–2):63–8.
Weiss LM. Microsporidia: emerging pathogenic protists. Acta Trop. 2001;78(2):89–102.
Kent ML. Marine netpen farming leads to infections with some unusual parasites. Int J Parasitol. 2000;30(3):321–6.
Matthews RA, Matthews BF. Cell and tissue reactions of turbot Scophthalmus maximus (L.) to Tetramicra brevifilum gen. n., sp. n. (Microspora). J Fish Dis. 1980;3(6):495–515.
Leiro J, Paramá A, Ortega M, Santamarina MT, Sanmartín ML. Redescription of Glugea caullery, a microsporidian parasite of the greater sand-eel, Hyperoplus lanceolatus (Le Sauvage), (Teleostei: Ammodytidae), as Micro Gemma caullery comb. nov. J Fish Dis. 1999;22(2):101–10.
de Kinkelin P. Occurrence of a microsporidian infection in zebra fish Brachydanio rerio (Hamilton-Buchanan). J Fish Dis. 1980;3(1):71–3.
Lom J, Noga E, Dykova I. Occurrence of a microsporean with characteristics of Glugea anomala in ornamental fish of the family Cyprinodontidae. Dis Aquat Org. 1995;21(3):239–42.
Constantine J. Estimating the cost of Loma salmonae to B.C. Aquaculture. British Columbia, Canada: Ministry of Agriculture and Food; 1999.
Canning EU. Phylum Microspora. In: Margulis L, Corliss JO, Melkonian M, Chapman DJ, editors. Handbook of protista. Boston, USA: Jones and Barlett Publishers; 1990. p. 53–72.
Al-Quraishy S, Abdel-Baki AS, Al-Qahtani H, Dkhil M, Casal G, Azevedo C. A new microsporidian parasite, Heterosporis saurida n. sp. (Microsporidia) infecting the lizardfish, Saurida undosquamis from the Arabian Gulf, Saudi Arabia: ultrastructure and phylogeny. Parasitology. 2012;139(4):454–62.
Lallo MA, Vidoto da Costa LF, Manoel de Castro J. Effect of three drugs against Encephalitozoon cuniculi infection in immunosuppressed mice. Antimicrob Agents Chemother. 2013;57(7):3067–71.
Chaloupka K, Malam Y, Seifalian AM. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28(11):580–8.
Gunalan S, Sivaraj R, Rajendran V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog Nat Sci Mater Int. 2012;22(6):693–700.
Ren G, Hu D, Cheng EW, Vargas-Reus MA, Reip P, Allaker RP. Characterization of copper oxide nanoparticles for antimicrobial applications. Int J Antimicrob Agents. 2009;33(6):587–90.
Ali DM, Thajuddin N, Jeganathan K, Gunasekaran M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf B. 2011;85(2):360–5.
Galdiero S, Falanga A, Vitiello M, Cantisani M, Marra V, Galdiero M. Silver nanoparticles as potential antiviral agents. Molecules. 2011;16(10):8894–918.
Nasrollahi A, Pourshamsian K, Mansourkiaee P. Antifungal activity of silver nanoparticles on some of fungi. Int J Nano Dim. 2011;1(3):233–9.
Seil JT, Webster TJ. Antimicrobial applications of nanotechnology: methods and literature. Int J Nanomed. 2012;7:2767–81.
Swain P, Nayak SK, Sasmal A, Behera T, Barik SK, Swain SK, et al. Antimicrobial activity of metal based nanoparticles against microbes associated with diseases in aquaculture. World J Microbiol Biotechnol. 2014;30(9):2491–502.
Chopra I. The increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern? J Antimicr Chem. 2007;59(4):587–90.
Rees EN, Tebbs SE, Elliott TSJ. Role of antimicrobial-impregnated polymer and Teflon in the prevention of biliary stent blockage. J Hosp Inf. 1998;39(4):323–9.
Haruta M, Tsubota S, Kobayashi T, Kageyama H, Genet M, Delmon B. Low temperature oxidation of CO over gold supported on TiO2, α-Fe2O3, and Co3O4. J Catal. 1993;144(1):175–92.
Huang J, Lima E, Akita T, Guzmán A, Qi C, Takei T, et al. Propene epoxidation with O2 and H2: Identification of the most active gold clusters. J Catal. 2011;278(1):8–15.
Sugunan A, Thanachayanont C, Dutta J, Hilborn JG. Heavy-metal ion sensors using chitosan-capped gold nanoparticles. Adv Mater. 2005;6:335–40.
Lima E, Guerra R, Lara V, Guzmán A. Gold nanoparticles as efficient antimicrobial agents for Escherichia coli and Salmonella typhi. Chem Cent J. 2013;7:11.
Soltani M, Ghodratnema M, Ahari H, Ebrahimzadeh Mousavi HA, Atee M, Dastmalchi F, et al. The inhibitory effect of silver nanoparticles on the bacterial fish pathogens, Streptococcus iniae, Lactococcus garvieae, Yersinia ruckeri and Aeromonas hydrophila. Int J Vet Res. 2009;3(2):137–42.
Vaseeharan B, Ramasamy P, Chen JC. Antibacterial activity of silver nanoparticles (AgNps) synthesized by tea leaf extracts against pathogenic Vibrio harveyi and its protective efficacy on juvenile Feneropenaeus indicus. Lett Appl Microbiol. 2010;50(4):352–6.
Ravikumar S, Gokulakrishnan R, Raj JA. Nanoparticles as a source for the treatment of fish diseases. Asian Pac J Trop Dis. 2012;2:S703–6.
Kumar G, Saleh M, Abdel-Baki AA, Al-Quraishy S, El-Matbouli M. In vitro cultivation model for Heterosporis saurida (Microsporidia) isolated from lizardfish, Saurida undosquamis (Richardson). J Fish Dis. 2014;37(5):443–9.
Saleh M, Kumar G, Abdel-Baki AA, El-Matbouli M, Al-Quraishy S. In vitro growth of the microsporidian Heterosporis saurida in the eel kidney EK-1 cell line. Dis Aquat Org. 2014;108(1):37–44.
Saleh M, Kumar G, Abdel-Baki AA, Dkhil M, El-Matbouli M, Al-Quraishy S. Development of a novel in vitro method for drug development for fish; application to test efficacy of antimicrosporidian compounds. Vet Rec. 2014;175(22):561.
Storhoff JJ, Elghanian R, Mucic RC, Mirkin CA, Letsinger RL. One-pot colorimetric differentiation of polynucleotides with single base imperfections using gold nanoparticle probes. J Am Chem Soc. 1998;120(9):1959–664.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Hobman JL, Wilson JR, Brown NL. Microbial mercury reduction. In: Lovley DR, editor. Environmental metal-microbe interactions. Washington: ASM Press; 2000. p. 177–90.
Lewis K, Klibanov AM. Surpassing nature: rational design of sterile-surface materials. Trends Biotechnol. 2005;23(7):343–8.
Rai A, Prabhune A, Perry CC. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J Mater Chem. 2010;20(32):6789–98.
Prema P, Thangapandiyan S. In-vitro antibacterial activity of gold nanoparticles capped with polysaccharides stabilizing agents. Int J Pharm Pharm Sci. 2013;5:310–4.
Bresee J, Bond CM, Worthington RJ, Smith CA, Gifford JC, Simpson CA, et al. Nonoscale structure-activity relationships, mode of action, and biocompatibility of gold nanoparticles antibiotics. J Am Chem Soc. 2014;136(4):5295–300.
Acknowledgments
This work was funded in part by King Saud University, Riyadh, Saudi Arabia; the research group projects number ARP-32-19 and by the Austrian Science Fund (FWF) project no. P 27489-B22.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MS designed and accomplished the study and drafted the manuscript. GK helped with the propagation of the spores, MTT assay, figures preparations and revision of the manuscript. AA and SA dissected the fish and collected the spores. MEL supervised the study and helped revised the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Saleh, M., Kumar, G., Abdel-Baki, AA. et al. In vitro antimicrosporidial activity of gold nanoparticles against Heterosporis saurida . BMC Vet Res 12, 44 (2016). https://doi.org/10.1186/s12917-016-0668-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-016-0668-x