Abstract
Background
Due to the abundant usage of chemotherapy in young triple-negative breast cancer (TNBC) patients, the unbiased prognostic value of BRCA1-related biomarkers in this population remains unclear. In addition, whether BRCA1-related biomarkers modify the well-established prognostic value of stromal tumor-infiltrating lymphocytes (sTILs) is unknown. This study aimed to compare the outcomes of young, node-negative, chemotherapy-naïve TNBC patients according to BRCA1 status, taking sTILs into account.
Methods
We included 485 Dutch women diagnosed with node-negative TNBC under age 40 between 1989 and 2000. During this period, these women were considered low-risk and did not receive chemotherapy. BRCA1 status, including pathogenic germline BRCA1 mutation (gBRCA1m), somatic BRCA1 mutation (sBRCA1m), and tumor BRCA1 promoter methylation (BRCA1-PM), was assessed using DNA from formalin-fixed paraffin-embedded tissue. sTILs were assessed according to the international guideline. Patients’ outcomes were compared using Cox regression and competing risk models.
Results
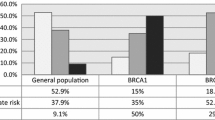
Among the 399 patients with BRCA1 status, 26.3% had a gBRCA1m, 5.3% had a sBRCA1m, 36.6% had tumor BRCA1-PM, and 31.8% had BRCA1-non-altered tumors. Compared to BRCA1-non-alteration, gBRCA1m was associated with worse overall survival (OS) from the fourth year after diagnosis (adjusted HR, 2.11; 95% CI, 1.18–3.75), and this association attenuated after adjustment for second primary tumors. Every 10% sTIL increment was associated with 16% higher OS (adjusted HR, 0.84; 95% CI, 0.78–0.90) in gBRCA1m, sBRCA1m, or BRCA1-non-altered patients and 31% higher OS in tumor BRCA1-PM patients. Among the 66 patients with tumor BRCA1-PM and ≥ 50% sTILs, we observed excellent 15-year OS (97.0%; 95% CI, 92.9–100%). Conversely, among the 61 patients with gBRCA1m and < 50% sTILs, we observed poor 15-year OS (50.8%; 95% CI, 39.7–65.0%). Furthermore, gBRCA1m was associated with higher (adjusted subdistribution HR, 4.04; 95% CI, 2.29–7.13) and tumor BRCA1-PM with lower (adjusted subdistribution HR, 0.42; 95% CI, 0.19–0.95) incidence of second primary tumors, compared to BRCA1-non-alteration.
Conclusions
Although both gBRCA1m and tumor BRCA1-PM alter BRCA1 gene transcription, they are associated with different outcomes in young, node-negative, chemotherapy-naïve TNBC patients. By combining sTILs and BRCA1 status for risk classification, we were able to identify potential subgroups in this population to intensify and optimize adjuvant treatment.
Similar content being viewed by others
Background
Pathogenic germline BRCA1 mutations (gBRCA1m) predispose women to breast cancer, especially triple-negative breast cancer (TNBC) [1]. Approximately 8.5 to 16% of unselected TNBC patients carry a pathogenic gBRCA1m [2,3,4,5]. This percentage is higher in those who are diagnosed at a younger age, ranging from 20 to 36% [2, 6,7,8]. Besides gBRCA1m, somatic BRCA1 mutations (sBRCA1m) and BRCA1 promoter methylation (BRCA1-PM) also alter the transcription of the BRCA1 gene. Since altered BRCA1 transcription hampers the homologous recombination pathway, leading to unrepaired DNA double-strand breaks and genomic instability, the affected tumors often present a typical profile of genomic aberrations [9,10,11]. In this study, we defined tumors with the typical genomic aberrations, which resemble the aberrations caused by gBRCA1m as BRCA1-like tumors [12]. In addition, increased genomic instability is suggested to promote anti-tumor immune response [13], which might be reflected by the abundance of tumor-infiltrating lymphocytes (TILs) in gBRCA1m or BRCA1-like tumors. Several studies have demonstrated that high TILs were associated with improved prognosis of TNBC patients [14,15,16,17]. However, whether TILs are more enriched in gBRCA1m or BRCA1-like tumors remains in dispute [18,19,20,21]. In addition, the prognostic value of TILs in gBRCA1m patients or in patients with other BRCA1-altered tumors is unclear.
Moreover, gBRCA1m or BRCA1-like tumors often present with aggressive phenotypes [22, 23] and are hypothesized to be associated with a worse prognosis compared to germline BRCA1 wild-type (gBRCA1wt) or non-BRCA1-like tumors. However, many studies observed that in chemotherapy-treated TNBC patients, those with gBRCA1m or BRCA1-like tumors had equivalent or even better survival compared to those with gBRCA1wt or non-BRCA1-like tumors [5, 6, 24,25,26,27]. This suggests that chemotherapy might obscure the worse survival of patients with gBRCA1m or BRCA1-like tumors [28]. However, robust evidence from studies with large sample sizes and minimal indication bias is scarce.
Few studies have directly compared the outcomes of patients with different BRCA1-related biomarkers, let alone in women who did not receive (neo)adjuvant chemotherapy. Investigating the disease course of these tumors, independent of the curative effects of chemotherapy, will help to understand the true prognostic value of BRCA1-related biomarkers. This study aimed to compare long-term outcomes of young, node-negative, (neo)adjuvant chemotherapy-naïve TNBC patients according to gBRCA1m, sBRCA1m, or tumor BRCA1-PM, or according to BRCA1-like status, taking into account TILs and other established clinicopathological characteristics.
Methods
Study population
All women with TNBC (n = 485; age at diagnosis ranged from 22 to 39 years) were selected from the nationwide, population-based PARADIGM cohort. The study design has been described elsewhere [29]. Briefly, the PARADIGM cohort included all (neo)adjuvant systemic therapy-naïve patients diagnosed under age 40 between 1989 and 2000 with non-metastatic, invasive breast cancer from the Netherlands Cancer Registry (Fig. 1). The final selection of the PARADIGM cohort only included node-negative patients, since adjuvant treatment allocation before 2000 was mostly based on nodal status [30]. Stromal TILs (sTILs) were assessed according to the international guideline [31] by an experienced pathologist using hematoxylin and eosin-stained, formalin-fixed, paraffin-embedded whole slides, as previously described [16]. Information on distant recurrences and incidence of second primary tumors was collected until June 2014; information on death was collected until January 2018. Among the TNBC patients, eight were lost to the follow-up. Further details are provided in Additional file 1: Supplementary Methods [12, 32,33,34,35,36,37,38].
Selection of young, chemotherapy-naïve triple-negative breast cancer patients. Abbreviations: NCR, Netherlands Cancer Registry; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2. aThe NCR provides nationwide registry since 1989. bThe exclusion steps are in subsequent order
Assessment of BRCA1-related biomarkers
BRCA1 status was determined according to the gBRCA1m, sBRCA1m, and tumor BRCA1-PM status. Tumor DNA and normal DNA were isolated from archived formalin-fixed, paraffin-embedded tumor and normal tissues, respectively, at the NKI. Multiplicom (Niel, Belgium), now incorporated into Agilent (Carpinteria, CA, USA), analyzed single-nucleotide variants (SNVs) and small insertions or deletions (indels) using the NGS SureSelect and/or SureMASTR HRR kit (Agilent Technologies). The hg19 human reference genome was used for the alignment. The results were analyzed in Bench Lab NGS v4.3.5 (Agilent Technologies) by an expert clinical molecular geneticist. In this study, we only referred to (likely) pathogenic (class 4/class 5) variants as mutations [39]. Tumor BRCA1-PM was analyzed using methylation-specific multiplex ligation-dependent probe amplification at the NKI. Tumors with neither a BRCA1 mutation (SNVs or indels) nor BRCA1-PM, or those with unknown BRCA1 mutation and/or BRCA1-PM status were additionally analyzed for Dutch founder mutations, i.e., BRCA1 exon 13 or 22 deletions, using deletion-specific PCR. Tumor BRCA1 mutations were confirmed with tumor DNA and matched normal DNA, using Sanger sequencing at the NKI, and were classified as somatic or germline (Additional file 2: Fig. S1).
The BRCA1-like classifier [12] was used to classify tumors with or without BRCA1-like genomic aberrations, using copy number profiles, obtained with low-coverage whole-genome sequencing [34, 35]. All BRCA1-related biomarkers were assessed blinded to the clinical outcomes. See Additional file 1: Supplementary Methods for more information on all biomarkers assessed, including BRCA1 mRNA expression levels.
Statistical analysis
sTILs and other clinicopathological characteristics, BRCA1-like status, BRCA1 mRNA expression, and treatment according to BRCA1 status were compared using the Kruskal–Wallis tests (continuous outcomes) and chi-square or Fisher’s exact tests (categorical outcomes). Similarly, we compared the clinicopathological characteristics, BRCA1 mRNA expression, and treatment according to BRCA1-like status using the aforementioned tests.
We assessed patients’ clinical outcomes, including overall survival (OS), distant recurrence-free survival (DRFS), and cumulative incidence of second primary tumors, stratified by BRCA1 status and BRCA1-like status. The five germline BRCA2 mutation carriers were excluded from clinical outcome analyses because previous studies have reported different associations of BRCA2 versus BRCA1 mutations with breast cancer prognosis [40,41,42,43,44,45]. Furthermore, the limited number of germline BRCA2 mutation carriers precluded from providing valid estimates. For OS, follow-up started at diagnosis and ended at death due to any cause or was administratively censored at 15 years because events occurring after this period were unlikely to be related to the initial TNBC diagnosis. For DRFS, follow-up started at diagnosis and ended at distant recurrence or death, or was censored at the incidence of second primary tumors, the last day of event collection, or at 15 years, whichever came first. For cumulative incidence of second primary tumors, the follow-up started at diagnosis and ended at the second primary tumor, or was censored at distant recurrence or death, or the last day of event collection, or at 15 years, whichever came first.
Absolute OS and DRFS were derived using the Kaplan–Meier method. Survival rates for different BRCA1 status were compared using log-rank tests. Hazard ratios (HR) for BRCA1 status on OS and DRFS were calculated using univariable and multivariable Cox regression models with adjustment for sTILs, other clinicopathological characteristics, and treatment. The cumulative incidence of second primary tumors was calculated using a non-parametric approach [46], with distant recurrence and death as competing events. The incidences for different BRCA1 status were compared using Gray’s tests. Subdistribution HRs for BRCA1 status on second primary tumors were calculated using univariable and multivariable Fine and Gray competing risk models with adjustment for sTILs, other clinicopathological characteristics, and treatment. Distant recurrence and death were considered competing events. Cause-specific HRs were calculated using cause-specific Cox regression models in case the subdistribution HRs reflected an indirect association through the competing events [47].
The proportionality of hazards was examined using Schoenfeld residuals. In cases where the assumption was violated, an interaction term of the variable of interest and follow-up periods was added. To test if the association between sTILs and each clinical outcome differed across BRCA1 status, we added interaction terms between BRCA1 status and sTILs in multivariable models. Only the significant interaction terms were kept in the final model. To assess whether second primary tumors mediated the relationship between BRCA1 status and OS, a time-varying covariate for second primary tumors was added in the multivariable model for OS.
Multiple imputation of missing values was performed using chained equations (MICE package, version 3.15.0, in R; see Additional file 1: Supplementary Methods). All regression models were performed using multiple-imputed data and cases with complete information separately. Sensitivity analyses were performed on patients with tumor ER and PR expression < 1% and on those diagnosed between 1989 and 1997, due to the recommendation of chemotherapy to some node-negative patients in the Netherlands after 1997. This led to a lower number of patients diagnosed after 1997 being included in this cohort. A post hoc sensitivity analysis was also conducted on patients with BRCA1-like tumors.
Detailed statistical analyses are described in Additional file 1: Supplementary Methods. All statistical tests were two-sided, and a P-value < 0.05 was considered statistically significant. All statistical analyses were performed using R version 4.1.3 in the R Studio environment [48].
Results
Of the 485 TNBC patients, 420 had valid results for both gBRCA1m and sBRCA1m status: 25.0% (105/420) carried a gBRCA1m, and 5.0% (21/420) carried only a sBRCA1m. Among the gBRCA1m carriers, one had an additional sBRCA1m and was considered as gBRCA1m in the analyses. We observed five patients with a germline BRCA2 mutation, all of whom were gBRCA1wt. Details of the BRCA1 mutations are shown in Additional file 3: Table S1. Tumor BRCA1-PM was present in 146 (36.5%) out of the 400 patients with available methylation status. Tumor BRCA1 mutation (gBRCA1m or sBRCA1m) and tumor BRCA1-PM were mutually exclusive. Therefore, if a patient had a tumor BRCA1 mutation and the methylation status was missing, we assumed the BRCA1 promoter to be unmethylated, and vice versa. In total, 399 patients were classified into four groups: BRCA1-non-alteration (31.8%), gBRCA1m (26.3%), sBRCA1m (5.3%), and tumor BRCA1-PM (36.6%) (Fig. 2). BRCA1-like status, which was determined using tumors’ copy number profiles based on low-coverage whole-genome sequencing, was analyzed in 418 patients; 352 passed quality control, and 304 (86.4%) had BRCA1-like tumors. Most patients with a gBRCA1m (87.1%; 74/85), a sBRCA1m (82.4%; 14/17), or tumor BRCA1-PM (92.6%; 113/122) had BRCA1-like tumors. BRCA1-PM tumors had significantly lower BRCA1 mRNA expression than gBRCA1m, sBRCA1m, or BRCA1-non-altered tumors (P < 0.001). We did not find any significant differences in sTILs, other clinicopathological characteristics, or treatment according to BRCA1 status (Table 1) or BRCA1-like status (see Additional file 4: Table S2).
Classification of BRCA1 mutation and tumor BRCA1 promoter methylation. In total, 399 patients were classified into four groups: BRCA1-non-altered (n = 127), tumor BRCA1-PM (n = 134 + 12 = 146), sBRCA1m (n = 19 + 2 = 21), and gBRCA1m (n = 94 + 11 = 105). Abbreviations: ER, estrogen receptor; PR, progesterone receptor; BRCA1-PM, BRCA1 promoter methylation; sBRCA1m, somatic BRCA1 mutation; gBRCA1m, germline BRCA1 mutation; BRCA1-non-altered, without germline BRCA1 mutation, without somatic BRCA1 mutation, and without tumor BRCA1 promoter methylation
During the 15-year follow-up, 137 patients died. Eighty-three patients first developed distant recurrence, 85 first developed second primary tumors, and 34 died without distant recurrence or second primary tumors. Eight patients were lost to the follow-up. Kaplan–Meier curves of OS and DRFS and cumulative incidence curves of second primary tumors stratified by BRCA1 status are depicted in Fig. 3. Patients with gBRCA1m and tumor BRCA1-PM showed significantly different OS (Benjamini-Hochberg-corrected pairwise P-value = 0.041) and cumulative incidence of second primary tumors (Benjamini-Hochberg-corrected pairwise P-value < 0.001), although no statistically significant difference was observed in DRFS. The clinical outcomes at different follow-up times stratified by BRCA1 status and sTIL levels are summarized in Table 2. Patients (n = 66) with tumor BRCA1-PM and sTILs ≥ 50% showed excellent 15-year OS (97.0%, 95% CI, 92.9–100%; Table 2), while patients (n = 61) with gBRCA1m and sTILs < 50% showed poor 15-year OS (50.8%; 95% CI, 39.7–65.0%; Table 2). The clinical outcomes stratified by BRCA1 status and by BRCA1-like status are summarized in Additional file 5: Table S3 and Additional file 6: Table S4.
Clinical outcomes according to BRCA1 status. Clinical outcomes include (A) overall survival, (B) distant recurrence-free survival, and (C) cumulative incidence of second primary tumors. Log-rank tests and Gray’s tests were used to compute the pairwise P-values. Comparison was only made among germline BRCA1-mutated, tumor BRCA1 promoter-methylated, or BRCA1-non-altered patients, as the number of somatic BRCA1-mutated patients was too low. Pairwise P-values were corrected for multiple testing using the Benjamini–Hochberg procedure. The uncorrected P-values for overall survival comparison are as follows: gBRCA1m vs. BRCA1-non-altered (P-value = 0.253), gBRCA1m vs. tumor BRCA1-PM (P-value = 0.014), and tumor BRCA1-PM vs. BRCA1-non-altered (P-value = 0.189). The uncorrected P-values for distant recurrence-free survival comparison are as follows: gBRCA1m vs. BRCA1-non-altered (P-value = 0.429), gBRCA1m vs. tumor BRCA1-PM (P-value = 0.079), and tumor BRCA1-PM vs. BRCA1-non-altered (P-value = 0.328). The uncorrected P-values for the incidence of second primary tumors comparison are as follows: gBRCA1m vs. BRCA1-non-altered (P-value < 0.001), gBRCA1m vs. tumor BRCA1-PM (P-value < 0.001), and tumor BRCA1-PM vs. BRCA1-non-altered (P-value = 0.043). Abbreviations: BRCA1-non-altered, without germline BRCA1 mutation, without somatic BRCA1 mutation, and without tumor BRCA1 promoter methylation; gBRCA1m, germline BRCA1 mutation; sBRCA1m, somatic BRCA1 mutation; tumor BRCA1-PM, tumor BRCA1 promoter methylation. Note that at time 0, the numbers at risk of tumor BRCA1 promoter methylated patients and BRCA1-non-altered patients were not 146 and 127, respectively, because five germline BRCA2-mutated patients were removed
The multivariable Cox regression model showed that gBRCA1m patients had a worse OS from the fourth year after diagnosis compared to BRCA1-non-altered patients (HR4–15 years, 2.11; 95% CI, 1.18–3.75; Table 3). After additional adjustment for second primary tumors, the HR for gBRCA1m was attenuated (HR4–15 years, 1.43; 95% CI, 0.77–2.66). Patients with a sBRCA1m or tumor BRCA1-PM did not have significantly different OS compared to BRCA1-non-altered patients. Higher sTILs were associated with better OS (HR 0.84; 95% CI, 0.78–0.90) in gBRCA1m, sBRCA1m, or BRCA1-non-altered patients. This association was significantly larger in tumor BRCA1-PM patients, as was reflected by a significant interaction term between sTILs and tumor BRCA1-PM (HRinteraction, 0.82; 95% CI, 0.68–0.98). This means that every 10% sTIL increment was associated with a 31% increase in OS for tumor BRCA1-PM patients and a 16% increase for patients with other BRCA1 status. The HRs for other covariates are in Additional file 7: Table S5.
Patients with gBRCA1m, sBRCA1m, or tumor BRCA1-PM did not have significantly different DRFS compared to BRCA1-non-altered patients (Table 3). Higher sTILs were associated with better DRFS in all patients. Although the interaction between sTILs and tumor BRCA1-PM was not statistically significant (the final model did not include this interaction term), the direction of the interaction was the same as in the model for OS. The HRs for other covariates are in Additional file 8: Table S6. Compared to BRCA1-non-altered patients, gBRCA1m patients had a higher incidence of second primary tumors (adjusted subdistribution HR, 4.04; 95% CI, 2.29–7.13; Table 3), while tumor BRCA1-PM patients had a lower incidence of second primary tumors (adjusted subdistribution HR, 0.42; 95% CI, 0.19–0.95; Table 3). There were no significant interaction terms between BRCA1 status and sTILs for the incidence of second primary tumors. Subdistribution HRs and cause-specific HRs for BRCA1 status (Additional file 9: Table S7 and Additional file 10: Table S8) were aligned.
Patients with BRCA1-like tumors did not have significantly different outcomes compared to patients with non-BRCA1-like tumors (Additional file 11: Table S9). Results from the sensitivity analyses (Additional file 7–10: Table S5–S8) aligned with the results from the main analysis. Results of the complete-case analysis (Additional file 12: Table S10) also aligned with the results using multiple-imputed data.
Discussion
In this population-based cohort of young, node-negative TNBC patients, we compared patients’ clinical outcomes independent of the curative effect of adjuvant chemotherapy across different BRCA1 status and BRCA1-like status. In addition, we investigated the prognostic value of sTILs in patients with different BRCA1 status and identified subgroups of patients with distinct risks. These findings have the potential to improve risk classification in young, node-negative TNBC patients.
Our study found that gBRCA1m was associated with worse OS in young, node-negative TNBC patients, consistent with several previous studies predominantly involving chemotherapy-naïve patients [23, 49, 50]. However, more recent data, including mainly chemotherapy-treated patients with or without risk-reducing surgeries, showed that germline BRCA1/2 mutations did not negatively impact the survival of TNBC patients [5, 6, 24, 25, 51,52,53,54]. When combined with the results of previous studies, our findings suggest that chemotherapy could considerably improve the OS of gBRCA1m patients.
Furthermore, we showed that young TNBC patients with a gBRCA1m had a significantly increased risk of second primary tumors, primarily contralateral breast tumors, which is consistent with a recent prospective cohort study [55]. Given that these second primary tumors contributed significantly to worse OS in our study population, it is necessary to consider risk-reducing surgery for young, node-negative TNBC patients who carry a gBRCA1m. However, it is important to note that the negative impact of second primary tumors on OS should not raise unnecessary anxiety to give risk-reducing surgery to young TNBC patients who have no genetic or familial risk factors [56]. We showed a relatively low incidence of second primary tumors in gBRCA1wt patients, especially in tumor BRCA1-PM patients. The incidence may have been lower after chemotherapy, as was shown by previous studies that chemotherapy reduces the risk of contralateral breast cancers [57,58,59]. Therefore, risk-reducing surgery should, in line with most guidelines, only be offered to patients with a predicted high risk of second primary tumors [60]. Nevertheless, our results, derived from this unique chemotherapy-naïve cohort with young, node-negative TNBC patients, can facilitate transparent risk communication and a shared treatment decision-making between oncologists and patients.
Results on the prognostic value of tumor BRCA1-PM in TNBC patients have been conflicting [26, 61,62,63,64,65], which may be due to different methods to analyze BRCA1-PM status [66, 67], different reference groups (including gBRCA1m patients or not), or different treatments [62, 64]. Our study found no significant difference in OS or DRFS between patients with tumor BRCA1-PM and BRCA1-non-altered patients. Interestingly, we found that tumor BRCA1-PM may modify the association between sTILs and OS, as shown by a nearly two-fold increase in OS for tumor BRCA1-PM patients with every 10% increment of sTILs, compared to those with other BRCA1 status. Combined with the result of the similar distribution of sTILs across the BRCA1 status, this stronger association suggests that sTIL compositions or spatial relationships with the tumor cells might differ between patients with and without tumor BRCA1-PM. Future research may consider using a comprehensive technique such as imaging mass cytometry [68] to compare the sTIL compositions and spatial relationships among TNBCs with different BRCA1 status.
Our previous study has shown that patients without tumor BRCA1 mutation and high sTILs may have the potential to forgo chemotherapy [16]. With further information on gBRCA1m and tumor BRCA1-PM, we redid the risk classification, and two distinct subgroups were identified. One group, characterized by high sTILs and tumor BRCA1-PM, showed excellent 15-year OS and DRFS, while the other group, characterized by low sTILs and gBRCA1m showed poor 15-year OS and DRFS. These results, once validated, have the potential to aid adjuvant treatment intensification and optimization in young, node-negative TNBC patients.
We found a lower incidence of second primary tumors in tumor BRCA1-PM patients, compared to BRCA1-non-altered patients. To date, we have no biological explanation for this novel association, and it might have been a chance finding. Preliminary analysis using DNA from tumor-free lymph nodes of 19 tumor BRCA1-PM patients showed no (constitutional) methylation of BRCA1. The association might have been overestimated due to the potential misclassification of gBRCA1m patients as BRCA1-non-altered, resulting in a higher incidence of second primary tumors in the BRCA1-non-altered group. However, the mutual exclusiveness between gBRCA1m and BRCA1-PM, which has been reported in many studies [22, 69,70,71], minimized the chance of misclassifying gBRCA1m patients into the BRCA1-PM group. Therefore, if validated, it would be interesting to further consider the clinical relevance of testing BRCA1-PM in young, node-negative TNBC patients.
The prevalence of gBRCA1m, sBRCA1m, and tumor BRCA1-PM in our cohort was similar to previous studies [6, 22, 26, 71, 72]. In addition, our study showed that young TNBC patients predominantly had BRCA1-like tumors, which aligns with other studies [22, 26, 73], regardless of different homologous recombination deficiency (HRD) classifiers being used. Although our study did not cross-validate tumors’ BRCA1-like status using other genomic measures, a recent study reported a 70% concordance between the BRCA-like classifier and the functional DNA repair capacity assays (RECAP), as well as the whole-genome sequencing-based Classifier of HOmologous Recombination Deficiency (CHORD) assay [74]. In addition, this study showed that BRCA-like tumors are enriched for tumor mutational signature 3 [74]. In our study, most tumors with gBRCA1m, sBRCA1m, or BRCA1-PM were classified as BRCA1-like, while only a small proportion were classified as non-BRCA1-like. These non-BRCA1-like tumors may have arisen sporadically, as was reported that the absence of locus-specific loss of heterozygosity was observed in 10% of gBRCA1m breast tumors and their HRD scores were similar to sporadic tumors [75]. However, our sensitivity analysis focusing only on patients with BRCA1-like tumors yielded similar results to the main analysis that included all patients. Moreover, the BRCA1-like classifier, as many other HRD classifiers, is not 100% accurate for detecting BRCA1-altered tumors [22, 26].
This study had several unique strengths. First, indication bias was minimized because all chemotherapy-naïve patients were treated according to the guidelines in the specific era of diagnosis. Including TNBC patients from a more recent era might lead to an underestimation of the negative impact of gBRCA1m, since currently, only those with an extremely low risk might forgo chemotherapy [76]. Second, immortal time bias was not an issue in this study since BRCA1 status was tested using archived tissues. Studies including prevalent patients who had to survive to be tested might have underestimated the effect of gBRCA1m. Third, the gBRCA1m patients in our study were unlikely to receive prophylactic mastectomy and salpingo-oophorectomy due to the lack of awareness of their mutation status at diagnosis. Although we lacked information on prophylactic surgery and subsequent surgery after the diagnosis of TNBC, BRCA1 mutation was only discovered in 1994, and genetic testing was not introduced in the Netherlands until 1995, followed by its implementation in the clinic.
Our study may not have been completely free of bias. One potential source of bias is that young patients with family histories may have been referred to clinical genetics after 1995, and those who were found to carry a gBRCA1m might have chosen risk-reducing treatments that could have improved their outcomes. However, gBRCA1m carriers were more likely to receive chemotherapy [50] and were excluded from our cohort, which may have partially counterbalanced such an impact on our findings. Besides, our study only focused on BRCA1 mutations, whereas other gene mutations associated with TNBC, such as BRCA2, RAD51C/D, BARD1, and PALB2 [8, 77], might also have influenced the outcomes. Nevertheless, the proportion of other germline mutations in young TNBC patients is very low [8]. Lastly, all patients were of European descent; thus, generalization to other ethnicities should be made carefully.
Conclusions
In conclusion, although both gBRCA1m and tumor BRCA1-PM alter BRCA1 gene transcription, they were associated with significantly different outcomes in young, node-negative TNBC patients. The prognostic value of sTILs remained across patients with different BRCA1 status, albeit this association was stronger in those with tumor BRCA1-PM. Combining sTILs and BRCA1 status has the potential to improve risk classification and tailored adjuvant treatment in this patient population. Furthermore, the high incidence of second primary tumors in young gBRCA1m carriers and its association with worse OS emphasize the importance of risk-reducing surgery or active monitoring. Such decisions should be discussed between physicians and patients with transparent information being provided, taking family planning into account [60].
Availability of data and materials
The clinical data in this study are available from the Netherlands Cancer Registry, hosted by the Netherlands Comprehensive Cancer Organization; however, restrictions apply to the availability of these data, which were used under license for the current study. Other data generated (BRCA1-related variables, sTILs) are available from the authors upon reasonable request and with permission from the Netherlands Comprehensive Cancer Organization.
Abbreviations
- BRCA1 non-alteration:
-
Without germline BRCA1 mutation, without somatic BRCA1 mutation, and without tumor BRCA1 promoter methylation
- BRCA1-PM:
-
BRCA1 Promoter methylation
- DRFS:
-
Distant recurrence-free survival
- gBRCA1m:
-
Germline BRCA1 mutation
- gBRCA1wt:
-
Germline BRCA1 wild-type
- HR:
-
Hazard ratio
- HRD:
-
Homologous recombination deficiency
- indels:
-
Insertions or deletions
- NKI:
-
Netherlands Cancer Institute
- OS:
-
Overall survival
- sBRCA1m:
-
Somatic BRCA1 mutation
- SNVs:
-
Single-nucleotide variants
- sTILs:
-
Stromal tumor-infiltrating lymphocytes
- TILs:
-
Tumor-infiltrating lymphocytes
- TNBC:
-
Triple-negative breast cancer
References
Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–30.
Fostira F, Tsitlaidou M, Papadimitriou C, Pertesi M, Timotheadou E, Stavropoulou AV, Glentis S, Bournakis E, Bobos M, Pectasides D, et al. Prevalence of BRCA1 mutations among 403 women with triple-negative breast cancer: implications for genetic screening selection criteria: a Hellenic Cooperative Oncology Group Study. Breast Cancer Res Treat. 2012;134(1):353–62.
Sharma P, Klemp JR, Kimler BF, Mahnken JD, Geier LJ, Khan QJ, Elia M, Connor CS, McGinness MK, Mammen JM, et al. Germline BRCA mutation evaluation in a prospective triple-negative breast cancer registry: implications for hereditary breast and/or ovarian cancer syndrome testing. Breast Cancer Res Treat. 2014;145(3):707–14.
Couch FJ, Hart SN, Sharma P, Toland AE, Wang X, Miron P, Olson JE, Godwin AK, Pankratz VS, Olswold C, et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol. 2015;33(4):304–11.
Hahnen E, Lederer B, Hauke J, Loibl S, Krober S, Schneeweiss A, Denkert C, Fasching PA, Blohmer JU, Jackisch C, et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: secondary analysis of the GeparSixto Randomized Clinical Trial. JAMA Oncol. 2017;3(10):1378–85.
Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG, Eccles B, Gerty S, Durcan LT, Jones L, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(2):169–80.
Breast Cancer Association Consortium, Dorling L, Carvalho S, Allen J, Gonzalez-Neira A, Luccarini C, Wahlstrom C, Pooley KA, Parsons MT, Fortuno C, et al. Breast cancer risk genes - association analysis in more than 113,000 women. N Engl J Med. 2021;384(5):428–39.
Breast Cancer Association Consortium, Mavaddat N, Dorling L, Carvalho S, Allen J, Gonzalez-Neira A, Keeman R, Bolla MK, Dennis J, Wang Q, et al. Pathology of tumors associated with pathogenic germline variants in 9 breast cancer susceptibility genes. JAMA Oncol. 2022;8(3):e216744.
Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27(3):247–54.
van Gent DC, Hoeijmakers JHJ, Kanaar R. Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet. 2001;2(3):196–206.
Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4(10):814–9.
Joosse SA, van Beers EH, Tielen IH, Horlings H, Peterse JL, Hoogerbrugge N, Ligtenberg MJ, Wessels LF, Axwijk P, Verhoef S, et al. Prediction of BRCA1-association in hereditary non-BRCA1/2 breast carcinomas with array-CGH. Breast Cancer Res Treat. 2009;116(3):479–89.
Loi S. Tumor-infiltrating lymphocytes, breast cancer subtypes and therapeutic efficacy. Oncoimmunology. 2013;2(7):e24720.
Loi S, Drubay D, Adams S, Pruneri G, Francis PA, Lacroix-Triki M, Joensuu H, Dieci MV, Badve S, Demaria S, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37(7):559–69.
Park JH, Jonas SF, Bataillon G, Criscitiello C, Salgado R, Loi S, Viale G, Lee HJ, Dieci MV, Kim SB, et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol. 2019;30(12):1941–9.
de Jong VMT, Wang Y, Ter Hoeve ND, Opdam M, Stathonikos N, Jozwiak K, Hauptmann M, Cornelissen S, Vreuls W, Rosenberg EH, et al. Prognostic value of stromal tumor-infiltrating lymphocytes in young, node-negative, triple-negative breast cancer patients who did not receive (neo)adjuvant systemic therapy. J Clin Oncol. 2022;40(21):2361–74.
Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–66.
Solinas C, Marcoux D, Garaud S, Vitoria JR, Van den Eynden G, de Wind A, De Silva P, Boisson A, Craciun L, Larsimont D, et al. BRCA gene mutations do not shape the extent and organization of tumor infiltrating lymphocytes in triple negative breast cancer. Cancer Lett. 2019;450:88–97.
Telli ML, Chu C, Badve SS, Vinayak S, Silver DP, Isakoff SJ, Kaklamani V, Gradishar W, Stearns V, Connolly RM, et al. Association of tumor-infiltrating lymphocytes with homologous recombination deficiency and BRCA1/2 status in patients with early triple-negative breast cancer: a pooled analysis. Clin Cancer Res. 2020;26(11):2704–10.
Grandal B, Evrevin C, Laas E, Jardin I, Rozette S, Laot L, Dumas E, Coussy F, Pierga JY, Brain E, et al. Impact of BRCA mutation status on tumor infiltrating lymphocytes (TILs), response to treatment, and prognosis in breast cancer patients treated with neoadjuvant chemotherapy. Cancers (Basel). 2020;12(12):3681.
Nolan E, Savas P, Policheni AN, Darcy PK, Vaillant F, Mintoff CP, Dushyanthen S, Mansour M, Pang JB, Fox SB, et al. Combined immune checkpoint blockade as a therapeutic strategy for BRCA1-mutated breast cancer. Sci Transl Med. 2017;9(393):eaal4922.
Lips EH, Mulder L, Oonk A, van der Kolk LE, Hogervorst FB, Imholz AL, Wesseling J, Rodenhuis S, Nederlof PM. Triple-negative breast cancer: BRCAness and concordance of clinical features with BRCA1-mutation carriers. Br J Cancer. 2013;108(10):2172–7.
Goffin JR, Chappuis PO, Begin LR, Wong N, Brunet JS, Hamel N, Paradis AJ, Boyd J, Foulkes WD. Impact of germline BRCA1 mutations and overexpression of p53 on prognosis and response to treatment following breast carcinoma: 10-year follow up data. Cancer. 2003;97(3):527–36.
Huzarski T, Byrski T, Gronwald J, Gorski B, Domagala P, Cybulski C, Oszurek O, Szwiec M, Gugala K, Stawicka M, et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J Clin Oncol. 2013;31(26):3191–6.
Tung N, Gaughan E, Hacker MR, Lee LJ, Alexander B, Poles E, Schnitt SJ, Garber JE. Outcome of triple negative breast cancer: comparison of sporadic and BRCA1-associated cancers. Breast Cancer Res Treat. 2014;146(1):175–82.
Sharma P, Barlow WE, Godwin AK, Pathak H, Isakova K, Williams D, Timms KM, Hartman AR, Wenstrup RJ, Linden HM, et al. Impact of homologous recombination deficiency biomarkers on outcomes in patients with triple-negative breast cancer treated with adjuvant doxorubicin and cyclophosphamide (SWOG S9313). Ann Oncol. 2018;29(3):654–60.
Lips EH, Benard-Slagter A, Opdam M, Scheerman CE, Wesseling J, Hogervorst FBL, Linn SC, Savola S, Nederlof PM. BRCAness digitalMLPA profiling predicts benefit of intensified platinum-based chemotherapy in triple-negative and luminal-type breast cancer. Breast Cancer Res. 2020;22(1):79.
Wang Y, van den Broek AJ, Schmidt MK. Letter to the editor regarding: ‘association between BRCA mutational status and survival in patients with breast cancer: a systematic review and meta-analysis.’ Breast Cancer Res Treat. 2021;188(3):821–3.
Dackus GM, Ter Hoeve ND, Opdam M, Vreuls W, Varga Z, Koop E, Willems SM, Van Deurzen CH, Groen EJ, Cordoba A, et al. Long-term prognosis of young breast cancer patients (≤40 years) who did not receive adjuvant systemic treatment: protocol for the PARADIGM initiative cohort study. BMJ Open. 2017;7(11):e017842.
Consensus conference. Adjuvant chemotherapy for breast cancer. JAMA. 1985;254(24):3461–3.
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an international TILs working group 2014. Ann Oncol. 2015;26(2):259–71.
Moelans CB, Atanesyan L, Savola SP, van Diest PJ. Methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA). Methods Mol Biol. 2018;1708:537–49.
Petrij-Bosch A, Peelen T, van Vliet M, van Eijk R, Olmer R, Drusedau M, Hogervorst FB, Hageman S, Arts PJ, Ligtenberg MJ, et al. BRCA1 genomic deletions are major founder mutations in Dutch breast cancer patients. Nat Genet. 1997;17(3):341–5.
Schouten PC, Grigoriadis A, Kuilman T, Mirza H, Watkins JA, Cooke SA, van Dyk E, Severson TM, Rueda OM, Hoogstraat M, et al. Robust BRCA1-like classification of copy number profiles of samples repeated across different datasets and platforms. Mol Oncol. 2015;9(7):1274–86.
de Boo LW, Jozwiak K, Joensuu H, Lindman H, Lauttia S, Opdam M, van Steenis C, Brugman W, Kluin RJC, Schouten PC, et al. Adjuvant capecitabine-containing chemotherapy benefit and homologous recombination deficiency in early-stage triple-negative breast cancer patients. Br J Cancer. 2022;126(10):1401–9.
Vollebergh MA, Lips EH, Nederlof PM, Wessels LF, Schmidt MK, van Beers EH, Cornelissen S, Holtkamp M, Froklage FE, de Vries EG, et al. An aCGH classifier derived from BRCA1-mutated breast cancer and benefit of high-dose platinum-based chemotherapy in HER2-negative breast cancer patients. Ann Oncol. 2011;22(7):1561–70.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
Lindor NM, Guidugli L, Wang X, Vallee MP, Monteiro AN, Tavtigian S, Goldgar DE, Couch FJ. A review of a multifactorial probability-based model for classification of BRCA1 and BRCA2 variants of uncertain significance (VUS). Hum Mutat. 2012;33(1):8–21.
Lee EH, Park SK, Park B, Kim SW, Lee MH, Ahn SH, Son BH, Yoo KY, Kang D, Group KR, et al. Effect of BRCA1/2 mutation on short-term and long-term breast cancer survival: a systematic review and meta-analysis. Breast Cancer Res Treat. 2010;122(1):11–25.
van den Broek AJ, Schmidt MK, van’t Veer LJ, Tollenaar RA, van Leeuwen FE. Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: what’s the evidence? A systematic review with meta-analysis. PLoS One. 2015;10(3):e0120189.
Zhong Q, Peng HL, Zhao X, Zhang L, Hwang WT. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clin Cancer Res. 2015;21(1):211–20.
Templeton AJ, Gonzalez LD, Vera-Badillo FE, Tibau A, Goldstein R, Seruga B, Srikanthan A, Pandiella A, Amir E, Ocana A. Interaction between hormonal receptor status, age and survival in patients with BRCA1/2 germline mutations: a systematic review and meta-regression. PLoS ONE. 2016;11(5):e0154789.
Zhu Y, Wu J, Zhang C, Sun S, Zhang J, Liu W, Huang J, Zhang Z. BRCA mutations and survival in breast cancer: an updated systematic review and meta-analysis. Oncotarget. 2016;7(43):70113–27.
Baretta Z, Mocellin S, Goldin E, Olopade OI, Huo D. Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine (Baltimore). 2016;95(40):e4975.
Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer. 2004;91(7):1229–35.
Latouche A, Allignol A, Beyersmann J, Labopin M, Fine JP. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol. 2013;66(6):648–53.
R Development Core Team: R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2022.
Robson ME, Chappuis PO, Satagopan J, Wong N, Boyd J, Goffin JR, Hudis C, Roberge D, Norton L, Begin LR, et al. A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res. 2004;6(1):R8–17.
Schmidt MK, van den Broek AJ, Tollenaar RA, Smit VT, Westenend PJ, Brinkhuis M, Oosterhuis WJ, Wesseling J, Janssen-Heijnen ML, Jobsen JJ et al: Breast cancer survival of BRCA1/BRCA2 mutation carriers in a hospital-based cohort of young women. J Natl Cancer Inst 2017;109(8):https://doi.org/10.1093/jnci/djw329(8)
Yadav S, Ladkany R, Yadav D, Alhalabi O, Khaddam S, Isaac D, Cardenas PY, Zakalik D. Impact of BRCA mutation status on survival of women with triple-negative breast cancer. Clin Breast Cancer. 2018;18(5):e1229–35.
Pogoda K, Niwinska A, Sarnowska E, Nowakowska D, Jagiello-Gruszfeld A, Siedlecki J, Nowecki Z. Effects of BRCA germline mutations on triple-negative breast cancer prognosis. J Oncol. 2020;2020:8545643.
Bayraktar S, Gutierrez-Barrera AM, Liu D, Tasbas T, Akar U, Litton JK, Lin E, Albarracin CT, Meric-Bernstam F, Gonzalez-Angulo AM, et al. Outcome of triple-negative breast cancer in patients with or without deleterious BRCA mutations. Breast Cancer Res Treat. 2011;130(1):145–53.
Maksimenko J, Irmejs A, Nakazawa-Miklasevica M, Melbarde-Gorkusa I, Trofimovics G, Gardovskis J, Miklasevics E. Prognostic role of BRCA1 mutation in patients with triple-negative breast cancer. Oncol Lett. 2014;7(1):278–84.
Yadav S, Boddicker NJ, Na J, Polley EC, Hu C, Hart SN, Gnanaolivu RD, Larson N, Holtegaard S, Huang H, et al. Contralateral breast cancer risk among carriers of germline pathogenic variants in ATM, BRCA1, BRCA2, CHEK2, and PALB2. J Clin Oncol. 2023;41(9):1703–13.
Hawley ST, Jagsi R, Morrow M, Janz NK, Hamilton A, Graff JJ, Katz SJ. Social and clinical determinants of contralateral prophylactic mastectomy. JAMA Surg. 2014;149(6):582–9.
Akdeniz D, van Barele M, Heemskerk-Gerritsen BAM, Steyerberg EW, Hauptmann M, Investigators H, van de Beek I, van Engelen K, Wevers MR, Gomez Garcia EB, et al. Effects of chemotherapy on contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers: a nationwide cohort study. Breast. 2022;61:98–107.
Kramer I, Schaapveld M, Oldenburg HSA, Sonke GS, McCool D, van Leeuwen FE, Van de Vijver KK, Russell NS, Linn SC, Siesling S, et al. The influence of adjuvant systemic regimens on contralateral breast cancer risk and receptor subtype. J Natl Cancer Inst. 2019;111(7):709–18.
Early Breast Cancer Trialists’ Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717.
Schmidt MK, Kelly JE, Bredart A, Cameron DA, de Boniface J, Easton DF, Offersen BV, Poulakaki F, Rubio IT, Sardanelli F, et al. EBCC-13 manifesto: balancing pros and cons for contralateral prophylactic mastectomy. Eur J Cancer. 2023;181:79–91.
Sharma P, Stecklein SR, Kimler BF, Sethi G, Petroff BK, Phillips TA, Tawfik OW, Godwin AK, Jensen RA. The prognostic value of BRCA1 promoter methylation in early stage triple negative breast cancer. J Cancer Ther Res. 2014;3(2):1–11.
Xu Y, Diao L, Chen Y, Liu Y, Wang C, Ouyang T, Li J, Wang T, Fan Z, Fan T, et al. Promoter methylation of BRCA1 in triple-negative breast cancer predicts sensitivity to adjuvant chemotherapy. Ann Oncol. 2013;24(6):1498–505.
Yamashita N, Tokunaga E, Kitao H, Hitchins M, Inoue Y, Tanaka K, Hisamatsu Y, Taketani K, Akiyoshi S, Okada S, et al. Epigenetic inactivation of BRCA1 through promoter hypermethylation and its clinical importance in triple-negative breast cancer. Clin Breast Cancer. 2015;15(6):498–504.
Jacot W, Lopez-Crapez E, Mollevi C, Boissiere-Michot F, Simony-Lafontaine J, Ho-Pun-Cheung A, Chartron E, Theillet C, Lemoine A, Saffroy R, et al. BRCA1 promoter hypermethylation is associated with good prognosis and chemosensitivity in triple-negative breast cancer. Cancers (Basel). 2020;12(4):828.
Ignatov T, Poehlmann A, Ignatov A, Schinlauer A, Costa SD, Roessner A, Kalinski T, Bischoff J. BRCA1 promoter methylation is a marker of better response to anthracycline-based therapy in sporadic TNBC. Breast Cancer Res Treat. 2013;141(2):205–12.
Vos S, van Diest PJ, Moelans CB. A systematic review on the frequency of BRCA promoter methylation in breast and ovarian carcinomas of BRCA germline mutation carriers: mutually exclusive, or not? Crit Rev Oncol Hematol. 2018;127:29–41.
Guo T, Ren Y, Wang B, Huang Y, Jia S, Tang W, Luo Y. Promoter methylation of BRCA1 is associated with estrogen, progesterone and human epidermal growth factor receptor-negative tumors and the prognosis of breast cancer: a meta-analysis. Mol Clin Oncol. 2015;3(6):1353–60.
Nederlof I, Horlings HM, Curtis C, Kok M. A high-dimensional window into the micro-environment of triple negative breast cancer. Cancers (Basel). 2021;13(2):316.
Stefansson OA, Hilmarsdottir H, Olafsdottir K, Tryggvadottir L, Sverrisdottir A, Johannsson OT, Jonasson JG, Eyfjord JE, Sigurdsson S. BRCA1 promoter methylation status in 1031 primary breast cancers predicts favorable outcomes following chemotherapy. JNCI Cancer Spectr. 2019;4(2):pkz100.
Tian T, Shan L, Yang W, Zhou X, Shui R. Evaluation of the BRCAness phenotype and its correlations with clinicopathological features in triple-negative breast cancers. Hum Pathol. 2019;84:231–8.
Glodzik D, Bosch A, Hartman J, Aine M, Vallon-Christersson J, Reutersward C, Karlsson A, Mitra S, Nimeus E, Holm K, et al. Comprehensive molecular comparison of BRCA1 hypermethylated and BRCA1 mutated triple negative breast cancers. Nat Commun. 2020;11(1):3747.
Gonzalez-Angulo AM, Timms KM, Liu S, Chen H, Litton JK, Potter J, Lanchbury JS, Stemke-Hale K, Hennessy BT, Arun BK, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res. 2011;17(5):1082–9.
Loibl S, Weber KE, Timms KM, Elkin EP, Hahnen E, Fasching PA, Lederer B, Denkert C, Schneeweiss A, Braun S, et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Ann Oncol. 2018;29(12):2341–7.
Meijer TG, Nguyen L, Van Hoeck A, Sieuwerts AM, Verkaik NS, Ladan MM, Ruigrok-Ritstier K, van Deurzen CHM, van de Werken HJG, Lips EH, et al. Functional RECAP (REpair CAPacity) assay identifies homologous recombination deficiency undetected by DNA-based BRCAness tests. Oncogene. 2022;41(26):3498–506.
Maxwell KN, Wubbenhorst B, Wenz BM, De Sloover D, Pluta J, Emery L, Barrett A, Kraya AA, Anastopoulos IN, Yu S, et al. BRCA locus-specific loss of heterozygosity in germline BRCA1 and BRCA2 carriers. Nat Commun. 2017;8(1):319.
Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S, Senkus E, Committee EG. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(10):1674.
Shimelis H, LaDuca H, Hu C, Hart SN, Na J, Thomas A, Akinhanmi M, Moore RM, Brauch H, Cox A, et al. Triple-negative breast cancer risk genes identified by multigene hereditary cancer panel testing. J Natl Cancer Inst. 2018;110(8):855–62.
Acknowledgements
We acknowledge the registration team of the Netherlands Comprehensive Cancer Organization for collecting patient data, the Dutch Pathology Registry and PALGA (the nationwide network and registry of histo- and cytopathology in the Netherlands) for providing the histopathological data and the formalin-fixed, paraffin-embedded tissues, the NKI Core Facility Molecular Pathology & Biobanking for analyzing normal and tumor DNA, and the Agilent Technologies Inc. for performing the tumor DNA variants analysis.
Funding
The Netherlands Cancer Institute was supported by an institutional grant of the Dutch Cancer Society and of the Dutch Ministry of Health, Welfare, and Sport. This study was supported by grants from the Dutch Cancer Society (KWF, grant No. 11655/2018–1 to Marjanka K. Schmidt), the Netherlands Organization for Health Research and Development (Project number 836021019, to Sabine C. Linn), A Sister’s Hope (to Sabine C. Linn), De Vrienden van UMC Utrecht (to Sabine C. Linn), and the Breast Cancer Research Foundation (BCRF, grant No. 17–194 to Roberto F. Salgado). The funders of this study had no role in the design and conduct of the study; collection, analysis, and interpretation of the data; writing of the manuscript; and the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
MS and SL conceptualized and designed the study. GD, ER, SC, LB, AB, WB, TC, PD, NH, OI, VJ, RK, MK, EK, PN, MO, PS, SS, CS, AV, WV, RS, SL, and MS provided patients’ data including clinical characteristics, BRCA1 status, and BRCA1-like status. YW and GD collected and assembled the data. YW, MH, and KJ analyzed the data. All authors interpreted the data. YW wrote the manuscript draft. All authors edited the manuscript draft and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of the Netherlands Cancer Institute (IRB code: CFMPB554). All retrospective medical data/biospecimen studies in the Netherlands are executed pursuant to Dutch legislation, international standards, and a self-regulatory Code of Conduct (https://www.coreon.org/gedragscode-gezondheidsonderzoek/). Prior to 25 May 2018, national legislation on data protection applied, as well as the International Guideline on Good Clinical Practice. From 25 May 2018, hospitals in the Netherlands also have to adhere to the General Data Protection Regulation. Within this framework, patients are informed and have the opportunity to object or actively consent to the (continued) use of their personal data and biospecimens in research. Hence, the procedures comply both with (inter-) national legislative and ethical standards.
Consent for publication
Not applicable.
Competing interests
SL has been an advisory board member for AstraZeneca, Cergentis, IBM, Novartis, Pfizer, Roche, and Sanofi and has received unrestricted institutional research support or unrestricted educational funding from Agendia, Amgen, AstraZeneca, Bayer, Daiichi Sankyo, Eurocept Pharmaceuticals, Genentech, Immunomedics, Merck, Roche, Sanofi, and TESARO. PS and SL have a pending patent application for a BRCA-like ovarian cancer classifier. PD has a pending patent application for DDX3 as a biomarker for cancer and its related methods. MK has been an advisory board member for Bristol Myers Squibb/Medarex, Roche, MSD, and AZ/Daiichi and has received research funding from Bristol Myers Squibb, Roche, and AstraZeneca/MedImmune. RS has received non-financial support from Merck and Bristol Myers Squibb (BMS); research support from Merck, Puma Biotechnology, and Roche; and personal fees from Roche, BMS, and Exact Sciences for advisory boards. The other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary Methods.
Additional file 2: Fig. S1.
Flow chart of tumor BRCA1 mutation testing.
Additional file 3: Table S1.
Locations of the germline and somatic BRCA1 mutations.
Additional file 4: Table S2.
Clinicopathological characteristics, BRCA1 mRNA expression, treatment, and follow-up events of patients with non- BRCA1-like or BRCA1-like tumors.
Additional file 5: Table S3.
3-, 5-, 10-, and 15-year overall survival rate, distant recurrence-free survival rate, and cumulative incidence of second primary tumors according to BRCA1 status.
Additional file 6: Table S4.
3-, 5-, 10-, and 15-year overall survival rate, distant recurrence-free survival rate, and cumulative incidence of second primary tumors according to BRCA1-like status.
Additional file 7: Table S5.
Hazard ratios for overall survival according to BRCA1 status, based on multiple-imputed data.
Additional file 8: Table S6.
Hazard ratios for distant recurrence-free survival according to BRCA1 status, based on multiple-imputed data.
Additional file 9: Table S7.
Subdistribution hazard ratios for second primary tumors according to BRCA1 status, based on multiple-imputed data, using Fine and Gray competing risk models with distant recurrence and death as competing events.
Additional file 10: Table S8.
Hazard ratios for second primary tumors according to BRCA1 status, based on multiple-imputed data, using cause-specific competing risk models with distant recurrence and death as competing events.
Additional file 11.
Univariable (subdistribution) hazard ratios according to BRCA1-like status.
Additional file 12.
(subdistribution) Hazard ratios according to BRCA1 status, based on cases with complete information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Y., Dackus, G.M.H.E., Rosenberg, E.H. et al. Long-term outcomes of young, node-negative, chemotherapy-naïve, triple-negative breast cancer patients according to BRCA1 status. BMC Med 22, 9 (2024). https://doi.org/10.1186/s12916-023-03233-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-023-03233-7