Abstract
Background
The introduction of multiparameter MRI and novel biomarkers has greatly improved the prediction of clinically significant prostate cancer (csPCa). However, decision-making regarding prostate biopsy and prebiopsy examinations is still difficult. We aimed to establish a quick and economic tool to improve the detection of csPCa based on routinely performed clinical examinations through an automated machine learning platform (AutoML).
Methods
This study included a multicenter retrospective cohort and two prospective cohorts with 4747 cases from 9 hospitals across China. The multimodal data, including demographics, clinical characteristics, laboratory tests, and ultrasound reports, of consecutive participants were retrieved using extract-transform-load tools. AutoML was applied to explore potential data processing patterns and the most suitable algorithm to build the Prostate Cancer Artificial Intelligence Diagnostic System (PCAIDS). The diagnostic performance was determined by the receiver operating characteristic curve (ROC) for discriminating csPCa from insignificant prostate cancer (PCa) and benign disease. The clinical utility was evaluated by decision curve analysis (DCA) and waterfall plots.
Results
The random forest algorithm was applied in the feature selection, and the AutoML algorithm was applied for model establishment. The area under the curve (AUC) value in identifying csPCa was 0.853 in the training cohort, 0.820 in the validation cohort, 0.807 in the Changhai prospective cohort, and 0.850 in the Zhongda prospective cohort. DCA showed that the PCAIDS was superior to PSA or fPSA/tPSA for diagnosing csPCa with a higher net benefit for all threshold probabilities in all cohorts. Setting a fixed sensitivity of 95%, a total of 32.2%, 17.6%, and 26.3% of unnecessary biopsies could be avoided with less than 5% of csPCa missed in the validation cohort, Changhai and Zhongda prospective cohorts, respectively.
Conclusions
The PCAIDS was an effective tool to inform decision-making regarding the need for prostate biopsy and prebiopsy examinations such as mpMRI. Further prospective and international studies are warranted to validate the findings of this study.
Trial registration
Chinese Clinical Trial Registry ChiCTR2100048428. Registered on 06 July 2021.
Similar content being viewed by others
Background
Prostate cancer (PCa) is a malignancy with the second highest incidence and the fifth highest mortality among tumors affecting males worldwide [1]. The early detection of PCa is particularly imperative for PCa patients. When the tumor spreads beyond the capsule or distant metastasis, therapeutic effectiveness remains limited, and the patient’s prognosis is devastatingly dismal [1,2,3,4]. Despite the widespread applications of prostate-specific antigen (PSA), the current detection modality of PCa has yielded huge overdiagnosis, overtreatment of indolent cases, and missing clinically significant cases [5]. Although multiparameter MRI (mp-MRI) has gained great importance in predicting the risk of PCa before biopsy, it is not possible for every man with elevated PSA levels to undergo mpMRI due to the limited recourses of MRI facilities and the high cost of mpMRI. The need for prebiopsy and prempMRI screening and selection is very urgent.
Artificial intelligence (AI) has the potential to revolutionize current clinical practice, such as diagnosis, identification of previously unrecognized images or genomic paradigms associated with disease phenotypes, adjuvant or incorporated singly surgical intervention [6, 7]. As reported, AI systems that integrate electronic case information present outstanding performance in diagnosing lung cancer compared with existing clinical diagnostic criteria and can reduce the missed diagnosis rate of lung cancer by 30.7% [8]. Therefore, AI, with its powerful capacity for information processing, can largely integrate clinical multimodal and multidimensional information resources and is expected to become a revolutionary milestone in the field of early detection and accurate diagnosis for PCa. Here, we established a prostate cancer artificial intelligence diagnostic system (PCAIDS) based on AutoML through processing, modeling, and verification of multimodal data, which might aid in the surveillance and early detection of PCa.
Methods
Study population
This is a multicenter, retrospective, diagnostic study that included consecutive clinical patients in nine tertiary medical centers in different regions of China. The study was approved by the local ethics committee (CHEC2021-092), registered in the Chinese Clinical Trial Registry (ChiCTR2100048428), and undertaken according to the Declaration of Helsinki. Informed consent from patients with PCa and controls was acquired in the prospective cohorts. All patients with PCa and controls were confirmed by pathological examination. Histological classification was established according to the WHO classification.
We extracted various features from the subjects, including demographics (height, weight, gender, etc.), imaging reports (abdominal B-ultrasound), and clinical laboratory tests (PSA, routine blood tests, routine urine tests, blood lipids, hepatic function, blood glucose, etc.). Inclusion criteria were (1) the subject scheduled to undergo the initial prostate biopsy; (2) PSA 4–20 ng/mL; (3) ultrasound examinations were completed and associated reports were processed by Natural Language Processing (NLP), with detailed information of upper and lower diameter and left and right diameters; and (4) patients with complete clinical information.
Study design
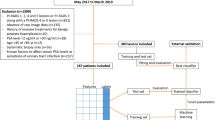
A summary of the workflow and an overview of the cohorts are shown in Fig. 1 according to the statement for transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) (http://www.equator-network.org/reportingguidelines/tripod-statement/). Specifically, we performed feature selection using data from 4312 biopsy-positive PCa patients and biopsy-negative control patients who underwent prostate biopsy at five clinical sites between May 2008 and December 2019. A total of 435 patients underwent prostate biopsy from January 2016 to December 2021 for subsequent analysis in the other 4 centers.
First, the retrospective data were divided into a training cohort (3230 patients, 80%) and an internal validation cohort (808 cases, 20%). The prospective cohorts included the Shanghai Changhai Hospital prospective cohort (519 cases) and Zhongda Hospital cohort (190 cases). The data of the training cohort were applied to construct models using four algorithms, including AutoML, logistic regression (LR), random forest (RF), and XGBoost. Then, the four models were evaluated, and the classifiers with the best prediction performances were chosen to establish the final diagnostic model. The parameters of the diagnostic model from the training cohort were applied to validate the diagnostic performance of the selected model. The discriminative power of the selected model with PCa was evaluated by the area under the curve (AUC) of the receiver operating characteristic curve (ROC). Then, the clinical value of the model was further evaluated.
Data preprocessing
First, demographics, imaging reports, and clinical laboratory tests of eligible participants were extracted from Shanghai Changhai Hospital, the First Affiliated Hospital of Soochow University, Zhongda Hospital Southeast University, West China Hospital of Sichuan University, and the First Affiliated Hospital of Xi’an Jiaotong University using extract-transform-load (ETL) tools. The NLP module was used to extract the “upper and lower diameters and left and right diameters” reported by ultrasound. Then, all the laboratory tests were subjected to quality control steps for further feature selection. The RF model-based method was used to select candidate features for modeling [9].
Furthermore, we collected the selected features of patients in the Ninth People’s Hospital Affiliated to Shanghai Jiaotong University School of Medicine, the Second Affiliated Hospital of Fujian Medical University, the First People Hospital of Yulin, and Nanjing University Jinling Hospital. All subjects in the abovementioned centers were randomly divided into the training cohort and internal validation cohort at a ratio of 8:2. Patients prospectively collected from Shanghai Changhai Hospital and Zhongda Hospital were used for independent prospective validation.
Feature transformation
For enumerated or categorical features, the missing values were filled with “NA”; some niche categories, such as “++, +++”, were classified and merged into one category; then, a one-hot encoding method was used to convert to a numeric vector. For continuous indicators, the mean was used to replace missing values, and then the MinMaxScaler method was used for regularization conversion [10]. The conversion formula is \({x}{\prime}=\frac{x-{x}_{min}}{{x}_{max}-{x}_{min}}\), \(x^{'}\) is the conversion result, and \({x}_{max}\) and \({x}_{min}\) are the maximum and minimum values of x, respectively.
AI-based feature selection
The random forest algorithm, a renowned machine learning method, was employed for feature selection. The dataset comprised 4312 biopsy cases, each characterized by 108 different features. This cohort was randomly partitioned into distinct training and validation subsets (8:2) to facilitate robust model training and subsequent performance evaluation. Utilizing the random forest algorithm, the importance of each feature was computed within the training subset. A cumulative contribution threshold of 95% was set to identify the most influential features. Finally, 36 features were selected. The selected features and corresponding contributions are summarized in Additional file 1: Table S1.
Model training and evaluation
The PCAIDS, an AutoML model, was redeveloped and trained based on Autogluon, one of the AutoML frameworks. We selected three commonly used algorithms in the field of machine learning: logistic regression (LR), random forest (RF), and extreme gradient boosting (XGBoost). Logistic regression is a statistical model used for predicting binary outcomes. Random forest is an ensemble learning method that operates by constructing a multitude of decision trees. Extreme gradient boosting, or XGBoost, is an ensemble tree method that utilizes a gradient boosting framework [11]. We used these algorithms to compare results in the internal validation cohort and two independent prospective cohorts. After data preprocessing and feature transformation, we also compared the predictive performance of PCAIDS with PSA and free PSA/total PSA (fPSA/tPSA). The whole project was implemented in Python 3 (Python 3.7.11) and partly conducted via the packages Scikit-learn (Scikit-learn 0.19.1) and autogluon (0.4.2).
Statistical analysis
All of the continuous features are presented as medians and interquartile ranges. Missing value cases and categorical variables are presented as numbers and percentages. We used the ROC curve to show the predictive ability of PCAIDS, and we calculated 95% confidence intervals (CIs) for sensitivity and specificity with the bootstrap method [12]. Then, the clinical value was evaluated by decision curve analysis (DCA) and a waterfall plot. We employed the SHAP tool to parse and evaluate the contribution of each predictor [13].
Results
Characteristics of the participants
A total of 108 variables from 4747 patients who underwent prostate biopsy were extracted from nine hospitals in China and randomly divided into a training cohort and a validation cohort at a ratio of 8:2. The demographics and clinical characteristics of patients in each center are shown in Additional file 2: Table S2. According to the feature selection results (Additional file 3: Table S3), 36 features were included in the study (Table 1).
The PCAIDS (AutoML based) showed the highest diagnostic efficacy compared to LR, RF, and XGBoost
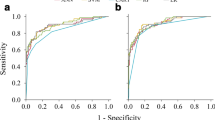
In the internal validation cohort, our results (Fig. 2, Table 2.) showed that AutoML manifested the highest diagnostic accuracy with an AUC of 0.820 (95% CI: 0.79–0.85) compared to LR of 0.816 (95% CI, 0.78–0.85), RF of 0.779 (95% CI, 0.74–0.82), and XGBoost of 0.795 (95% CI, 0.76–0.83) when distinguishing clinically significant prostate cancer (csPCa) from benign disease and insignificant PCa. Similarly, in two prospective cohorts, AutoML showed an ideal diagnostic performance. In the Changhai prospective cohort, AutoML had the highest AUC of 0.807 (95% CI: 0.76–0.85) versus LR of 0.793 (95% CI: 0.75–0.83), RF of 0.766 (95% CI: 0.72–0.81) and XGBoost of 0.763 (95% CI: 0.71–0.81). In the Zhongda prospective cohort, AutoML also had the highest AUC of 0.850 (95% CI: 0.80–0.89) versus LR of 0.848 (95% CI: 0.80–0.90), RF 0.844 (95% CI: 0.79–0.90) and XGBoost of 0.817 (95% CI: 0.76–0.87). Based upon the above results, AutoML was used to construct the final model.
Diagnostic efficacy of the PCAIDS
The PCAIDS had a higher diagnostic efficacy than PSA and fPSA/tPSA, with AUCs of 0.820 (95% CI: 0.79–0.85), 0.616 (95% CI: 0.57–0.66) and 0.675 (95% CI: 0.63–0.71) in distinguishing csPCa, respectively (Fig. 3, Table 2). In the Changhai prospective cohort and the Zhongda prospective cohort, the PCAIDS was superior to PSA and fPSA/tPSA, with higher AUC values (Fig. 3).
The clinical benefits of the PCAIDS
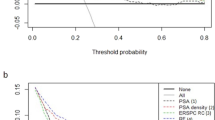
We also investigated the net clinical value of the PCAIDS using DCA by comparing PSA and fPSA/PSA over a range of probabilities. In this analysis, the PCAIDS had the highest net benefit in the validation cohort, and the Changhai prospective cohort and Zhongda prospective cohort demonstrated significant clinical utility when compared with PSA and fPSA/PSA (Fig. 4).
DCA for AutoML, PSA, fPSA/tPSA, and SOC in an internal validation cohort and two prospective cohorts, Chinghai and Zhongdu. a DCA shows that AutoML presented the highest net benefit across all threshold probabilities for PCa. The horizontal gray‒green lines parallel to the x-axis represent no patient undergoing a biopsy (Treat None). The red line indicates that all the patients will have PCa (Treat All). b AutoML outperformed PSA, fPSA/tPSA, and SOC in net reduction per 100 patient interventions at all thresholds. DCA, decision curve analysis; AutoML, automated machine learning; PSA, prostate cancer-specific antigen; fPSA/tPSA, free PSA/total PSA; SOC, standard of care
In the validation cohort, the distribution of biopsy results was depicted in a waterfall plot (Fig. 5). The cutoff values were set according to the sensitivity of 90% and 95%. When the cutoff value with 95% sensitivity was applied (23.8%), the PCAIDS indicated a negative predictive value (NPV) of 96.15% and PPV of 35.58%, preventing 32.18% of unnecessary biopsies at risk and missing only 4.88% of cases of csPCa (Table 3).
Waterfall plot of AutoML related to prostate biopsy results in an internal validation cohort and two prospective cohorts, Chinghai and Zhongdu. Each bar represents an individual. Red indicates ISUP grade ≥ two tumors (Gleason score ≥ 7); blue indicates ISUP grade of one tumor (Gleason score < 7). Two black horizontal lines represent the cutoff points of 26.4 at a sensitivity of 90% and 23.8 at a sensitivity of 95%. AutoML, automated machine learning; ISUP, International Society of Urological Pathology
In the Changhai prospective cohort, when the cutoff value with 95% sensitivity was applied (21.5%), the PCAIDS showed an NPV of 89.9% and PPV of 45.3%, preventing 17.6% of unnecessary biopsies and missing 4.5% of csPCa cases. In the Zhongda prospective cohort, the PCAIDS showed an NPV of 94.1% and PPV of 48.7% at the cutoff value of 95% sensitivity (24.3%), contributing to the reduction of 26.3% unnecessary biopsies with 4.1% csPCa missed.
Discussion
We proposed the PCAIDS, an AutoML-based model, for the prediction of csPCa based on quick and economic routinely performed clinical examinations. The PCAIDS incorporated multimodal and multidimensional data, including laboratory tests, imaging tests, and demographic data, revealing encouraging discriminative power with AUCs of 0.820 in the validation cohort and 0.807 and 0.850 in the two prospective test cohorts.
Compared with previous prediction models, such as the ERSPC-RC [14], PCPT-RC [15] and CPCC-RC [16], the PCAIDS, for the first time, evaluated over 100 multimodal features with AI-based algorithms. These features, including demographics, laboratory tests, and imaging examinations, were assessed by a series of AI algorithms. Among these AI algorithms, AutoML outperformed logistic regression, random forest, and XGBoost. AutoML has become a popular and efficient modeling tool for data science that uses k-fold cross-validation through varying optimization algorithms, such as grid search, random search, and genetic algorithm (GA), to scan different feature combinations, feature transformations, supervised algorithms, and their corresponding hyperparameter combinations implemented in AutoWEKA [17], Autogluon [18], AutoSklearn 2.0 [19], and TPOT, [20] thereby identifying the optimal machine learning pipeline.
Additionally, AI-based methods have the potential to analyze high-volume data and to discover nonlinear and interactive prediction information. For cancer diagnosis, there were huge possibilities that currently applied predictive models only included a proportion of effective predictors. Although the application of AI-based methods may not always outperform linear models, the advantage of involving more features could help the models to be more stable and more applicable for different populations.
In this aspect, Jungyo Suh et al. proposed the possibility of applying AI-based algorithms in the prediction of prostate biopsy. They developed an AI-based prediction tool with PSA, total prostate volume, age, hypoechoic lesion on ultrasonography, transitional zone volume, testosterone, and fPSA [21]. This study showed the promising future of using AI-based algorithms in predicting PCa; however, the investigated features were of limited number. To some extent, AI-based algorithms were not ideal for the analysis of limited features, which could have been done by traditional methods. In predicting colon cancer, researchers applied AI-based methods to data from health maintenance organizations by evaluating analytes from standard laboratory records, including hematology, liver function, and metabolism [22]. In breast cancer, the notion of applying AI-based methods to diagnose breast cancer was validated, and age, body mass index (BMI), glucose, insulin, homeostasis model assessment (HOMA), leptin, adiponectin, resistin and chemokine monocyte chemoattractant protein 1 (MCP1) attributes were used in the prediction model [23]. Further studies validated that routine blood analysis features had a boosted performance for breast cancer diagnosis and supported the notion that this approach is of great potential to be used in a widespread manner to detect cancers [24]. These studies suggested the possibility of using routine health examinations to predict cancer based on AI algorithms.
The clinical scenario for the application of PCAIDS is between PSA-based screening and novel tests predicting PCa, including mpMRI, urinary PCA3 test, 4kScore, and Prostate Health Index. MpMRI, a potent modality in predicting biopsy results, is of great importance in patients who are at high risk of PCa. However, the application of mpMRI is limited by the accessibility of MRI machines and the professionalism of the radiologists who interpret the images. Meanwhile, these biomarkers were only available for patients in some countries and regions. In addition, mpMRI and these novel biomarkers are associated with high costs in most countries. The application of PCAIDS, on the other hand, does not require special examination equipment. The features included in the model were common, routinely performed, quick, and economic tests, which were also needed for a general health check-up. The application of B-ultrasound in evaluating the size of the prostate is also accessible for almost every hospital. In general, this AI-based modality is not here to perfect the diagnostic modality with mpMRI and novel biomarkers, rather than replacing them.
AutoML has the flaw of interpretability, which is consistently met with skepticism, similar to other complex models, especially in the medical field. To this end, we applied the SHAP [13] tool to explore the contribution of individual features to the model. To explore the rationality of this contribution, we also examined the interpretability of the LR compared to SHAP (Additional file 4: Figure S1). First, the contribution of the key variables (the cross-sectional area of the prostate (B_AREA), AGE, and fPSA) is basically the same in the two prediction modalities. This is similar to the previous conclusions obtained by the RF model (Additional file 1: Table S1). Second, the SHAP value from AutoML is roughly the same as the importance of LR calculated by model coefficients. Third, B_AREA is the most important variable. Significantly, the risk of PCa did not increase with B_AREA, which may be due to the increased concertation of PSA produced by a larger prostate, misstating that the risk of PCa and fPSA/tPSA are similar. In addition, age played the second most important role in the prediction model. Thereafter, the risk of PCa increases with age, which is intuitive, and the same holds true for other clinical indicators, although no direct cause can be inferred.
One of the limitations of this study is the lack of a head-to-head comparison with mpMRI or other novel biomarkers. However, the clinical scenario of this prediction mode is not to replace novel diagnostic methods but to assist in decision-making for novel diagnostic methods. In addition, we introduced the dimensions of the prostate from the B ultrasound in the model, and there might be inter- and intrarater differences among different centers in terms of ultrasound results. Furthermore, ultrasound images were not included in this study due to the lack of image storage in all centers. We believe that future studies may incorporate the images captured during ultrasound examinations. The findings of this study are applicable primarily to Asian populations due to the vast discrepancy between Asian and Caucasian patients. In the future, we intend to collect data from various populations to adapt our model to different ethnic groups. Finally, the performance of the PCAIDS is not better than that of the other algorithms, including LR. However, it is important to note that in the study, given the serious implications of missing a prostate cancer diagnosis, prioritizing sensitivity rather than specificity was chosen. This decision was made understanding that it might increase the false positive rate, but it's a reasonable trade-off given the potential severity of a missed diagnosis, where high sensitivity can often lead to lower specificity. We consider that further validation studies may help us to show its wide applicability.
Conclusion
The AutoML-based PCAIDS was a real-time, noninvasive, easy-to-use tool that could be applied for the prediction of csPCa in multimodal routine clinical examinations. The system presented greater diagnostic performance and clinical utility in detecting csPCa than traditional predictors. The PCAIDS was an effective tool for physicians to decide the need for prostate biopsy and prebiopsy examinations such as mpMRI. Further perspective and international studies are warranted to validate the findings of this study.
Availability of data and materials
The relevant data have been presented in the article. Correspondence and requests for materials should be addressed to F.W. and R.C.
Abbreviations
- AI:
-
Artificial intelligence
- AUC:
-
Area under the curve
- AutoML:
-
Automated machine learning
- csPCa:
-
Clinically significant prostate cancer
- DCA:
-
Decision curve analysis
- ETL:
-
Extract-transform-load
- fPSA/tPSA:
-
Free PSA/total PSA
- GA:
-
Genetic Algorithm
- LR:
-
Logistic regression
- mp-MRI:
-
Multiparameter MRI
- NLP:
-
Natural Language Processing
- PCa:
-
Prostate cancer
- PCAIDS:
-
Prostate Cancer Artificial Intelligence Diagnostic System
- PSA:
-
Prostate-specific antigen
- RF:
-
Random forest
- ROC:
-
Receiver operating characteristic
- TRIPOD:
-
Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis
- XGBoost:
-
Extreme gradient boosting
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32.
Shao Y-HJ, Kim S, Moore DF, Shih W, Lin Y, Stein M, et al. Cancer-specific survival after metastasis following primary radical prostatectomy compared with radiation therapy in prostate cancer patients: results of a population-based, propensity score-matched analysis. Eur Urol. 2014;65:693–700.
Fenton JJ, Weyrich MS, Durbin S, Liu Y, Bang H, Melnikow J. Prostate-specific antigen-based screening for prostate cancer: evidence report and systematic review for the us preventive services task force. JAMA. 2018;319:1914–31.
Yu K-H, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2:719–31.
Zhu L, Mou W, Chen R. Can the ChatGPT and other large language models with internet-connected database solve the questions and concerns of patient with prostate cancer and help democratize medical knowledge? J Transl Med. 2023;21:269.
Lu MT, Raghu VK, Mayrhofer T, Aerts HJWL, Hoffmann U. Deep Learning Using Chest Radiographs to Identify High-Risk Smokers for Lung Cancer Screening Computed Tomography: Development and Validation of a Prediction Model. Ann Internal Med. 2020;173:704–13.
Menze BH, Kelm BM, Masuch R, Himmelreich U, Bachert P, Petrich W, et al. A comparison of random forest and its Gini importance with standard chemometric methods for the feature selection and classification of spectral data. BMC Bioinformatics. 2009;10:213.
Tipping ME. Sparse bayesian learning and the relevance vector machine. J Mach Learn Res. 2001;1:211–44.
Chen T, Guestrin C. XGBoost: A Scalable Tree Boosting System. In: Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. New York: Association for Computing Machinery; 2016. p. 785–94.
DiCiccio TJ, Efron B. Bootstrap Confidence Intervals. Statist Sci. 1996;11:189–212.
Lundberg SM, Lee S-I. A Unified Approach to Interpreting Model Predictions. In: Advances in Neural Information Processing Systems. California: Curran Associates Inc; 2017.
Roobol MJ, Steyerberg EW, Kranse R, Wolters T, van den Bergh RCN, Bangma CH, et al. A Risk-Based Strategy Improves Prostate-Specific Antigen-Driven Detection of Prostate Cancer. Eur Urol. 2010;57:79–85.
Thompson IM, Ankerst DP, Chi C, Goodman PJ, Tangen CM, Lucia MS, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98:529–34.
Chen R, Xie L, Xue W, Ye Z, Ma L, Gao X, et al. Development and external multicenter validation of Chinese Prostate Cancer Consortium prostate cancer risk calculator for initial prostate biopsy. Urol Oncol. 2016;34(416):e1-7.
Kotthoff L, Thornton C, Hoos HH, Hutter F, Leyton-Brown K. Auto-WEKA: Automatic Model Selection and Hyperparameter Optimization in WEKA. In: Hutter F, Kotthoff L, Vanschoren J, editors. Automated Machine Learning: Methods, Systems, Challenges. Cham: Springer International Publishing; 2019. p. 81–95.
Erickson N, Mueller J, Shirkov A, Zhang H, Larroy P, Li M, et al. AutoGluon-Tabular: Robust and Accurate AutoML for Structured Data. 2020.
Feurer M, Eggensperger K, Falkner S, Lindauer M, Hutter F. Auto-Sklearn 2.0: Hands-free AutoML via Meta-Learning. J Mach Learn Res. 2022;23:1–61.
Olson RS, Moore JH. TPOT: A Tree-Based Pipeline Optimization Tool for Automating Machine Learning. In: Hutter F, Kotthoff L, Vanschoren J, editors. Automated Machine Learning: Methods, Systems, Challenges. Cham: Springer International Publishing; 2019. p. 151–60.
Suh J, Yoo S, Park J, Cho SY, Cho MC, Son H, et al. Development and validation of an explainable artificial intelligence-based decision-supporting tool for prostate biopsy. BJU Int. 2020;126:694–703.
Goshen R, Mizrahi B, Akiva P, Kinar Y, Choman E, Shalev V, et al. Predicting the presence of colon cancer in members of a health maintenance organisation by evaluating analytes from standard laboratory records. Br J Cancer. 2017;116:944–50.
Aslan MF, Celik Y, Sabanci K, Durdu A. Breast Cancer Diagnosis by Different Machine Learning Methods Using Blood Analysis Data. Int J Intell Syst Appl Eng. 2018;6:289–93.
Yavuz E, Eyupoglu C. An effective approach for breast cancer diagnosis based on routine blood analysis features. Med Biol Eng Comput. 2020;58:1583–601.
Acknowledgements
We acknowledge all the donors, collaborators, and healthcare workers who supported this study.
Funding
This work was supported by grants from the National Natural Science Foundation of China (NSFC) (81902616), Science and Technology Major Project of Guangxi (AA22398), and Raising Star Program of Shanghai Science and Technology Commission (21QA1411500).
Author information
Authors and Affiliations
Contributions
HZ, JJ, ZL, HL, CQ, and CMW: conceptualization, methodology, project administration, resources, writing — original draft. SHC, WHL, and CBW: conceptualization, methodology, project administration, resources, investigation. HX, YLX, XC, XH, ZHW, and XDZ: data curation, formal analysis. WC, XFC, GJP, GPY, YG, and KXJ: data curation, resources. BX, JYC, BX, XDW, and MC: conceptualization, data curation, formal analysis, investigation, methodology, project administration. RC, JC, and FW: conceptualization, data curation, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, writing — review and editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the local ethics committee (CHEC2021-092), registered on the Chinese Clinical Trial Registry (ChiCTR2100048428), and undertaken according to the Declaration of Helsinki.
Consent to participate
The patients/participants provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Handling Editor: Layla Mohammad Hadi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Selected featuresin the Prostate Cancer Artificial Intelligence Diagnostic System.
Additional file 2: Table S2.
Demographics andclinical characteristics of participants.
Additional file 3: Table S3.
Clinical researchcenters involved in this study.
Additional file 4: Figure S1.
Featureimportance analysis uses AutoML. (a) SHAP summary plot. SHAP feature importancemeasured as the mean absolute Shapley values. (b).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, H., Ji, J., Liu, Z. et al. Artificial intelligence for the diagnosis of clinically significant prostate cancer based on multimodal data: a multicenter study. BMC Med 21, 270 (2023). https://doi.org/10.1186/s12916-023-02964-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-023-02964-x