Abstract
Background
Childhood maltreatment is associated with depression and cardiometabolic disease in adulthood. However, the relationships with these two diseases have so far only been evaluated in different samples and with different methodology. Thus, it remains unknown how the effect sizes magnitudes for depression and cardiometabolic disease compare with each other and whether childhood maltreatment is especially associated with the co-occurrence (“comorbidity”) of depression and cardiometabolic disease. This pooled analysis examined the association of childhood maltreatment with depression, cardiometabolic disease, and their comorbidity in adulthood.
Methods
We carried out an individual participant data meta-analysis on 13 international observational studies (N = 217,929). Childhood maltreatment comprised self-reports of physical, emotional, and/or sexual abuse before 18 years. Presence of depression was established with clinical interviews or validated symptom scales and presence of cardiometabolic disease with self-reported diagnoses. In included studies, binomial and multinomial logistic regressions estimated sociodemographic-adjusted associations of childhood maltreatment with depression, cardiometabolic disease, and their comorbidity. We then additionally adjusted these associations for lifestyle factors (smoking status, alcohol consumption, and physical activity). Finally, random-effects models were used to pool these estimates across studies and examined differences in associations across sex and maltreatment types.
Results
Childhood maltreatment was associated with progressively higher odds of cardiometabolic disease without depression (OR [95% CI] = 1.27 [1.18; 1.37]), depression without cardiometabolic disease (OR [95% CI] = 2.68 [2.39; 3.00]), and comorbidity between both conditions (OR [95% CI] = 3.04 [2.51; 3.68]) in adulthood. Post hoc analyses showed that the association with comorbidity was stronger than with either disease alone, and the association with depression was stronger than with cardiometabolic disease. Associations remained significant after additionally adjusting for lifestyle factors, and were present in both males and females, and for all maltreatment types.
Conclusions
This meta-analysis revealed that adults with a history of childhood maltreatment suffer more often from depression and cardiometabolic disease than their non-exposed peers. These adults are also three times more likely to have comorbid depression and cardiometabolic disease. Childhood maltreatment may therefore be a clinically relevant indicator connecting poor mental and somatic health. Future research should investigate the potential benefits of early intervention in individuals with a history of maltreatment on their distal mental and somatic health (PROSPERO CRD42021239288).
Similar content being viewed by others
Background
Childhood maltreatment is a major public health concern [1]. Robust evidence shows that childhood maltreatment is associated with a twofold to threefold increased risk of depression in adulthood [2, 3], and this association is likely causal [4]. Depression risk is increased after experiencing any type of maltreatment, although emotional abuse and neglect seem to be particularly strong predictors [5]. The effect of childhood maltreatment on depression has also been suggested to be sex-specific, with stronger associations in females than in males [6], yet evidence is limited.
Beyond mental health, childhood maltreatment is also linked to cardiometabolic diseases in adulthood. Exposure to maltreatment in childhood is associated with an increased incidence of cardiovascular diseases (incidence rate ratio = 1.71) and type 2 diabetes (incidence rate ratio = 2.13) [7]. There seems to be a dose–response relationship; the greater the number of experienced maltreatment types, the higher the risk of cardiometabolic diseases [8]. Although this association is mostly similar across sexes, emotional neglect appears more strongly related to cardiovascular disease in females [9].
Extensive evidence shows that cardiometabolic disease and depression co-occur and are bidirectionally linked [10, 11]. Meta-analyses indicate that 29% of patients with myocardial infarction [12] and 18–32% of those with diabetes have comorbid depression [13]. Depressed individuals also have a 64–80% higher risk of developing cardiovascular disease [14, 15]. The high risk of co-occurrence, or comorbidity, is possibly explained by common underlying mechanisms. Childhood maltreatment could be a shared risk factor prompting a cascade of mechanisms leading to these diseases. In fact, depression and cardiovascular disease have a shared genetic vulnerability [16], possibly associated to biological pathways [17] such as inflammation, hypothalamic–pituitary–adrenal (HPA) axis dysregulations, and dysfunction of the autonomic nervous system; and behavioral pathways such as physical inactivity, unhealthy diet, smoking, and drinking, that may be further stimulated by childhood maltreatment, giving rise to more comorbidity between depression and cardiometabolic diseases.
Despite this evidence, no one has yet directly compared the increase in depression prevalence with the increase in cardiometabolic disease prevalence after childhood maltreatment. Investigating the associations of childhood maltreatment with depression and cardiometabolic disease in the same samples with harmonized variable definitions and a uniform handling of covariates is novel and enables the comparison of effect sizes. Additionally, although childhood maltreatment potentially activates biological and behavioral risk pathways that are shared for depression and cardiometabolic disease, no one has yet established whether childhood maltreatment is associated with the comorbidity of these diseases in adulthood. Because comorbid depression and cardiometabolic disease involve a heavier disease burden and mortality than each disease individually [18], characterizing the association with comorbidity would facilitate efforts to develop appropriate and efficient approaches to this major public health issue.
In this study, we conducted a large-scale individual participant data (IPD) meta-analysis to investigate the association of childhood maltreatment with depression, cardiometabolic disease and their comorbidity in adults. We also explored the role of childhood maltreatment type, lifestyle factors, and sex in these associations.
Methods
Cohorts and participants
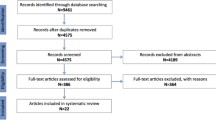
This IPD meta-analysis is an effort of the EarlyCause consortium [19], which investigates the association between early-life stress and comorbid depression and cardiometabolic outcomes. Studies within and outside the consortium were selected based on the consortium network. Study inclusion criteria were as follows: having retrospective reports on childhood maltreatment (at least physical and emotional abuse) before the age of 18 and having data on depression and/or cardiometabolic diseases in adulthood. Studies were excluded if participants were younger than 18 years at assessment time. In total, 13 studies from Germany, the Netherlands, the UK, and the USA were included.
Three studies were case–control studies with an overrepresentation of individuals with affective disorders: the Marburg-Münster Affective Disorders Cohort Study (MACS) [20], the Netherlands Study of Depression and Anxiety (NESDA) [21], and the Netherlands Study of Depression in Older persons (NESDO) [22]. Ten studies were population-based cohort studies: the mothers and partners of the Avon Longitudinal Study of Parents and Children (ALSPAC, see Additional file 1: Sect. 1) [23,24,25], the mothers of Generation R Study (GenR, see Additional file 1: Sect. 2) [26], the Healthy Life in an Urban Setting (HELIUS) study [27], the first wave of the Midlife in the United States (MIDUS) study [28], the first and second Netherlands Mental Health Survey and Incidence Studies (NEMESIS-1 and NEMESIS-2) [29, 30], two independent cohorts of the Study of Health In Pomerania (SHIP-Trend baseline assessment and SHIP-Legend) [31], and the UK Biobank (UKBB) [32, 33]. Each cohort study was approved by local ethics committees and all participants provided informed consent. This research project was pre-registered on the international prospective register of systematic reviews PROSPERO in March 2021 (reference CRD42021239288). The PRISMA-IPD guidelines [34] were followed except for the systematic review-related steps which were not applicable in this research.
Measures
Childhood maltreatment
The main exposure was the presence of childhood maltreatment in any of the following categories: physical, emotional, and/or sexual abuse, before the age of 18. Physical and emotional maltreatment were defined by the following: (1) self-reported history of regular or more frequent abuse (“often true”, “very often true”, “regularly”, “often”, or “very often” frequency ratings depending on the instrument) when a frequency assessment was available or (2) self-reported history of abuse in case of a dichotomous assessment. Sexual abuse was defined by the report of at least one occurrence of sexual abuse in childhood. Cases of childhood maltreatment were identified when criteria were met for either maltreatment type. Neglect was not included in the definition of childhood maltreatment because participating studies either did not assess physical and emotional neglect (n = 6) or assessed them in discrepant manners. Specific measures and criteria used to code childhood maltreatment (absent vs. present) in each study are described in Additional file 1: Table S1 [35,36,37,38].
Psycho-cardiometabolic outcomes
Depression
The presence of depression was defined by the following: (1) the presence of a lifetime (eight cohorts) or current (one cohort) diagnosis of major depressive disorder assessed with (semi-)structured clinical interviews or (2) current depressive symptomatology (four cohorts) assessed with self-report scales using validated clinical cut-offs. Cohort-specific measures and criteria used to identify depression cases (absent vs. present) are described in Additional file 1: Table S2 [39,40,41,42,43,44,45,46,47,48].
In sensitivity analyses, the depression definition was extended, including information on self-reported current use of antidepressants (Anatomical Therapeutic Chemical (ATC) codes starting with N06A, N06AA, N06AB, N06AF, N06AG, and N06AX) to identify additional depression cases.
Cardiometabolic disease
The presence of cardiometabolic disease was based on self-reports of a lifetime clinical diagnosis of a non-congenital cardiovascular disease (see Additional file 1: Table S3 for a complete list) and/or diabetes mellitus (absent vs. present). Cardiovascular disease and diabetes were selected as cardiometabolic diseases because of their known co-occurrence with depression [15, 49], their high prevalence and major impact on public health [50, 51], and their consistent assessment across cohorts.
In sensitivity analyses, we additionally tested strict (limited to heart/cardiac diseases) and broad (also including blood pressure and other heart and peripheral vascular problems; see Additional File 1: Table S3 for complete list) definitions of cardiovascular disease. Furthermore, the cardiometabolic disease definition was extended, including information on self-reported current use of related medications (ATC codes C01, C03, C04, C05, C07, C08, C09, and C10) to identify additional cases of cardiometabolic disease.
Comorbidity status
Comorbidity status was based on depression and cardiometabolic disease status. It comprised four levels: 0 = absence of depression and cardiometabolic disease (heathy controls), 1 = depression only, 2 = cardiometabolic disease only, 3 = comorbidity of depression and cardiometabolic disease.
In sensitivity analyses, the definition of comorbidity status was adjusted based on the strict (model 8) and broad (model 9) definitions of cardiovascular disease and on definitions of depression and cardiometabolic disease incorporating current medication use (model 10).
Covariates
Sociodemographic covariates sex, age, and educational attainment were assessed at the earliest timepoint available. Sex and age were based on either self-reports or municipal registries, and educational attainment was based exclusively on self-reports. Educational attainment was harmonized across cohorts and countries by using the International Standard Classification of Education (ISCED) 2011 [52] and categorized in three levels: ISCED 0–2 corresponds to no education, early childhood education, primary and lower secondary education; ISCED 3–4 corresponds to upper secondary education and post-secondary non-tertiary education; and ISCED 5–6-7–8 corresponds to short-cycle tertiary education, bachelor, master, and doctor or equivalent levels. In addition, ethnicity was entered as a sociodemographic covariate in all analyses of HELIUS due to its design-specific oversampling of participants from different ethnic groups (Dutch, Ghanaian, Moroccan, Surinamese, and Turkish). Lifestyle covariates included self-reported current smoking status, weekly alcohol consumption, and weekly physical activity. Smoking status was assessed consistently across cohorts (current smoking vs. no current smoking). Alcohol consumption and physical exercise assessments varied across cohorts and specifications are described in Additional File 1: Table S4 [53].
Statistical analyses
A two-step IPD meta-analysis was carried out [54]. In the first step, cohorts applied a standardized protocol for data harmonization to create the required variables and carry out statistical analyses estimating the associations between childhood maltreatment and the different outcomes. In the second step, we meta-analyzed cohorts’ aggregate effect sizes with random-effects models using inverse-variance weighting. We chose random-effects models to pool the aggregate effect sizes because these effect sizes are drawn from different populations. Cohorts with cell count < 5 across exposure and outcome categories were excluded from the meta-analyses. Heterogeneity parameters Q, I2, and \({\tau }^{2}\) were calculated. Scripts of the two steps can be found on the Github EarlyCause repository (see Additional File 1: Sect. 3).
Main models
The main models assessed the association of childhood maltreatment with (model 1) depression (vs. no depression) and (model 2) cardiometabolic disease (vs. no cardiometabolic disease) using binomial logistic regressions and (model 3) comorbidity status (absence of disease vs. depression only vs. cardiometabolic disease only vs. comorbidity) using multinomial logistic regression. Subgroup analyses were carried out to explore whether differences in cohorts’ depression assessments possibly explained effect size heterogeneity in model 3. All models were adjusted for sociodemographic covariates. Lifestyle factors were additionally included in the model to examine their impact on the association of childhood maltreatment with comorbidity status (model 4). Analyses were then stratified by sex to check the consistency of results in males and females (models 5a and 5b). Additionally, the association of types of childhood maltreatment (physical abuse, emotional abuse, and sexual abuse) with the four-level comorbidity status was investigated in a multinomial logistic multiple regression model (model 6). Finally, we examined the role of maltreatment severity by creating a new variable, number of maltreatment types (0 type vs. 1 type vs. 2 or more types of childhood maltreatment) and testing its association with comorbidity status in a multinomial logistic regression model (model 7).
Sensitivity analyses
We carried out sensitivity analyses to check the consistency of the results obtained in the main model 3. First, we alternatively applied different definitions (strict and broad) of cardiovascular disease (models 8 and 9, respectively). Then, we extended the definition of depression and cardiometabolic disease incorporating information on the use of related medications (model 10).
Analyses were conducted on participants with complete data on childhood physical and emotional abuse, as well as on depression and/or cardiometabolic disease. For cohorts with 20% or more cases with missing lifestyle values in model 4 compared to the sample used in model 3, missing lifestyle values were imputed (see Additional File 1: Sect. 4 and Table S5 for detailed explanations). For cohorts with less than 20% missingness on lifestyle factors, cases with missing lifestyle values were excluded from the model. High missingness in lifestyle covariates (in particular smoking status) applied in model 4 was limited to two out of nine total cohorts. Although participants with these missing covariates represented only 2.1% of the total sample size of the pooled model 4, we decided a priori to impute lifestyle covariates when their missingness caused an important loss of data since we aimed to compare estimated associations from models with (model 4) and without lifestyle covariates (model 3). The statistical software R version 4.0.5. (packages “metafor” version 3.0–2 [55] and “meta” version 5.2–0 [56]) was used to carry out the analyses. Statistical significance level was set at p < 0.05, two-sided.
Results
This study includes 13 cohorts, with a combined sample size of 217,929 participants. Weighted mean age across studies was 52.4 years. Three studies were case–control studies with a higher prevalence of depression only (weighted mean 52.4%) compared to the 10 population-based cohort studies (weighted mean 19.1%). The weighted mean prevalence of cardiometabolic disease was 5.1%, and the weighted mean prevalence of comorbidity was 2.1%. Cohort-specific information can be found in Table 1.
Association of childhood maltreatment with depression, cardiometabolic disease, and comorbidity
The odds of having depression increased almost three folds in those with a history of childhood maltreatment compared to those without (model 1, OR [95% CI] = 2.82 [2.40; 3.30], Fig. 1A). This positive association was seen in all cohorts, albeit with significant heterogeneity across studies (Q = 70.44, p < .001, I2 = 91.8%, \({\tau }^{2}\) = 0.07). Adults with a history of childhood maltreatment, as compared to those without, were also more likely to have a cardiometabolic disease (model 2, OR [95% CI] = 1.34 [1.23; 1.46], Fig. 1B). Effect sizes were relatively homogeneous across studies (Q = 18.09, p = 0.113, I2 = 27.1%, \({\tau }^{2}\) = 0.01). The results of all meta-analyzed models are shown in Table 2.
Forest plots of the random-effects models of the association of childhood maltreatment with depression (A) and cardiometabolic disease (B). CM, childhood maltreatment. Dep., depression. Card., cardiometabolic disease. OR, odds ratio. CI, confidence interval. Note. Squares represent effect sizes of individual studies. Their size reflects the precision of the estimate based on the random-effect model. The diamond represents the pooled effect size across studies in the center of the diamond, and the lower and upper 95% confidence interval limits at the left and right side of the diamond
The analysis of the association of childhood maltreatment with comorbidity status (model 3) was restricted to nine cohorts (n = 146,167) due to crosstab cells with less than five cases for some outcomes in four cohorts. In these nine cohorts, adults with a history of childhood maltreatment had twice higher odds of depression only (OR [95% CI] = 2.68 [2.39; 3.00], Fig. 2) and also higher odds of cardiometabolic disease only (OR [95% CI] = 1.27 [1.18; 1.37], Fig. 2). The effect size for cardiometabolic disease was around half of the effect size for depression only. The strongest association was found for comorbid depression and cardiometabolic disease (OR [95% CI] = 3.04 [2.51; 3.68]), Fig. 2), and this positive association was statistically significant in most cohorts. Since the UKBB represented the largest dataset in the pooled analysis (n = 98,619, 67.5% of participants, see Additional File 1: Table S7 for weights in pooled estimate), we re-ran the meta-analysis excluding the UKBB and observed that results remained largely similar (OR [95% CI] depression only = 2.67 [2.33; 3.06], OR [95% CI] cardiometabolic disease only = 1.29 [1.15; 1.44], OR [95% CI] comorbidity = 2.82 [2.43; 3.27]). These results were mostly consistent with results of the UKBB only (OR [95% CI] depression only = 2.66 [2.54; 2.78], OR [95% CI] cardiometabolic disease only = 1.26 [1.13; 1.40], OR [95% CI] comorbidity = 4.11 [3.73; 4.53]), although the odds of comorbidity seemed to be slightly higher in the UKBB than in the other cohorts. Subgroup analyses showed that results were largely unaffected by depression assessment type (see Additional File 1: Table S8) and by current vs. lifetime depression (see Additional File 1: Sect. 5). Additionally, we exploratively ran a post hoc test to evaluate whether childhood maltreatment was more strongly associated with comorbidity than with the individual diseases. Since the UKBB was the largest sample for which we had direct access to the individual-level data, we used that sample to calculate the following two odds ratios after childhood maltreatment: depression only vs. comorbidity and cardiometabolic disease only vs. comorbidity. Instead of using the outcome level “absence of depression and cardiometabolic disease” as reference category as in the previous calculations of odds ratios, we used the outcome level “comorbidity” as new reference category to statistically test whether childhood maltreatment was more strongly associated with comorbidity than with the single diseases. We found that the association of childhood maltreatment with depression only (OR [95% CI] = 0.65 [0.59; 0.71]) and cardiometabolic disease only (OR [95% CI] = 0.31 [0.27; 0.35]) were significantly smaller than with comorbidity.
Forest plot of the random-effects model of the association of childhood maltreatment with depression only, cardiometabolic disease only, and comorbidity. Note. Number of cases, weights and odds ratios of each cohort can be found in Additional file 1: Table S7. OR: odds ratio. CI, confidence interval. Note. Squares represent effect sizes of individual studies. Their size reflects the precision of the estimate based on the random-effect model. The diamond represents the pooled effect size across studies in the center of the diamond, and the lower and upper 95% confidence interval limits at the left and right side of the diamond
Additional adjustment for smoking status, alcohol consumption, and physical activity (model 4) did not substantially change the associations of childhood maltreatment with depression only (OR [95% CI] = 2.57 [2.28; 2.89]), cardiometabolic disease only (OR [95% CI] = 1.25 [1.15; 1.36]), and comorbidity (OR [95% CI] = 2.99 [2.46; 3.63]), highlighting the independence of these associations from lifestyle factors. Similar associations to those obtained in the main model 3 were observed for males (model 5a) and females (model 5b). However, the association with cardiometabolic disease only seemed stronger in females than in males (OR [95% CI] in females = 1.41 [1.26; 1.57], in males = 1.13 [1.01; 1.27], with OR difference z = 2.77, p = .006; Table 2, models 5a and 5b). All types of childhood maltreatment (physical, emotional, and sexual abuse) were associated with increased odds of developing depression only, cardiometabolic disease only, and comorbidity, although physical and emotional abuse were particularly strong predictors of the comorbidity (Table 2, model 6). Finally, although all numbers of maltreatment types were related to comorbidity status, there seemed to be a dose–response relationship: When two or more types of childhood maltreatment were experienced, the odds of (comorbid) depression and cardiometabolic disease exceeded the odds found after one type of maltreatment only (Table 2, model 7).
Sensitivity analyses
Results were similar in sensitivity analyses applied to comorbidity status model 3 when using different operational definitions of cardiovascular disease based on stricter or broader definition (models 8 and 9; Table 2) or different definitions of cardiometabolic diseases and depression additionally including information on medication (model 10; Table 2).
Discussion
Association of childhood maltreatment with (comorbid) depression and cardiometabolic disease
This study used data from 13 international cohorts involving 217,929 persons to systematically investigate the association of childhood maltreatment with (comorbid) depression and cardiometabolic disease in adulthood. In order to obtain a consistent set of aggregate data across cohorts, individual participant data were harmonized and cohort-level analyses were standardized. Main findings show that adults with a history of childhood maltreatment, as compared to those without, are 1.27 times more likely to have cardiometabolic disease only and 2.68 times more likely to have depression only. The largest difference between maltreated and non-maltreated individuals was found for the co-occurrence of both conditions: Maltreated individuals were 3.04 times more likely to suffer from comorbid depression and cardiometabolic disease in adulthood. Post hoc analyses showed that this association was larger than the ones for either disease alone. Results remain similar in sensitivity analyses using different outcome ascertainment definitions, suggesting findings are robust.
Our results are in line with findings from existing meta-analyses. Two relatively recent meta-analyses [2, 3] evaluated the association of childhood maltreatment history with depression and found that childhood maltreatment was associated with 2.03 (95% CI = [1.37; 3.01]) and 2.81 (95% CI = [2.35; 3.36]) increased odds of depression in adulthood, using pooled samples of 4579 [2] and 26,536 [3] participants, respectively. The current study found similar heightened odds of depression (OR [95% CI] = 2.82 [2.40; 3.30]) after childhood maltreatment, based on by far the largest sample size (n = 155,869). Additionally, although age was demonstrated to moderate this association [3], pooled effect sizes from the previous meta-analyses were either based on study-level effect sizes with inconsistent handling of covariates or on raw data excluding covariate adjustments. In contrast, the current research facilitated cohort-level analyses in a systematic manner, enabling the estimation of pooled effect sizes adjusting for important sociodemographic and lifestyle covariates. Although the effect size reported in Li et al. [2] is slightly smaller than the one in the current study, the difference may be explained by the definition of Li et al.’s exposure variable: Childhood maltreatment was based on official records, which are more likely to suffer from underreporting than retrospective self-reports. Despite this difference in assessment, the consistent direction of findings increase confidence in the validity of maltreatment self-reports. Further, a previous meta-analysis [57] of 29 studies (N = 247,393) showed that cumulative childhood adversity was moderately related to cardiometabolic disease in adulthood (OR [95% CI] = 1.36 [1.27; 1.46]). Although the exposure (an index including at least two adverse childhood experiences) and outcome (cardiometabolic disease including metabolic syndrome) definitions slightly differ from the ones of the current meta-analysis, results align closely (our findings: OR [95% CI] = 1.34 [1.23; 1.46]). Finally, because the current meta-analysis consistently adjusted associations for the same covariates, it provides the unique possibility to directly compare the increased odds of each disease after maltreatment. The results show that, compared to non-maltreated individuals, maltreated adults are 2.68 times more likely to suffer from depression and “only” 1.27 times more from cardiometabolic disease. Although linked to both, childhood maltreatment is therefore more strongly related to depression than to cardiometabolic disease in adulthood.
A striking result is that the odds of comorbid depression and cardiometabolic disease after childhood maltreatment (OR [95% CI] = 3.04 [2.51; 3.68]) are higher than for each disease alone. Although previous studies report that depression and cardiometabolic disease tend to co-occur, the current meta-analysis is the first study to investigate and support the relationship between childhood maltreatment and the co-occurrence of depression and cardiometabolic disease in adulthood. This association is possibly explained by the early-life stress triggering mechanisms common to both depression and cardiometabolic disease. Previous research suggests that childhood maltreatment activates interrelated biological and behavioral pathways [17] potentially leading to adverse health outcomes. Because childhood maltreatment occurs during a critical period for brain neuroplasticity, it may dysregulate stress-related neural circuits [58, 59]. Longitudinal studies show that childhood maltreatment is associated with structural and functional neural changes [60]. Among others, these changes may subsequently dysregulate neuroendocrine and immune systems. The HPA axis may be hyper- or hypo-activated due to impaired glucocorticoid receptor function and inflammation levels may be elevated [1, 61]. Although behavioral pathways are also hypothesized to contribute to poor health outcomes in people with childhood maltreatment [17], our results show that the associations of childhood maltreatment with comorbidity status survive adjustment for smoking, alcohol consumption and physical activity; suggesting that the increased likelihood of (comorbid) depression and cardiometabolic disease after maltreatment does not exclusively depend on one’s lifestyle. Additionally, other non-biological factors (i.e., disease severity, age at diagnosis, working conditions) may also explain the strong association observed between childhood maltreatment and comorbidity and should be investigated. Lastly, the higher odds of comorbidity than single diseases after childhood maltreatment may be explained by the fact that depression and cardiometabolic disease have a bidirectional feedforward loop [10]. Both diseases likely magnify each other in a reinforcing vicious cycle, which is further stimulated by childhood maltreatment and its related biological, psychosocial and behavioral consequences.
Differential effects of sex and maltreatment types
We carried out additional analyses to explore how the associations between childhood maltreatment and (comorbid) depression and cardiometabolic disease varied across sex and maltreatment types. Both in males and females, childhood maltreatment was associated with more (comorbid) depression and cardiometabolic disease. Associations between childhood maltreatment and comorbidity status were mostly similar across males and females. However, females showed a slightly stronger association than males for cardiometabolic disease only (males: OR [95% CI] = 1.13 [1.01; 1.27], females: OR [95% CI] = 1.41 [1.26; 1.57]). Evidence from the literature on that matter is inconsistent [9, 57], and conclusions should therefore be drawn carefully. Further analyses were carried out to test the relationship between maltreatment types and (comorbid) depression and cardiometabolic disease. Because multi-type maltreatment is common [62], the different maltreatment types were entered as multiple predictors within the same model to obtain average estimates of the association between each maltreatment type with comorbidity status while accounting for the co-occurring experience of other types of maltreatment. Findings show that all maltreatment types were independently associated with (comorbid) depression and cardiometabolic disease. Zooming in on specific outcomes, depression only was particularly strongly associated with emotional abuse. Cardiometabolic disease only and comorbidity were particularly strongly associated with physical and emotional abuse. Previous research findings support our results: Physical and emotional abuse are stronger predictors of depression and cardiovascular disease than sexual abuse [3, 9]. Alternatively, the estimated associations of sexual abuse with the disease outcomes may be harder to detect because of the relatively low prevalence of sexual abuse compared to the other types of abuse [63] or because milder forms of sexual abuse are picked up, for instance from the population-based studies. Lastly, findings endorse a dose–response relationship of childhood maltreatment severity—here operationalized as the number of maltreatment types—with (comorbid) depression and cardiometabolic disease. This converges with previous evidence on various health outcomes [64, 65].
Strengths and limitations
This study has several strengths. First, the meta-analysis gathered 13 international cohorts including 217,929 individuals from European countries and the USA. Second, the systematic methodology used with the two-step individual participant data design has essential advantages [54]. It enables the standardization of analyses across studies (i.e., harmonization of variables and consistent covariate adjustment of estimates) and increases the quality of aggregate data entering the meta-analysis. Third, the variety of cohorts involved (e.g., case–control and population-based studies; cohort oversampling persons with migration background) and comprehensiveness of the analyses carried out (e.g., sensitivity analyses with different outcome definitions, effects of different maltreatment types, stratified analyses across sex) strengthens the robustness of the findings across settings.
This study also has limitations. In some cohorts, especially those with younger samples, the prevalence of comorbid depression and cardiometabolic disease was low (weighted mean 2.1%), leading to some studies being excluded of the multinomial regression analyses due to small cell count. This is likely because cardiometabolic events usually happen in later life [66]. The prevalence of comorbidity increases with age as seen in the oldest cohort NESDO with the highest rate of comorbidity (22.6%). The relatively high average age across cohorts (52.4 years old) may have thereby facilitated finding existing associations. Despite the difference in outcome prevalence across cohorts of different ages, the associations found for depression and cardiometabolic disease are consistent across younger (e.g., ALSPAC mothers and partners) and older (e.g., NESDO and SHIP-Legend) cohorts. Therefore, there is no obvious indication of a differential effect of age. An alternative explanation for the low prevalence of comorbidity may be survival bias where patients with severe depression and cardiometabolic disease have died or are too ill to participate in the studies. Nevertheless, even after excluding studies with too few comorbidity cases from the multinomial regression analyses, the total sample used to investigate the association with comorbidity status still amounted to 146,167 individuals. Another limitation is that meta-analyzed associations of childhood maltreatment with depression and comorbidity showed non-negligible heterogeneity. However, we used random-effect models which, by definition, assume the included studies have different true effect sizes, and thereby account for heterogeneity in calculating pooled estimates. The heterogeneity could not be explained by differences in depression assessment but other factors could have possibly caused this divergence (e.g., study design, age, cultural differences in stigma reporting childhood maltreatment) and should be further investigated. Additionally, as with every assessment type, the reliance on self-reports has its set of limitations. Cardiometabolic diseases were assessed with self-reported diagnoses, which may be perceived as biased. However, previous research show that cardiometabolic disease assessment (self-reports vs. medical records) does not influence the association found between childhood maltreatment and cardiometabolic disease [9]. Childhood maltreatment was also assessed with self-reports. It has been suggested that depression may negatively bias someone’s recall of their childhood experiences [67] in which case, self-reports may spuriously inflate the association found between childhood maltreatment and depression as well as comorbidity. Recent evidence from published and unpublished research [67, 68] highlights the marginal susceptibility of maltreatment self-reports to negative recall bias as well as their temporal stability irrespective of depression diagnosis. In order to test this in the current study, we compared the associations found in population-based cohorts using lifetime vs. current depression assessments (see Additional file 1: Sect. 5) and found no evidence of negative recall bias. In addition, analyses were exclusively carried out on individuals with available data on childhood maltreatment. This may have introduced some bias as maltreatment non-response might be associated with the disease outcomes [8]. Moreover, the current study’s assessment of maltreatment was limited to experiences of abuse because neglect was assessed so differently across cohorts that we could not harmonize. Yet, childhood neglect is an important early-life stressor potentially affecting depression and cardiometabolic outcomes in adulthood and should be investigated in future studies. Furthermore, although the current study focusses on the comorbidity of depression with cardiovascular disease and diabetes, other comorbid psychiatric and somatic diseases may also be activated by early-life stress pathways and warrant further investigation. Another limitation concerns the fact that the current study did not test the role of maltreatment timing. Although a recent meta-analysis shows no evidence of consistent sensitive periods of childhood maltreatment linked to various health outcomes [69], future studies with detailed timing information are needed to determine with more certainty whether timing of childhood maltreatment exposure matters for (comorbid) depression and cardiometabolic disease. Finally, a last limitation concerns the unknown timeline of events. Although depression and cardiometabolic disease likely have their onset after—and we believe are caused by mechanisms stemming from—childhood maltreatment, the current study only articulates associations and does not inform about causality.
Implications
The current findings have clinical implications. First, our results raise awareness on the association between early-life stress and distal psychiatric and somatic health. Second, this study may be a first step in the process of preserving the health of individuals with a history of childhood maltreatment. If future evidence supports that childhood maltreatment triggers a cascade of mechanisms leading to (comorbid) depression and cardiometabolic disease, early intervention could prevent the dysregulation of biological stress systems and preserve the health of individuals with a history of childhood maltreatment. For instance, standard psychotherapy has been shown to effectively reduce depression severity in individuals with a history of childhood maltreatment [70]. One could therefore consider providing trauma-focused psychotherapy to help victims of maltreatment process the stress evoked by the trauma, or pharmacotherapy aiming to regulate biological stress systems, subsequently promoting somatic and mental health. In addition to individual interventions, societal action is an opportunity to prevent these comorbid diseases. Recent influential work emphasizes that promoting fair distribution of income, protecting work conditions, fostering gender equity, decreasing discrimination, and improving social cohesion/support have a great potential to prevent early-life stress, and in turn (comorbid) depression and cardiometabolic disease [71, 72].
Conclusions
In sum, adults with a history of childhood maltreatment are more likely to suffer from depression and cardiometabolic disease than those without a history of childhood maltreatment. Notably, childhood maltreatment is more strongly associated with the comorbidity of the two diseases than with each disease alone suggesting shared mechanisms. Since childhood maltreatment appears to be a relevant indicator linking poor mental and somatic adult health, the findings emphasize the need for the fields of pediatrics, psychiatry, cardiology, and endocrinology to collaborate in efforts to improve health outcomes.
Availability of data and materials
The code used to carry out statistical analyses and extract aggregate data from each of the included cohort studies, as well as to synthesize the aggregate data are available on the GitHub repository of EarlyCause. It can be accessed from the EarlyCause portal (portal.earlycause.eu/tools) or directly from the GitHub repository (github.com/camillesouama/earlycause-tools/tree/main/Amsterdam%20UMC/Meta-analysis%20on%20childhood%20maltreatment%20and%20(comorbid)%20depression%20and%20cardiometabolic%20disease). Individual participant data from the included cohorts are available from management teams of ALSPAC, GenR, HELIUS, MACS, NESDA, NESDO, NEMESIS, SHIP, and UKBB but restrictions apply to the availability of these data, which were used under license for the current study and are not publicly available. For ALSPAC specifically, please note that the study website contains details of all the data that is available through a fully searchable data dictionary and variable search tool: http://www.bristol.ac.uk/alspac/researchers/our-data/. Derived aggregate data are available from the authors upon reasonable request and with permission of the management teams of ALSPAC, GenR, HELIUS, MACS, NESDA, NESDO, NEMESIS, SHIP, and UKBB. Individual participant data from MIDUS is publicly available (28).
Abbreviations
- ALSPAC:
-
Avon Longitudinal Study of Parents And Children
- ATC:
-
Anatomical therapeutic chemical
- Card.:
-
Cardiometabolic disease
- CC:
-
Case–control
- CI:
-
Confidence interval
- CM:
-
Childhood maltreatment
- Dep.:
-
Depression
- GenR:
-
Generation R
- HELIUS:
-
Healthy Life In Urban Setting
- HPA:
-
Hypothalamic–pituitary–adrenal
- IPD:
-
Individual participant data
- ISCED:
-
International Standard Classification of Education
- k:
-
Number of studies
- MACS:
-
Marburg-Münster Affective Disorders Cohort Study
- MIDUS:
-
Midlife in the United States
- N:
-
Sample size
- NEMESIS:
-
Netherlands Mental Health Survey and Incidence Study
- NESDA:
-
Netherlands Study of Depression and Anxiety
- NESDO:
-
Netherlands Study of Depression in Older Persons
- OR:
-
Odds ratio
- PB:
-
Population-based
- PRISMA-IPD:
-
Preferred reporting items for a systematic review and meta-analysis of individual participant data
- ref:
-
Reference category
- SHIP:
-
Study of Health in Pomerania
- UK:
-
United Kingdom
- UKBB:
-
UK Biobank
- USA:
-
United States of America
References
Danese A, Moffitt TE, Harrington HL, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse childhood experiences and adult risk factors for age-related disease: Depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135–43.
Li M, D’Arcy C, Meng X. Maltreatment in childhood substantially increases the risk of adult depression and anxiety in prospective cohort studies: Systematic review, meta-analysis, and proportional attributable fractions. Psychol Med. 2016;46:717–30.
Nelson J, Klumparendt A, Doebler P, Ehring T. Childhood maltreatment and characteristics of adult depression: Meta-analysis. Br J Psychiatry. 2017;210:96–104.
Warrier V, Kwong ASF, Luo M, Dalvie S, Croft J, Sallis HM, et al. Gene–environment correlations and causal effects of childhood maltreatment on physical and mental health: a genetically informed approach. Lancet Psychiatry. 2021;8:373–86.
Humphreys KL, LeMoult J, Wear JG, Piersiak HA, Lee A, Gotlib IH. Child maltreatment and depression: a meta-analysis of studies using the Childhood Trauma Questionnaire. Child Abus Negl. 2020;102:104361.
Gallo EAG, Munhoz TN, Loret de Mola C, Murray J. Gender differences in the effects of childhood maltreatment on adult depression and anxiety: a systematic review and meta-analysis. Child Abus Negl. 2018;79:107–14.
Chandan JS, Okoth K, Gokhale KM, Bandyopadhyay S, Taylor J, Nirantharakumar K. Increased cardiometabolic and mortality risk following childhood maltreatment in the United Kingdom. J Am Heart Assoc. 2020;9:e015855.
Bakema MJ, van Zuiden M, Collard D, Zantvoord JB, de Rooij SR, Elsenburg LK, et al. Associations between child maltreatment, autonomic regulation, and adverse cardiovascular outcome in an urban population: the HELIUS study. Front Psychiatry. 2020;11:1–11.
Soares ALG, Hammerton G, Howe LD, Rich-Edwards J, Halligan S, Fraser A. Sex differences in the association between childhood maltreatment and cardiovascular disease in the UK Biobank. Heart. 2020;106:1310–6.
Gold SM, Köhler-Forsberg O, Moss-Morris R, Mehnert A, Miranda JJ, Bullinger M, Steptoe A, Whooley MA, Otte C. Comorbid depression in medical diseases. Nat Rev Dis Prim. 2020;6:69.
Penninx BWJH, Milaneschi Y, Lamers F, Vogelzangs N. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 2013;11:1–14.
Feng L, Li L, Liu W, Yang J, Wang Q, Shi L, Luo M. Prevalence of depression in myocardial infarction; A PRISMA-compliant meta-analysis. Medicine. 2019;98:e14596.
Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–78.
Rugulies R. Depression as a predictor for coronary heart disease: a review and meta-analysis. Am J Prev Med. 2002;23:51–61.
Penninx BWJH. Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev. 2017;74:277–86.
de Geus EJC. Mendelian randomization supports a causal effect of depression on cardiovascular disease as the main source of their comorbidity. J Am Heart Assoc. 2021;10:1–4.
Sowder KL, Knight LA, Fishalow J. Trauma exposure and health: a review of outcomes and pathways. J Aggress Maltreatment Trauma. 2018;27:1041–59.
Nemeroff CB, Goldschmidt-Clermont PJ. Heartache and heartbreak-the link between depression and cardiovascular disease. Nat Rev Cardiol. 2012;9:526–39.
Mariani N, Borsini A, Cecil CAM, Felix JF, Sebert S, Cattaneo A, et al. Identifying causative mechanisms linking early-life stress to psycho-cardio-metabolic multi-morbidity: The EarlyCause project. PLoS ONE. 2021;16:1–18.
Kircher T, Wöhr M, Nenadic I, Schwarting R, Schratt G, Alferink J, et al. Neurobiology of the major psychoses: a translational perspective on brain structure and function—the FOR2107 consortium. Eur Arch Psychiatry Clin Neurosci. 2019;269:949–62.
Penninx B, Beekma A, Smit J, Frans Z, Nolen W, Spinhoven P, et al. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. Int J Methods Psychiatr Res. 2008;17:121–40.
Comijs HC, Van Marwijk HW, Van Der Mast RC, Naarding P, Oude Voshaar RC, Beekman ATF, Boshuisen M, Dekker J, Kok R, De Waal MWM, Penninx BWJH, Stek ML, Smit JH. The Netherlands study of depression in older persons (NESDO); a prospective cohort study. BMC Res Notes. 2011;4.
Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort profile: The ’Children of the 90s’-the index offspring of the avon longitudinal study of parents and children. Int J Epidemiol. 2013;42:111–27.
Golding G, Pembrey P, Jones J. ALSPAC - The Avon Longitudinal Study of Parents and Children I. Study methodology Paediatr Perinat Epidemiol. 2001;15:74–87.
Fraser A, Macdonald-wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, Henderson J, Macleod J, Molloy L, Ness A, Ring S, Nelson SM, Lawlor DA. Cohort profile: The avon longitudinal study of parents and children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42:97–110.
Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH, van IJzendoorn MH, de Jongste JC, Klaver CCW, van der Lugt A, Mackenbach JP, Moll HA, Peeters RP, Raat H, Rings EHHM, Rivadeneira F, van der Schroeff MP, Steegers EAP, Tiemeier H, Uitterlinden AG, Verhulst FC, Wolvius E, Felix JF, Jaddoe VWV. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31:1243–64.
Snijder MB, Galenkamp H, Prins M, Derks EM, Peters RJG, Zwinderman AH, et al. Cohort profile: The Healthy Life in an Urban Setting (HELIUS) study in Amsterdam, the Netherlands. BMJ Open. 2017;7:1–11.
Brim OG, Baltes PB, Bumpass LL, Cleary PD, Featherman DL, Hazzard WR, et al. Midlife in the United States (MIDUS 1), 1995-1996. 2020; Available from: https://doi.org/10.3886/ICPSR02760.v19
Bijl RV, Van Zessen G, Ravelli A, De Rijk C, Langendoen Y. The Netherlands Mental Health Survey and Incidence Study (NEMESIS): objectives and design. Soc Psychiatry Psychiatr Epidemiol. 1998;33:581–6.
De Graaf R, Ten Have M, Van Dorsselaer S. The Netherlands Mental Health Survey and Incidence Study-2 (NEMESIS-2): design and methods. Int J Methods Psychiatr Res. 2010;19:125–41.
Völzke H, Schössow J, Schmidt CO, Jürgens C, Richter A, Werner A, Werner N, Radke D, Teumer A, Ittermann T, Schauer B, Henck V, Friedrich N, Hannemann A, Winter T, Nauck M, Dörr M, Bahls M, Felix SB, Stubbe B, Ewert R, Frost F, Lerch MM, Grabe HJ, Bülow R, Otto M, Hosten N, Rathmann W, Schminke U, Großjohann R, Tost F, Homuth G, Völker U, Weiss S, Holtfreter S, Bröker BM, Zimmermann K, Kaderali L, Winnefeld M, Kristof B, Berger K, Samietz S, Schwahn C, Holtfreter B, Biffar R, Kindler S, Wittfeld K, Hoffmann W, Kocher T. Cohort Profile Update: The Study of Health in Pomerania (SHIP). Int J Epidemiol. 2022;51:e372–83.
Allen N, Sudlow C, Downey P, Peakman T, Danesh J, Elliott P, et al. UK Biobank: current status and what it means for epidemiology. Heal Policy Technol. 2012;1:123–6.
Davis KAS, Coleman JRI, Adams M, Allen N, Breen G, Cullen B, et al. Mental health in UK Biobank – development, implementation and results from an online questionnaire completed by 157 366 participants: a reanalysis. BJPsych Open. 2020;6:1–8.
Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred reporting items for a systematic review and meta-analysis of individual participant data: The PRISMA-IPD statement. JAMA - J Am Med Assoc. 2015;313(16):1657–65.
Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abus Negl. 2003;27:169–90.
De Graaf R, Bijl RV, Smit F, Vollebergh WAM, Spijker J. Risk factors for 12-month comorbidity of mood, anxiety, and substance use disorders: findings from the Netherlands Mental Health Survey and Incidence Study. Am J Psychiatry. 2002;159:620–9.
Straus MA. Measuring intrafamily conflict and violence: The Conflict Tactics (CT) Scales. J Marriage Fam. 1979;41:75–88.
Straus MA, Hamby SL, Boney-McCoy SUE, Sugarman DB. The revised conflict tactics scales (CTS2) development and preliminary psychometric data. J Fam Issues. 1996;17:283–316.
Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression scale. Br J Psychiatry. 1987;150:782–6.
Murray L, Carothers A. The validation of the Edinburgh Post-natal Depression Scale on a Community Sample. Br J Psychiatry. 1990;157:288–90.
Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605.
Manea L, Gilbody S, McMillan D. A diagnostic meta-analysis of the Patient Health Questionnaire-9 (PHQ-9) algorithm scoring method as a screen for depression. Gen Hosp Psychiatry. 2015;37:67–75.
Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13.
Wittchen HU, Zaudig M, Fydrich T. SKID. Strukturiertes Klinisches Interview für DSM-IV. Achse I und II. Handanweisung. Göttingen: Hogrefe; 1997.
Kessler RC, Andrews G, Mroczek D, Ustun B, Wittchen HU. The World Health Organization composite international diagnostic interview short-form (CIDI-SF). Int J Methods Psychiatr Res. 1998;7:171–85.
Kessler RC, Üstün TB. The world mental health (WMH) survey initiative version of the world health organization (WHO) composite international diagnostic interview (CIDI). Int J Methods Psychiatr Res. 2004;13:93–121.
Wittchen HU, Lachner G, Wunderlich U, Pfister H. Test-retest reliability of the computerized DSM-IV version of the Munich-Composite International Diagnostic Interview (M-CIDI). Soc Psychiatry Psychiatr Epidemiol. 1998;33:568–78.
Coleman JRI, Davis K. Code for use in R statistics with UK Biobank Mental Health Questionnaire data. Mendeley Data. 2019;v3.
Berge LI, Riise T. Comorbidity between type 2 diabetes and depression in the adult population: directions of the association and its possible pathophysiological mechanisms. Int J Endocrinol. 2015;164760.
World Health Organization. Cardiovascular diseases (CVDs). 2021; Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
World Health Organization. Diabetes. 2022; Available from: www.who.int/news-room/fact-sheets/detail/diabetes
United Nations Educational Scientific and Cultural Organization. The International Standard Classification of Education (ISCED 2011). Montreal, Quebec: UNESCO Institute for Statistics; 2012.
Stevens M. De Nederlandse norm gezond bewegen: Verslag van een expertmeeting. Trendrapport bewegen en gezondheid 1998/1999. Lelystad: Koninklijke Vermande; 1999.
Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:521–5.
Viechtbauer W. Conducting meta-analyses in R with the metafor Package. J Stat Softw. 2010;36:1–48.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60.
Jakubowski KP, Cundiff JM, Matthews KA. Cumulative childhood adversity and adult cardiometabolic disease: a meta-analysis. Heal Psychol. 2018;37:701–15.
Agorastos A, Pervanidou P, Chrousos GP, Kolaitis G. Early life stress and trauma: developmental neuroendocrine aspects of prolonged stress system dysregulation. Hormones. 2018;17:507–20.
Malave L, Van Dijk MT, Anacker C. Early life adversity shapes neural circuit function during sensitive developmental periods. Transl Psychiatry. 2022;12:1–14.
Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71:286–93.
Tran MLM, Kuhlman KR. The impact of childhood maltreatment on biological systems: the role of childhood maltreatment subtypes. Psychoneuroendocrinology. 2020;119:104935.
Kim K, Mennen FE, Trickett PK. Patterns and correlates of co-occurrence among multiple types of child maltreatment. Child Fam Soc Work. 2017;22:492–502.
Scher CD, Forde DR, McQuaid JR, Stein MB. Prevalence and demographic correlates of childhood maltreatment in an adult community sample. Child Abus Negl. 2004;28(2):167–80.
McKay MT, Cannon M, Chambers D, Conroy RM, Coughlan H, Dodd P, et al. Childhood trauma and adult mental disorder: a systematic review and meta-analysis of longitudinal cohort studies. Acta Psychiatr Scand. 2021;143:189–205.
Hemmingsson E, Johansson K, Reynisdottir S. Effects of childhood abuse on adult obesity: a systematic review and meta-analysis. Obes Rev. 2014;15:882–93.
North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–108.
Goltermann J, Meinert S, Hülsmann C, Dohm K, Grotegerd D, Redlich R, Waltemate L, Lemke H, Thiel K, Mehler DMA, Enneking V, Borgers T, Repple J, Gruber M, Winter N, Hahn T, Brosch K, Meller T, Ringwald KG, Schmitt S, Stein F, Pfarr JK, Krug A, Nenadic I, Kircher T, Opel N, Dannlowski1 U. Temporal stability and state-dependence of retrospective self-reports of childhood maltreatment in major depression: a two-year longitudinal analysis of the childhood trauma questionnaire. medRxiv. 2021.
Spinhoven P, Penninx BW, Hickendorff M, van Hemert AM, Bernstein D, Elzinga BM. Childhood Trauma Questionnaire: factor structure, measurement invariance, and validity across emotional disorders. Psychol Assess. 2014;26:717–29.
Schaefer JD, Cheng TW, Dunn EC. Sensitive periods in development and risk for psychiatric disorders and related endpoints: a systematic review of child maltreatment findings. Lancet Psychiatry. 2022;9:978–91.
Childhood Trauma Meta-Analysis Study Group, Kuzminskaite E, Gathier AW, Cuijpers P, Penninx BWJH, Ammerman RT, Brakemeier EL, Bruijniks S, Carletto S, Chakrabarty T, Douglas K, Dunlop BW, Elsaesser M, Euteneuer F, Guhn A, Handley ED, Heinonen E, Huibers MJH, Jobst A, Johnson GR, Klein DN, Kopf-Beck J, Lemmens L, Lu XW, Mohamed S, Nakagawa A, Okada S, Rief W, Tozzi L, Trivedi MH, van Bronswijk S, van Oppen P, Zisook S, Zobel I, Vinkers CH. Treatment efficacy and effectiveness in adults with major depressive disorder and childhood trauma history: a systematic review and meta-analysis. The Lancet Psychiatry. 2022;9:860–73.
Kivimäki M, Bartolomucci A, Kawachi I. The multiple roles of life stress in metabolic disorders. Nat Rev Endocrinol. 2023;19:10–27.
Herrman H, Patel V, Kieling C, Berk M, Buchweitz C, Cuijpers P, et al. Time for united action on depression: a Lancet-World Psychiatric Association Commission. Lancet. 2022;399:957–1022.
Acknowledgements
We are extremely grateful to the EarlyCause consortium for their contribution to the conceptualization of this project. We also thank all the participants, children, parents and families that took part in the studies, general practitioners, hospitals, midwives, pharmacies, management teams, interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, receptionists, nurses, medical specialists, and other staff and stakeholders who have taken part in the studies included in this meta-analysis.
Funding
This study has received funding from the European Union’s Horizon 2020 research and innovation program under Grant Agreement N° 848158 (EarlyCause). In addition, included studies received their own funding. The UK Medical Research Council and Wellcome (Grant ref: 217065/Z/19/Z) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors, and CS will serve as a guarantor for the contents of this paper. The general design of the Generation R Study is made possible by financial support from the Erasmus MC, Erasmus University Rotterdam, the Netherlands Organization for Health Research and Development and the Ministry of Health, Welfare and Sport. This project received funding from the European Union’s Horizon 2020 research and innovation program (733206, LifeCycle; 874739, LongITools; 874583, ATHLETE; 824989, EUCAN-Connect). HELIUS is conducted by the Amsterdam University Medical Centers, location AMC and the Public Health Service of Amsterdam. Both organizations provided core support for HELIUS. In addition, HELIUS is also funded by the Dutch Heart Foundation (2010T084), the Netherlands Organization for Health Research and Development (ZonMw, 200500003), the European Union (FP-7, 278901), and the European Fund for the Integration of non-EU immigrants (EIF, 2013EIF013). For MACS, Tilo Kircher received unrestricted educational grants from Servier, Janssen, Recordati, Aristo, Otsuka, neuraxpharm. MACS was funded by the German Research Foundation (DFG grant FOR2107, KI588/14–1 and KI588/14–2 to Tilo Kircher, Marburg, Germany; DFG grant DA1151/5–1 and DA1151/5–2 to Udo Dannlowski, Münster, Germany). MIDUS has received funding from John D. and Catherine T. MacArthur foundation Research Network on Successful Midlife Development. NEMESIS-1 is conducted by the Netherlands Institute of Mental Health and Addiction (Trimbos Institute) in Utrecht. Financial support has been received from the Netherlands Ministry of Health, Welfare and Sport (VWS), the Medical Sciences Department of the Netherlands Organization for Scientific Research (NWO), and the National Institute for Public Health and Environment (RIVM). NEMESIS-2 is conducted by the Netherlands Institute of Mental Health and Addiction (Trimbos Institute) in Utrecht. Financial support has been received from the Ministry of Health, Welfare and Sport, with supplementary support from the Netherlands Organization for Health Research and Development (ZonMw) and the Genetic Risk and Outcome of Psychosis (GROUP) investigators. The infrastructure of NESA (www.nesda.nl) is funded through the Geestkracht program of the Netherlands Organization for Health Research and Development (Grant No. 10–000-1002) and financial contributions by participating universities and mental health care organizations (VU University Medical Center, GGZ inGeest, Leiden University Medical Center, Leiden University, GGZ Rivierduinen, University Medical Center Groningen, University of Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Dimence, Rob Giel Onderzoekscentrum). The infrastructure for NESDO is funded through the Fonds NutsOhra, Stichting tot Steun VCVGZ, NARSAD The Brain and Behavior Research Fund, and the participating universities and mental health care organizations (VU University Medical Center, Leiden University Medical Center, University Medical Center Groningen, Radboud University Nijmegen Medical Center, and GGZ inGeest, GGZ Nijmegen, GGZ Rivierduinen, Lentis, and Parnassia). The SHIP project is part of the Community Medicine Research Network of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (grants no. 01ZZ9603, 01ZZ0103, and 01ZZ0403), the Ministry of Cultural Affairs, and the Social Ministry of the Federal State of Mecklenburg-West Pomerania. This study was further supported by the German Research Foundation (GR 1912/5–1, GR 1912/13–1 and grant no. 403694598). UKBB was established by the Wellcome Trust medical charity, Medical Research Council, Department of Health, Scottish Government and the Northwest Regional Development Agency. It has received funding from the Welsh Government, British Heart Foundation, Cancer Research UK and Diabetes UK. It is further supported by the National Health Service and core funding continues to be received from the Wellcome Trust, the Medical Research Council, and, more recently, from Cancer Research UK and NIHR.
Author information
Authors and Affiliations
Consortia
Contributions
CS coordinated the project administration, analyzed and interpreted cohort-level and aggregate data, and was a major contributor in writing the original manuscript. BP, YM, and FL substantively participated in the supervision and conceptualization and methodology of the project as well as extensively revised the manuscript for critical review. CC, EW, JF, and KL participated in the conceptualization of the research project and monitored progress. SD, LG, FS, and TW substantially contributed to the data analysis of cohorts’ datasets. KL contributed to the acquisition of the financial support for the project leading to this publication. MH and KB contributed to the data curation of cohorts’ datasets. CC, EW, JF, KL, SD, LG, FS, TW, CV, UD, HG, RG, VJ, AL, BR, HV, HG, and TL contributed to the critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Cohort | Considerations |
|---|---|
ALSPAC, mothers | Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees. Informed consent for the use of data collected via questionnaires and clinics was obtained from participants following the recommendations of the ALSPAC Ethics and Law Committee at the time. |
ALSPAC, partners | |
GenR, mothers | The Generation R Study has been approved by the Medical Ethical Committee of the Erasmus University Medical Center in Rotterdam, the Netherlands. Participants provided written informed consent. |
HELIUS | The HELIUS study has been approved by the Institutional Review Board of the AMC at the University of Amsterdam. All participants gave written informed consent prior study participation. |
MACS | All procedures were approved by the local Ethics Committees of Marburg (AZ:07/14) and Münster (AZ:2014-422-b-S), Germany according to the Declaration of Helsinki. Participants gave written informed consent prior study participation and received financial compensation. |
MIDUS | The study was approved by the Harvard ethics committee. Participants provided verbal informed consent. |
NESDA | The protocol of the Netherlands Study of Depression and Anxiety was approved centrally by the Ethical Review Board of the VU University Medical Centre and subsequently by local review boards of each participating center. All participants provided written informed consent. |
NESDO | The study protocol of NESDO has been approved centrally by the Ethical Review Board of the VU University Medical Center and subsequently by the local ethical review boards of the Leiden University Medical Center, University Medical Center Groningen, and the Radboud University Medical Center in Nijmegen. All participants provided written informed consent. |
NEMESIS-1 | The study was approved by the internal review board of the Trimbos institute, Utrecht. Respondents provided verbal informed consent according to the prevailing Dutch law of 1996. |
NEMESIS-2 | The study was approved by the Medical Ethics Review Committee for Institutions on Mental Health Care. After having been informed about the study aims, respondents provided written informed consent at each wave. |
SHIP-Legend SHIP-Trend | Written informed consent was obtained from all participants according to the principles of the Declaration of Helsinki. SHIP-Legend and SHIP-Trend were approved by the Ethics Committee at the University Medicine Greifswald, Germany. |
UKBB | This research was conducted using the UK Biobank resource, application number 65769. The UK Biobank study was conducted under generic approval from the National Health Service, National Research Ethics Service (approval letter dated 17 June 2011, Ref 11/NW/0382). Participants gave full informed written consent. |
Consent for publication
Not applicable.
Competing interests
HG has received travel grants and speakers’ honoraria from Fresenius Medical Care, Neuraxpharm, Servier, and Janssen Cilag as well as research funding from Fresenius Medical Care. The other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Section 1. ALSPAC participants. Section 2. GenR additional information. Section 3. R-script of analyses. Section 4. Imputation of lifestyle variables. Section 5. Associations with current vs. lifetime depression diagnoses. TableS1. Childhood maltreatment assessment overview. Table S2. Depressionassessment overview. Table S3. Definition of cardiovascular disease. Table S4. Alcohol consumption and physical activity assessment overview. Table S5. Pooled associations of childhood maltreatment with comorbidity status after adjusting for lifestyle factors (model 4), according to three different imputation strategies. Table S6. Overview of cohorts included in each meta-analyzed model. Table S7. Number of cases, weights and odds ratios of thecohorts in meta-analyzed model 3. Table S8. Results of meta-analyzed model 3 per subgroup of studies based on depression assessment type.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Souama, C., Lamers, F., Milaneschi, Y. et al. Depression, cardiometabolic disease, and their co-occurrence after childhood maltreatment: an individual participant data meta-analysis including over 200,000 participants. BMC Med 21, 93 (2023). https://doi.org/10.1186/s12916-023-02769-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-023-02769-y