Abstract
Background
Metabolic-associated fatty liver disease (MAFLD) encompasses diverse disease groups with potentially heterogeneous clinical outcomes. We investigated the risk of all-cause and disease-specific mortality in MAFLD subgroups.
Methods
Using the Korean National Health Insurance Service database, participants were divided into four subgroups: no MAFLD, MAFLD-diabetes, MAFLD-overweight/obese, and MAFLD-lean. Hazard ratios (HRs) and 95% confidence interval (CI) values for all-cause and disease-specific mortality according to MAFLD subgroups were analyzed using Cox proportional hazards models.
Results
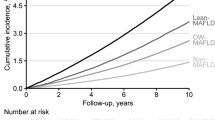
Among 9,935,314 participants, those with MAFLD-diabetes showed the highest risk of all-cause and disease-specific mortality. The HRs (95% CI) for all-cause mortality were 1.61 (1.59–1.63), 1.36 (1.34–1.38), and 1.19 (1.18–1.20) in the MAFLD-diabetes, MAFLD-lean, and MAFLD-overweight/obese groups, respectively. The magnitude of cardiovascular disease and cancer-related risk showed the same pattern. The risk of liver-related mortality in the MAFLD-lean group (HR: 2.84, 95% CI: 2.72–2.97) was comparable with that in the MAFLD-diabetes group (HR: 2.85, 95% CI: 2.75–2.95). When stratified by body mass index, liver-related mortality was the highest in MAFLD-lean individuals in the underweight group (HR, 5.03, 95% CI: 4.23–5.97).
Conclusions
The MAFLD-lean and MAFLD-diabetes groups had a higher risk of all-cause and disease-specific mortality than did the MAFLD-overweight/obese group. Classifying MAFLD subgroups based on metabolic phenotypes might help risk stratification of patients with MAFLD.
Similar content being viewed by others
Background
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a new conceptual definition of fatty liver disease that is based on metabolic abnormalities (regardless of the etiology of chronic liver disease) that emphasizes the role of metabolic dysfunction in the clinical outcomes of patients with hepatic steatosis [1, 2]. MAFLD is defined according to the presence of hepatic steatosis along with one or more of the following criteria: (i) overweight or obesity, (ii) diabetes mellitus (DM), or (iii) ≥ 2 metabolic abnormalities [3]. Recent studies have investigated the predictive role of MAFLD in cardiovascular disease (CVD) [4, 5] and all-cause mortality [6,7,8].
However, MAFLD includes a heterogeneous group of patients, and the following subgroups should be recognized: lean and non-lean patients, alcoholic and nonalcoholic patients, and patients with isolated hepatic steatosis (vs. those with underlying liver disease) [9]. A previous study reported that MAFLDs are heterogeneous in terms of mortality outcomes and cardiovascular risk and that these outcomes vary according to the accompanying metabolic dysfunctions [10]. A previous study demonstrated that MAFLD better identifies cardiovascular risk than nonalcoholic fatty liver disease (NAFLD), which was attributed to the inclusion criteria of metabolic dysfunctions [11]. Moreover, a statistically significant difference in the degree of liver fibrosis has been reported among MAFLD subgroups, suggesting differential risks of advanced liver disease across MAFLD subgroups [12].
Since overweight/obesity is typically defined according to body mass index (BMI), it does not always entail metabolic abnormalities [13]. As MAFLD is a spectrum encompassing diverse disease groups defined according to metabolic abnormalities and overweight/obesity, there may be heterogeneity in clinical outcomes according to disease subcategorizations. Therefore, we aimed to investigate the differential risk of all-cause and disease-specific mortality according to MAFLD subgroups divided by metabolic risk factors and overweight/obesity status within a nationally representative Korean study population.
Methods
Data source
The present study obtained data from the Korean National Health Insurance System (NHIS). This is a national insurer managed by the Korean government, and approximately 97% of the Korean population subscribe to it [14]. The NHIS database contains health records, including sociodemographic data (age, sex, income level), anthropometric measurements, laboratory tests (e.g., lipid profiles, blood glucose levels), lifestyle behaviors (smoking, alcohol consumption, regular exercise), medical diagnoses (based on the 10th revision of the International Classification of Diseases [ICD-10]), and treatment data [15]. This database has been widely used for conducting previous epidemiologic studies [16, 17].
Study sample
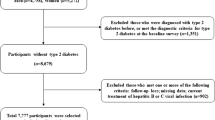
This investigation included 10,585,844 adults aged ≥ 20 who underwent health screening examinations between January 1, 2009, and December 31, 2009. We ascertained outcome events after a lag of 1 year; those with outcome events within 1 year were excluded (n = 28,478). After excluding participants with incomplete information (n = 622,052), 9,935,314 subjects were analyzed.
The study protocol was approved by the Institutional Review Board of Soongsil University (SSU-202007-HR-236–01). This work conformed to the ethical guidelines delineated within the Declaration of Helsinki and its later amendments. The requirement for patient informed consent was waived as this was a retrospective study conducted exclusively using de-identified secondary data.
Measurement of hepatic steatosis
Although ultrasonography is a first-line screening technique used in clinical practice [18], it is not included in the NHIS mass screening program. Therefore, the fatty liver index (FLI), as a surrogate marker of hepatic steatosis, was used to assess hepatic steatosis. The calculation of FLI was based on the following equation, using BMI, waist circumference (WC), triglyceride, and gamma-glutamyl transferase (GGT) data [19]. The lower cut-off of FLI ≥ 30 was used in this study [4].
Definitions of MAFLD and subgroups
MAFLD was defined as the presence of metabolic risk factors in hepatic steatosis, not excluding other concomitant liver diseases and significant alcohol consumption, based on diagnostic criteria proposed in an international expert consensus statement in 2020 [3]. MAFLD was diagnosed as the presence of hepatic steatosis and one or more of the following criteria: (i) overweight or obesity (BMI ≥ 23 kg/m2), (ii) DM, and (iii) ≥ 2 metabolic abnormalities. The cut-off value of BMI for overweight and obesity was determined based on the criteria for the Asia–Pacific region. Metabolic abnormalities were as follows: (i) WC of ≥ 90 cm for men and ≥ 80 cm for women, (ii) blood pressure ≥ 130/85 mmHg or specific drug treatment for elevated blood pressure, (iii) fasting plasma triglycerides ≥ 150 mg/dL or specific drug treatment for elevated triglycerides, (iv) plasma high-density lipoprotein-cholesterol < 40 mg/dL for men and < 50 mg/dL for women or specific drug treatment for high cholesterol, and (v) fasting glucose ≥ 100 mg/dL. As the homeostasis model assessment of insulin resistance scores and high-sensitivity C-reactive protein levels were not available through the NHIS screening program, these criteria were not implemented in this study.
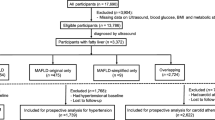
In the present study, participants identified as affected by MAFLD were classified into three groups, as described previously [12, 20]. First, we determined the diabetic MAFLD group based on the presence of DM regardless of BMI; subsequently, in subjects without DM, we classified MAFLD subgroups according to BMI: (i) overweight/obese (BMI ≥ 23 kg/m2) or (ii) lean or normal weight (BMI < 23 kg/m2). Finally, the study population was divided into four subgroups: no MAFLD, MAFLD-diabetes, MAFLD-overweight/obese, and MAFLD-lean (Fig. 1).
Study outcomes
The primary outcomes evaluated in this study were all-cause and disease-specific mortality. Information on mortality and cause of death was available for all subjects in the cohort; cause-of-death variables were classified according to the Korean Standard Classification of Diseases and Causes of Death, based on the ICD-10 (with data provided by the Korean National Statistical Office) [21].
Based on the ICD-10 diagnostic codes, the cause of death was categorized into CVD mortality (I00-I99), cancer mortality other than hepatocellular carcinoma (C00-C97, except for C22), and liver-associated mortality (K70-76, C22) [22]. The study population was followed from baseline to the date of death or until December 31, 2019, allowing for a minimum of 12 months of follow-up for each individual.
Covariates
As described previously [23], standardized self-reported questionnaires were used to collect data during enrollment. Briefly, smoking status (non-smokers, ex-smokers, current smokers), alcohol consumption (none, mild, heavy [≥ 30 g for males and ≥ 20 g for females per day]), and regular exercise were evaluated using a self-administered questionnaire. When participants exercised at a high intensity at least three times per week or at a moderate intensity at least five times per week, they were defined as undertaking regular physical exercise. Household income was dichotomized at the lowest 20%. Comorbidities were defined using ICD-10 diagnosis codes, prescription information in the year prior to the health screening, and health screening results. Criteria for hypertension were I10–13 or I15 claim codes in addition to ≥ 1 prescription for an antihypertensive agent or a systolic/diastolic blood pressure reading of ≥ 140/90 mmHg. Criteria for diabetes were E11–14 claim codes in addition to ≥ 1 prescription for an antidiabetic medication per year or a fasting glucose level of ≥ 126 mg/dL. The criteria for dyslipidemia were the E78 claim code and ≥ 1 prescription for a lipid-lowering agent or a total cholesterol level of ≥ 240 mg/dL. The Charlson Comorbidity Index (CCI) [24] was determined from claims data during the period spanning 2 years prior to baseline. K74 and B15–B19 claim codes were the criteria used to determine liver cirrhosis and viral hepatitis.
Height, weight, and WC were measured and evaluated by healthcare professionals during nationally mandated health screenings. BMI was calculated as the participant’s weight in kilograms divided by their height in meters squared. Blood specimens were obtained from each participant after an overnight fast. The estimated glomerular filtration rate (eGFR) was calculated from serum creatinine using an equation determined by the Modification of Diet in Renal Disease Study [25].
Statistical analyses
Data are presented as means ± standard deviations for normally distributed continuous variables and as proportions for categorical variables (unless otherwise indicated). Comparisons of baseline characteristics were conducted using independent t-tests and analysis of variance for continuous variables and chi-square tests for categorical variables.
All-cause and disease-specific mortality rates were calculated by dividing the number of incident cases by the total follow-up period and were presented as rates per 1000 person-years. Hazard ratios (HRs) and 95% confidence interval (CI) values for all-cause and disease-specific mortality according to MAFLD subgroups were analyzed using Cox proportional hazards models. We selected potential prognostic factors a priori based on clinical relevance and a comprehensive literature review; potential prognostic factors included age, sex, BMI, income, smoking, alcohol consumption, regular exercise, and CCI scores. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Two-sided P-values of < 0.05 were considered the threshold for statistical significance.
Results
Baseline characteristics
This analysis included 9,935,314 participants (median age [interquartile range] 46 [36–57] years; mean age, 47.2 years; 56.0% males). The prevalence of MAFLD was 35.8%. The prevalence of each MAFLD subtype was 5.5% (MAFLD-diabetes), 28.4% (MAFLD-overweight/obese), and 1.9% (MAFLD-lean). The baseline characteristics for the study population are shown in Table 1. Compared with the no MAFLD group, people with MAFLD were more likely to be male and current smokers and had higher alcohol consumption (P < 0.001 for all). Additionally, subjects in the MAFLD-diabetes group and the MAFLD-lean group were more likely to have hypertension and dyslipidemia than those in the no MAFLD group (P < 0.001). Most anthropometric and laboratory variables (including systolic/diastolic blood pressure, fasting glucose, total cholesterol, triglycerides, and eGFR) were less metabolically favorable in the MAFLD-diabetes and MAFLD-lean groups than in the no MAFLD group (all P < 0.001).
All-cause and disease-specific mortality by MAFLD subgroup
A total of 417,593 of the 9,935,314 subjects (4.2%) died during the median follow-up of 9.3 (interquartile range: 9.1, 9.6) years. Table 2 summarizes the observed associations between MAFLD subgroups and all-cause or cause-specific mortality. In the age- and sex-adjusted model, individuals in the MAFLD-diabetes group had the highest all-cause mortality among the four evaluated groups (HR: 1.43, 95% CI: 1.42–1.45). In the multivariable model adjusting for age, sex, BMI, income, smoking, alcohol consumption, exercise, and CCI score, individuals with MAFLD-diabetes had the highest increased risk of all-cause mortality (HR: 1.61, 95% CI: 1.59–1.63) followed by the MAFLD-lean and MAFLD-overweight/obese groups (HR: 1.36, 95% CI: 1.34–1.38 and HR: 1.19, 95% CI: 1.18–1.20, respectively, Table 2). When further adjusting for serum glucose, cholesterol, alanine aminotransferase, eGFR, liver cirrhosis, and hepatitis, these associations persisted. (Additional file 1: Table S1).
Overall, cancer and CVD represented the two most common causes of mortality in the current study; there were a total of 152,212, 80,262, and 31,290 cancer-associated, CVD-associated, and liver disease-associated deaths, respectively. In the multivariate model, individuals in the MAFLD-diabetes group had the highest CVD-specific mortality (HR 1.61, 95% CI: 1.58–1.65). Individuals in the MAFLD-lean and MAFLD-overweight/obese groups also had higher CVD-specific mortality than those without MAFLD (HR 1.45, 95% CI: 1.40–1.51 and HR 1.22, 95% CI: 1.19–1.25, respectively). Cancer-specific mortality increased significantly in all MAFLD subgroups compared to those without MAFLD. Individuals in the MAFLD-diabetes group showed the highest HR (1.31, 95% CI: 1.29–1.33), followed by the MAFLD-lean and MAFLD-overweight/obese groups (HR 1.27, 95% CI: 1.23–1.30 and HR 1.11, 95% CI: 1.09–1.13, respectively). Individuals in the MAFLD-diabetes group had a 2.85-fold higher liver disease-related mortality (HR: 2.85, 95% CI: 2.75–2.95), with similar risk for those in the MAFLD-lean group (HR: 2.84, 95% CI: 2.72–2.97), followed by those in the MAFLD-overweight/obese group (HR 1.76, 95% CI: 1.70–1.82), compared to those without MAFLD.
Because FLI has different cut-offs for men and women and bears a large gray zone of undetermined presence/absence of hepatic steatosis, we conducted analysis using two different sex-specific cut-offs [26, 27]. Similar findings were observed in the increase of HR according to the MAFLD subtypes (Additional file 1: Table S2).
To minimize the effect of age difference among the MAFLD subtypes, we performed sensitivity analysis according to subjects aged 60 years or older, and the results were similar to those of the entire population (Additional file 1: Table S3 and Table S4).
Stratified analyses
We performed secondary analyses stratified by BMI. The increased risk for all-cause, cardiovascular, cancer, and liver-related mortality in the MAFLD-diabetes group was most prominent in individuals with underweight (P for interaction < 0.05). Liver-related mortality was the highest in MAFLD-lean individuals in the underweight group (HR, 5.03, 95% CI: 4.23–5.97, Table 3).
Discussion
In this large nationwide, population-based cohort study, we calculated all-cause and disease-specific mortality in MAFLD subgroups using a database of health insurance claims in Korea. All-cause and disease-specific mortality were highest in the MAFLD-diabetes group, followed by the MAFLD-lean and MAFLD-overweight/obese groups, suggesting differential prognoses according to the MAFLD subtype. Interestingly, the liver-related mortality in the MAFLD-lean group was comparable to that in the MAFLD-diabetes group, emphasizing the poor liver-related prognosis in the MAFLD-lean group. The classification of MAFLD subtypes based on the metabolic phenotypes used in this study might help risk stratification of MAFLD patients.
Among the MAFLD subtypes, MAFLD-diabetes showed the highest increased risk for all-cause and disease-specific mortality. Diabetes is associated with a thrombotic and inflammatory condition and a higher incidence of atherosclerosis and carcinogenesis due to sustained hyperglycemia [28, 29]. As a consequence, diabetes has been recognized as a risk factor for all-cause and cause-specific mortality [30,31,32]. The results of this study showing that MAFLD-diabetes can be a strong predictor of all-cause and disease-specific mortality are in line with previous literature.
In addition to diabetes, MAFLD subcategorizations according to overweight/obesity may help elucidate the differential mortality risk among MAFLD subtypes. However, since the proportion of lean MAFLD is relatively low in real clinical practice and it is difficult to collect enough samples, the clinical and prognostic features of lean MAFLD have not been defined well in prior work. In the current study, although the prevalence of MAFLD-lean was 1.9% of the total population, the characteristics of the nationwide population study design enabled reliable analysis by securing a significant sample size.
In this study, individuals in the MAFLD-lean group were at a higher risk of all-cause and disease-specific mortality than those in the MAFLD-overweight/obese group. Similar to our results, several studies have reported that the cumulative CVD incidence rate was higher in lean MAFLD than in overweight/obese MAFLD [4, 19], and the risk of all-cause mortality was higher in those with lean MAFLD without diabetes (HR: 2.34) than in overweight/obese MAFLD subjects without diabetes (HR: 1.23) [10]. These findings are in line with poor clinical outcomes previously documented in patients with lean NAFLD [33,34,35]. A recent study reported that NAFLD subjects with normal BMI had a higher risk of all-cause mortality than those with obese NAFLD and that the major causes of death in this subgroup were cancer and CVD [36]. Similarly, people with lean NAFLD show a comparable risk of advanced liver disease, metabolic comorbidities, CVD, and liver-associated mortality to those with non-lean NAFLD without weight gain during follow-up and independent of genotype [37].
As lean NAFLD represents a unique subset related to the interaction of genetics, underlying medical conditions, and environmental exposures [38], the mechanism-related poor prognosis in lean MAFLD is not completely elucidated. Although lean and obese NAFLD share common altered metabolic and cardiovascular profiles, NAFLD patients having normal body weight show excess abdominal adipose tissue and metabolic abnormalities [39]. Recent studies reported that the progression of lean NAFLD is affected by multiple epigenetic mechanisms such as modification of cytosine and histones and changes in nucleosome localization [40], and circulating histones may discriminate the severity of steatosis in lean MAFLD patients [41]. In addition, a previous study reported that lean MAFLD subjects had lower fatty tissue index, lean tissue index, and total body water than did non-lean MAFLD subjects, which means that lean MAFLD subjects had less adiposity and less muscle mass compared to obese MAFLD subjects [42]. These results suggest that sarcopenia, which is the loss of muscle mass and function with aging, is a possible underlying reason for poor prognosis in people with lean MAFLD.
Because MAFLD is closely related to metabolic diseases, various pharmacological approaches that have been attempted to manage NAFLD’s metabolic traits, such as anti-obesity, antidiabetic, antioxidants, and cytoprotective agents, may also be effective in MAFLD. Emerging data from various clinical trials with drugs targeting diverse molecular mechanisms show promising results [43], and a recent phase II trial reported that semaglutide might be an attractive potential therapeutic option for NASH [44]. Future potential treatments for NAFLD include agents that act through peroxisomal proliferator-activated receptors, glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter 2 inhibitors, or farnesoid X receptor agonists, and several investigating agents have entered phase III clinical trials [45].
Overweight/obesity increases the risk for CVD, cancer, and liver-associated adverse outcomes. However, subjects in the MAFLD-overweight/obese group showed better prognoses than did those in the MAFLD-lean group in the present study. This phenomenon may be associated with the obesity paradox (i.e., elevated BMI may improve survival in individuals with CVD, primarily those with congestive heart failure [46, 47], cancer [48, 49], and type 2 DM [50]). Potential explanations for the obesity paradox include reverse causation, the inability to distinguish fat tissue from muscle mass using BMI, and even the possibility that adipose tissue may provide some level of protection. Although the evidence for an obesity paradox is mixed for individuals with a BMI between 22 and 24.9 kg/m2 [51, 52], the findings of increased mortality in individuals with lower BMI values are indisputable.
Our study presents the substantial strengths of a large sample size, a population-based design, and the categorization of MAFLD considering diabetes and metabolic risk factors in addition to BMI. This study provides new insights into the understanding of mortality-related outcomes of MAFLD, which can be used for risk stratification strategies in patients with MAFLD. However, we also acknowledge some limitations of the present work. First, although histological or radiological methods are more accurate for detecting hepatic steatosis than FLI, we used FLI as a surrogate marker of fatty liver and, thus, cannot accurately quantify the presence and severity of steatosis [53]. In addition, FLI has different cut-offs for men and women and bears a large gray zone of undetermined presence/absence of hepatic steatosis [26, 27]. However, similar results were observed when we used two different sex-specific cut-off values of FLI. Moreover, the use of FLI is practical for screening the general population in epidemiologic studies [10, 54], and FLI is regarded as an acceptable surrogate for diagnosing hepatic steatosis in MAFLD [55]. Second, our study cannot establish a causal relationship because of its population-based observational design. Third, there might be residual confounding factors such as family history or occupational exposures related to cancer development since the NHIS does not collect these data. Fourth, although most studies used BMI to define lean NAFLD/MAFLD [38], total body fat quantity (assessed based on BMI) does not reflect regional body fat distribution. Because visceral adiposity can be a target for interventions in the treatment of MAFLD, future studies need to consider visceral adiposity. Finally, it is possible that the evaluated conditions were under- or overestimated because the diagnoses were based on claims data (ICD-10 codes). However, the definitions we used in this study were validated in several previous studies [21, 22]. Further replicative research is warranted to validate and elucidate our results’ underlying mechanisms.
Conclusions
Lean or diabetic MAFLD showed a poor prognosis for all-cause and disease-specific mortality. Classifying MAFLD subgroups based on metabolic phenotypes that account for integrated metabolic disorders might help risk stratification of patients with MAFLD.
Availability of data and materials
The dataset (NHIS) supporting the conclusions of this article is available in the homepage of National Health Insurance Sharing Service [http://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do]. To gain access to the data, a completed application form, a research proposal, and the applicant’s approval document from the institutional review board should be submitted to and reviewed by the inquiry committee of research support in NHIS. Currently, the use of NHIS data is allowed only for Korean researchers.
Abbreviations
- BMI:
-
Body mass index
- CCI:
-
Charlson Comorbidity Index
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular disease
- DM:
-
Diabetes mellitus
- eGFR:
-
Estimated glomerular filtration rate
- FLI:
-
Fatty liver index
- GGT:
-
Gamma-glutamyl transferase
- HR:
-
Hazard ratio
- ICD-10:
-
International Classification of Diseases, 10th revision
- MAFLD:
-
Metabolic (dysfunction)-associated fatty liver disease
- NAFLD:
-
Nonalcoholic fatty liver disease
- NHIS:
-
National Health Insurance System
- WC:
-
Waist circumference
References
Eslam M, Sanyal AJ, George J, International Consensus Panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999-20141 e1.
Kang SH, Cho Y, Jeong SW, et al. From nonalcoholic fatty liver disease to metabolic-associated fatty liver disease: Big wave or ripple? Clin Mol Hepatol. 2021;27:257–69.
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–9.
Lee H, Lee YH, Kim SU, Kim HC. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: a nationwide cohort study. Clin Gastroenterol Hepatol. 2021;19:2138-2147 e10.
Niriella MA, Ediriweera DS, Kasturiratne A, De Silva ST, Dassanayaka AS, De Silva AP, et al. Outcomes of NAFLD and MAFLD: results from a community-based, prospective cohort study. PLoS ONE. 2021;16:e0245762.
Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential clinical characteristics and mortality outcomes in persons with NAFLD and/or MAFLD. Clin Gastroenterol Hepatol. 2021;19:2172–81.
Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. 2021;75:1284–91.
Huang Q, Zou X, Wen X, Zhou X, Ji L. NAFLD or MAFLD: Which has closer association with all-cause and cause-specific mortality?-Results from NHANES III. Front Med (Lausanne). 2021;8:693507.
Younossi ZM, Rinella ME, Sanyal AJ, Harrison SA, Brunt EM, Goodman Z, et al. From NAFLD to MAFLD: implications of a premature change in terminology. Hepatology. 2021;73:1194–8.
Moon JH, Kim W, Koo BK, Cho NH, Innovative Target Exploration of NAFLD (ITEN) consortium. Metabolic dysfunction-associated fatty liver disease predicts long-term mortality and cardiovascular disease. Gut Liver. 2022;16:433–42.
Tsutsumi T, Eslam M, Kawaguchi T, Yamamura S, Kawaguchi A, Nakano D, et al. MAFLD better predicts the progression of atherosclerotic cardiovascular risk than NAFLD: generalized estimating equation approach. Hepatol Res. 2021;51:1115–28.
Sohn W, Kwon HJ, Chang Y, Ryu S, Cho YK. Liver fibrosis in asians with metabolic dysfunction-associated fatty liver disease. Clin Gastroenterol Hepatol. 2022;20:e1135–48.
Iacobini C, Pugliese G, Blasetti Fantauzzi C, Federici M, Menini S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism. 2019;92:51–60.
Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC). South Korea Int J Epidemiol. 2017;46:e15.
Shin DW, Cho J, Park JH, Cho B. National General Health Screening Program in Korea: history, current status, and future direction. Precis Future Med. 2022;6:9–31.
Chung GE, Cho EJ, Yoon JW, Yoo JJ, Chang Y, Cho Y, et al. Nonalcoholic fatty liver disease increases the risk of diabetes in young adults: a nationwide population-based study in Korea. Metabolism. 2021;123:154866.
Chung GE, Cho EJ, Yoo JJ, Chang Y, Cho Y, Park SH, et al. Nonalcoholic fatty liver disease is associated with the development of obstructive sleep apnea. Sci Rep. 2021;11:13473.
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609.
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33.
Lee H, Lim TS, Kim SU, et al. Long-term cardiovascular outcomes differ across metabolic dysfunction-associated fatty liver disease subtypes among middle-aged population. Hepatol Int. 2022;16:1308–17.
Kim YS, Park YM, Han KD, Yun JS, Ahn YB, Ko SH. Fasting glucose level and all-cause or cause-specific mortality in Korean adults: a nationwide cohort study. Korean J Intern Med. 2021;36:647–58.
Yang Y, Han K, Park SH, Kim MK, Yoon KH, et al. High-density lipoprotein cholesterol and the risk of myocardial infarction, stroke, and cause-specific mortality: A nationwide cohort study in Korea. J Lipid Atheroscler. 2021;10:74–87.
Cho Y, Cho EJ, Yoo JJ, Chang Y, Chung GE, Jeong SM, et al. Association between lipid profiles and the incidence of hepatocellular carcinoma: a nationwide population-based study. Cancers (Basel). 2021;13:1599.
Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–82.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Wu J, Tian S, Li H, Xu Z, Li S, Chen YL, et al. Population-specific cut-off points of fatty liver index: a study based on the national health and nutrition examination survey data. BMC Gastroenterol. 2022;22:265.
Cho EJ, Jung GC, Kwak MS, Yang JI, Yim JY, Yu SJ, et al. Fatty liver index for predicting nonalcoholic fatty liver disease in an asymptomatic Korean population. Diagnostics (Basel). 2021;11:2233.
Poznyak A, Grechko AV, Poggio P, et al. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. 2020;21:1835. https://pubmed.ncbi.nlm.nih.gov/32155866/.
Wojciechowska J, Krajewski W, Bolanowski M, et al. Diabetes and cancer: a review of current knowledge. Exp Clin Endocrinol Diabetes. 2016;124:263–75.
Yang JJ, Yu D, Wen W, et al. Association of diabetes with all-cause and cause-specific mortality in Asia: a pooled analysis of more than 1 million participants. JAMA Netw Open. 2019;2:e192696.
Tseng CH. Mortality and causes of death in a national sample of diabetic patients in Taiwan. Diabetes Care. 2004;27:1605–9.
Kang YM, Kim YJ, Park JY, et al. Mortality and causes of death in a national sample of type 2 diabetic patients in Korea from 2002 to 2013. Cardiovasc Diabetol. 2016;15:131.
Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:739–52.
Das K, Chowdhury A. Lean NASH: distinctiveness and clinical implication. Hepatol Int. 2013;7(Suppl 2):806–13.
Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: a long-term follow-up study. Hepatol Commun. 2018;2:48–57.
Ahmed OT, Gidener T, Mara KC, Larson JJ, Therneau TM, Allen AM. Natural history of nonalcoholic fatty liver disease with normal body mass index: a population-based study. Clin Gastroenterol Hepatol. 2021;S1542–3565(21):00747–53.
Younes R, Govaere O, Petta S, Miele L, Tiniakos D, Burt A, et al. Caucasian lean subjects with non-alcoholic fatty liver disease share long-term prognosis of non-lean: time for reappraisal of BMI-driven approach? Gut. 2022;71:382–90.
Maier S, Wieland A, Cree-Green M, Nadeau K, Sullivan S, Lanaspa MA, et al. Lean NAFLD: an underrecognized and challenging disorder in medicine. Rev Endocr Metab Disord. 2021;22:351–66.
Sookoian S, Pirola CJ. Systematic review with meta-analysis: risk factors for non-alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment Pharmacol Ther. 2017;46:85–95.
Xu R, Pan J, Zhou W, Ji G, Dang Y. Recent advances in lean NAFLD. Biomed Pharmacother. 2022;153:113331.
Buzova D, Maugeri A, Liguori A, Napodano C, Lo Re O, Oben J, et al. Circulating histone signature of human lean metabolic-associated fatty liver disease (MAFLD). Clin Epigenetics. 2020;12:126.
Cheng YM, Kao JH, Wang CC. The metabolic profiles and body composition of lean metabolic associated fatty liver disease. Hepatol Int. 2021;15:405–12.
Negi CK, Babica P, Bajard L, Bienertova-Vasku J, Tarantino G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metabolism. 2022;126:154925.
Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–24.
Kuchay MS, Martínez-Montoro JI, Choudhary NS, Fernández-García JC, Ramos-Molina B. Non-alcoholic fatty liver disease in lean and non-obese individuals: current and future challenges. Biomedicines. 2021;9:1346.
Lavie CJ, Sharma A, Alpert MA, De Schutter A, Lopez-Jimenez F, Milani RV, et al. Update on obesity and obesity paradox in heart failure. Prog Cardiovasc Dis. 2016;58:393–400.
Lavie CJ, Milani RV, Artham SM, Patel DA, Ventura HO. The obesity paradox, weight loss, and coronary disease. Am J Med. 2009;122:1106–14.
Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep. 2016;18:56.
Park Y, Peterson LL, Colditz GA. The plausibility of obesity paradox in cancer-point. Cancer Res. 2018;78:1898–903.
Han SJ, Boyko EJ. The evidence for an obesity paradox in type 2 diabetes mellitus. Diabetes Metab J. 2018;42:179–87.
Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, et al. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308:581–90.
Tobias DK, Pan A, Jackson CL, O’Reilly EJ, Ding EL, Willett WC, et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370:233–44.
Fedchuk L, Nascimbeni F, Pais R, Charlotte F, Housset C, Ratziu V, et al. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40:1209–22.
Chung GE, Jeong SM, Cho EJ, Yoo JJ, Cho Y, Lee KN, et al. Association of fatty liver index with all-cause and disease-specific mortality: A nationwide cohort study. Metabolism. 2022;133:155222.
Xu Z, Li H, Tian S, et al. Blood biomarkers for the diagnosis of hepatic steatosis in metabolic dysfunction-associated fatty liver disease. J Hepatol. 2020;73:1264–5.
Acknowledgements
This study was performed using a database from the Korean National Health Insurance System (NHIS).
Funding
This work was supported in part by grants from the Seoul National University Hospital (04–2022-3140 and 30–2022-0340) and the Liver Research Foundation of Korea (Bio Future Strategies Research Project).
Author information
Authors and Affiliations
Contributions
The corresponding authors (EJC and KH) had full access to all study data in the study and took responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: EJC and KH. Acquisition, analysis: GEC, SJY, J-JY, YC, KH, KNL, and DWS. Interpretation of data: GEC, SJY, YC, KNL, DWS, DK, YJK, and J-HY. Manuscript writing: GEC, SJY, and EJC. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This was a retrospective study with clinical data collection, and the Institutional Review Board of Soongsil University (SSU-202007-HR-236–01) approved to waive the patient consent.
Consent for publication
All the authors consented to publish work.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
All-cause and cause-specific mortality by MAFLD subgroup. Table S2. All-cause and cause-specific mortality by MAFLD subgroup with sex-specific FLI cut-offs. Table S3. Baseline characteristics of the study population with aged ≥60 years. Table S4. All-cause and cause-specific mortality by MAFLD subgroup in individuals with aged ≥60 years.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chung, G.E., Yu, S.J., Yoo, JJ. et al. Lean or diabetic subtypes predict increased all-cause and disease-specific mortality in metabolic-associated fatty liver disease. BMC Med 21, 4 (2023). https://doi.org/10.1186/s12916-022-02716-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02716-3